Abstract
BACKGROUND
Several studies have addressed short-term admission rates after bariatric surgery. However, studies on long-term admission rates are few and population based studies are even scarcer.
OBJECTIVE
The aim of this study was to assess short and long-term admission rates for gastrointestinal surgery after gastric bypass in Sweden compared to admission rates in the general population.
SETTING
Swedish healthcare system.
METHODS
The surgery cohort consisted of adults with Body Mass Index (BMI) ≥35 identified in the Scandinavian Obesity Surgery Registry (SOReg; n=28,331; mean age 41y; 76% women; Roux-en-Y gastric bypass performed 2007–2012). For each individual, up to 10 comparators from the general population were matched on birth year, sex, and place of residence (n=274,513). The primary outcome was inpatient admissions due to gastrointestinal surgery retrieved from the National Patient Register through December 31, 2014. Conditional hazard ratios (HR) were estimated using Cox regression.
RESULTS
All-cause admission rates were 6.5%, 21.4% and 65.9% during 30d, 1, and 6 years after surgery. The corresponding rates for gastrointestinal surgery were 1.8%, 6.8%, and 24.4%. Compared to the general population, there was an increased risk of all-cause hospital admission at 1 year (HR 2.6 [2.5–2.6]) and 6 years (HR 2.7 [2.6–2.7]). The risk of hospital admission for any gastrointestinal surgical procedure was greatly increased throughout the study period (HR 8.6 [8.4–8.9]). Female sex, psychiatric disease, and low education were risk factors.
CONCLUSION
We found a significant risk of admission to hospital over more than 6 years after gastric bypass surgery.
BACKGROUND
In light of the increasing number of bariatric surgical procedures performed world-wide, unplanned admissions place a large economic burden on health care systems. The 30-day admission rates after bariatric surgery have been reported to range between 0.6 to 11.3% [1–4]. The wide range reflects that the majority of studies are single-center and may not take into account admissions at other hospitals. In a recent study from New York State based on a longitudinal administrative database, the 30-day admission rate was 5.3% [5]. In the same study, the two-year risk of admission was 26%. Most patients were only admitted once, but a subgroup of individuals were admitted on several occasions. The existence of comorbid diseases was a risk factor for hospital admission, and patients who had undergone laparoscopic sleeve gastrectomy had the highest risk and frequency of admission [6]. However, another US study including some 35 000 patients showed higher 30-day admission rates after gastric bypass compared to sleeve gastrectomy [7].
When studying admission rates after surgical procedures, there is a need to take into account the underlying hospital admission rates for the whole population. Therefore, the aim of this study was to examine the short- and long-term risk of hospital admission for gastrointestinal surgery after gastric bypass in Sweden compared to the risk among matched general population comparators.
METHODS
This study was conducted using a register linkage based on the Scandinavian Obesity Surgery Register (SOReg) [8]. Using the Swedish personal identity number, unique for each resident, SOReg participants were linked to nationwide health registers, and comparators from the general population were identified and matched to the bariatric surgery patients.
The register linkage was approved by the regional ethics committee in Stockholm, Sweden. All analyses were conducted on de-identified data.
Study Population & Intervention
Between 2007 and 2012, 29,474 adults who underwent primary Roux-en-Y gastric bypass were included from SOReg, a nationwide, prospective quality of care register for bariatric surgery started in 2007. The register has been estimated to cover 98.5% of all bariatric surgery procedures in Sweden. Data are stored electronically and recorded as part of clinical practice.
Inclusion and Exclusion Criteria
There were no mandatory national eligibility criteria for bariatric surgery during the study period, although most counties used BMI ≥35 with or without obesity-related comorbidity. Adults ≥21 years of age with a BMI equal or greater than 35 were included.
For each patient who had bariatric surgery, up to 10 comparators from the general population identified via the Total Population Register [9] were matched by birth year, sex, and place of residence (county, municipality, and parish).
Covariates
Data were available on baseline BMI and HbA1c from SOReg but not for the general population comparators. Patients were categorized into euglycemic (HbA1c<5.7), prediabetes (HbA1c 5.7 to <6.5%) and diabetes (HbA1c≥6.5%) based on the American Diabetes Association guidelines[10].
Dispensed prescription drugs were retrieved from the nationwide Prescribed Drug Register and inpatient care admissions from the National Patient Register. The International Classification of Diseases (ICD) codes, NOMESCO surgical codes, and Anatomical Therapeutical Chemical (ATC) classification system codes used are provided in supplement tables (Supplementary table 1&2). Data on educational attainment were retrieved from Statistics Sweden.
Outcome & Follow-Up
Inpatient care admissions were retrieved from the nationwide National Patient Register until December 31, 2014. The primary outcome was inpatient admission for gastrointestinal surgery. As secondary outcomes, we analyzed admissions for gallstones and internal hernia. Patients were followed from the surgery date until admission, death, emigration, or end of follow-up, whichever came first. During follow-up, 163 participants emigrated making register-based follow-up complete for 99.4% (28 168/28 331).
Statistical Analysis
The risk of admission was analyzed using survival analysis both comparing gastric bypass patients with matched general population comparators, and within the gastric bypass group. For comparison versus matched general population comparators, conditional hazard ratios were conditioned on the matching set with each set containing 1 surgery patient and up to 10 general population comparators.
For analyses within the gastric bypass group, multivariable hazard ratios were estimated for age, sex, education, BMI, glycemic status, cardiovascular hospitalization, psychiatric hospitalization, other hospitalization, and year of surgery.
Statistical analyses were performed using SAS (version 9.4) and Stata (version 14). Two-sided P-values of <0.05 were considered to indicate statistical significance.
RESULTS
During the study period 2007–2012, a total of 28,331 patients where identified in SOReg having a primary gastric bypass procedure, of which more than 95% were performed laparoscopically. Up to 10 comparators from the general population were matched by birth year, sex, and place of residence (n=274,513). Basic patient and general population characteristics at baseline are presented in Table 1, including data on 1-year weight loss in the surgical cohort.
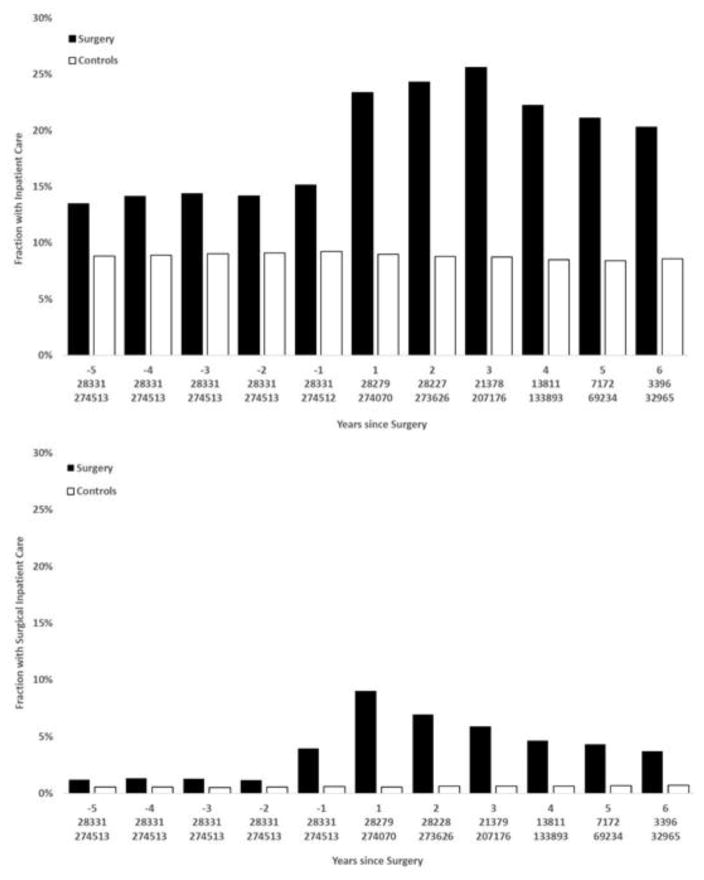
Median follow-up for any hospitalization was 2.5 years (range 0 to 8 years) and for gastrointestinal surgery 3.6 years (range 0 to 8 years; eTable 3). Figure 1 shows all-cause and gastrointestinal surgery related admissions by year, from 5 years preoperatively until 6 years postoperatively for the surgical and reference cohort. The surgical group had higher all-cause admission rates postoperatively than preoperatively, partly driven by gastrointestinal surgery (eFigures 1–3). Compared to matched general population comparators, surgery patients had consistently higher admission rates preoperatively and the gap increased further in the postoperative period. Admission for any gastrointestinal surgical procedure was substantially increased from one year preoperatively and throughout the follow up period.
Figure 1.
Admission to inpatientcare for any cause1(top panel) and inpatient care admissions due to gastrointestinal surgery in relation to time of bariatric surgery, and in matched controls from the general population2
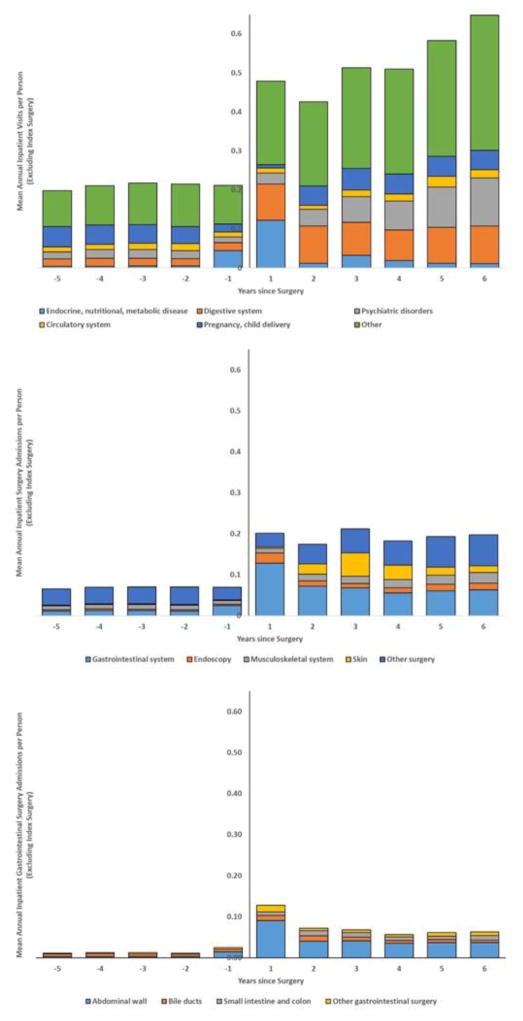
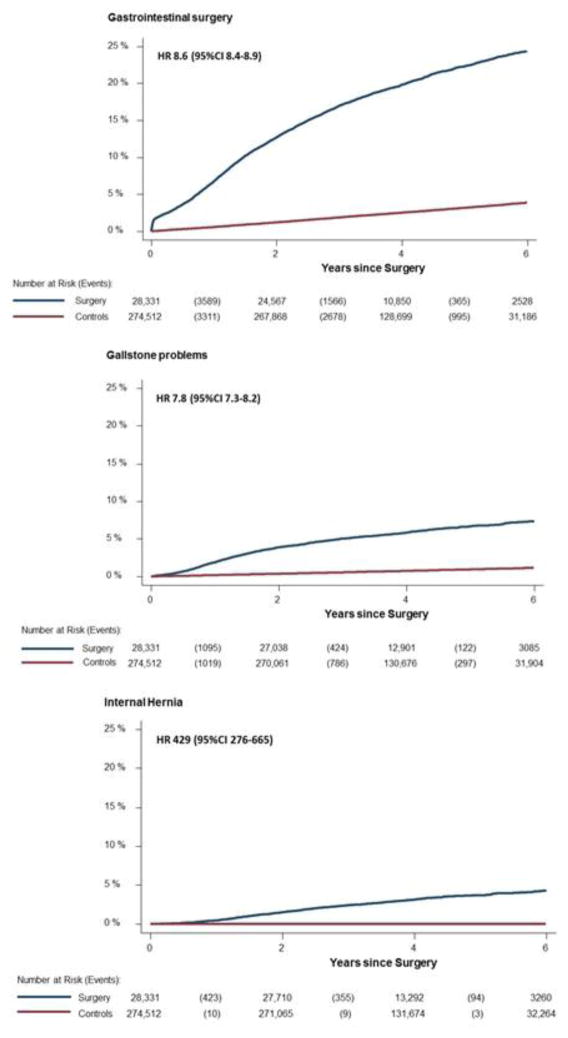
Admission rates for the surgical cohort for five major diagnostic groups and four major surgical groups are shown in Figure 2. A substantial increase is seen for admissions related to gastrointestinal and psychiatric diagnoses during the postoperative period. An increase is seen for gastrointestinal surgical procedures including endoscopy during the follow-up. Cumulative admissions during the postoperative period for admissions due to gastrointestinal surgery, gallstone problems, and internal hernia are presented in Figure 3. The risk of having any gastrointestinal surgery procedure was twelve times higher during the first postoperative year (6.8% vs 0.58%; HR 12.2 [95%CI 11.5–13.0]) in the bariatric group compared with the matched general population cohort. During the full observation period, there was an increased risk for gastrointestinal surgery admissions (24.4% vs 3.9%; HR 8.6 [95%CI 8.4–8.9]), admissions for gallstone problems (7.3% vs 1.2%; HR 7.8 [95%CI 7.3–8.2]), and internal hernia (4.3% vs 0.01%; HR 429 [95%CI 276–665]).
Figure 2.
Admissions to inpatient care for any cause (upper panel), due to any surgery (middle panel) and due to gastrointestinal surgery (lower panel) split into categories3,4
Figure 3.
Kaplan-Meier failure functions for admissions into inpatient care for gastrointestinal surgery, admissions listing gallstone, and admissions listing internal hernia. Controls are comparators sampled from the general population matched up to 10:1 for age, sex and place of residence.
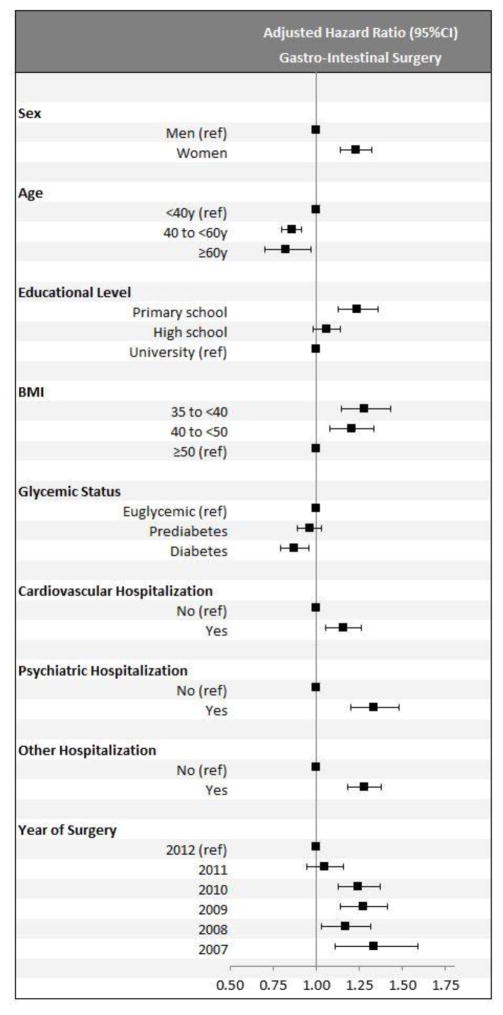
Figure 4 shows patient demographics and comorbid diseases as predictors of hospital admission due to gastrointestinal surgery for the gastric bypass cohort. In adjusted analysis, being a woman, having lower education, and a having a history of psychiatric inpatient care were independent and statistically significant risk factors.
Figure 4.
Adjusted hazard ratios for admission into inpatient care for gastrointestinal surgery after the index surgery (within-cohort analysis of patients undergoing gastric bypass; n=28,331)5
DISCUSSION
Bariatric surgery is today a common surgical procedure and considered as the main treatment option for treatment of morbid obesity when non-surgical interventions have failed. Postoperative admission rates are important since they place a burden on the health care system as well as stress on the patients.
Compared to other surgical procedures, bariatric surgery has admission rates in the midrange [11]. The 30-day admission rate for bariatric surgery was 5.3%, while ventral hernia repair was found to have a 30-day admission rate of 4.6%, compared to colectomy or proctectomy for which an admission rate of 11.1% was reported. The most common reason for admission in the bariatric surgical group was ileus or obstruction (24.5%). Clearly, it would be advantageous for the individual patient and society if some of these admissions could be avoided.
We investigated admission rates among Swedish patients having a gastric bypass procedure for morbid obesity compared with a sex, age, and place of residence matched sample from the general population. It would also be of interest to compare these two groups with an obese non-surgical cohort. In a previous study from the Swedish Obese Study group, inpatient care for any cause for the surgical patients were in the same range as found in our data, and the morbidly obese control group had significantly lower inpatient admission rates during the first years after surgery [12].
Studies on hospital admission after bariatric procedures often focus on admissions within 30-days [4, 5, 7], capturing most of the early postoperative complications, but missing potential later complications such as internal hernia and gallstone disease [13, 14]. A previous study has shown increased healthcare utilization during a 20-year period following bariatric surgery, but the majority of those patients had restrictive procedures such as vertical banded gastroplasty or gastric banding [12] which are hardly performed in Sweden today.
This study shows a 30-day all-cause admission rate after gastric bypass of 6.5 % and a rate of 1.8% for gastrointestinal surgery. This is in accordance with previously published series [6, 7, 15]. The 1-year and 6-year all-cause admissions rates were 21.4% and 65.9%, respectively. The corresponding admission rates for gastrointestinal surgery were 6.8% and 24.4%, respectively. Few series have reported outcomes beyond 30 days. Telem et al. reported an admission rate of 26% after 2 years of various bariatric procedures [6], which is well in line with our results. The admission rate during the first year is more than twice that of the matched control group from the general population, and increases during the first year beyond the period where one would expect complications due to the index procedure. There seems to be an increase in admissions during the first three years, then followed by a slight decrease. The gastric bypass cohort was found to be at a 3-fold higher risk for inpatient care compared to the general population throughout the follow-up period. More in-depth studies are needed to elucidate the reasons for the increased demand for healthcare.
The risk of having any gastrointestinal surgical procedure is highest during the first year with a 12-fold risk increase. The risk increase for the whole 6-year follow-up period is more than 8-fold. This increased surgical risk beyond the immediate postoperative period is, in part, due to internal hernias, or fear thereof. Internal hernia is a well-known complication after gastric bypass. In this series, 4.3% of the gastric bypass patients were operated with a procedural code suggesting internal herniation during the follow-up period. This will increase with a longer follow-up time. During the studied time period 2007–2012, closure of the mesenteric defects at the time of surgery was not mandatory in Sweden. Since the Swedish randomized trial of closure vs non-closure of the mesenteric defects was published [14], stressing the importance of closure, most Swedish centers close the mesenteric defects, which will lower the risk of subsequent surgery. The fear of internal herniation and the difficulties in obtaining a proper diagnosis will also lead to many diagnostic laparoscopies in the surgical group.
Another cause of increased surgery in the postoperative period in the surgical group is gallstones. Obesity is a risk factor for development of gallstone disease [16] and periods of weight loss further increase the risk of gall stone formation [17]. A population based Swedish study has shown a fivefold increase in cholecystectomy among bariatric surgical patients compared to the general population with a peak in cholecystectomies being performed in the second postoperative year [13]. Screening for gallstones is not routine during the preoperative workup before gastric bypass surgery in Sweden. Symptomatic patients are examined with an ultrasound and patients with known gallstones will generally be operated on before their gastric bypass. Ursodiol is not used postoperatively on a routine basis. Our study show an almost 8-fold risk for inpatient care in the operated group for gallstone disease (see Figure 3).
Bariatric surgery dramatically improves several comorbid diseases, most notably diabetes but also hypertension and dyslipidemia [18–21]. The presence of diabetes preoperatively does not increase the risk of hospital admission, perhaps due to the large proportion of patients going into remission. However, previous cardiovascular hospitalization does increase the risk. Female sex is a risk factor for hospital admission. This has been shown in other bariatric studies [6, 22] and is of special relevance since most of the bariatric surgery patients are women. The reason for this is not clear.
Psychiatric comorbidity is also a known risk factor. Inability to cope with the dietary and lifestyle changes after surgery might be one reason. One recent study has also questioned whether bariatric surgery has a positive effect on depression [20]. Low education may be a risk factor for several different reasons, possibly for the same reasons that admission rates are higher among Medicare/Medicaid patients in the United States [6]. In this series, patients with admissions preoperatively are at risk for admission postoperatively.
Some of the risk factors may not be modifiable but are still of interest. By identifying patients at risk, preoperative education could be targeted towards the right individuals and more intense postoperative follow-up protocols could be implemented, potentially resulting in lower admission rates. Further studies are needed on how best to identify patients at risk of increased readmissions postoperatively and, more importantly, how to tailor preoperative education to reduce admissions after surgery.
Strengths of this study include the large sample size and the use of validated nationwide health registers enabling us to track patients regardless of where in Sweden they seek inpatient care. Limitations are that we only cover hospital admissions. We only investigated inpatient care, while we did not characterize use of outpatient care. Thus, the data underestimate the patients’ total contacts with the healthcare system. Another limitation of the study is the lack of a morbidly obese control group.
CONCLUSION
Gastric bypass patients are at a significant risk of admission to hospital after surgery, both in the short- and long term. Even after 6 years, they are admitted to hospital almost three times as often as an age, sex, and place of residence matched control group from the general population. Being a woman, having lower educational level, and having a history of psychiatric inpatient care were significant risk factors within the gastric bypass group.
Supplementary Material
Table 1.
Participant characteristics at baseline
| Surgery | Matched General Population Controls | |
|---|---|---|
| n=28,331 | n=274,513 | |
|
| ||
| Women, n (%) | 21,378 (76%) | 206,759 (75%) |
| Age (Years), Mean (SD) | 41 (11.0) | 41 (11.0) |
| University Education, n (%) | 5916 (21%) | 97,357 (36%) |
| Married, n (%) | 11,909 (42%) | 120,569 (44%) |
| Type of Surgery | ||
| Laparascopic | 27,036 (95.4%) | - |
| Open | 1018 (3.6%) | - |
| Converted | 277 (1.0%) | - |
| BMI (kg/m2), Mean (SD) | 43.0 (5.3) | - |
| 35–39.9, n (%) | 8899 (31%) | - |
| 40–49.9, n (%) | 16,537 (58%) | - |
| ≥50, n (%) | 2895 (10%) | - |
| 1-Year Weight Loss, Mean (SD) | ||
| Kg | 33.0 (11.1) | - |
| % | 28.1% (8.1%) | - |
| Anti-Hypertensive Drugs, n (%) | 9543 (34%) | 32,304 (12%) |
| Lipid Modifying Drugs, n (%) | 4543 (16%) | 12,899 (4.7%) |
| Antidiabetic Drugs, n (%) | ||
| Any | 4773 (17%) | 6827 (2.5%) |
| Non-Insulins | 4488 (16%) | 4581 (1.7%) |
| Insulins | 1942 (6.9%) | 3745 (1.4%) |
| Hospitalization Previous 5 years | ||
| Circulatory Causes (ICD10: I00–I99) | 5458 (19%) | 7615 (2.8%) |
| Psychiatric Causes (ICD10: F00–F99) | 2042 (7.2%) | 8174 (3.0%) |
Acknowledgments
Funding: National Institutes of Health (R01DK105948) and Swedish Research Council
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R01DK105948. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Excluding admissions due to plastic surgery for removal of excess skin and child birth
Matched 10:1 on age, sex, place of residence and treatment year
Further subcategorization into gastrointestinal surgery and into JA, JF and JK can be found in the supplementary appendix eFigure 1–4
Corresponding graphs for the matched general population comparators are provided in eFigure 5
Underlying numerical data provided in eTable 4
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorman RB, Miller CJ, Leslie DB, Serrot FJ, Slusarek B, Buchwald H, et al. Risk for hospital readmission following bariatric surgery. PLoS One. 2012;7:e32506. doi: 10.1371/journal.pone.0032506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellogg TA, Swan T, Leslie DA, Buchwald H, Ikramuddin S. Patterns of readmission and reoperation within 90 days after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009;5:416–23. doi: 10.1016/j.soard.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Paya M, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240:586–93. doi: 10.1097/01.sla.0000140752.74893.24. discussion 93–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders JK, Ballantyne GH, Belsley S, Stephens D, Trivedi A, Ewing DR, et al. 30-day readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-en-Y gastric bypass. Obes Surg. 2007;17:1171–7. doi: 10.1007/s11695-007-9210-3. [DOI] [PubMed] [Google Scholar]
- 5.Telem DA, Yang J, Altieri M, Patterson W, Peoples B, Chen H, et al. Rates and Risk Factors for Unplanned Emergency Department Utilization and Hospital Readmission Following Bariatric Surgery. Ann Surg. 2016;263:956–60. doi: 10.1097/SLA.0000000000001536. [DOI] [PubMed] [Google Scholar]
- 6.Telem DA, Talamini M, Gesten F, Patterson W, Peoples B, Gracia G, et al. Hospital admissions greater than 30 days following bariatric surgery: patient and procedure matter. Surg Endosc. 2015;29:1310–5. doi: 10.1007/s00464-014-3834-x. [DOI] [PubMed] [Google Scholar]
- 7.Sippey M, Kasten KR, Chapman WH, Pories WJ, Spaniolas K. 30-day readmissions after sleeve gastrectomy versus Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:991–6. doi: 10.1016/j.soard.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Hedenbro JL, Naslund E, Boman L, Lundegardh G, Bylund A, Ekelund M, et al. Formation of the Scandinavian Obesity Surgery Registry, SOReg. Obes Surg. 2015;25:1893–900. doi: 10.1007/s11695-015-1619-5. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–36. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313:483–95. doi: 10.1001/jama.2014.18614. [DOI] [PubMed] [Google Scholar]
- 12.Neovius M, Narbro K, Keating C, Peltonen M, Sjoholm K, Agren G, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308:1132–41. doi: 10.1001/2012.jama.11792. [DOI] [PubMed] [Google Scholar]
- 13.Jonas E, Marsk R, Rasmussen F, Freedman J. Incidence of postoperative gallstone disease after antiobesity surgery: population-based study from Sweden. Surg Obes Relat Dis. 2010;6:54–8. doi: 10.1016/j.soard.2009.03.221. [DOI] [PubMed] [Google Scholar]
- 14.Stenberg E, Szabo E, Agren G, Ottosson J, Marsk R, Lonroth H, et al. Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial. Lancet. 2016;387:1397–404. doi: 10.1016/S0140-6736(15)01126-5. [DOI] [PubMed] [Google Scholar]
- 15.Garg T, Rosas U, Rogan D, Hines H, Rivas H, Morton JM, et al. Characterizing Readmissions After Bariatric Surgery. J Gastrointest Surg. 2016 doi: 10.1007/s11605-016-3247-3. [DOI] [PubMed] [Google Scholar]
- 16.Amaral JF, Thompson WR. Gallbladder disease in the morbidly obese. Am J Surg. 1985;149:551–7. doi: 10.1016/s0002-9610(85)80055-6. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000–5. [PubMed] [Google Scholar]
- 18.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–25. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundbom M, Hedberg J, Marsk R, Boman L, Bylund A, Hedenbro J, et al. Substantial Decrease in Comorbidity 5 Years After Gastric Bypass: A Population-based Study From the Scandinavian Obesity Surgery Registry. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001920. [DOI] [PubMed] [Google Scholar]
- 21.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–77. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of Risk Factors for Patient Readmission 30 Days Following Discharge From General Surgery. JAMA Surg. 2016;151:855–61. doi: 10.1001/jamasurg.2016.1258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.