Abstract
Background
Bariatric surgery provides durable weight loss and decreases the incidence of comorbid conditions for people with obesity. Most patients benefit from resultant weight loss, but some are at risk for postoperative refractory malnutrition, a serious but poorly understood complication.
Objective
To evaluate differences in bariatric surgery patients who received a feeding tube postoperatively for malnutrition compared with other indications
Setting
Retrospective cohort study at an academic bariatric surgery center (1985-2015)
Methods
All bariatric surgery patients that received a feeding tube postoperatively over a 30-year period were identified. Data abstraction from the medical record was performed to assess demographics, operative details, tube indication, and resultant body mass index (BMI) changes.
Results
From a total of 3,487 patients who underwent bariatric surgery during the study period, 139 (3.9%) required placement of a feeding tube postoperatively. Refractory malnutrition was the indication in 24 patients, all following Roux-en-Y gastric bypass. There were no significant differences between these patients and other bariatric surgery patients in terms of mean age (40.6±9.9 vs. 43.1±13.4 years, p=0.4) and preoperative BMI (47.5±10.5 vs. 51.0±9.6 kg/m2, p=0.1). The median time from surgery to tube placement for malnutrition patients was 4 years. Compared with other feeding tube indications, malnutrition patients had higher percent excess BMI lost after surgery (126.2±31.9 vs. 52.5±44.3%, p<0.0001). After tube placement, malnutrition patients had a significant increase in mean BMI compared to other indications (14.5±20.9 vs. -13.0±14.0%, p<0.001).
Conclusions
Patients with refractory malnutrition benefit from feeding tube placement, which results in a significant increase in BMI.
Keywords: bariatric surgery, refractory malnutrition, feeding tube, gastrostomy, jejunostomy
Introduction
Bariatric surgery provides patients with durable weight loss, a reduction in associated comorbidities, and improved overall survival. (1-4) More than half of the United States population will be diagnosed with obesity by year 2030, with an estimated healthcare cost of $60 billion. (5, 6) As the number of bariatric surgeries performed increases, improving the management of postoperative complications is necessary to ensure long-lasting, sustainable health benefits. (7) Malnutrition after bariatric surgery is a poorly characterized, late postoperative complication that can abrogate the benefits associated with significant weight loss.
Patients are at risk for malnutrition following bariatric surgery due to anatomic and metabolic changes induced by the operation, along with required dietary changes. (8, 9) If nutrition counseling and dietary changes do not ameliorate malnutrition, patients may require feeding tube placement or parenteral nutrition. (10, 11) There is little data available that evaluates this unique cohort of bariatric surgery patients. (12-14)
The objective of this study was to identify differences between patients who need feeding tubes for refractory malnutrition after bariatric surgery compared to patients needing feeding tubes for other reasons. Better understanding of this uncommon but significant late complication is necessary to help clinicians provide better care and education to affected patients. We hypothesized that feeding tube placement would be beneficial and increase body mass index (BMI) in patients with refractory malnutrition after bariatric surgery.
Patients and Methods
Patients
The Institutional Review Board approved this study and waiver of consent was granted for retrospective chart review. A prospectively collected database was used to identify patients undergoing bariatric surgery at our academic medical center from 1985 through 2015. This database has been maintained over the past 30 years and includes age, sex, preoperative weight and comorbidities, postoperative complications and comorbidities, and annual postoperative weights recorded at follow-up appointments. To identify patients with feeding tube placement after surgery, we queried our prospectively collected Clinical Data Repository for all procedure codes related to gastrostomy and jejunostomy tubes in our bariatric surgery population.
Definitions
Each patient undergoing bariatric surgery and having feeding tube placement postoperatively was reviewed using the electronic medical record. Patients were identified as possibly having a feeding tube based on the Current Procedural Terminology (CPT) codes listed in Table 1. All CPT codes that could be associated with feeding tubes were used initially to identify the target cohort, followed by confirmation of feeding tube placement in each patient using the electronic medical record.
Table 1. Current Procedural Terminology codes used to identify all bariatric surgery patients from January 1, 1985 through December 31, 2015 who had a postoperative feeding tube placed.
| CPT1 Code | Procedural Detail |
|---|---|
| 43246 | UGI ENDO; W/PLCMT2 GASTROSTOMY TUBE |
| 43653 | LAP SURG3; GASTROS W/O TUBE-SEP PROC4 |
| 43760 | CHANGE OF GASTROSTOMY TUBE |
| 44372 | SM INTEST ENDO5; W/PLCMT JEJUNO6 TUBE |
| 44373 | SM INTEST ENDO; W/GASTRO TO JEJUNO7 |
| 49440 | PLACE GASTROSTOMY TUBE PERC8 |
| 49441 | PLACE DUOD/JEJ9 TUBE PERC |
| 49446 | CHANGE G-TUBE TO G-J10 PERC |
| 49450 | REPLACE G/C11 TUBE PERC |
| 49451 | REPLACE DUOD/JEJ TUBE PERC |
CPT = Current Procedural Terminology
UGI ENDO; W/PLCMT = Upper gastrointestinal endoscopy; with placement
LAP SURG = Laparoscopic surgery
GASTROS W/O TUBE-SEP PROC = Gastrostomy without tube-separate procedure
SM INTEST ENDO = Small intestine endoscopy
W/PLCMT JEJUNO = With placement of jejunostomy
W/GASTRO TO JEJUNO = With gastrostomy to jejunostomy
PERC = Percutaneous
DUOD/JEJ = Duodenostomy/jejunostomy
G-TUBE TO G-J = Gastrostomy tube to gastrojejunostomy
G/C = Gastrostomy/cecostomy
Detailed chart review was performed to assess demographics, operative details, feeding tube indications, and resultant weight/BMI changes. Patients were categorized into nine groups based on their indication for feeding tube placement: refractory malnutrition, access for endoscopic retrograde cholangiopancreatography, dysphagia or dehydration, obstruction decompression, hypoglycemia, anastomotic leak diversion, marginal ulcer or perforation, superior mesenteric artery syndrome, and non-gastrointestinal indications such as trauma or stroke. Refractory malnutrition was defined as any malnutrition requiring feeding tube placement post-bariatric surgery as intensive dietary modifications and counseling are standard first line treatments at our institution. Feeding tube removal was defined as documentation of feeding tube removal prior to last medical center follow-up. There was no missing preoperative data and available follow-up data was used.
Statistics
Univariate statistical analysis was performed using χ2 for categorical variables. For normally distributed continuous variables, t test was performed and the results reported as mean ± standard deviation. For continuous variables that were not normally distributed, Mann-Whitney U test was performed and the results reported as median with interquartile range. Hierarchical regression modeling was performed to obtain adjusted odds ratios with year of surgery as a fixed effect. Alpha less than 0.05 was used for statistical significance. SAS version 9.4 (SAS Institute, Cary NC) was used for analyses.
Results
A total of 3,487 patients underwent bariatric surgery during the 30-year study period, of which 139 (3.9%) required placement of a feeding tube postoperatively. These patients were predominantly female (82.7%) and had a Roux-en-Y gastric bypass (RYGB) (92.1%). The mean age was 44.5±9.9 years, with a pre-bariatric surgery BMI of 50.8±9.7 and a median time from bariatric surgery to feeding tube placement of 9.2 (IQR 1.3 – 73.6) months.
The indications for and the characteristics of the feeding tubes placed are shown in Table 2. The most common indications for feeding tube placement were decompression of obstruction (21.1%), anastomotic leak diversion (19.4%), and refractory malnutrition (17.3%). The majority of patients received laparoscopically-placed feeding tubes (68.4%) and most were placed into the gastric remnant (79.8%).
Table 2. Indications for and characteristics of feeding tube placement in patients following bariatric surgery (n=139).
| Indications | |
| Refractory malnutrition | 17.3 (24)1 |
| Access for ERCP2 | 5.0 (7) |
| Dysphagia or dehydration | 14.4 (20) |
| Obstruction decompression | 21.1 (30) |
| Hypoglycemia | 2.2 (3) |
| Anastomotic leak diversion | 19.4 (27) |
| Marginal ulcer or perforation | 10.1 (14) |
| SMA3 syndrome | 1.4 (2) |
| Non-GI4 (trauma, neurologic status) | 8.6 (12) |
| Characteristics | |
| Placement Method | |
| Open | 22.3 (31) |
| Laparoscopic | 68.4 (95) |
| Endoscopic | 9.3 (13) |
| Tube Location | |
| Jejunum | 13.7 (19) |
| Gastric pouch | 6.5 (9) |
| Gastric remnant | 79.8 (111) |
| Tube Removed | 54.7 (76) |
% (n), all such values
ERCP = Endoscopic retrograde cholangiopancreatography
SMA = Superior mesenteric artery
GI = Gastrointestinal
Comparing patients who received tubes for refractory malnutrition with all other bariatric surgery patients in the database, there were no differences in age, sex, preoperative BMI, or comorbidities (gastroesophageal reflux disease, degenerative joint disease, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, and psychiatric history) (Table 3). Of the 24 patients that received tubes for refractory malnutrition, all underwent RYGB, which resulted in a significantly higher rate of malabsorptive procedures compared with the overall cohort (100% vs. 81%, p=0.02). These patients also had a higher rate of open surgery (67% vs. 24%, p<0.001). Compared to other indications for tube placement, the malnutrition patients were younger (40.6±9.9 vs. 45.3±8.6 years, p=0.032), but had similar preoperative BMIs and rates of comorbidities (Table 3).
Table 3. Demographic and perioperative differences between patients who received feeding tubes after bariatric surgery for refractory malnutrition compared with all other bariatric surgery patients, and compared with patients who received feeding tubes for other indications.
| Malnutrition (n=24) | All other patients (n=3463) | p-value | Other tube indications (n=115) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 40.6±9.91 | 43.1±13.4 | 0.4 | 45.3±8.6 | 0.03 |
| Female | 91.7 (22)2 | 81.2 (2812) | 0.2 | 80.9 (93) | 0.2 |
| Preoperative BMI3 (kg/m2) | 47.5±10.5 | 51.0±9.6 | 0.1 | 51.4±9.5 | 0.08 |
| GERD4 | 20.8 (5) | 27.7 (960) | 0.5 | 26.1 (30) | 0.6 |
| Degenerative joint disease | 50.0 (12) | 32.0 (1107) | 0.06 | 33.0 (38) | 0.1 |
| Diabetes mellitus | 20.8 (5) | 29.3 (1013) | 0.4 | 33.0 (38) | 0.2 |
| Hypertension | 54.2 (13) | 51.6 (1787) | 0.8 | 59.1 (68) | 0.7 |
| COPD5 | 8.3 (2) | 3.4 (119) | 0.2 | 1.7 (2) | 0.08 |
| Psychiatric history | 20.8 (5) | 15.2 (527) | 0.5 | 11.3 (13) | 0.2 |
| Type of Procedure | 0.02 | 0.02 | |||
| Malabsorptive | 100 (24) | 80.9 (2800) | 90.4 (104) | ||
| Restrictive | 0 (0) | 19.2 (663) | 9.6 (11) | ||
| Surgical Approach | < 0.001 | 0.004 | |||
| Open | 66.7 (16) | 23.7 (822) | 34.7 (40) | ||
| Laparoscopic | 33.3 (8) | 76.3 (2641) | 65.2 (75) | ||
| Operative Procedure | < 0.001 | 0.02 | |||
| Malabsorptive | |||||
| Open gastric bypass | 54.2 (13) | 17.5 (605) | 20.9 (24) | ||
| Gastric bypass revision | 4.2 (1) | 0.4 (14) | 1.7 (2) | ||
| Lap6 gastric bypass | 33.3 (8) | 58.9 (2040) | 60.9 (70) | ||
| Lap to open gastric bypass | 8.3 (2) | 4.1 (141) | 7.0 (8) | ||
| Restrictive | |||||
| Gastric sleeve | 0 (0) | 7.8 (270) | 4.4 (5) | ||
| Vertical band gastroplasty | 0 (0) | 1.8 (62) | 5.2 (6) | ||
| Adjustable gastric banding | 0 (0) | 9.6 (331) | 0 (0) |
Mean ± standard deviation, all such values
% (n), all such values
BMI = Body mass index
GERD = Gastroesophageal reflux disease
COPD = Chronic obstructive pulmonary disease
Lap = Laparoscopic
Feeding tube differences between malnutrition patients compared with other tube indications are shown in Table 4. Malnutrition patients had lower BMIs at the time of tube placement (20.2±6.0 vs. 38.2±13.6, p<0.001) and required their feeding tube later after bariatric surgery (median [IQR]: 48.5 [16.9 – 109.0] vs. 3.8 [1.0 – 50.6] months, p<0.001). Malnutrition patients required their feeding tube for a longer period of time, which trended toward significance (median [IQR]: 5.3 [2 – 45.8] vs. 4.6 [1.4 – 12.8] months, p=0.056).
Table 4. Feeding tube differences between malnutrition patients compared with other tube indications.
| Malnutrition (n=24) | Other tube indications | p-value | |
|---|---|---|---|
| BMI1 before tube placement (kg/m2) | 20.2±6.02 | 38.2±13.6 | < 0.001 |
| BMI after tube removal (kg/m2) | 24.7±6.9 | 36.4±10.6 | 0.004 |
| Time from bariatric surgery to tube placement (months) | 48.5 (16.9 – 109.0)3 | 3.8 (1.0 – 50.6) | < 0.001 |
| Time from tube placement to removal (months) | 5.3 (2 – 45.8) | 4.6 (1.4 – 12.8) | 0.056 |
| %EBMIL4 prior to tube placement | 126.2±31.9 | 52.5±44.3 | < 0.001 |
| %BMI increase after tube placement | 14.5±20.9 | -13.0±14.0 | < 0.001 |
| Placement Method | 0.4 | ||
| Open | 20.8 (5)5 | 22.6 (26) | |
| Laparoscopic | 62.5 (15) | 69.6 (80) | |
| Endoscopic | 16.7 (4) | 7.8 (9) | |
| Tube Location | 0.026 | ||
| Jejunum | 29.2 (7) | 10.4 (12) | |
| Gastric pouch | 0 (0) | 7.8 (9) | |
| Gastric remnant | 70.8 (17) | 81.7 (94) | |
| Tube Removed | 37.5 (9) | 58.3 (67) | 0.063 |
BMI = Body mass index
Mean ± standard deviation, all such values
Median (Interquartile range), all such values
%EBMIL = Percent excess BMI lost
% (n), all such values
There was no significant difference in pre-bariatric surgery BMI between malnutrition patients and those receiving tubes for other indications (47.5±10.5 vs 51.4±9.5, p=0.075). However, malnutrition patients gained significantly more weight after tube placement compared to other indications (14.5±20.9 vs. -13.0±14.0% increase in BMI, p<0.001). Likewise, compared with mean BMI before tube placement, malnutrition patients had a significant increase in BMI after tube placement (before: 20.2±6.0 vs. after: 24.7±6.9 kg/m2, p<0.001), which was not observed in other indications (38.2±13.6 vs. 36.4±10.6 kg/m2, p=0.11) (Figure 1a). The difference in percent excess BMI lost from before tube placement to after is shown in Figure 1b.
Figure 1.
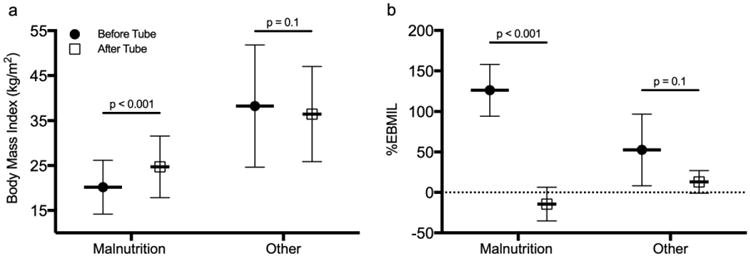
(a) Body mass index and (b) percent excess body mass index lost (%EBMIL) before and after feeding tube placement for bariatric surgery patients with refractory malnutrition compared with other indications. All values shown as mean ± standard deviation.
Based on hierarchical regression modeling, risk factors for feeding tube placement due to malnutrition included preoperative BMI and surgical approach (open vs. laparoscopic). Every one-point increase in preoperative BMI was associated with an adjusted odds ratio of 0.92 (95% CI 0.87 – 0.97) and for patients undergoing an open operation, the odds ratio was 9.79 (95% CI 0.04 – 999.99). The model had poor discriminatory power (AIC 85.91, BIC 95.26).
Discussion
Refractory malnutrition is a late complication after bariatric surgery that is not well characterized in the literature. Poor compliance with dietary and lifestyle recommendations along with malabsorption induced by surgery can contribute to postoperative caloric, protein, and vitamin deficiencies. (15) The present study sought to evaluate patients with malnutrition after bariatric surgery that progress until feeding tube placement was required. Over a 30-year period, 139 patients (3.9%) were identified who received a feeding tube after bariatric surgery. Of those, 24 patients (17.3%) required tube placement to manage refractory malnutrition, all of which underwent RYGB. Compared with patients who received feeding tubes for other indications (82.7%) such as dysphagia and dehydration, malnutrition patients lost significantly more weight after their bariatric surgery and had lower BMIs at the time of feeding tube placement. Although malnutrition was less common than surgical complications as an indication for tube placement, these patients benefited from feeding tube placement as indicated by a significant increase in BMI.
RYGB is associated with consistent weight loss and improved overall health in patients with obesity, but can occasionally lead to significant nutritional deficiencies. (16) The most common deficiencies seen after bariatric surgery include protein malnutrition, low levels of iron and calcium, and deficiencies in vitamin B1, vitamin B12, folate, and fat-soluble vitamins. (15, 17, 18) A case report by Wade AN et al. describes a 48-year-old female who developed unrecoverable malnutrition 6 years after RYGB and died. (19) The patient presented initially with severe protein calorie malnutrition, which is reported to occur in approximately 4.7% of patients after RYGB. (20) Although not well understood, protein malnutrition may lead to reduced protein catabolism, decreased availability of amino acids, and hepatic dysfunction characterized by reduced levels of acute phase proteins. (21) In the present study, patients with malnutrition required feeding tube placement years after bariatric surgery (median time from surgery to tube placement of 4 years), similar to the amount of time described in the aforementioned case report. Future studies are needed to identify any trends related to macro and micronutrient deficiencies and the development of refractory malnutrition requiring feeding tube placement.
In a case series of 12 patients in France who required enteral or parenteral nutrition after bariatric surgery, the authors identified “one-anastomosis gastric bypass” (OAGB) procedures as potentially associated with increased malabsorption and nutritional complications. (11) OAGB is a relatively new bariatric surgery procedure first reported in the early 2000s that consists of reducing the size of the “working” stomach and then performing a single latero-lateral gastro-jejunal anastomosis to bypass a segment of small intestine. (22, 23) This combined restrictive and malabsorptive procedure is effective at causing significant weight loss and has high rates of diabetes remission. (24) OAGB is currently not performed at our institution but the potential postoperative malnutrition further highlights the need for close nutritional support following gastric bypass surgery.
Of the 3.9% of bariatric surgery patients in this 30-year observational study that required postoperative feeding tube placement, it appears as though patients who received a feeding tube for refractory malnutrition benefited significantly from the intervention based on a mean BMI increase of 4.5 kg/m2 and a switch from continued weight loss to an appreciable weight gain. There is little data available in the bariatric surgery literature to help guide clinicians on how to best manage severe postoperative malnutrition, and whether enteral or parenteral nutrition is preferred. A study published by Hamilton C et al. describes 23 patients who were managed with hypocaloric home parenteral nutrition after presenting with anastomotic leak, fistula, or bowel obstruction after bariatric surgery. (25) While appropriate for these indications, feeding tube placement and initiation of enteral feeding is reasonable for patients with malnutrition and no anatomic abnormalities that preclude enteral nutrition. Enteral nutrition may help reduce mucosal atrophy and prevent loss of epithelial barrier function, both of which can exacerbate malabsorption and lead to subsequent malnutrition. (26-29) Further research is needed to determine the ideal management strategy for refractory malnutrition that does not resolve with dietary changes and vitamin supplementation. As demonstrated by this study, feeding tube placement should be considered.
Limitations
Although the present study describes the largest cohort of bariatric surgery patients with postoperative refractory malnutrition requiring feeding tube placement in the literature, the findings are limited by the observational design, the relatively small sample size, and the lack of specific data points (such as level of compliance with nutrition recommendations) that may be important for completely understanding this complication. As bariatric surgery continues to be performed more frequently worldwide, improved understanding of late complications will be necessary. Considering that nutritional counseling is part of the recommended multidisciplinary approach to the surgical management of obesity, appropriate strategies for treating refractory malnutrition should be developed and included in comprehensive care recommendations.
Conclusions
Using a 30-year prospectively collected bariatric surgery database, the present study identified the unique characteristics associated with refractory malnutrition after bariatric surgery requiring feeding tube placement. Malnutrition requiring tube placement was uncommon in the overall cohort, and compared with other indications for tube placement, malnutrition was less common than surgical complications. Malnutrition patients lost significantly more weight after bariatric surgery compared with patients receiving feeding tubes for other indications, and did not require tube placement until years after their initial operation. As demonstrated by the poor discriminative power of our model, this rare complication after bariatric surgery is difficult to predict and may be related to compliance with postoperative nutrition recommendations as opposed to operative-specific factors. Long-term follow-up with a physician or nutritionist may allow for early identification of patients who will benefit from nutritional support. If intensive counseling and noninvasive nutritional support fail, our study demonstrates that patients with refractory malnutrition may benefit from feeding tube placement and can expect a significant increase in BMI.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute (grants T32 HL007849 and UM1 HL088925).
This work was supported by the National Heart, Lung and Blood Institute (grants T32 HL007849 and UM1 HL088925). The authors had full control of the design of the study, methods used, results, data analysis and production of the written manuscript.
Footnotes
This work was presented at Obesity Week 2016 in New Orleans, LA.
None of the authors report any financial or personal conflicts of interest.
Disclosure Statement: The authors have no financial or personal conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guidry CA, Davies SW, Sawyer RG, Schirmer BD, Hallowell PT. Gastric bypass improves survival compared with propensity-matched controls: a cohort study with over 10-year follow-up. Am J Surg. 2015;209:463–7. doi: 10.1016/j.amjsurg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehaffey JH, LaPar DJ, Clement KC, et al. 10-Year Outcomes After Roux-en-Y Gastric Bypass. Ann Surg. 2016;264:121–6. doi: 10.1097/SLA.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 4.Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–70. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen NT, Vu S, Kim E, Bodunova N, Phelan MJ. Trends in utilization of bariatric surgery, 2009-2012. Surg Endosc. 2016;30:2723–7. doi: 10.1007/s00464-015-4535-9. [DOI] [PubMed] [Google Scholar]
- 8.Shikora SA, Kim JJ, Tarnoff ME. Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract. 2007;22:29–40. doi: 10.1177/011542650702200129. [DOI] [PubMed] [Google Scholar]
- 9.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013;9:159–91. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Mundi MS, Vallumsetla N, Davidson JB, McMahon MT, Bonnes SL, Hurt RT. Use of Home Parenteral Nutrition in Post-Bariatric Surgery-Related Malnutrition. JPEN J Parenter Enteral Nutr. 2016 doi: 10.1177/0148607116649222. [DOI] [PubMed] [Google Scholar]
- 11.Betry C, Disse E, Chambrier C, et al. Need for Intensive Nutrition Care After Bariatric Surgery: Is Mini Gastric Bypass at Fault? JPEN J Parenter Enteral Nutr. 2016 doi: 10.1177/0148607116637935. [DOI] [PubMed] [Google Scholar]
- 12.Dodell GB, Albu JB, Attia L, McGinty J, Pi-Sunyer FX, Laferrere B. The bariatric surgery patient: lost to follow-up; from morbid obesity to severe malnutrition. Endocr Pract. 2012;18:e21–5. doi: 10.4158/EP11200.CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushner R. Managing the obese patient after bariatric surgery: a case report of severe malnutrition and review of the literature. JPEN J Parenter Enteral Nutr. 2000;24:126–32. doi: 10.1177/0148607100024002126. [DOI] [PubMed] [Google Scholar]
- 14.Pelizzo G, Calcaterra V, Fusillo M, et al. Malnutrition in pregnancy following bariatric surgery: three clinical cases of fetal neural defects. Nutr J. 2014;13:59. doi: 10.1186/1475-2891-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handzlik-Orlik G, Holecki M, Orlik B, Wylezol M, Dulawa J. Nutrition management of the post-bariatric surgery patient. Nutr Clin Pract. 2015;30:383–92. doi: 10.1177/0884533614564995. [DOI] [PubMed] [Google Scholar]
- 16.Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–93. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka K, DiBaise JK, Martindale RG. Nutrition and metabolic complications after bariatric surgery and their treatment. JPEN J Parenter Enteral Nutr. 2011;35:52S–9S. doi: 10.1177/0148607111413600. [DOI] [PubMed] [Google Scholar]
- 18.Gletsu-Miller N, Wright BN. Mineral malnutrition following bariatric surgery. Adv Nutr. 2013;4:506–17. doi: 10.3945/an.113.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade AN, Dolan JM, Cambor CL, Boullata JI, Rickels MR. Fatal malnutrition 6 years after gastric bypass surgery. Arch Intern Med. 2010;170:993–5. doi: 10.1001/archinternmed.2010.164. [DOI] [PubMed] [Google Scholar]
- 20.Faintuch J, Matsuda M, Cruz ME, et al. Severe protein-calorie malnutrition after bariatric procedures. Obes Surg. 2004;14:175–81. doi: 10.1381/096089204322857528. [DOI] [PubMed] [Google Scholar]
- 21.Jahoor F, Badaloo A, Reid M, Forrester T. Protein metabolism in severe childhood malnutrition. Ann Trop Paediatr. 2008;28:87–101. doi: 10.1179/146532808X302107. [DOI] [PubMed] [Google Scholar]
- 22.Carbajo MA, Luque-de-Leon E. Differentiating mini-gastric bypass/one-anastomosis gastric bypass from the single-anastomosis duodenoileal bypass procedures. Surg Obes Relat Dis. 2016;12:933–4. doi: 10.1016/j.soard.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Musella M, Milone M, Deitel M, Kular KS, Rutledge R. What a Mini/One Anastomosis Gastric Bypass (MGB/OAGB) Is. Obes Surg. 2016;26:1322–3. doi: 10.1007/s11695-016-2168-2. [DOI] [PubMed] [Google Scholar]
- 24.Musella M, Apers J, Rheinwalt K, et al. Efficacy of Bariatric Surgery in Type 2 Diabetes Mellitus Remission: the Role of Mini Gastric Bypass/One Anastomosis Gastric Bypass and Sleeve Gastrectomy at 1 Year of Follow-up. A European survey. Obes Surg. 2016;26:933–40. doi: 10.1007/s11695-015-1865-6. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton C, Dasari V, Shatnawei A, Lopez R, Steiger E, Seidner D. Hypocaloric home parenteral nutrition and nutrition parameters in patients following bariatric surgery. Nutr Clin Pract. 2011;26:577–82. doi: 10.1177/0884533611416125. [DOI] [PubMed] [Google Scholar]
- 26.Goichon A, Bertrand J, Chan P, et al. Enteral delivery of proteins enhances the expression of proteins involved in the cytoskeleton and protein biosynthesis in human duodenal mucosa. Am J Clin Nutr. 2015;102:359–67. doi: 10.3945/ajcn.114.104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groos S, Hunefeld G, Luciano L. Parenteral versus enteral nutrition: morphological changes in human adult intestinal mucosa. J Submicrosc Cytol Pathol. 1996;28:61–74. [PubMed] [Google Scholar]
- 28.Yang H, Feng Y, Sun X, Teitelbaum DH. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:338–46. doi: 10.1111/j.1749-6632.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Jiang X. Effects of enteral nutrition on the barrier function of the intestinal mucosa and dopamine receptor expression in rats with traumatic brain injury. JPEN J Parenter Enteral Nutr. 2015;39:114–23. doi: 10.1177/0148607113501881. [DOI] [PubMed] [Google Scholar]


