Abstract
Three decades ago Tony Sclafani proposed the existence of a polysaccharide taste quality that was distinguishable from the taste generated by common sweeteners and that it was mediated by a separate receptor mechanism. Since that time, evidence has accumulated, including psychophysical studies conducted in our laboratory, buttressing this hypothesis. The use of knockout (KO) mice that lack functional T1R2 + T1R3 heterodimers, the principal taste receptor for sugars and other sweeteners, have been especially informative in this regard. Such KO mice display severely diminished electrophysiological and behavioral responsiveness to sugars, artificial sweeteners, and some amino acids, yet display only slightly impaired concentration-dependent responsiveness to a representative polysaccharide, Polycose. Moreover, although results from gene deletion experiments in the literature provide strong support for the primacy of the T1R2 + T1R3 heterodimer in the taste transduction of sugars and other sweeteners, there is also growing evidence suggesting that there may be T1R-independent receptor mechanism(s) activated by select sugars, especially glucose. The output of these latter receptor mechanisms appears to be channeled into brain circuits subserving various taste functions such as cephalic phase responses and ingestive motivation. This paper highlights some of the findings from our laboratory and others that lend support for this view, while emphasizing the importance of considering the multidimensional nature of taste function in the interpretation of outcomes from experiments involving manipulations of the gustatory system.
Keywords: Sweet taste, Insulin, Cephalic phase reflexes, T1R heterodimers, Glucose receptor, Polysaccharide taste
The publication of Tony Sclafani’s impressive compendium of papers published in a dedicated issue of Neuroscience and Biobehavioral Reviews (Nissenbaum & Sclafani, 1987) had a tremendous influence on experimental pursuits of carbohydrate taste. Along that theme, this paper deals with saccharide sensing by the gustatory system - a topic for which Tony Sclafani’s and Karen Ackroff’s interests intersect with our own. We do this as an homage for the significant contributions that both of them have made to the science of ingestive behavior. The work presented in the following pages is not meant to provide a comprehensive review of the issue, but to merely highlight a few key findings from our laboratory and Tony Sclafani’s, as well as some others, that provide significant support for the view that select carbohydrate stimuli, including some sugars, engage more than one taste receptor mechanism.
1. A heuristic framework of taste function
At the outset, it would be worthwhile highlighting a heuristic multidimensional framework for understanding taste function [see (Spector, 2000) for more detail]. First, taste serves a sensory-discriminative role that helps animals identify stimuli. Perceptions of quality and basic stimulus strength fall under this functional domain. Second, taste serves to promote or discourage the consumption of foods and fluids. The motivational, reward, and hedonic properties of a taste stimulus are part and parcel of this domain of function that we refer to as ingestive motivation [see (Spector, 2000)]. Students of motivational processes further divide such behavior into an appetitive and a consummatory component. Appetitive behavior refers to the approach toward, or away from in the case of avoidance, a taste stimulus. Consummatory behavior refers to the oromotor actions supporting the ingestion, or rejection in the case of aversion, of the taste stimulus triggered by the activation of oral receptors. Finally, taste plays a role in physiological processes that prepare the body for the arrival of food and fluids via so-called cephalic phase reflexes. A clear example of a cephalic phase reflex is the salivation elicited by the “sour” taste of a lemon. Importantly, in the context of this framework, the input from a given taste receptor could, by virtue of its neuronal connections, be channeled into circuits that subserve one (or more) of these functions, but not another. Given the multidimensional nature of gustatory function, the types of tasks used to assess taste responsiveness must be considered in the interpretation of experimental outcomes.
2. The multiple taste receptor model for carbohydrates
Three decades ago, Tony Sclafani hypothesized the existence of a polysaccharide taste that was discriminable from that of sugars and other sweeteners and mediated through a separate receptor mechanism in rodents (Nissenbaum & Sclafani, 1987). This hypothesis was based on a series of clever behavioral experiments demonstrating that rats treat the taste of Polycose (a prototypical maltodextrin with an average molecular weight of 1000) differently from other sweeteners despite the fact that these animals find all of these stimuli palatable. At the time, there was also growing evidence suggesting that sucrose was discernable from the glucose disaccharide, maltose. For example, Spector and Grill (Spector & Grill, 1988), as well as Nissenbaum and Sclafani (Nissenbaum & Sclafani, 1987), demonstrated that although rats will cross-generalize taste aversions conditioned to maltose and sucrose, indicative of some degree of qualitative similarity, they display a greater aversion to the sugar that serves as the conditioned stimulus, which suggests there must be some discriminable feature between the two sugars.
Consistent with this implication, using a gustometer in which small volumes of taste stimuli are delivered and immediate responses are measured, Spector et al. (Spector, Markison, St. John, & Garcea, 1997) trained “thirsty” rats to suppress their licking when they sampled sucrose to avoid a brief foot-shock, but to maintain licking when they sampled maltose. Concentration was varied to render intensity an irrelevant cue. The sugar (sucrose or maltose) that signaled shock was counterbalanced across rats. All animals learned to perform this discrimination with great competence. Performance was unaffected by sham surgery, or bilateral transection of the chorda tympani nerve (innervating taste buds in the front of the tongue), or bilateral transection of the glossopharyngeal nerve (innervating taste buds in the back of the tongue). However, when the chorda tympani nerve was transected in combination with the greater superficial petrosal nerve (innervating the taste buds of the palate), the rats were severely impaired. Thus, rats can discriminate orally sampled sucrose from maltose, and this ability is dependent, in part, on signals arising from the combined gustatory branches of the seventh cranial nerve. The fact that transection of gustatory nerves led to this impairment offers strong support that the behavior was under discriminative control on the basis of taste signals.
Dotson and Spector (Dotson & Spector, 2007) used a similar gustometer to test whether C57BL/6J (B6) mice could discriminate sucrose from glucose, maltose, and fructose. The “thirsty” mice were initially trained in a two-response operant taste discrimination procedure to lick a left (or in other mice a right) response spout after sampling sucrose from a center spout and to lick a right (or in other mice a left) response spout after sampling NaCl. Correct responses were reinforced by the delivery of water and incorrect responses were punished with a time-out. Concentration was varied to render intensity an irrelevant cue. After competent performance was achieved, the mice were tested for their ability to discriminate sucrose from other compounds. With respect to the sugars tested, mice could not discriminate sucrose from glucose. Although mice appeared to discriminate fructose from sucrose slightly (but significantly) above chance level, a close inspection of the data suggested that these animals were potentially using intensity cues. Interestingly, the mice did perform the sucrose vs. maltose discrimination above chance and although performance was modest at best, it could not easily be explained by intensity cues. A human psychophysical study employing a forced-choice sugar discrimination procedure reached a similar conclusion (Breslin, Beauchamp, & Pugh, 1996). In that study, a fixed concentration of one standard sugar was pitted against various concentrations of a comparison sugar to control for intensity differences. There was always a concentration of the comparison sugar that could not be discriminated from the standard sugar. The only exception was maltose, which, while indiscriminable from low concentrations of a fructose standard, was discernable from higher concentrations of a fructose standard. Overall, the results from both human and rodent studies suggest that most sugars, aside from maltose, generate a unitary qualitative taste sensation (i.e., “sweetness”).
A mechanistic basis underlying the qualitative similarity of sugars was finally discovered at the start of the new millennium with the identification of the T1R family of taste receptors (Bachmanov et al., 2001; Hoon et al., 1999; Kitagawa, Kusakabe, Miura, Ninomiya, & Hino, 2001; Max et al., 2001; Montmayeur, Liberles, Matsunami, & Buck, 2001; Nelson et al., 2001; Sainz, Korley, Battey, & Sullivan, 2001). It is composed of three members: T1R1, T1R2, and T1R3. The T1R1 combines with T1R3 to form a heterodimer that binds with L-amino acids, and the T1R2 combines with the T1R3 to form a heterodimer that binds with sweeteners. Very strong support for the T1R2 + T1R3 as the principal receptor for natural and artificial sweeteners has been derived from knockout (KO) experiments in which one or both of the subunits have been genetically silenced in mice leading to severe reductions or abolition of behavioral and electrophysiological responses to these stimuli [e.g., (Bachmanov et al., 2001; Hoon et al., 1999; Kitagawa et al., 2001; Max et al., 2001; Montmayeur et al., 2001; Nelson et al., 2001; Sainz et al., 2001)].
The severe effects that genetic ablation of either T1R2 or T1R3 has on the capacity of mice to respond to sugars has provided compelling evidence of the importance of the T1R2 + T1R3 for “sweet” taste. Indeed, polymorphisms in the Tas1r3 gene in various congenic and inbred strains of mice are associated with differences in responsiveness to sweeteners, corroborating the gene deletion findings (Bachmanov et al., 2016; Eylam & Spector, 2004; Inoue et al., 2004, 2007). For example, Eylam and Spector (Eylam & Spector, 2004) used the gustometer mentioned above along with the two-response operant taste detection procedure to psychophysically measure taste detection thresholds for sucrose, glucose, and the sweet-tasting amino acid glycine, in several strains of inbred mice, some of which are subsensitive to sweeteners due to variation in the Tas1r3 gene. Interestingly, across all of these mice, sucrose and glucose thresholds correlated quite highly with one another, but did not correlate as well with thresholds for glycine, which is a ligand that also activates the T1R1 + T1R3 heterodimer (Nelson et al., 2002). Importantly, responsiveness to glycine is not thought to be affected by the T1R3 polymorphism (Bachmanov et al., 2016; Inoue et al., 2004, 2007). Thus, with respect to sensory-discriminative taste function as assessed by a signal detection task, sugars (at least sucrose and glucose) appear to activate a common taste receptor(s) consistent with the properties of the T1R2 + T1R3 heterodimer. That said, it is important to note that these findings do not necessarily preclude the existence of T1R-independent taste receptors that are activated by one or more of these sugars, the output of which may be channeled into circuits subserving other taste functions such as ingestive motivation of cephalic phase responses (more on this in later sections).
Although sugars seem to activate the T1R2 + T1R3 receptor, Polycose, for the most part, does not. Genetic deletion of the T1R2 or T1R3 subunit in mice, while severely disrupting concentration-dependent responding to sucrose, has only minor effects on responding to Polycose in brief access taste tests (Treesukosol, Blonde, & Spector, 2009; Zukerman, Glendinning, Margolskee, & Sclafani, 2009), lending significant support for the polysaccharide taste receptor hypothesis initially proposed by Tony Sclafani. These gene deletion results were presaged by the finding that polymorphisms in the T1R3 receptor in mice affected behavioral and electrophysiologically assessed nerve responsiveness to many common sweeteners, but not to Polycose (Inoue et al., 2007).
Our group followed up on this work in several ways. First we tested T1R2 KO, T1R3 KO, and T1R2 + T1R3 double KOs for their responsiveness to glucose, maltose (2 glucose moieties), maltotriose (3 glucose moieties), and Polycose in a brief access test in which mice were presented with randomized blocks of 5-s trials of taste stimuli (and water) and licking was quantified (Treesukosol, Smith, & Spector, 2011). As expected, the wild-type (WT) mice displayed concentration-dependent licking to all of the carbohydrate solutions on the first session. In contrast, the three KO groups did so only for Polycose. From this, we inferred that the ideal stimulus for the proposed polysaccharide receptor was an oligomer greater than 3 glucose units. This was consistent with the finding published by Sclafani et al. (Sclafani, Hertwig, Vigorito, Sloan, & Kerzner, 1987) suggesting that the optimal chain length for maximal preference and acceptability in short-term intake tests was between 4 and 8 glucose units in rats Together, these findings confirm that input arising from the T1R2 + T1R3 receptor contributes to ingestive motivation for the taste of sugars while importantly also showing that the taste of Polycose must motivate ingestive responding through a T1R2 + T1R3-independent pathway.
Second, we conducted psychophysical experiments in a newer version of the gustometer (Spector et al., 2015). Treesukosol and Spector (Treesukosol & Spector, 2012) used the two-response operant taste detection procedure to measure sensitivity to Polycose and a variety of sugars. The T1R2 KO and T1R3 KO mice displayed clear concentration-dependent detection of Polycose, but their performance was not quite as good as WT mice. This difference may be due to the fact that control mice can take advantage of the low molecular weight sugars in the mixture, whereas KO mice cannot. In contrast, the performance of these same groups of KO mice was severely impaired when sucrose, glucose, and maltose were tested. Thus, in this task, that involves the use of taste as a cue to guide behavior for a reinforcing event (water) and does not rely on the hedonic characteristics of the stimulus to drive responses, T1R2 KO and T1R3 KO mice could competently detect Polycose albeit not as well as WT mice. In contrast, these KO mice could only poorly detect the sugars tested, if at all. Simply put, whereas the ability to detect sugars largely depends on the T1R2 + T1R3 heterodimer, the ability to detect Polycose does not.
Through gene deletion approaches, Sclafani and colleagues (Sclafani & Ackroff, 2014; Sclafani, Zukerman, Glendinning, & Margolskee, 2007) have shown that the ability to display normal preferences for Polycose solutions depends on the presence of TRPM5, α-gustducin, and the purinergic P2X2/P2X3 receptor; the fact that these proteins are all critical components in some taste receptor cell signaling cascades or neurotransmission strongly implicates the importance of the gustatory system in the maintenance of behavioral responsiveness to Polycose. It is also worth noting that recent psychophysical studies suggest that humans can detect the taste of maltodextrin solutions even when the T1R2 + T1R3 receptor is inhibited by lactisole treatment which is known to attenuate the taste of sweeteners (Lapis, Penner, & Lim, 2014; Lapis, Penner, & Lim, 2016).
Polycose is clearly capable of activating an orosensory mechanism(s) that is independent of the T1R2 + T1R3 heterodimer, but what about sugars? Although the underlying mechanism remains to be identified, the finding that rats, mice, and humans, as noted above, appear to be able to qualitatively discriminate, at least to some extent, between sucrose and maltose when taste intensity is controlled implies that these two sugars are not activating an identical set of sensory receptors. There are also some hints from studies with T1R KO mice that suggest the possibility that there is residual nerve and behavioral responses to simple sugars (Damak et al., 2003; Delay, Hernandez, Bromley, & Margolskee, 2006; Ohkuri et al., 2009; Zhao et al., 2003; Zukerman et al., 2009). Some of these results can be possibly explained by methodology and some might be explained by the potential capacity of the remaining subunit in single KO mice to serve as a homodimer. We believe that disparities in the literature regarding the contribution of the T1R2 + T1R3 heterodimer to sugar taste have arisen precisely because some taste functions are more dependent on input from this receptor and others are not.
3. Support for a T1R2 + T1R3-independent sugar receptor
Indeed, there is evidence, old and new, with respect to the physiological domain of gustatory function that not all sugars stimulate the same taste receptors. In 1984, Harvey Grill and his colleagues found that glucose was the only sweetener tested that, when orally delivered in a small volume, was capable of stimulating an early rise in plasma levels of insulin without any detectable rise in blood glucose (Grill, Berridge, & Ganster, 1984).
More recently, John Glendinning and Tony Sclafani along with their coworkers found that in B6 mice, glucose and sucrose, but not equimolar fructose, were capable of triggering an early rise (at 5 min) in serum insulin when orally ingested; no such early insulin response was seen when these sugars were intragastrically infused (Glendinning et al., 2015). Importantly, deletion of the T1R3 subunit did not curtail the effectiveness of glucose and sucrose to elicit an orally triggered early rise in insulin levels. These results, coupled with the fact that all three sugars were effective at stimulating vigorous licking in a brief access taste test in B6 mice but not in T1R3 KO mice, provides an elegant dissociation of taste function between the ingestive motivation and physiological domains.
A final experiment that is relevant to this topic was recently published (Schier & Spector 2016). The design took advantage of the fact that the postingestive consequences of glucose stimulate ingestion and reinforce preferences for novel flavors more readily than those of equicaloric fructose, as was shown by Tony Sclafani and Karen Ackroff (Sclafani & Ackroff, 1994, 2012). One group of food-deprived rats received a single solution of glucose or fructose in 30-min sessions across 18 days. Concentration was varied and the sugar offered in a given session was randomized in blocks. A second group received the same concentrations of only glucose solutions across the 18 days (glucose-only group), while a third group only received the fructose solutions (fructose-only group). A fourth group was kept sugar-naïve and was given concentrations of corn oil equicaloric with the sugar solutions. When all four groups were tested in a brief access licking assay in which all 3 concentrations of glucose and fructose were presented in blocks of six 15-s trials in the same test session, the glucose-only, fructose-only, and sugar-naïve groups treated the fructose concentrations almost identically to the glucose concentrations. However, the group that was exposed to glucose and fructose on separate days during the 30-min sessions displayed enhanced licking responses to the glucose solutions compared with the fructose solutions in the brief access taste test.
While still speculative, our favored interpretation of the findings from this study coupled with the results regarding cephalic phase insulin release discussed above is that in addition to the T1R2 + T1R3 heterodimer, there is a taste receptor (though we cannot yet entirely exclude the contribution of other sensory modalities) that responds to one of these sugars (presumably glucose) and not the other (see Fig. 1). Sucrose may also be effective because of the presence of sucrase on the apical membranes of a subset of taste receptor cells thus providing a source of glucose (Sukumaran et al., 2016). Under naïve conditions, activation of the glucose receptor is channeled into circuits that subserve the cephalic phase insulin release, but perhaps only weakly activates circuits that subserve ingestive motivation. Meanwhile, the positive hedonic responses normally elicited by various simple sugars are likely dominated by the signals that arise from the T1R2 + T1R3 receptor. However, when rats are given significant ingestive experience with both solutions one at a time, they learn that the input arising from the common receptor (i.e., T1R2 + T1R3) is not a reliable a predictor of the positive postingestive outcome, and, instead, input arising from the alternative (glucose) receptor is a better predictor. Accordingly, experience modifies the relative strengths of the T1R-dependent and T1R-independent orosensory inputs for sugars, at least with respect to the circuits that give rise to ingestive motivation (Fig. 2). Whether the same is true for the other domains of taste function (e.g., sensory-discriminative) remains to be determined.
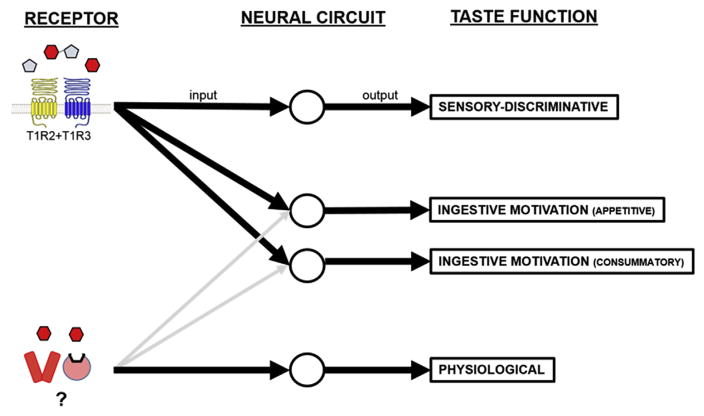
Fig. 1.
Schematic depiction of the hypothesized gustatory pathways that contribute to perceptual/behavioral and physiological responses to sugars in rodents. Taste function can be divided into three primary domains—sensory-discriminative, ingestive motivation (appetitive and consummatory), and physiological reflexes. Separate, or at least partially separate, central circuitries are proposed to mediate each of these functional domains. Accumulating evidence suggests that at least two different classes of taste receptors for sugars exist in the periphery (see text). Our model posits that these receptors are differentially channeled into circuits subserving different taste functions. Accordingly, one class—the canonical T1R2 + T1R3 heterodimeric receptor—binds with many of the common sugars [glucose (red), fructose (grey), sucrose (red + grey)] and other sweeteners (not shown) and predominately feeds into sensory-discriminative and ingestive motivation pathways. The other class, which has yet to be identified, binds with glucose and sucrose (possibly as a result of glucose product from enzymatic breakdown via sucrase in the apical membranes of some taste receptor cells), but not fructose, and predominately feeds into pathways involved in the generation of physiological preparatory reflexes, such as the cephalic phase insulin response. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
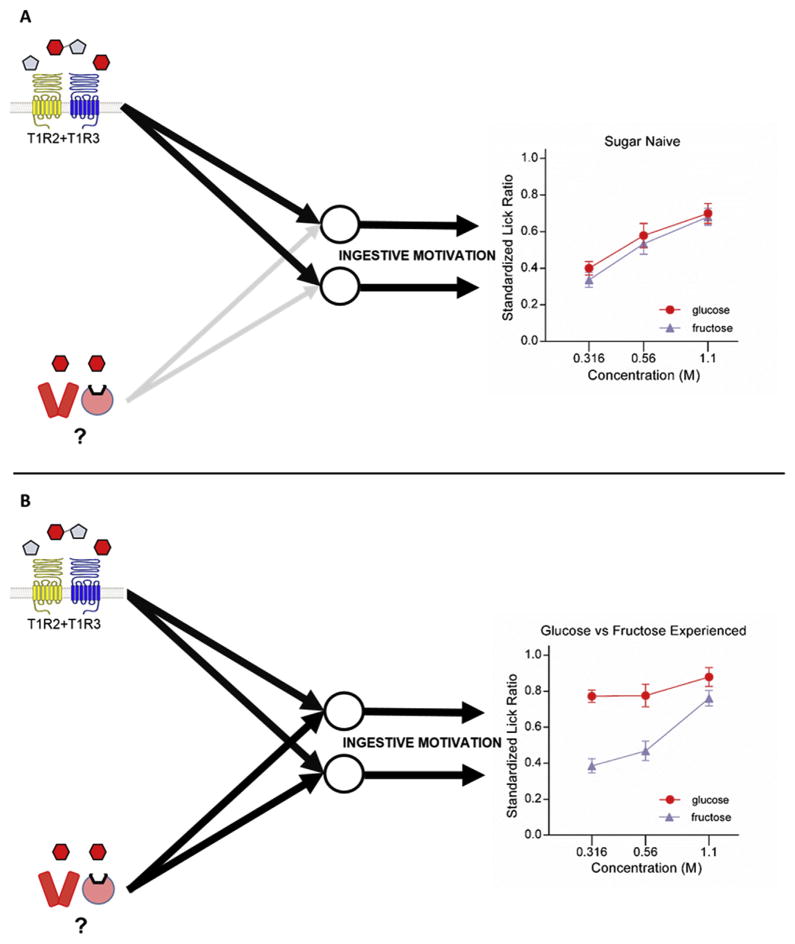
Fig. 2.
Consistent with the model presented in Fig. 1, Schier and Spector (2016) found that sugar-naïve rats (as well as other control groups, not shown) licked at near identical rates at each of three test concentrations of glucose and fructose—presented in discrete 15-s trials (order randomized) in a brief access taste test (see line graph in panel A). However, this same study found that rats that had prior experience consuming these two metabolically distinct sugars in separate single bottle access sessions, licked substantially more for glucose over fructose in the same test (see line graph in panel B). Accordingly, then, our model further posits that certain circumstances (e.g., experience) can modify the strength of inputs (either peripherally or centrally) arising from the yet to be identified alternative sugar receptor into the circuits that subserve ingestive motivation, ultimately permitting the orosensory properties of some sugars (e.g., glucose) to become more motivationally salient than that of other sugars. The T1R-independent stimulus features that underlie this differential enhanced responsivity to glucose is still unknown (e.g., taste, olfactory, somatosensory). Whether these alternative sugar receptors differentially contribute to other aspects of taste function (e.g., sensory-discriminative) also awaits investigation.
4. Final remarks
Thus, when considered on the whole, the evidence suggests that there are T1R-independent receptor mechanisms that are activated by select carbohydrate stimuli and that this not only includes the polysaccharide receptor that was proposed by Tony Sclafani 30 years ago, but may also involve receptors responsive to select sugars such as glucose. Like the polysaccharide receptor, the proposed selective oral glucose sensing mechanism remains to be identified, but some glucose transporters and the ATP-sensitive K+ channel (known to serve as a metabolic sensor) have been proposed as candidates (Liu, Liu, Zhou, Feng, & Zhang, 2011; Merigo, Benati, Cristofoletti, Osculati, & Sbarbati, 2011; Sukumaran et al., 2016; Toyono, Seta, Kataoka, Oda, & Toyoshima, 2011; Yee, Sukumaran, Kotha, Gilbertson, & Margolskee, 2011; Yoshida et al., 2015). From a broader perspective, the set of results discussed above highlights the importance of accounting for the multidimensional nature of taste function in the interpretation of experimental outcomes of manipulations of the gustatory system (Spector, 2000).
Acknowledgments
A portion of the work presented in this article was supported, in part, by grants from the National Institute on Deafness and Other Communication Disorders: R01-DC01628 (ACS), R01-DC004574 (ACS), and F32-DC-013494 (LAS).
References
- Bachmanov AA, Bosak NP, Glendinning JI, Inoue M, Li X, Manita S, et al. Genetics of amino acid taste and appetite. Advances in Nutrition. 2016;7:806S–822S. doi: 10.3945/an.115.011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, et al. Positional cloning of the mouse saccharin preference (sac) locus. Chemical Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin PAS, Beauchamp GK, Pugh EN. Monogeusia for fructose, glucose, sucrose, and maltose. Perception & Psychophysics. 1996;58:327–341. doi: 10.3758/bf03206809. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chemical Senses. 2006;31:351–357. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: Implications for the neural coding of T1R ligands. Journal of Neuroscience. 2007;27:11242–11253. doi: 10.1523/JNEUROSCI.1227-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylam S, Spector AC. Stimulus processing of Glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in sac ‘taster’ and ‘Non-taster’ mice. Chemical Senses. 2004;29:639–649. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, et al. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2015;309:R552–R560. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1984;246:R88–R95. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, et al. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: Evidence from 129.B6-Tas1r3 congenic mice. Physiological Genomics. 2007;32:82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. Journal of Neuroscience. 2004;24:2296–2303. doi: 10.1523/JNEUROSCI.4439-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochemical and Biophysical Research Communications. 2001;283:236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- Lapis TJ, Penner MH, Lim J. Evidence that humans can taste glucose polymers. Chemical Senses. 2014;39:737–747. doi: 10.1093/chemse/bju031. [DOI] [PubMed] [Google Scholar]
- Lapis TJ, Penner MH, Lim J. Humans can taste glucose oligomers independent of the hT1R2/hT1R3 sweet taste receptor. Chemical Senses. 2016;41:755–762. doi: 10.1093/chemse/bjw088. [DOI] [PubMed] [Google Scholar]
- Liu DX, Liu XM, Zhou LH, Feng XH, Zhang XJ. Expression of sulfonylurea receptors in rat taste buds. Acta Histochemica. 2011;113:489–492. doi: 10.1016/j.acthis.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nature Genetics. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. Journal of Anatomy. 2011;219:243–252. doi: 10.1111/j.1469-7580.2011.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nature Neuroscience. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neuroscience and Biobehavioral Reviews. 1987;11:187–196. doi: 10.1016/s0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;296:R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. Journal of Neurochemistry. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- Schier LA, Spector AC. Behavioral evidence for more than one taste signaling pathway for sugars in rats. Journal of Neuroscience. 2016;36:113–124. doi: 10.1523/JNEUROSCI.3356-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiology and Behavior. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiology and Behavior. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Maltodextrin and fat preference deficits in “taste-blind” P2X2/P2X3 knockout mice. Chemical Senses. 2014;39:507–514. doi: 10.1093/chemse/bju019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Hertwig H, Vigorito M, Sloan H, Kerzner B. Influence of saccharide length on polysaccharide appetite in the rat. Neuroscience and Biobehavioral Reviews. 1987;11:197–200. doi: 10.1016/s0149-7634(87)80026-x. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: The contribution of alpha-gustducin and Trpm5 taste-signaling proteins. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2007;293:R1504–R1513. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neuroscience and Biobehavioral Reviews. 2000;24:391–416. doi: 10.1016/s0149-7634(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Spector AC, Blonde GD, Henderson RP, Treesukosol Y, Hendrick P, Fletcher FH, et al. A new gustometer for taste testing in rodents. Chemical Senses. 2015;40:187–196. doi: 10.1093/chemse/bju072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Grill HJ. Differences in the taste quality of maltose and sucrose in rats: Issues involving the generalization of conditioned taste aversions. Chemical Senses. 1988;13:95–113. [Google Scholar]
- Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1997;41:R1210–R1228. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- Sukumaran SK, Yee KK, Iwata S, Kotha R, Quezada-Calvillo R, Nichols BL, et al. Taste cell-expressed alpha-glucosidase enzymes contribute to gustatory responses to disaccharides. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:6035–6040. doi: 10.1073/pnas.1520843113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell and Tissue Research. 2011;345:243–252. doi: 10.1007/s00441-011-1210-x. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: Implications for saccharide taste receptors in mice. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. Journal of Neuroscience. 2011;31:13527–13534. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2012;303:R218–R235. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Noguchi K, Shigemura N, Jyotaki M, Takahashi I, Margolskee RF, et al. Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes. 2015;64:3751–3762. doi: 10.2337/db14-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]