Abstract
PURPOSE
Low amplitude rhythmic contractions (LARC) occur in detrusor smooth muscle and may play a role in storage disorders such as overactive bladder (OAB) and detrusor overactivity (DO). The purpose of this study was to determine whether LARC frequencies identified in vitro from strips of human urinary bladder tissue correlate with in vivo LARC frequencies, visualized as phasic intravesical pressure (pves) waves during urodynamics (UD).
METHODS
After IRB approval, fresh strips of human urinary bladder were obtained from patients. LARC was recorded with tissue strips at low tension (<2g) and analyzed by fast Fourier transform (FFT) to identify LARC signal frequencies. Blinded UD tracings were retrospectively reviewed for signs of LARC on the pves tracing during filling and were analyzed via FFT.
RESULTS
Distinct LARC frequencies were identified in 100% of tissue strips (n=9) obtained with a mean frequency of 1.97±0.47 cycles/min (33±8 mHz). Out of 100 consecutive UD studies reviewed, 35 visually displayed phasic pves waves. In 12/35 (34%), real pves signals were present that were independent of abdominal activity. Average UD LARC frequency was 2.34±0.36 cycles/min (39±6 mHz) which was similar to tissue LARC frequencies (p=0.50). A majority (83%) of the UD cohort with LARC signals also demonstrated detrusor overactivity.
CONCLUSIONS
During UD, a subset of patients displayed phasic pves waves with a distinct rhythmic frequency similar to the in vitro LARC frequency quantified in human urinary bladder tissue strips. Further refinements of this technique may help identify subsets of individuals with LARC–mediated storage disorders.
Keywords: Fourier analysis, smooth muscle, urinary bladder, overactive
INTRODUCTION
Low amplitude rhythmic contractions (LARC) are observed during the filling phase of micturition, characterized as phasic contractions having lower amplitude than observed with electrical or potassium stimuli [1]. LARC occur in mammalian detrusor smooth muscle (DSM), including human [1–4], although their function and exact stimulus (or stimuli) remain to be elucidated [5]. One suggested functional role of LARC is to maintain preload (bladder tone), allowing for active detrusor contraction at a continuum of urinary bladder volumes [1,6]. In this regard, LARC may provide a mechanism through which the bladder is primed to empty completely at any volume.
The human urinary bladder consists mainly of DSM which participates in complex signaling pathways between nerves, interstitial cells (IC) and the urothelium [7,8]. IC in the lower urinary tract of mammals demonstrate spontaneous periodic calcium transients that may generate or modulate LARC [9,10]. Additionally, DSM cells are able to myogenically generate spontaneous activity and the frequency of this activity is increased in DSM samples taken from patients with overactive bladder syndrome (OAB) [11]. Previous studies demonstrate an increase in bladder micromotion in patients with urinary urgency and LARC with increased frequency and amplitude has been observed in vitro in urinary bladder tissue strips obtained from patients with detrusor overactivity, thereby suggesting dysregulation of LARC in patients with OAB [3,12].
LARC can be represented mathematically as a complex signal with periodic changes in amplitude. Fast Fourier transforms (FFT) process a time-dependent signal and outputs the power density spectrum and additional analysis allows for discrimination of real signals from background noise. FFT has been used previously to analyze LARC observed in rabbit and mouse DSM and in phasic pressure waves in ex vivo pig bladders [2,13,14].
A quantitative correlation of in vitro human urinary bladder LARC and LARC observed during human filling cystometry has never been performed. In the present study we aim to identify and characterize LARC frequencies from isolated strips of full thickness (urothelium, lamina propria and DSM) human urinary bladder tissue via FFT analysis. Furthermore, we aim to identify LARC frequencies evidenced by phasic intravesical pressure (pves) waves during in vivo human urodynamics (UD) via FFT and correlate these frequencies with LARC identified from human bladder tissue strip analysis. We propose that FFT may prove to be a useful tool in characterizing subtypes of storage disorders mediated by LARC.
MATERIAL AND METHODS
Human tissue preparation
All experiments involving human bladders were approved by the institutional review boards at Virginia Commonwealth University and the Hunter Holmes McGuire Veterans Affairs Medical Center. Human urinary bladder tissue was obtained from adults 21 years and older undergoing cystectomy or other open bladder operation for indicated clinical conditions. No patient who provided tissue for the study had UD performed. Chart review was performed at time of tissue harvest to document use of antimuscarinic agents approved for OAB. Tissue specimens were transported on ice immediately after resection to the surgical pathologist who provided tissue that was deemed discardable. A full-thickness section of supratrigonal tissue, as near as possible to the dome and not adjacent to visible tumor (if present), was cut by the surgical pathologist. The tissue was then placed in cold (0–4 °C) Dulbecco’s modified Eagle’s medium/F12 (DMEM) nutrient mixture (HyClone®, Thermo Scientific) or Krebs buffer for transport. Thin strips (approximately 1–2 mm thick) of human bladder tissue were cut and attached by small clips to a micrometer and force transducer (159901a, Radnoti Glass Technology, Inc). Tissues were then submerged in a 30mL water-jacketed, aerated (95%/5% O2/CO2) tissue bath containing 37°C DMEM or Krebs buffer at a slack length of 3mm and stretched to induce low tension (<2g, or 19mN). Tissues were equilibrated for 15–30 minutes to allow LARC development.
Human tissue data analysis
Voltage signals from isometric force transducers were digitized (NI USB-6341, National Instruments) with data acquisition occurring at 10Hz using multichannel data-integration software (DASYLab ®, National Instruments). A segment of 4096 consecutive data points of steady state LARC tension data were normalized to the maximum tension during this time (example tracing, Figure 1A). A Hanning window was applied to filter out very low frequency signal (i.e. stress relaxation) and prevent discontinuity artifact during FFT analysis. FFT was then performed using Excel Analysis ToolPak (Microsoft Corp). Frequency domain output was in ~2.4mHz intervals. A frequency range of interest (0.005–0.200 Hz or 0.3–12 cycles/min) was determined by preliminary data of carbachol-induced LARC in human DSM (not shown) and prior studies of DSM LARC in both humans and other species [2,1,15–18]. A real signal was defined as having an associated amplitude on the power spectrum which was the maximum as well as being greater than 2 standard deviations (SD) above the mean amplitude over the aforementioned frequency range (example spectrum, Figure 1B).
Figure 1.
A Example of human urinary bladder tissue strip LARC B Power density spectrum of signal in A after FFT. Real signal (arrow) has an associated amplitude which is both the maximum and greater than two standard deviations above the average amplitude of the spectrum of interest.
UD data analysis
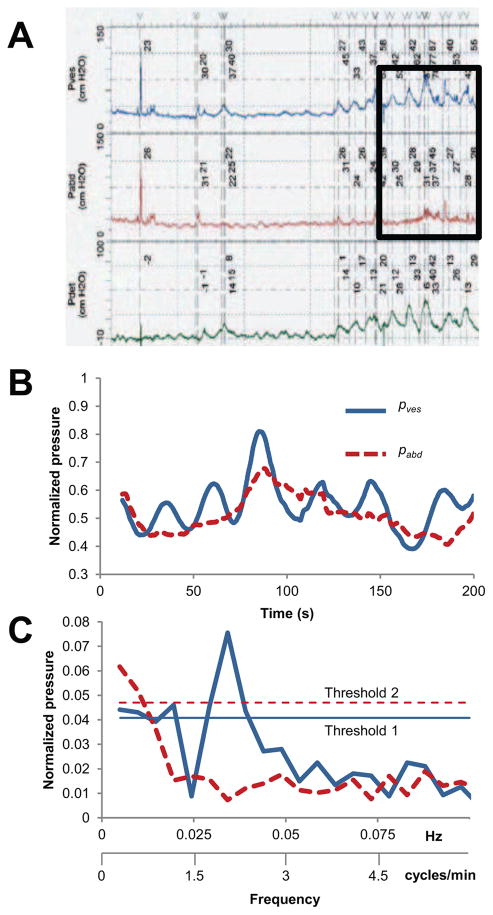
All UD studies were performed by a trained nurse urodynamicist in compliance with best practice guidelines [19]. As a standardized control measure, a cough test was performed at the beginning of each UD study to confirm an equal pressure rise (within 10cm H20) in both the bladder (pves) and abdominal (pabd) pressures. Multichannel digital pressure and flow data acquisition was performed at 10 Hz via an Aquarius TT™ system (Laborie, Toronto). Blinded multichannel digital data from the 100 most recently performed consecutive UD studies was imported into Excel for retrospective analysis directly from the UD computer. Only measured pressures (pves and pabd) were analyzed due to potential confounding from pdet which has been shown in previous publications[20]. Studies with a constant (pabd) value suggesting that a rectal or vaginal catheter was not used were excluded. Also, studies with a phasic pabd activity (suggesting rhythmic rectal contractions) were excluded from analysis. Tracings were visually inspected for phasic pves as signs of LARC (example, Figure 2A), and if present, further analysis was conducted. Next, pves and pabd were normalized to their respective maximum recorded pressures during 2048 or 4096 consecutive data points of filling where LARC was visualized without provocative maneuvers or voiding/incontinent episodes (example, Figure 2B). FFT was then performed on both pves and pabd to identify the real signals in the range of 0.010–0.100 Hz (0.6–6 cycles/min). This frequency range was chosen because of overlap with the human urinary bladder tissue strip LARC frequency range but remained below any physiologic respiratory rate or heart rate as well as UD pump cycling rate which may cause artifact on pressure tracings. Two thresholds were conservatively set and both were required to identify a real pves signal seen on FFT power spectrum thought to be originating from LARC (example spectrum, Figure 2C). Threshold 1: the pves signal must have an associated amplitude greater than 2 Standard Deviations (SD) above the mean normalized pves amplitude over the analyzed frequency range. Threshold 2: the pves signal must have an associated amplitude greater than 3 SD above the mean normalized pabd amplitude over the analyzed frequency range to be considered exclusive of any intra-abdominal process. A higher threshold (3 SD) was used in human UD studies as compared to human urinary bladder tissue strip studies (2 SD) to account for increased artifact that is inherent during in vivo human studies. After all data analysis, UD studies were un-blinded to collect demographic data.
Figure 2.
A Example of UD LARC B Superimposed pves and pabd from UD tracing in A (inset) demonstrating periodic frequency in pves not present in pabd. C Power density spectrum of signals in B after FFT demonstrating real signal in pves. The real signal in pves has an associated amplitude greater than both predefined thresholds (see Methods).
Statistical analysis
Human tissue LARC signal frequencies were compared to UD LARC signal frequencies using a two-tailed Student’s t-test with p<0.05 being considered statistically significant. Frequency values are reported as means ± standard error.
RESULTS
Human tissue
Urinary bladder tissue strip data were obtained from 9 patients. Average age was 60±5 years and 78% were male. Urothelial carcinoma was the underlying pathology in 6/9 of tissue samples. No patients had indwelling catheters or performed intermittent catheterization. Antimuscarinc use was noted in 2/9 patients (22%). All tissue samples obtained demonstrated LARC and mean LARC signal frequency identified after FFT signal analysis was 0.033±0.008 Hz (1.97±0.47 cycles/min). Power spectrum analysis performed on tension data obtained from the experimental apparatus without tissue confirmed that all LARC signal amplitudes were greater than two standard deviations above the average amplitude of any inherent noise. Urodynamics had not been performed on any of the nine patients providing tissue for analysis.
UD
Of the 100 blinded UD studies obtained, 9 were excluded due to corrupted files which could not be imported. Of the remaining studies, 56 (62%) did not demonstrate any visual signs of LARC on the pves tracing (vLARC−). Thirty five studies (38%) demonstrated visual LARC as a phasic pves tracing during filling cystometry (vLARC+) and were analyzed via FFT. Patient characteristics for these two groups are listed in Table 1. A significantly higher proportion of vLARC+ patients did not void spontaneously and performed intermittent catheterization. Of vLARC+ patients, 12/35 (34%) had pves signals on FFT analysis that were exclusive of any pabd signal. The majority of these patients (83%) with real pves signals on FFT analysis also had evidence of detrusor overactivity with 33% being neurogenic and 50% being idiopathic. Mean pves signal frequency on analysis was 0.039±0.006 Hz (2.34±0.36 cycles/min) which was not significantly different compared to the mean pves signal frequency observed in the in vitro human tissues (p=0.50).
Table I.
UD Patient Characteristics
| vLARC+ | vLARC− | p | |
|---|---|---|---|
| N | 35 | 56 | |
| Male (%) | 16 (46) | 23 (41) | 0.67 |
| Female (%) | 19 (54) | 33 (49) | 0.11 |
| Age, years | 42±4 | 34±4 | 0.20 |
| Neurologic injury (%) | 19 (54) | 29 (52) | |
| Indication for UD (%) | |||
| Retention | 11 (31) | 7 (13) | 0.03* |
| Irritative symptoms | 4 (11) | 1 (2) | 0.07 |
| Incontinence | 8 (23) | 24 (43) | 0.07 |
| Neurogenic surveillance | 5 (14) | 12 (21) | 0.58 |
| Other/unknown | 7 (20) | 12 (21) | 0.79 |
| Bladder management (%) | |||
| Spontaneous void | 16 (46) | 39 (70) | 0.03* |
| CIC | 13 (37) | 7 (12) | 0.01* |
| Indwelling catheter | 1 (3) | 5 (9) | 0.40 |
| Unknown | 5 (14) | 5 (9) | 0.50 |
| Detrusor overactivity (%) | 18 (51) | 17 (30) | 0.05 |
| Neurogenic | 12 (34) | 11 (19) | 0.14 |
| Idiopathic | 6 (17) | 6 (11) | 0.53 |
v = visual, LARC = Low Amplitude Rythmic Contractions, + = positive, − = negative, UD =Urodynamics, CIC = clean intermittent catheterization.
= statistically significant finding.
DISCUSSION
The present study reveals for the first time that the phasic pves frequency measured in vivo during UD filling cystometry in human subjects is similar to the LARC frequency measured in vitro generated by isolated strips of human bladder tissue. Analysis of UD LARC frequencies showed that a majority of the patients identified with real phasic pves signals had detrusor overactivity. Recognizing that all DSM strips in our study were observed to have an identifiable LARC frequency, we hypothesize that a subset of patients with detrusor overactivity may have an amplified LARC (higher amplitude but similar baseline frequency).
Our findings suggest that this phasic activity in pves may be due to LARC originating from elements within the detrusor. Potential physiological roles for LARC in acute length adaptation and adjustable preload have been identified in rabbit DSM [6,21]. Length adaptation and adjustable preload have also been identified in the human detrusor, and the clinical correlate of adjustable preload, dynamic elasticity, has been identified during human urodynamics [20,22]. Thus, LARC could function as an intrinsic regulator of bladder tension. Because the bladder wall is effectively in-series with pelvic sensory nerves [23], increased tension during filling generated by higher amplitude or faster frequencies of LARC could illicit increased urgency.
In addition, the identification of a unique frequency of approximately 33 mHz (2 cycles/min) observed both in vitro in human urinary bladder tissue strips and in vivo in human UD studies suggests that a LARC pacemaker may arise from within the bladder itself. Several investigators have shown that IC exist within DSM bundles of various mammalian species [7–9]. Previous work with rabbit DSM suggests that cyclooxygenase-mediated signaling between IC and adjacent DSM bundles may play a role in the generation of LARC via prostaglandin signaling with these findings supporting the fact that increased prostaglandin levels are seen in patients with OAB-type symptoms [24]. Others have shown that urinary bladder IC generate calcium transients that have similar frequencies to those observed in the current study suggesting IC within the bladder may be a potential source for an intrinsic bladder pacemaker [9,10].
Periodic fluctuations in pves have been described previously as phasic detrusor overactivity. Even at low amplitudes, these phasic pressure waves can illicit patient symptoms [25]. The predominant UD LARC frequencies seen in this study are lower than other physiologic periodic pressure fluctuations (aside from rectal contractions) that could have provided artifact during the study such as respiratory rate (18/min = 300 mHz), heart rate (70/min = 1.2 Hz) or non-physiologic rates from bladder filling by UD pump (>100 cycles/min for 40 mL/s fill rate = >1.7 Hz). Frequency ranges for analysis were consistent with prior published studies [1,2,15–18] and an initial analysis of all FFT data from both human urinary bladder tissue strip LARC and UD LARC showed noise only above the frequency ranges used for analysis (not shown), ensuring that relevant frequencies were not omitted. However, lack of literature demonstrating quantitative analysis of LARC during UD may reflect difficulty in separating signal from noise. Using selective criteria and high exclusion thresholds may have provided the ability to discern these signals in our series.
Studies of LARC have been previously performed in animal models and these studies tempt speculation that alterations in LARC may cause or worsen overactive bladder symptoms [23,2,26]. Prior in vitro animal models have demonstrated that LARC directly causes an increase in detrusor tension [6]. Because DSM is in-series with mechanoreceptors coupled to Aδ afferent nerve fibers, increased detrusor tension (generated by LARC) may contribute to the sensation of urgency. Therefore, it is imperative to develop techniques to objectively analyze LARC during in vivo human UD studies to diagnose patients that lack visibly obvious pves fluctuations.
The current study is limited by the fact that the majority of tissue used was from patients with known pathologies. However, one bladder tissue specimen, obtained from a healthy 27 year-old during organ harvest, exhibited LARC at a similar frequency to the remainder of the cohort. Furthermore, the use of retrospective UD data makes interpretation more difficult. However, we have accounted for some of these issues by performing analysis in a blinded fashion and describing a detailed selection process for data analysis. We have also begun prospectively collecting LARC data during UD in which an extended 5-minute hold period is employed at defined percentages of bladder capacity. Future research will hopefully define normal ranges for LARC frequency and amplitude with development of computerized algorithms to integrate FFT analysis into UD. Further investigation into LARC and its regulation may ultimately lead to the identification of storage disorders mediated by LARC.
CONCLUSIONS
LARC can be identified and characterized by FFT during in vivo UD studies. The LARC frequency identified in vitro from human DSM was found to be similar to the LARC frequency identified during in vivo UD studies evidenced by phasic pves waves. Refinements of FFT as a means to objectively analyze signals during UD represents an important step toward characterization of storage disorders mediated by LARC. Future studies of LARC may lead to a better mechanistic understanding of storage disorders and other forms of voiding dysfunction.
Acknowledgments
FUNDING
Research funding for this study was provided by the Virginia Commonwealth University Presidential Research Quest Fund and National Institute of Health grant R01DK101719
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All experiments involving human bladder tissue were approved by the institutional review boards at Virginia Commonwealth University and the Hunter Holmes McGuire Veterans Affairs Medical Center. Informed consent was obtained from all patients involved in this study.
Author’s Contribution:
AF Colhoun: protocol/project development, data collection, data analysis, manuscript writing/editing
JE Speich: protocol/project development, data analysis, manuscript writing/editing
LF Cooley: data collection, data analysis
ED Bell: data collection
RW Barbee: manuscript writing/editing
G Guruli: data collection
PH Ratz: protocol/project development, manuscript writing/editing
AP Klausner: protocol/project development, data analysis, manuscript writing/editing
Conflicts of Interest: Drs. Speich and Klausner report funding from the National Institutes of Health. Drs. Colhoun, Cooley, Bell, Barbee, Guruli and Ratz have no disclosures.
References
- 1.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. The Journal of physiology. 2006;570(Pt 1):13–22. doi: 10.1113/jphysiol.2005.097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne MD, Klausner AP, Speich JE, Southern JB, Habibi JR, Ratz PH. Fourier transform analysis of rabbit detrusor autonomous contractions reveals length dependent increases in tone and slow wave development at long lengths. J Urol. 2013;190(1):334–340. doi: 10.1016/j.juro.2013.02.071S0022-5347(13)00347-9. [pii] [DOI] [PubMed] [Google Scholar]
- 3.Drake MJ, Harvey IJ, Gillespie JI, Van Duyl WA. Localized contractions in the normal human bladder and in urinary urgency. BJU international. 2005;95(7):1002–1005. doi: 10.1111/j.1464-410X.2005.05455.x. [DOI] [PubMed] [Google Scholar]
- 4.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. British journal of pharmacology. 2003;140(1):159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourology and urodynamics. 2010;29(1):97–106. doi: 10.1002/nau.20784. [DOI] [PubMed] [Google Scholar]
- 6.Almasri AM, Ratz PH, Bhatia H, Klausner AP, Speich JE. Rhythmic contraction generates adjustable passive stiffness in rabbit detrusor. Journal of applied physiology. 2010;108(3):544–553. doi: 10.1152/japplphysiol.01079.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birder L, Andersson KE. Urothelial signaling. Physiological reviews. 2013;93(2):653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCloskey KD. Bladder interstitial cells: an updated review of current knowledge. Acta physiologica. 2013;207(1):7–15. doi: 10.1111/apha.12009. [DOI] [PubMed] [Google Scholar]
- 9.Hashitani H, Lang RJ. Functions of ICC-like cells in the urinary tract and male genital organs. Journal of cellular and molecular medicine. 2010;14(6A):1199–1211. doi: 10.1111/j.1582-4934.2010.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray SM, McGeown JG, McMurray G, McCloskey KD. Functional innervation of Guinea-pig bladder interstitial cells of cajal subtypes: neurogenic stimulation evokes in situ calcium transients. PloS one. 2013;8(1):e53423. doi: 10.1371/journal.pone.0053423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui G, Fry CH, Malone-Lee J, Wu C. Aberrant Ca2+ oscillations in smooth muscle cells from overactive human bladders. Cell calcium. 2009;45(5):456–464. doi: 10.1016/j.ceca.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Kinder RB, Mundy AR. Pathophysiology of idiopathic detrusor instability and detrusor hyper-reflexia. An in vitro study of human detrusor muscle. British journal of urology. 1987;60(6):509–515. doi: 10.1111/j.1464-410x.1987.tb05031.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith PP, DeAngelis A, Simon R. Evidence of increased centrally enhanced bladder compliance with ageing in a mouse model. BJU international. 2015;115(2):322–329. doi: 10.1111/bju.12669. [DOI] [PubMed] [Google Scholar]
- 14.Lentle RG, Reynolds GW, Janssen PW, Hulls CM, King QM, Chambers JP. Characterisation of the contractile dynamics of the resting ex vivo urinary bladder of the pig. BJU Int. 2015;116(6):973–983. doi: 10.1111/bju.13132. [DOI] [PubMed] [Google Scholar]
- 15.Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285(4):R809–816. doi: 10.1152/ajpregu.00641.2002. [DOI] [PubMed] [Google Scholar]
- 16.Potjer RM, Constantinou CE. Frequency of spontaneous contractions in longitudinal and transverse bladder strips. The American journal of physiology. 1989;257(4 Pt 2):R781–787. doi: 10.1152/ajpregu.1989.257.4.R781. [DOI] [PubMed] [Google Scholar]
- 17.Sibley GN. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. The Journal of physiology. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. British journal of pharmacology. 2002;135(3):639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winters JC, Dmochowski RR, Goldman HB, Herndon CD, Kobashi KC, Kraus SR, Lemack GE, Nitti VW, Rovner ES, Wein AJ American Urological A, Society of Urodynamics FPM Urogenital R. Urodynamic studies in adults: AUA/SUFU guideline. The Journal of urology. 2012;188(6 Suppl):2464–2472. doi: 10.1016/j.juro.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 20.Colhoun AF, Klausner AP, Nagle AS, Carroll AW, Barbee RW, Ratz PH, Speich JE. A pilot study to measure dynamic elasticity of the bladder during urodynamics. Neurourology and urodynamics. 2016 doi: 10.1002/nau.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speich JE, Wilson CW, Almasri AM, Southern JB, Klausner AP, Ratz PH. Carbachol-induced volume adaptation in mouse bladder and length adaptation via rhythmic contraction in rabbit detrusor. Ann Biomed Eng. 2012;40(10):2266–2276. doi: 10.1007/s10439-012-0590-8. [DOI] [PubMed] [Google Scholar]
- 22.Colhoun AF, Speich JE, Dolat MT, Habibi JR, Guruli G, Ratz PH, Barbee RW, Klausner AP. Acute length adaptation and adjustable preload in the human detrusor. Neurourology and urodynamics. 2015 doi: 10.1002/nau.22820. [DOI] [PubMed] [Google Scholar]
- 23.De Wachter S. Afferent signaling from the bladder: species differences evident from extracellular recordings of pelvic and hypogastric nerves. Neurourology and urodynamics. 2011;30(5):647–652. doi: 10.1002/nau.21135. [DOI] [PubMed] [Google Scholar]
- 24.Rahnama’i MS, Van Koeveringe GA, Van Kerrebroeck PE. Overactive bladder syndrome and the potential role of prostaglandins and phosphodiesterases: an introduction. Nephro-urology monthly. 2013;5(4):934–945. doi: 10.5812/numonthly.14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrams P. Describing bladder storage function: overactive bladder syndrome and detrusor overactivity. Urology. 2003;62(5 Suppl 2):28–37. doi: 10.1016/j.urology.2003.09.050. discussion 40–22. [DOI] [PubMed] [Google Scholar]
- 26.Chacko S, Cortes E, Drake MJ, Fry CH. Does altered myogenic activity contribute to OAB symptoms from detrusor overactivity? ICI-RS 2013. Neurourology and urodynamics. 2014;33(5):577–580. doi: 10.1002/nau.22599. [DOI] [PubMed] [Google Scholar]