Abstract
Despite promising trends of the efficacy of mobile health (mHealth) based strategies to a broad range of health conditions, very few if any studies have been done in terms of the examining the use of mHealth in HIV prevention efforts among people who use drugs in treatment. Thus, the goal of this study was to gain insight into the real-world acceptance of mHealth approaches among high-risk people who use drugs in treatment. A convenience sample of 400 HIV-negative drug users, who reported drug- and/or sex-related risk behaviors, were recruited from a methadone clinic in New Haven, Connecticut. Participants completed standardized assessments of drug- and sex-related risk behaviors, neurocognitive impairment (NCI), and measures of communication technology access and utilization, and mHealth acceptance. We found a high prevalence of current ownership and use of mobile technologies, such as cell phone (91.5%) including smartphone (63.5%). Participants used mobile technologies to communicate mostly through phone calls (M = 4.25, SD = 1.24), followed by text messages (M = 4.21, SD = 1.29). Participants expressed interest in using mHealth for medication reminders (72.3%), receive information about HIV (65.8%), and to assess drug-related (72.3%) and sex-related behaviors (64.8%). Furthermore, participants who were neurocognitively impaired were more likely to use cell phone without internet and show considerable interest in using mHealth as compared to those without NCI. The findings from this study provide empirical evidence that mHealth-based programs, specifically cell phone text messaging-based health programs, may be acceptable to this high-risk population.
Keywords: mHealth, text messaging, substance abuse, people who use drugs, neurocognitive impairment
INTRODUCTION
Substance use disorders have been inextricably linked with HIV with a wide range of social and health consequences [1]. Despite recent evidence showing that the number of diagnosed HIV infections attributed to people who use drugs has decreased [2], they still remain a priority population for the prevention of HIV. People who use drugs represent a population vulnerable to new HIV infections mostly as a result of preventable drug-related (e.g., sharing of injection equipment) and sex-related (e.g., condomless sex) HIV risk behaviors [3–7].
Additionally, recent studies have shown that a disproportionate percentage of people who use drugs (30% – 40%) display a wide range of cognitive deficits – such as difficulties in executive function, memory, attention, new learning, information-processing speed – that have significant impact on HIV risk behaviors and risk-reduction intervention outcomes [8–15]. For example, deficits in executive function influences rational decision-making, which may impede individuals from making safer sexual choices. Similarly, slowed information processing function may prevent the timely, appropriate consideration of risk variables during decision-making instances [15]. As a result of chronic drug use, related lifestyle experiences, and relatively poor health, a disproportionate percentage of people who use drugs experience NCI to the extent that it may be disruptive to their participation in treatment services, including decreased treatment engagement, poor treatment retention, and suboptimal medication adherence [16–21].
Considerable progress has been made in developing and expanding the evidence-based HIV prevention interventions [22]. The available evidence-based interventions, however, do not adequately address the unique cognitive needs of people who use drugs and, thus, have resulted in this high risk population deriving less benefit from existing interventions [23]. Failing to properly intervene with this high risk population has resulted in poor individual outcomes, and also threatens public health by increasing the likelihood of HIV transmission via people who use drugs. These individuals – and the communities in which they live – would greatly benefit from improving and expanding existing EBIs.
The recent availability of pre-exposure prophylaxis (PrEP) has provided unprecedented opportunities in the public health response to curtail the HIV epidemic. Findings from recent PrEP trials have demonstrated that taking PrEP daily significantly reduces HIV transmission among those who are at substantial risk of acquiring HIV infection, including people who use drugs [24–27]. Early optimism related to the potential benefits of PrEP may be diminished with regard to certain risk populations, however, due to evidence suggesting that even modest or occasional non-adherence can greatly lessen the effects of PrEP [25, 27, 28]. Particularly with regard to high-risk people who use drugs, the combination of substance abuse and higher levels of neuropsychological symptoms may significantly affect their adherence to PrEP, engagement and retention in HIV interventions as well as their ability to acquire and retain the knowledge and skills necessary to modify their risky behaviors [16–20]. Therefore, incorporation of innovative tools to improve medication adherence, health-promoting behaviors, and access to and utilization of health care are urgently needed, particularly tailored toward people who use drugs with NCI.
There has been growing interest in the use of mobile technologies for health (mHealth) in health care and public health practice to facilitate delivery of health promotion and disease prevention initiatives [29–33]. mHealth interventions (e.g., phone calls or text messaging reminders, boosters to reinforce risk reduction skills) have been shown to have a positive impact on medication adherence, appointment attendance, and health-promoting behaviors (e.g., modify risky behaviors) [30–37]. Furthermore, growth in the number of health-related ‘apps’ and steady reduction in cost of mobile devices have created the potential for mHealth to add considerable value to medical care and public health programs, particularly among high risk populations with limited resources. As mobile technology access, utilization, and services become increasingly prevalent, this seems to be an ideal tool to incorporate within existing EBIs to facilitate self-monitoring, positive reinforcement, and remote coaching to improve medication (e.g., PrEP) adherence and to modify risk behaviors.
Over the past few decade, the adoption of mobile technologies throughout the United States has grown significantly. Mobile device sales are expected to grow from 172 million in 2009 to 215 million in 2016, an increase of 25% [38]. Recent estimate showed that 31% of cell phone users use their phones to look up health information, almost double that of those who did this in 2010 (17%) [39]. Despite these promising trends, there is a dearth of evidence specific to on the use of mHealth tools in addressing the needs of high risk people who use drugs who are enrolled in substance abuse treatment settings (e.g., methadone maintenance programs: MMP). To our knowledge, only one study has assessed the utilization of communication technology [40]; however, no published data on the acceptability of mHealth-based interventions in high-risk people who use drugs exist. Furthermore, although the popularity of using mHealth has increased among the general population [29, 30], previous research has expressed concerns that certain characteristics, such as cognitive deficits, may present barrier towards wide scale use of mHealth [41]. No studies to date, however, have assessed the influence of NCI on mHealth utilization, particularly among people who use drugs in treatment. Thus, the goal of this study was to gain insight into the real-world acceptance of mHealth approaches among high-risk people who use drugs in treatment. More specifically, we wanted to examine: 1) the current ownership and utilization of communication technology (e.g., landline phone, internet, cell phone), 2) acceptability of future mHealth-based approaches, and 3) whether ownership and utilization of communication technology and acceptability of mHealth differs between individuals with and without significant NCI. The findings from this study will help to gauge the possibility of incorporating mHealth-based approaches in HIV-risk reduction programs to enhance medication (e.g., PrEP) adherence and to modify risky behaviors.
METHODS
Participants
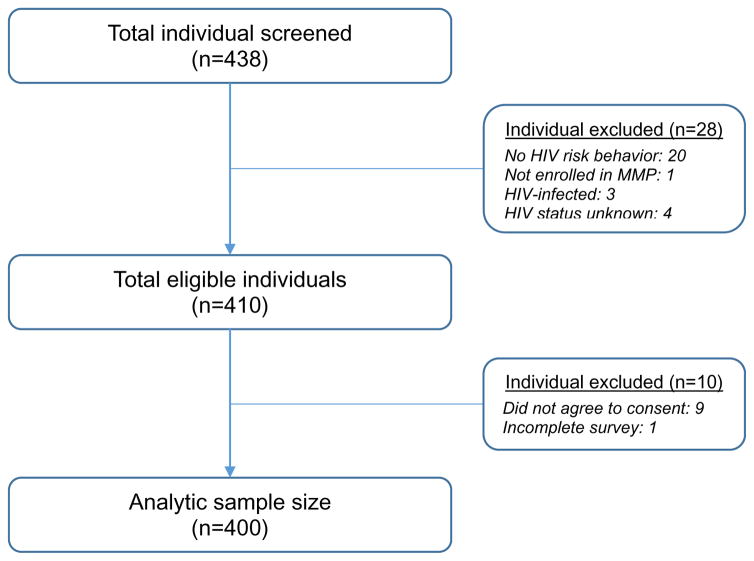
A total of 400 participants were recruited using a convenience sampling process between June and July 2016. Individuals were eligible if they were: i) 18 years or older, ii) HIV-negative, iii) reported drug- or sex-related HIV risk behaviors in the past 6 months, iv) met DSM-V criteria for opioid use disorders, v) enrolled in MMP, vi) able to understand, speak, and read English, and vii) able to provide informed consent. Figure 1 shows the schematic representation of sampling plan for this study.
Figure 1.
Schematic representation of sampling plan for the study
A total of 438 people completed the initial screening form; however, 28 were screened out because they did not meet eligibility criteria. Of those who were eligible to participate (n=410), one participant did not complete the survey and nine did not agree to participate in the study. Therefore, the final sample size included 400 participants.
Study setting and procedures
This cross-sectional study was conducted among high-risk people who use drugs within APT Foundation, which is a community-based facility in New Haven, Connecticut that provides drug treatment and clinical care to opioid-dependent drug users. The MMP is offered by the APT Foundation and is dispensed daily by MMP nurses; case management and counseling is provided by counselors certified by the State of Connecticut to provide substance abuse counseling.
Participants were recruited via flyers, peers, word-of-mouth, and direct referral from counselors at the APT Foundation MMP. Potential participants were screened in-person in a private room or by phone using a standard screening form. Individuals who met inclusion criteria, and who were willing to participate, were provided a description of the study and invited to provide informed consent, followed by the survey. Participants were assessed using audio computer assisted self-interview (ACASI) that has demonstrated sound psychometric properties in prior studies [42, 43] and controlled trials [44]. All participants were reimbursed for the time and effort needed to participate in the survey. The study protocol was approved by the Investigational Review Board (IRB) at the University of Connecticut and received board approval from APT Foundation Inc.
Measures
Participant characteristics
Data were collected regarding a number of participant characteristics, including age, gender, sexual orientation, ethnicity, marital status, educational status, primary language, employment status, income, health insurance, visit to psychiatrist, homeless status, and methadone dose. We also assessed whether participants were prescribed any medication (other than methadone) in the past 30 days, and for those who were, we assessed medication adherence (range: 0 – 100) with a self-reported, validated three-item scale [45].
Drug- and sex-related risk behaviors
Participants’ self-report measure of drug use and sex-related HIV risk behaviors during the past 30 days were assessed using an adapted version of the HIV risk-taking behavior scale (HRBS) [46]. We utilized seven out of the eleven items from the original scale to measure risk behaviors among our sample (Table 2).
Table 2.
Frequency of use of communication technology stratified by neurocognitive impairment status of the participants (N=400)
| Variable | Overall Mean (SD) | NCI |
Cohen’s d | t | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Basic cell phone | 2.75 (1.84) | 2.59 (1.86) | 3.13 (1.72) | −0.30 | −2.74 | 0.006 | (−0.93, −0.15) |
| Smartphone | 3.38 (1.89) | 3.58 (1.83) | 2.93 (1.94) | 0.34 | 3.16 | 0.002 | (0.24, 1.04) |
| Landline telephone | 1.61 (1.45) | 1.55 (1.40) | 1.76 (1.54) | −0.14 | −1.34 | 0.178 | (−0.52, 0.09) |
| Tablet | 1.72 (1.69) | 1.66 (1.68) | 1.87 (1.72) | −0.12 | −1.15 | 0.251 | (−0.57, 0.15) |
| Laptop | 1.66 (1.65) | 1.60 (1.67) | 1.78 (1.61) | −0.10 | −0.97 | 0.332 | (−0.52, 0.17) |
| Personal Computer | 1.40 (1.49) | 1.37 (1.51) | 1.46 (1.44) | −0.06 | −0.60 | 0.549 | (−0.41, 0.22) |
Note:
SD: Standard deviation
NCI: Neurocognitive impairment
CI: Confidence interval
1 = ‘never’, 2 = ‘rarely’, 3 = ‘sometimes’, 4 = ‘Often’, 5 = ‘all the time’
Access to and frequency of use of communication technology
We utilized an adapted version of an existing scale [47] to measure participants’ access to and frequency of use of various types of communication devices (e.g., landline telephone, cell phone, tablet, laptop, personal computer). Participants were asked how often they use various types of communication technologies on a 5-point Likert scale (ranging from 1 = ‘never’ to 5 = ‘all the time’).
Utilization of cell phone/smartphone
Participants’ utilization of cell phone, including smartphone, for various activities (e.g., make or receive phone calls, send or receive text messages, take a picture, record video, access internet, send or receive emails, download applications, listen to music, watch videos, play games, use health-related apps, etc.) was assessed using a 5-point Likert scale (ranging from 1 = ‘never’ to 5 = ‘all the time’) questions [47].
Acceptability of mHealth
Participants were asked whether they would be willing to use mHealth in several ways in order to promote better health outcomes, such as to receive reminders to take medication, to receive information about HIV, and to assess drug and sexual behaviors. In this context, we used three items, which were measured on a 5-point Likert scale (ranging from 1 = ‘not interested at all’ to 5 = ‘extremely interested’). Additionally, participants’ frequency (i.e., daily, weekly, and monthly) and preference of type of mHealth use (i.e., phone call, text message, email, or any methods) were also assessed [47].
Neurocognitive impairment (NCI)
NCI was measured using the Brief Inventory of Neurocognitive Impairment (BINI), which is a 54-item, brief, self-report measure of neuropsychological symptoms [48]. The BINI was developed as a quick and convenient way to help elicit diagnostically relevant information about both general NCI and specific symptom (e.g., attention, memory, linguistic functioning, etc.). The overall BINI score was obtained by summing responses to all items. Scores were then converted to age-adjusted standardized scores (i.e., z-scores) based on normative data. Participants with an age-adjusted z-score of ≥ 0.5 were classified as neurocognitively “impaired”(at least mild impairment), whereas, those with < 0.5 were classified as “not impaired” [49, 50].
Data analysis
All data analyses were performed using SPSS v. 22 [51]. We computed descriptive statistics, including frequencies and percentages for categorical variables and means, standard deviations, and ranges for continuous variables. We used chi-square tests to examine the statisitical differences by NCI status based on participants ownership or access to communication technologies. Additionally, two-sided t-tests were conducted to determine whether differences existed in mHealth acceptance between participants with and without NCI. Estimates were evaluated for statistical significance based on 95% confidence intervals using p < .05.
RESULTS
Participant characteristics
Participants were mostly in their early 40s (mean=40.9; SD=11.1 years), male (58.5%), White (63.2%), and single (48.3%). The majority of the participants identified as heterosexual or straight (86.5%), reported to have English as a primary language (92.3%) and to have graduated from high school (73.2%). Only 17.3% of the participants reported being currently employed and 78% were earning less than $10,000 per year. Over 95% of participants reported to have health insurance, 91.8% reported to have seen a healthcare provider in the past 12 months, and more than half of the participants (50.2%) reported to have been homeless in the past 12 months.
All participants were enrolled in an inner-city MMP and were maintained on a stable dose of methadone. The mean daily methadone dose was 81.5 (SD = 28.4) mg. Almost one-third of the participants (30.3%) were classified as being neurocognitively impaired. In addition, the majority reported taking prescribed medication (other than methadone) in the past 30 days. The average total score for medication adherence was 73.3 (SD = 15.4) on a scale of 0 – 100.
Drug- and sex-related risk behaviors
Self-reported recent HIV risk behaviors were highly prevalent among study samples. Over half of the participants (57.5%) reported current drug injection in the past 30 days. Of those, two-thirds reported having shared injection equipment while only 21.3% reported having always cleaned their needles with bleach before re-using. Of those who were sexually active (82.0%), 40% reported having sex with more than one sexual partner and only 7.9%, 14.9%, and 9.1% reported always using condoms with regular, casual, and paid sexual partners, respectively. Of the total sample, 13.5% reported having been diagnosed with a sexually transmitted infection.
Access to and frequency of use of communication technology
Data in Table 1 show that 91.5% of participants reported to owning/having daily access to cell phone: 63.5% reported owning/having daily access to a cell phone with internet access (i.e., smartphone), 42.5% owing a phone without internet access (i.e., basic cell phone), and 14.5% owning both (i.e., smartphone and basic cell phone). Rates of ownership were fairly consistent across other devices, such as landline phones (22.8%), tablet (21.5%), and laptops (21.5%). Also, 14.2% regularly accessed a personal computer and 6% reported having access to other devices (e.g., Personal Digital Assistant, Google Glass, Samsung Smartwatch). The results of the chi-square suggested that participants with NCI were significantly (2.20 times) less likely to own a cell phone (either smartphone or basic phone: (OR = 0.45, χ2 = 4.97, p = 0.026) and 2.10 times less likely (OR = 0.47, χ2 = 11.25, p < 0.001) to report having daily access to smartphone.
Table 1.
Ownership or access to communication technology stratified by neurocognitive impairment status of the participants (N=400)
| Variables | Total n(%) |
NCI |
OR | χ2 | p | |
|---|---|---|---|---|---|---|
| No N=279 (69.7%) |
Yes N=121 (30.3%) |
|||||
| Cell phone | ||||||
| With internet access (Smartphone) | 254 (63.5) | 192 (48.0) | 62 (15.5) | 0.47 | 11.25 | 0.001 |
| Without internet access (Basic phone) | 170 (42.5) | 114 (28.5) | 56 (14.0) | 1.24 | 1.01 | 0.314 |
| Either (Smartphone or basic phone) | 366 (91.5) | 261 (65.3) | 105 (26.3) | 0.45 | 4.97 | 0.026 |
| Both (Smartphone and basic phone) | 58 (14.5) | 45 (11.3) | 13 (3.3) | 0.62 | 1.97 | 0.160 |
| Landline telephone | 91 (22.8) | 67 (16.8) | 24 (6.0) | 0.78 | 0.83 | 0.360 |
| Tablet | 86 (21.5) | 64 (16.0) | 22 (5.5) | 0.74 | 1.13 | 0.287 |
| Laptop | 85 (21.3) | 63 (15.8) | 22 (5.5) | 0.76 | 0.97 | 0.323 |
| Personal Computer | 57 (14.2) | 41 (10.3) | 16 (4.0) | 0.88 | 0.15 | 0.699 |
| Other devices | 24 (6.0) | 17 (4.3) | 7 (1.8) | 0.94 | 0.01 | 0.905 |
Note:
NCI: Neurocognitive impairment
OR: Odds ratio
Other devices includes Personal Digital Assistant, Google Glass, Samsung Smartwatch
As shown in Table 2, the most frequently used communication device among participants was a smartphone (M = 3.38, SD = 1.89), followed by basic cell phone (M = 2.75, SD = 1.84), and tablet (M = 1.72, SD = 1.69). Among the least frequently used devices included laptop (M = 1.66, SD = 1.65), landline telephone (M = 1.61, SD = 1.45), and personal computer (M = 1.40, SD = 1.49). Interestingly, t-test results showed that those who were neurocognitively impaired were significantly more likely to use a basic cell phone [t(398) = −2.74, p = 0.006, d = −0.30] but less likely to use a smartphone [t(398) = 3.16, p = 0.002, d = 0.34] as compared to those without NCI.
Utilization of cell phone/smartphone
The most frequent activity participants engaged on their cell phone was making or receiving phone calls (M = 4.25, SD = 1.24), followed by sending or receiving text messages (M = 4.21, SD = 1.29). The least frequent activities were online banking (M = 1.50, SD = 1.28) and reading e-books (M = 1.26, SD = 1.26). An independent sample t-test showed that the difference in the utilization of a cell phone to make or receive phone calls, [t(398) = 1.71, p = 0.084, d = 0.19], and to send or receive text messages, [t(398) = 1.38, p = 0.166, d = 1.38], was not statistically significant between participants with and without NCI; however, a significant difference was observed with regard to taking photos, [t(398) = 2.08, p = 0.038, d = 2.08], and accessing the internet, [t(398) = 2.00, p = 0.046, d = 2.00] (Table 3).
Table 3.
Communication technology utilization stratified by neurocognitive impairment status of the participants (N=400)
| Variable | Overall Mean (SD) | NCI |
Cohen’s d | t | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Number of cell phone owned (Currently) | 1.55(1.60) | ||||||
|
| |||||||
| How often do you engage in the following activities on your cell phone or smartphone? | |||||||
|
| |||||||
| Make or receive phone calls | 4.25 (1.24) | 4.32 (1.18) | 4.08 (1.35) | 0.18 | 1.73 | 0.084 | (−0.03, 0.49) |
| Send or receive text messages | 4.21 (1.29) | 4.27 (1.22) | 4.07 (1.44) | 0.15 | 1.38 | 0.166 | (−0.08, 0.47) |
| Access the internet | 3.41 (1.72) | 3.52 (1.91) | 3.15 (1.73) | 0.21 | 2.01 | 0.046 | (0.01, 0.74) |
| Listen to music | 3.34 (1.64) | 3.40 (1.64) | 3.18 (1.64) | 0.13 | 1.23 | 0.218 | (−0.13, 0.57) |
| Take a picture | 3.34 (1.56) | 3.44 (1.52) | 3.09 (1.65) | 0.22 | 2.08 | 0.038 | (0.02, 0.68) |
| Watch videos | 3.12 (1.64) | 3.20 (1.64) | 2.93 (1.65) | 0.16 | 1.51 | 0.131 | (−0.08, 0.62) |
| Online social networking | 2.94 (1.71) | 3.01 (1.73) | 2.77 (1.67) | 0.14 | 1.29 | 0.195 | (−0.12, 0.60) |
| Send or receive emails | 2.78 (1.67) | 2.80 (1.66) | 2.74 (1.69) | 0.03 | 0.33 | 0.741 | (−0.29, 0.41) |
| Download applications | 2.78 (1.67) | 2.85 (1.65) | 2.61 (1.69) | 0.14 | 1.31 | 0.190 | (−0.11, 0.59) |
| Record video | 2.63 (1.58) | 2.63 (1.55) | 2.62 (1.64) | 0.01 | 0.08 | 0.932 | (−0.32, 0.35) |
| Play games | 2.62 (1.63) | 2.62 (1.63) | 2.62 (1.64) | 0.01 | 0.01 | 0.999 | (−0.35, 0.35) |
| Use health-related apps | 1.84 (1.28) | 1.87 (1.29) | 1.78 (1.27) | 0.07 | 0.67 | 0.501 | (−0.18, 0.36) |
| Make a purchase | 1.82 (1.34) | 1.88 (1.36) | 1.68 (1.29) | 0.15 | 1.40 | 0.161 | (−0.12, 0.60) |
| Reading e-books | 1.65 (1.26) | 1.64 (1.25) | 1.68 (1.29) | −0.03 | −0.26 | 0.793 | (−0.30, 0.23) |
| Online banking | 1.50 (1.28) | 1.52 (1.29) | 1.46 (1.24) | 0.04 | 0.40 | 0.683 | (−0.21, 0.33) |
Note:
SD: Standard deviation
NCI: Neurocognitive impairment
CI: Confidence interval
Acceptability of mHealth
Table 4 depicts participants’ interest in and potential acceptance of mHealth. The majority of the participants (72.3%) indicated that they would like to receive reminders to take their medication(s). Participants were interested in receiving electronic medication reminders mostly on a daily basis (43.5%), followed by a weekly basis (22.3%), and monthly (5.8%). Additionally, there was considerable interest in mHealth among participants to receive information about HIV (65.8%) and to assess drug-related (72.3%) and sex-related risk behaviors (64.8%). In terms of receiving information about HIV, participants mostly preferred to receive information on a weekly basis (27.0%). The most preferred method of mHealth intervention was via text messages for both to receive medication reminders (54.5%) and to receive information about HIV (35.3%).
Table 4.
Interest in and acceptance of mHealth among participants (N=400)
| Variable | Interest in mHealth
|
|
|---|---|---|
| No | Yes | |
| Interested would you be in using mHealth to | ||
| Remind to take medication | 111 (27.8) | 289 (72.3) |
| Frequency | ||
| Daily | 175 (43.5) | |
| Weekly | 89 (22.3) | |
| Monthly | 23 (5.8) | |
| Method | ||
| Phone calls | 41 (10.3) | |
| Text messages | 218 (54.5) | |
| Emails | 13 (3.3) | |
| They are all equally fine | 17 (4.3) | |
| Receive information about HIV | 137 (34.3) | 263 (65.8) |
| Frequency | ||
| Daily | 46 (11.5) | |
| Weekly | 108 (27.0) | |
| Monthly | 107 (26.8) | |
| Method | ||
| Phone calls | 37 (9.3) | |
| Text messages | 141 (35.3) | |
| Emails | 51 (12.8) | |
| They are all equally fine | 34 (8.5) | |
| Assess health behaviours | ||
| Drug use behaviors | 17 (4.3) | 289 (72.3) |
| Sexual behaviors | 47 (11.8) | 259 (64.8) |
The results of an independent samples t-test showed that there were significant differences in terms of all aspects of mHealth acceptance among study participants. Participants who were cognitively impaired were significantly more likely than those without NCI to show interest in using mHealth to remind them to take medication(s), [t(398) = −2.49, p = 0.013, d = −0.26], to receive information about HIV, [t(398) = −4.22, p < 0.001, d = −0.43], to assess drug-related risk behaviors, [t(398) = −2.00, p = 0.046, d = −0.24], and sex-related risk behaviors, [t(398) = −2.61, p = 0.009, d = −0.31] (Table 5).
Table 5.
Interest in and acceptance of mHealth stratified by neurocognitive impairment status of the participants (N=400)
| Variable | Overall Mean (SD) | NCI |
Cohen’s d | t | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| How interested would you be in using mHealth to | |||||||
| Remind to take medication | 2.60 (1.35) | 2.49 (1.29) | 2.85 (1.46) | −0.26 | −2.49 | 0.013 | (−0.65, −0.07) |
| Receive information about HIV | 2.28 (1.22) | 2.11 (1.09) | 2.66 (1.40) | −0.43 | −4.22 | <0.001 | (−0.80, −0.29) |
| Assess health behaviours | |||||||
| Drug use behaviors | 3.28 (1.18) | 3.19 (1.13) | 3.48 (1.26) | −0.24 | −2.00 | 0.046 | (−0.57, −0.00) |
| Sexual behaviors | 2.94 (1.28) | 2.81 (1.24) | 3.21 (1.33) | −0.31 | −2.61 | 0.009 | (−0.71, −0.10) |
Note:
SD: Standard deviation
NCI: Neurocognitive impairment
CI: Confidence interval
DISCUSSION
The use of mobile communication technologies (e.g., cell phone, smartphone) is growing rapidly to supplement traditional public health programs to promote health and healthy behaviors, raise awareness of health risks, and manage treatment and medication adherence [29, 30, 52]. To our knowledge, however, there is a lack of empirical evidence on the utilization of communication technologies and the potential acceptability of mHealth that is needed to guide the integration and implementation of mHealth strategies targeting toward high-risk drug users in treatment settings. Our study evaluated the current ownership and use of communication technology as well as the potential mHealth acceptance among high-risk people who use drugs. The findings from this study provide preliminary evidence of a previously unexplored area, supporting the integrated mHealth-based approach in HIV-risk reduction programs to enhance medication adherence (e.g., PrEP) and to support risk reduction behaviors among this underserved population.
Among high-risk people who use drugs in treatment, we found a high prevalence of current ownership and use of mobile technologies, particularly cell phone. The overall rate of cell phone ownership among participants was 91.5%, with 63.5% reported to have smartphone ownership, which is slightly lower than the US national ownership rate of 68% [53]. Furthermore, the findings showed that more participants used mobile technologies (i.e., cell phone) to communicate (e.g., primarily through phone calls and text messages) compared to landline phones, tablets, laptops, and personal computers. This likely reflects the digital revolution and the fast-paced growth of mobile technologies and adoption of such technologies among our sampled population. This prevalent use of mobile technologies among people who use drugs may be viewed as an opportunity to integrate mHealth into existing EBI tools.
A novel aspect of this study was that our study indicated considerable interest among high-risk people who use drugs in specific mHealth strategies, such as receiving reminders to take medication(s), receiving information related to HIV, and assessing drug- and sex-related risk behaviors. These findings are comparable to those from prior studies with other risk populations [37, 54, 55]. Participants preferred daily text reminder to remind them to take medication(s); however, they preferred weekly text messages to receive information about HIV. Cell phone calls and text message reminders have been already used successfully in other settings to improve medication adherence by direct reminders and to disseminate health-related information [34, 37, 56, 57]. The high penetration of mobile technologies (i.e., cell phone and smartphone) and its potential acceptability suggest its utility to improve health outcomes among this high-risk population. The findings from this study, thus, provide important insights into how cell phone messages can similarly be utilized in this high-risk population for medication (e.g., PrEP) reminders and additionally to target them with HIV-prevention or risk-reduction health campaigns.
As an extension of prior literature, we explored the differential pattern of the use of communication technologies and mHealth acceptance based on participants’ NCI status in this at-risk population. Our findings indicate that participants with higher levels of cognitive impairment were less likely to own a cell phone, particularly smartphones. Furthermore, those with NCI were more likely to use a basic cell phone but less likely to use smartphone as compared to their counterparts. This may be because impairment in cognitive functioning may act as a barrier for these participants to navigate through a more complex and multifaceted smartphone interface [58, 59] and, thus, these participants may prefer a basic phone. In terms of utilization of cell phone, participants with NCI were less likely to access the internet, which could be driven by the fact that they were less likely to own smartphone, which may be associated with these participants belonging to low socio-economic status (e.g., low income). Alternatively, participants with NCI may lack necessary skills or understanding to benefit maximally from mHealth technology as compared to those without NCI [40]. Whereas, both participants with and without NCI were equally likely to use cell phone for a number of activities, such as phone calls, text messaging, watching videos, and recording videos. Overall, these findings lend support to the notion that cell phone could be used as a tool to accommodate NCI through its use as a new modality of monitoring and treatment support that may include text messaging [37, 56, 60], phone calls [57, 60], video-based eHealth interventions [61–63], video (or virtually) observed therapy (VOT) [64, 65] targeted specifically for this at-risk populations.
Importantly, findings revealed a greater interest and willingness of participants with cognitive deficits to use mHealth technology, particularly text messaging, for medication reminder (e.g., PrEP), receiving HIV-related knowledge, and assessing health behaviors. This could be because neurocognitively impaired participants are not only concerned about their risk of HIV infection but also motivated to engage in treatment by using innovative tools (e.g., use of mHealth) as opposed to their counterparts. This highlights a possibility of incorporating mHealth as an additional strategy to remediate the negative impacts that NCI may cause in various aspects of HIV intervention (e.g., medication adherence, information acquisition and retention, treatment follow-up). It should be noted, however, that careful consideration needs to be given during the design and implementation of such mHealth tools among this high-risk population, including the content of the text messages, the frequency and timing, type of communication (i.e., one-way or two-way communication), the cultural competency of content, the ethical considerations, and the motivation to engage in communication technology [32, 33, 41, 66].
Implications and future studies
Over the past decade, the enthusiasm of clinicians, researchers, and policy makers has led to a rapid proliferation of various mHealth strategies for improving health outcomes [29–31, 33, 52], especially because the Affordable Care Act is pushing practices toward population health management and performance-based payment models [67]. The integration of mHealth tools within existing EBIs has the potential to bridge systemic gaps needed to improve medication adherence as well as more effective utilization of health services, particularly among underserved populations. Furthermore, it can not only gain wider geographical access, but also surmount barriers such as transportation limitations, stigma, and privacy loss associated with traditional intervention strategies. Despite this increasing trend, the research literature investigating the use of mHealth in HIV prevention interventions remains in its infancy. Indeed, very little has been done on the use of mHealth tools in addressing the needs of high-risk, underserved, drug users in treatment setting. We hope the findings from this study will serve as empirical evidence to inform clinicians and researchers how best to integrate mHealth approaches to support existing evidence-based HIV prevention programs tailored towards underserved high-risk people who use drugs.
With the recent emergence of PrEP as an innovative strategy to reduce new HIV infections, results from PrEP trials have also highlighted the relationship between adherence to PrEP and its efficacy [25, 27, 28]. Therefore, as PrEP rolls out in the United States and elsewhere, research and demonstration projects are testing intervention strategies (i.e., the use of mHealth) that have effectively supported adherence to other medications (e.g., antiretroviral therapy; ART) among people living with HIV) [31, 34, 37, 56]. With the wide-scale availability of mobile technologies and high acceptability of mHealth-based approaches, as demonstrated in this study, mobile text messaging may serve as a feasible option to supplement traditional HIV prevention approaches (i.e., behavioral interventions) to improve PrEP adherence and treatment retention and, thus, to help prevent HIV acquisition and transmission among high-risk HIV-negative individuals. Overall, this study demonstrated that high-risk people who use drugs are interested in using mHealth-based HIV prevention approach; formative research on preferences for design and functionality of mHealth-based HIV prevention are now needed, followed by practical development, implementation, and evaluation of these new intervention strategies.
Limitations
The results should be interpreted in light of a few inherent methodological limitations. First, as with all cross-sectional studies, we are constrained in our ability to conclude cause and-effect relations. Second, we used self-report measures, which may have resulted participants underreporting socially undesirable behaviors (e.g., drug- and sex-related risk behaviors). This may have been reduced, however, by the use of an ACASI approach, which provided participants with a high level of response privacy. Third, the BINI, while a very user-friendly and convenient screening instrument for difficult-to-reach populations, has not been validated using a gold standard battery and is not designed to measure all possible cognitive domains as would be the case in conducting a comprehensive neuropsychological battery. For a brief self-report measure, however, it does include a diverse set of factors with excellent overall reliability. Fourth, although the participants in this study showed significant interest in using text-messing services, the study did not look at whether they preferred one-way or two-way texting. Finally, the results of this study are specific to high-risk drug users in a substance abuse treatment setting. Therefore, future research that expands to include different study samples (e.g., individuals out of treatment and/or not enrolled in MMP) or locations may be needed before the findings can be generalized to other risk populations. Nonetheless, the results provide clear guidance for the development and implementation of mHealth-based strategies as part of existing evidence-based HIV prevention efforts that target medication (e.g., PrEP) adherence and HIV risk reduction strategies.
CONCLUSIONS
Despite promising trends of the efficacy of mHealth-based strategies to a broad range of health conditions [29, 30, 52], very little if any studies have been done in terms of the examining the use of mHealth in HIV prevention efforts among high-risk people who use drugs in treatment. The findings from this study provide empirical evidence that mHealth-based programs, specifically cell phone text messaging-based health programs, may be acceptable to this high-risk population. Researchers are encouraged to conduct formative research to explore opportunities to integrate cell phone text messaging into HIV prevention programs designed for implementation, followed by practical development, implementation, and evaluation among people who use drugs in treatment.
Acknowledgments
Source of Funding: This work was supported by grants from the National Institute on Drug Abuse for research (R01 DA025943 to FLA) and for career development (K24 DA017072 to FLA; K02 DA033139 to MMC).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Compliance with Ethical Standards
Ethical approval
The study protocol was approved by the Investigational Review Board (IRB) at the University of Connecticut and received board approval from APT Foundation Inc. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382(9904):1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. Vol. 26. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 3.Arasteh K, Jarlais DCD, Perlis TE. Alcohol and HIV sexual risk behaviors among injection drug users. Drug & Alcohol Dependence. 2008;95(1):54–61. doi: 10.1016/j.drugalcdep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall BDL, Friedman SR, Monteiro JFG, Paczkowski M, Tempalski B, Pouget ER, et al. Prevention And Treatment Produced Large Decreases In HIV Incidence In A Model Of People Who Inject Drugs. Health Affairs. 2014;33(3):401–409. doi: 10.1377/hlthaff.2013.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noar SM. Behavioral interventions to reduce HIV-related sexual risk behavior: review and synthesis of meta-analytic evidence. AIDS Behav. 2008;12(3):335–353. doi: 10.1007/s10461-007-9313-9. [DOI] [PubMed] [Google Scholar]
- 6.Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. The Lancet. 2010;376(9737):268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health affairs (Project Hope) 2011;30(8):1411–1419. doi: 10.1377/hlthaff.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Meade CS, Towe SL, Skalski LM, Robertson KR. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend. 2015;149:128–135. doi: 10.1016/j.drugalcdep.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. Journal of addiction medicine. 2014;8(5):368–376. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 11.Ezeabogu I, Copenhaver MM, Potrepka J. The influence of neurocognitive impairment on HIV treatment outcomes among drug-involved people living with HIV/AIDS. AIDS Care. 2012;24(3):386–393. doi: 10.1080/09540121.2011.608794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AM, Higgins MK, Ownby RL, Waldrop-Valverde D. Changes in neurocognition and adherence over six months in HIV-infected individuals with cocaine or heroin dependence. AIDS Care. 2015;27(3):333–337. doi: 10.1080/09540121.2014.985183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attonito JM, Devieux JG, Lerner BD, Hospital MM, Rosenberg R. Exploring Substance Use and HIV Treatment Factors Associated with Neurocognitive Impairment among People Living with HIV/AIDS. Frontiers in Public Health. 2014;2:105. doi: 10.3389/fpubh.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS and Behavior. 2010;14(6):1213–1226. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha R, Huedo-Medina TB, Copenhaver MM. Sex-Related Differences in Self-Reported Neurocognitive Impairment among High-Risk Cocaine Users in Methadone Maintenance Treatment Program. Substance Abuse: Research and Treatment. 2015;9:17–24. doi: 10.4137/SART.S23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2006;20(3):241–253. doi: 10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190(4):517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, et al. Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug Alcohol Depend. 2007;90(1):25–38. doi: 10.1016/j.drugalcdep.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vo HT, Schacht R, Mintzer M, Fishman M. Working memory impairment in cannabis- and opioid-dependent adolescents. Substance Abuse. 2014;35(4):387–390. doi: 10.1080/08897077.2014.954027. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha R, Copenhaver M. The Influence of Neurocognitive Impairment on HIV Risk Behaviors and Intervention Outcomes among High-Risk Substance Users: A Systematic Review. Frontiers in Public Health. 2016:4. doi: 10.3389/fpubh.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Effective interventions: HIV prevention that works. Atlanta, GA: CDC; 2016. [Google Scholar]
- 23.Huedo-Medina TB, Shrestha R, Copenhaver M. Modeling a theory-based approach to examine the influence of neurocognitive impairment on HIV risk reduction behaviors among drug users in treatment. AIDS and Behavior. 2016 doi: 10.1007/s10461-016-1394-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. New England Journal of Medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 25.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. New England Journal of Medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 28.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa. PLoS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. mHealth: New horizons for health through mobile technologies: second global survey on eHealth. Geneva, Switzerland: 2011. [Google Scholar]
- 30.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The Effectiveness of Mobile-Health Technology-Based Health Behaviour Change or Disease Management Interventions for Health Care Consumers: A Systematic Review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. The Lancet. 376(9755):1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 32.Milward J, Lynskey M, Strang J. Solving the problem of non-attendance in substance abuse services. Drug and Alcohol Review. 2014;33(6):625–636. doi: 10.1111/dar.12194. [DOI] [PubMed] [Google Scholar]
- 33.Mbuagbaw L, van der Kop ML, Lester RT, Thirumurthy H, Pop-Eleches C, Ye C, et al. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): an individual patient data meta-analysis of randomised trials. BMJ Open. 2013;3(12) doi: 10.1136/bmjopen-2013-003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, De Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS (London, England) 2011;25(6):825. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiologic reviews. 2010;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Militello LK, Kelly SA, Melnyk BM. Systematic review of text-messaging interventions to promote healthy behaviors in pediatric and adolescent populations: implications for clinical practice and research. Worldviews on Evidence-Based Nursing. 2012;9(2):66–77. doi: 10.1111/j.1741-6787.2011.00239.x. [DOI] [PubMed] [Google Scholar]
- 37.Finitsis DJ, Pellowski JA, Johnson BT. Text Message Intervention Designs to Promote Adherence to Antiretroviral Therapy (ART): A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenspun H, Coughlin S. mHealth in an mWorld: How mobile technology is transforming health care. Deloitte Center for Health Solutions; 2012. [Google Scholar]
- 39.Fox S, Duggan M. Mobile health 2012. Washington, DC: Pew Internet & American Life Project; 2012. [Google Scholar]
- 40.McClure EA, Acquavita SP, Harding E, Stitzer ML. Utilization of communication technology by patients enrolled in substance abuse treatment. Drug Alcohol Depend. 2013;129(1–2):145–150. doi: 10.1016/j.drugalcdep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firth J, Cotter J, Torous J, Bucci S, Firth JA, Yung AR. Mobile Phone Ownership and Endorsement of “mHealth” Among People With Psychosis: A Meta-analysis of Cross-sectional Studies. Schizophrenia Bulletin. 2015 doi: 10.1093/schbul/sbv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copenhaver, Lee IC, Margolin A. Successfully integrating an HIV risk reduction intervention into a community-based substance abuse treatment program. The American Journal of Drug and Alcohol Abuse. 2007;33(1):109–120. doi: 10.1080/00952990601087463. [DOI] [PubMed] [Google Scholar]
- 43.Copenhaver MM, Lee IC. Optimizing a community-friendly HIV risk reduction intervention for injection drug users in treatment: a structural equation modeling approach. Journal of urban health: bulletin of the New York Academy of Medicine. 2006;83(6):1132–1142. doi: 10.1007/s11524-006-9090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher JD, Cornman DH, Osborn CY, Amico KR, Fisher WA, Friedland GA. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: Formative Research, Acceptability, and Fidelity of the Options Project. Journal of acquired immune deficiency syndromes (1999) 2004;37(Suppl 2):S78–87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 45.Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a New Three-Item Self-Report Measure for Medication Adherence. AIDS and Behavior. 2016:1–9. doi: 10.1007/s10461-016-1406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward J, Darke S, Hall W. The HIV risk-taking behaviour scale (HRBS) manual. National Drug and Alcohol Research Centre, University of New South Wales Sydney; 1990. [Google Scholar]
- 47.Krishnan A, Ferro EG, Weikum D, Vagenas P, Lama JR, Sanchez J, et al. Communication technology use and mHealth acceptance among HIV-infected men who have sex with men in Peru: implications for HIV prevention and treatment. AIDS Care. 2014:1–10. doi: 10.1080/09540121.2014.963014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Copenhaver M, Shrestha R, Wickersham JA, Weikum D, Altice FL. An Exploratory Factor Analysis of a Brief Self-Report Scale to Detect Neurocognitive Impairment among Participants Enrolled in Methadone Maintenance Therapy. Journal of Substance Abuse Treatment. 2016;63:61–65. doi: 10.1016/j.jsat.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; 1998. [Google Scholar]
- 50.Dwan TM, Ownsworth T, Chambers S, Walker DG, Shum DHK. Neuropsychological Assessment of Individuals with Brain Tumor: Comparison of Approaches Used in the Classification of Impairment. Frontiers in Oncology. 2015;5:56. doi: 10.3389/fonc.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.IBM Corp. IBM SPSS Statistics for Windows, Version 23. Armonk, NY: IBM Corp; 2015. [Google Scholar]
- 52.Free C, Phillips G, Felix L, Galli L, Patel V, Edwards P. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Research Notes. 2010;3(1):1–7. doi: 10.1186/1756-0500-3-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pew Research Center. Technology device ownership: 2015. Washington, DC: Pew Research Center; 2015. [Google Scholar]
- 54.Chang LW, Njie-Carr V, Kalenge S, Kelly JF, Bollinger RC, Alamo-Talisuna S. Perceptions and acceptability of mHealth interventions for improving patient care at a community-based HIV/AIDS clinic in Uganda: a mixed methods study. AIDS Care. 2013;25(7):874–880. doi: 10.1080/09540121.2013.774315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Zhang W, Nyonyitono M, Lourenco L, Nanfuka M, Okoboi S, et al. Feasibility and acceptability of mobile phone short message service as a support for patients receiving antiretroviral therapy in rural Uganda: a cross-sectional study. Journal of the International AIDS Society. 2015;18(1):20311. doi: 10.7448/IAS.18.1.20311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;(3):Cd009756. doi: 10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang D, Sangthong R, McNeil E, Chongsuvivatwong V, Zheng W, Yang X. Effects of a Phone Call Intervention to Promote Adherence to Antiretroviral Therapy and Quality of Life of HIV/AIDS Patients in Baoshan, China: A Randomized Controlled Trial. AIDS Research and Treatment. 2013;2013:580974. doi: 10.1155/2013/580974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallace SE, Graham C, Saraceno A. Older Adults’ Use of Technology. SIG 15. Perspectives on Gerontology. 2013;18(2):50–59. [Google Scholar]
- 59.Charness N, Boot WR. Aging and Information Technology Use: Potential and Barriers. Current Directions in Psychological Science. 2009;18(5):253–258. [Google Scholar]
- 60.Yasmin F, Banu B, Zakir SM, Sauerborn R, Ali L, Souares A. Positive influence of short message service and voice call interventions on adherence and health outcomes in case of chronic disease care: a systematic review. BMC Medical Informatics and Decision Making. 2016;16(1):1–14. doi: 10.1186/s12911-016-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calderon Y, Cowan E, Nickerson J, Mathew S, Fettig J, Rosenberg M, et al. Educational Effectiveness of an HIV Pretest Video for Adolescents: A Randomized Controlled Trial. Pediatrics. 2011;127(5):911–916. doi: 10.1542/peds.2010-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirshfield S, Downing JM, Jr, Parsons TJ, Grov C, Gordon JR, Houang TS, et al. Developing a Video-Based eHealth Intervention for HIV-Positive Gay, Bisexual, and Other Men Who Have Sex with Men: Study Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2016;5(2):e125. doi: 10.2196/resprot.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuong W, Larsen ER, Armstrong AW. Videos to influence: a systematic review of effectiveness of video-based education in modifying health behaviors. Journal of Behavioral Medicine. 2014;37(2):218–233. doi: 10.1007/s10865-012-9480-7. [DOI] [PubMed] [Google Scholar]
- 64.Chuck C, Robinson E, Macaraig M, Alexander M, Burzynski J. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int J Tuberc Lung Dis. 2016;20(5):588–593. doi: 10.5588/ijtld.15.0738. [DOI] [PubMed] [Google Scholar]
- 65.Goggin K, Liston RJ, Adelson Mitty J. Modified Directly Observed Therapy for Antiretroviral Therapy: A Primer from the Field. Public Health Reports. 2007;122(4):472–481. doi: 10.1177/003335490712200408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wald DS, Butt S, Bestwick JP. One-way Versus Two-way Text Messaging on Improving Medication Adherence: Meta-analysis of Randomized Trials. The American Journal of Medicine. 128(10):1139.e1131–1139.e1135. doi: 10.1016/j.amjmed.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 67.Rosenbaum S. The Patient Protection and Affordable Care Act: Implications for Public Health Policy and Practice. Public Health Reports. 2011;126(1):130–135. doi: 10.1177/003335491112600118. [DOI] [PMC free article] [PubMed] [Google Scholar]