SUMMARY
Human neural progenitor cell (NPC) migration within the subventricular zone (SVZ) of the lateral ganglionic eminence is an active process throughout early brain development. The migration of human NPCs from the SVZ to the olfactory bulb during fetal stages resembles what occurs in adult rodents. As the human brain develops during infancy, this migratory stream is drastically reduced in cell number and becomes barely evident in adults. The mechanisms regulating human NPC migration are unknown. The Slit-Robo signaling pathway has been defined as a chemorepulsive cue involved in axon guidance and neuroblast migration in rodents. Slit and Robo proteins expressed in the rodent brain help guide neuroblast migration from the subventricular zone (SVZ) through the rostral migratory stream to the olfactory bulb. Here, we present the first study on the role that Slit and Robo proteins play in human-derived fetal neural progenitor cell migration (hfNPC). We describe that Robo1 and Robo2 isoforms are expressed in the human fetal SVZ. Furthermore, we demonstrate that Slit2 is able to induce a chemorepellent effect on the migration of hfNPCs derived from the human fetal SVZ. In addition, when Robo1 expression is inhibited, hfNPCs are unable to migrate to the olfactory bulb of mice when injected in the anterior SVZ. Our findings indicate that the migration of human NPCs from the SVZ is partially regulated by the Slit-Robo axis. This pathway could be regulated to direct the migration of NPCs in human endogenous neural cell therapy.
Keywords: Human subventricular zone, neural stem cells, Slit2, cell migration
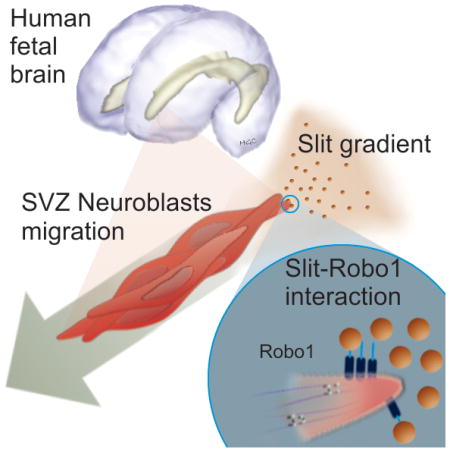
Graphical Abstract
Human fetal subventricular zone (SVZ) cells, expressing robo1 receptor, migrate in response to slit proteins in a chemorepellant manner.
INTRODUCTION
The subventricular zone (SVZ) is one of the most active neurogenic niches in adult mammals [1]. SVZ neural stem cells are slowly proliferating cells which divide asymmetrically to give rise to transient amplifying cells, which subsequently turn into migratory neuroblasts. The migration of neuroblasts (also known as type A cells) from the SVZ to the olfactory bulb (OB) along the rostral migratory stream (RMS) is maintained throughout the adult life of rodents [2]. In humans, a similar migratory pattern exists during fetal development, which then shifts and becomes barely evident during early childhood [3–9]. Although some of the mechanisms regulating neuroblast migration in rodents have been described, little is known about the process in the human brain. The regulation of SVZ neuroblast migration in the rodent brain involves a complex balance of extracellular chemorepellent and chemoattracant signals as well as intracellular regulators [10–14]. In this context, the family of Slit chemorepellent factors and their Robo receptor proteins participate in the guidance of neuroblasts from the SVZ to the OB [15]. Slits released from the choroid plexus and the septum into the cerebrospinal fluid induce a chemorepellent effect on migratory neuroblasts and send them away from their site of origin in the SVZ [11, 16]. Additionally, migratory neuroblasts can also release Slit1 to open a tunnel through astrocytes, which allows for formation of the RMS as they migrate to the OB [10]. Here we demonstrate, for the first time, the presence of Robo receptors in the human fetal SVZ and elucidate the effects of Robo1 expression on the migratory behavior of primary-cultured human-derived fetal neural progenitor cells (hfNPCs). We demonstrate that Slit2 exerts a chemorepellent effect and an increase in cell migration persistence on hfNPCs. We observe that these effects are partially due to the activation of the Robo1 receptor, as its knockdown decreases the response of hfNPCs to human Slit2. Furthermore, xenografted hfNPCs are unable to reach the OB when Robo1 expression is knocked down prior to their implantation into the rodent’s SVZ.
METHODS
Detailed description of experimental procedures is included in supplemental data.
Specimen Collection
Human fetal brains for immunohistochemistry were collected and processed as described previously [4]. Specimens for cell culture were obtained within 4 hours after surgery (18–20 gestational weeks). All procedures were approved by the Johns Hopkins Internal review board.
Tissue sectioning and immunohistochemistry
Fixed tissue was cryosectioned as reported previously by our group [4]. Sections were immunostained against: Robo1, Robo2, GFAP, and Nestin.
Human fetal neural progenitor cell culture
Cell cultures of hfNPCs were established from freshly dissociated brain tissue as described [17–20]. Cell culture media was supplemented with 5 μg/ml of heparin, 10ng/ml of Leukemia inhibitory factor, 20ng/ml Epithelial Growth Factor, and 20ng/ml of Fibroblast Growth Factor. To determine the progenitor cell features of primary cell cultures, we evaluated the neurosphere formation, differentiation, and self-renewal capacity (Figure S3).
Transwell migration assay
The migratory response of hfNPCs to Slit2 was evaluated via transwell migration assays with or without Slit2 (200ng/ml Peprotech 150-11) added to the bottom well, following manufacturer instructions (Costar).
Chemotaxis assay
To evaluate the migratory response of hfNPCs to a gradient of Slit2 we used a μ-Slide chemotaxis 2D chamber (Ibidi), with or without Slit2 (200ng/ml Peprotech 150-11), following the instructions of the manufacturer.
Active small Rho GTPase Pulldown
The GTP-bound fraction of Rac1 and CDC42 in response to Slit2 was evaluated using a Rac1/Cdc42 Activation Assay Combo Kit (Cell Biolabs, inc. STA-404).
Lentiviral transduction of hfNPCs
Robo1 knockdown was induced in hfNPCs by lentiviral vectors carrying shRNA sequences specific to human Robo1 (Sigma Aldrich Mission shRNA TRCN00000414 and TRCN00000417).
In vivo cell injection and animal sacrifice
All animal experiments were performed under Johns Hopkins University institutionally approved protocols as described previously by our group[21]. Empty vector control and shRNA transduced cells were injected in 4 week-old male mice at the most anterior region of the SVZ under stereotactic control. Mice were sacrificed 1 or 5 weeks after injection.
RESULTS AND DISCUSSION
Robo1 and Robo2 isoforms are expressed in the human SVZ
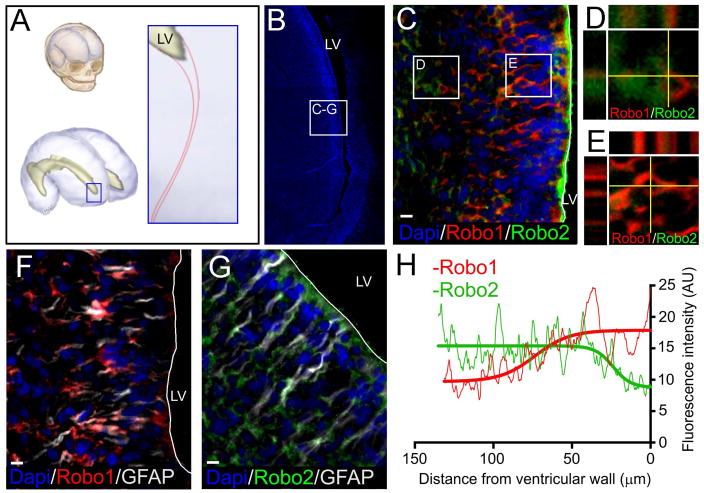
We have previously demonstrated the existence of an anterior extension of the ventricular lateral ganglionic eminence (LGE) in second trimester human fetal brains to the olfactory tract [4]. Here we evaluated the expression of Robo1 and Robo2 in the LGE of human fetal brains (21–23 gestational weeks). Interestingly, Robo1 was found close to the ventricular wall, where more GFAP positive cells are observed. Conversely, Robo2 was localized deeper in the parenchyma among DCx positive cells. Very sparse cells were found co-expressing the isoforms (Figures 1 and S1). The isoform distribution is in accordance with previous studies of the LGE of embryonic mice (E15.5), where Robo1 expression occurs at the intermediate and sub-ventricular zones, closer to the ventricle, while Robo2 expression occurs at the subplate and marginal zone, closer to the cortical plate [22]. We next evaluated the expression of Robo1 and Robo2 in vitro in hfNPCs obtained from the human fetal SVZ (21–23 gestational weeks) (Figure 2). hfNPCs were cultured in conditions that promote the presence of undifferentiated progenitor cells [17, 23]. The expression of Robo receptors by hfNPCs suggests that the migration of human fetal SVZ cells could be influenced by Slit2 signals as it occurs in rodents [10, 11].
Figure 1.
Expression of Robo1 and Robo2 in human fetal SVZ at the LGE. A, Schematic representation of the area studied at the anterior horn of the lateral ventricle in the human fetal brain. B, Dapi stained image showing the anterior extension of the lateral ventricle and the area observed in the following frames. C-E, Co-staining of Robo1 and Robo2 isoforms shows that while Robo1 appears closer to the ventricular wall, Robo2 appears deeper into the parenchyma. F, Robo1 co-staining with GFAP shows expression of Robo1 in areas close to the ventricle where GFAP cells are present. G, Robo2 co-staining with GFAP shows a higher concentration of Robo1 deeper in the parenchyma. H, Fluorescence intensity analysis of Robo1 (red) and Robo2 (green) signals. Graph shows a higher intensity of Robo1 in areas close to the ventricle, which decreases as the distance to the ventricular wall increases. Robo2 signal intensity shows an opposite distribution, showing higher intensity in areas farther from the ventricular wall. Scale bar: 10μm
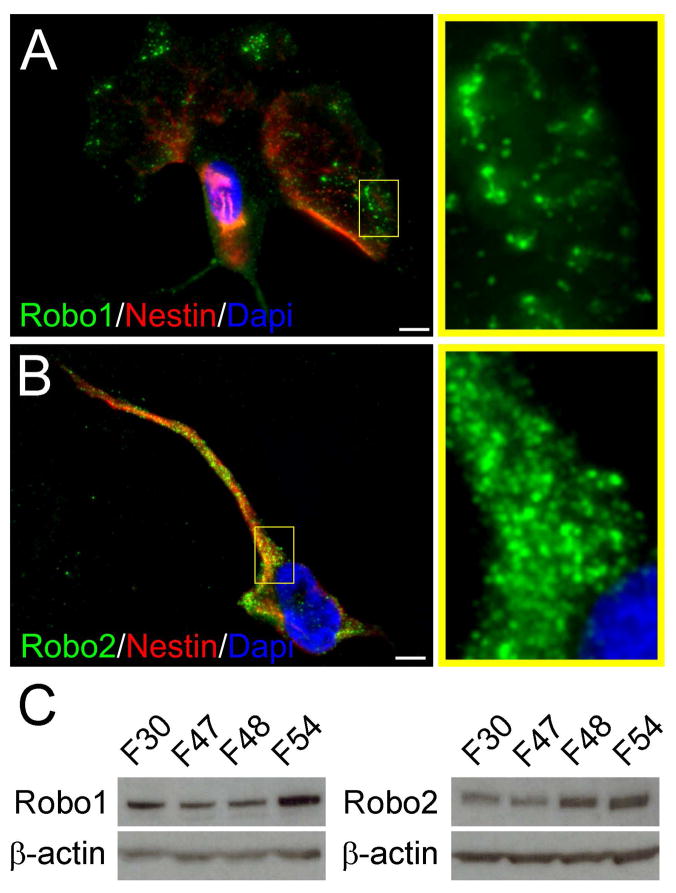
Figure 2.
Human fetal neural progenitor cells (hfNPCs) express Robo1 and Robo2 isoforms in vitro. A ubiquitous distribution of both isoforms was observed. A-Robo1 was observed at the distal cellular projections of Nestin, expressing cells. B, Robo2 had broader distribution in the cell body but was also observed in all evaluated cell types. C, Multiple primary cultures of hfNPCs were positive for Robo1 and Robo2 isoforms by western blot analysis. Dapi was used to counterstain cell nuclei. Scale bar: 5μm.
Slit2 exerts a chemorepellent effect on the migration of human fetal neural progenitor cells
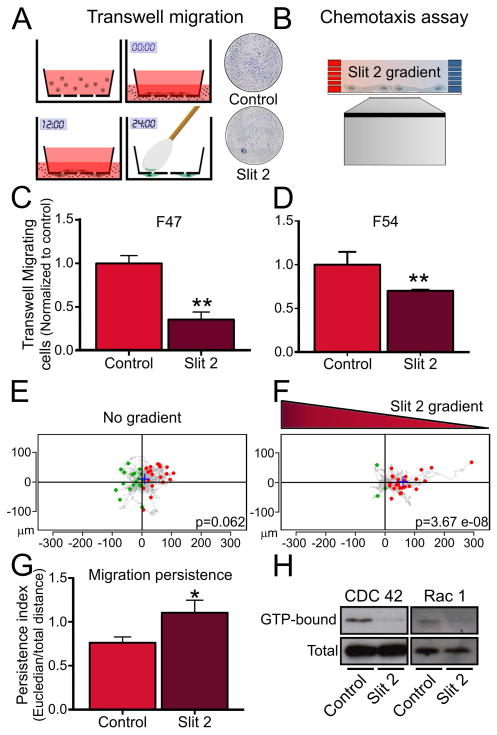
To determine whether the migration of hfNPCs is affected by the Robo ligand Slit2 we performed in vitro cell migration assays. When Slit2 was added to the bottom well of a transwell migration assay we observed a decrease in the number of cells migrating through the porous membrane (Figure 3A, C, D). We observed that 200ng/ml was the minimum concentration that achieved a significant chemorepellant effect on hfNPCs (Figure S2). We then evaluated the cell migration in response to a gradient of Slit2 using a chemotaxis migration assay. We observed that hfNPCs migrate away from higher concentrations of Slit2 (Figure 3B, E, F and supplemental videos S1 and S2) and their overall migration persistence is increased (Figure 3G), suggesting that cell migration itself is not inhibited but directionally regulated. In addition, we observed a decrease in the active (GTP-bound) form of CDC42 and Rac1 (Figure 3H) upon 18 hours of Slit2 stimulation. Cell viability and proliferation were not affected, as evaluated by MTT assay and EdU incorporation, respectively (Figure S3). In addition, we observed an increase in the expression of Robo1 upon differentiation. Slit2 exerted a chemorepellant effect on differentiated cells expressing the glial marker GFAP or the neuronal marker TuJ1 (Figure S4).
Figure 3.
Effects of Slit2 stimulation on hfNPCs migration. A and B, Schematic representation of a transwell migration and chemotaxis assay, respectively. C–D, Quantification of transwell migrated cells shows a reduced number of cells when Slit2 (200ng/ml) was present in the bottom well using two different primary cell cultures. E, Basal cell migration in a 2D chemotaxis assay shows aleatory migration distribution. F, hfNPCs show a biased cell migration pattern in the presence of a Slit2 gradient, indicating a chemorepellent response. G, hfNPC migration persistence is increased in response to a gradient of Slit2. H, The active forms of the small Rho-GTPases CDC42 and Rac1 are decreased upon Slit2 stimulation. **p<0.01, *p<0.05.
Robo1 KD hfNPCs are unable to reach the olfactory bulb after implantation in the SVZ of mice
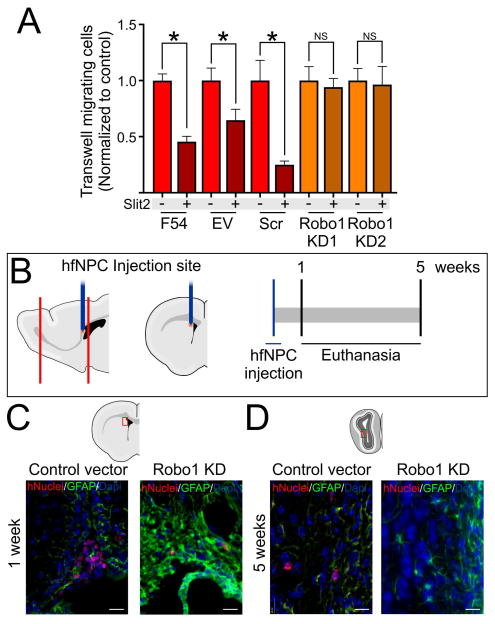
The in vitro cell migration response of hfNPCs to Slit2 was decreased after Robo1 knockdown with shRNA (Figure 4A–B). We next tested the effects of Robo1 expression on the migration of hfNPCs in vivo. Empty vector (EV) or Robo1-KD hfNPCs were stereotactically implanted in the SVZ of immunosuppressed adult mice (4–6 weeks old) (Figure 4C). 1-week post cell implantation, we observed that both EV and Robo1-KD cells incorporated into the SVZ of mice (Figure 4D). 5 weeks after implantation, we observed that 6±1 EV-transduced hfNPCs per mouse were present in the OB (Figure 4E). However, no Robo1-KD cells were observed in the OB (Figure 4E), supporting the hypothesis that Robo1 plays an important role in the migration of human progenitor cells. Intriguingly, no hfNPCs accumulation was observed at the SVZ of mice injected with EV or Robo1-KD cells. In contrast to what we observed in vitro, this in vivo result could indicate that Robo1 expression is not only important for cell migration but also for cell survival. Whether Robo1 expression directly impacts survival, or indirectly results in cell death due to the inability of Robo1-KD cells to migrate out of the SVZ, these findings merit further studies.
Figure 4.
Effects of Robo1-KD on the migration of hfNPCs. A, Robo1-KD hfNPCs do not respond to Slit2 in a transwell migration assay. C, Schematic representation of in vivo experiment where control vector or Robo1-KD hfNPCs were injected in the anterior SVZ of 4 week old mice. D, hfNPCs at the SVZ of injected mice 1 week post injection. D, hfNPCs at the OB 5 weeks post injection. No Robo1-KD hfNPCs were observed in the OB after this term. *p<0.05 Scale bar: 20μm
These results are in accordance with studies on rodent cells [10, 11, 15, 16, 24, 25] and are the first demonstration that Slit2 elicits a chemorepulsive response in human SVZ-derived cells. The decrease in cell migration response to Slit2 upon Robo1 KD indicates the importance of this receptor in the regulation of SVZ cell migration during fetal development [10, 11, 15, 16, 24, 25]. Our observations suggest that Robo1 regulates SVZ cell migration in response to Slits during human fetal development. Further studies are necessary to elucidate the specific role of Robo2 in this process during development and in adult human samples.
CONCLUSION
There is a potential use for endogenous neural progenitor cells as cellular therapy in neurodegenerative diseases. In order to evaluate the feasibility of this application in humans, a thorough understanding of the mechanisms regulating neural progenitor cell migration is needed. Therefore, processes that have been identified in rodents must be evaluated using human cells in clinically relevant models. Here we describe how the expression of Robo1 is necessary for hfNPCs to migrate in response to Slit2 stimulation in vitro and in vivo. We propose that the Slit-Robo axis is one potential target for directing progenitor cells to damaged brain areas.
Supplementary Material
Acknowledgments
H.G.C., A.Q.H., V.C.G., and P.S. were supported by the National Institutes of Health (R01NS070024) H.G.C. and E.L. were supported by the National Cancer Institute (R21CA199295) and the Maryland Stem Cells Research Fund. L.C. was supported by the Howard Hughes Medical Institute. V.C.G. was supported by the Fundacion Progreso y Salud of the Andalusian Regional Ministry of Health (PI01092014). Authors report no conflict of interest in this work. We would like to thank Sural Ranamukhaarachchi, Raisa Rauf, and Matt Sklar for their help in this work and Dr Oscar Gonzalez-Perez for his critical comments.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, H.G.C. and A.Q.H. Methodology, H.G.C., E.L., and L.C. Formal Analysis, H.G.C., P.S. and V.C.G. Investigation, H.G.C., E.L., V.C.G., G.D., M.L.V., A.C.C., and L.N. Resources, K.T., A.B., and A.Q.H. Writing, H.G.C., and E.L. Supervision, A.Q.H. Funding Acquisition, H.G.C. and A.Q.H.
References
- 1.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–81. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 3.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–9. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero-Cazares H, et al. Cytoarchitecture of the lateral ganglionic eminence and rostral extension of the lateral ventricle in the human fetal brain. J Comp Neurol. 2011;519(6):1165–80. doi: 10.1002/cne.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 6.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–6. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011;21(11):1534–50. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paredes MF, et al. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354(6308) doi: 10.1126/science.aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko N, et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 2010;67(2):213–23. doi: 10.1016/j.neuron.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311(5761):629–32. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 12.Leong SY, Turnley AM. Regulation of adult neural precursor cell migration. Neurochem Int. 2011;59(3):382–93. doi: 10.1016/j.neuint.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Mejia-Gervacio S, Murray K, Lledo PM. NKCC1 controls GABAergic signaling and neuroblast migration in the postnatal forebrain. Neural Dev. 2011;6:4. doi: 10.1186/1749-8104-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capilla-Gonzalez V, et al. Regulation of Subventricular Zone-Derived Cells Migration in the Adult Brain. In: Moneeb Ehtesham M, editor. Stem Cell Neoplasms of the Central Nervous System. Springer Science; 2015. [DOI] [PubMed] [Google Scholar]
- 15.Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23(4):703–11. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Ba-Charvet KT, et al. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24(6):1497–506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaichana KL, et al. Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J Neurosci Methods. 2009;180(1):116–25. doi: 10.1016/j.jneumeth.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy AF, et al. Influence of basement membrane proteins and endothelial cell-derived factors on the morphology of human fetal-derived astrocytes in 2D. PLoS One. 2014;9(3):e92165. doi: 10.1371/journal.pone.0092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Placone AL, et al. Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials. 2015;42:134–43. doi: 10.1016/j.biomaterials.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravin R, et al. Shear forces during blast, not abrupt changes in pressure alone, generate calcium activity in human brain cells. PLoS One. 2012;7(6):e39421. doi: 10.1371/journal.pone.0039421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Perez O, Guerrero-Cazares H, Quinones-Hinojosa A. Targeting of deep brain structures with microinjections for delivery of drugs, viral vectors, or cell transplants. J Vis Exp. 2010;(46) doi: 10.3791/2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews W, et al. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313(2):648–58. doi: 10.1016/j.ydbio.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero-Cazares H, Chaichana KL, Quinones-Hinojosa A. Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. Methods Mol Biol. 2009;568:73–83. doi: 10.1007/978-1-59745-280-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu W, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400(6742):331–6. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu H, Rutishauser U. A septum-derived chemorepulsive factor for migrating olfactory interneuron precursors. Neuron. 1996;16(5):933–40. doi: 10.1016/s0896-6273(00)80116-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.