Abstract
False memories are often demonstrated using the misinformation paradigm, in which a person's recollection of a witnessed event is altered after exposure to misinformation about the event. The neural basis of this phenomenon, however, remains unknown. We used fMRI to investigate encoding processes during the viewing of an event and misinformation to see whether neural activity during either encoding phase could predict what would be remembered. fMRI data were collected as participants studied eight vignettes (Original Event phase). Shortly afterward, participants studied the same vignettes during scanning, but with changes to several details, serving as the misinformation (Misinformation phase). Two days later, their memories for the Original Event were assessed. Activity that subsequently led to true and false memories was examined during both encoding phases. Two interaction patterns between encoding phase (Original Event and Misinformation) and type of memory (true and false) were observed in MTL and PFC regions. In the left hippocampus tail and perirhinal cortex, a predictive item-encoding pattern was observed. During the Original Event phase, activity was greater for true than false memories, whereas during the Misinformation phase, activity was greater for false than true memories. In other regions, a pattern suggestive of source encoding was observed, in which activity for false memories was greater during the Original Event phase than the Misinformation phase. Together, these results suggest that encoding processes play a critical role in determining true and false memory outcome in misinformation paradigms.
Forgetting is a normal, well-accepted behavior in the human memory system. Somehow, through the passage of time or learning of new information, memories are lost. While normal forgetting is a failing or “sin” (Schacter 1999) of memory, it is one that is rather benign and readily forgiven. Other sins of memory in which information is not lost, but is distorted, are not as benign. For example, you may have a memory of witnessing an important event such as your wedding day, or the birth of a child, or even a traumatic crime or tragedy such as September 11, 2001. You feel you remember these events, but the memories are most likely distorted to some degree. These distortions, or false memories, represent significant flaws in our memory system, but just as visual illusions have helped us understand the processes underlying visual perception, these memory distortions or illusions may help us understand the processes underlying normal memory.
False memories manifest themselves in various forms (for review, see Schacter 1999), from changes in the context of a memory (e.g., believing you saw something that was imagined or believing you heard about an event on the television news rather than from a friend) to changes in the content of the memory itself (e.g., believing a criminal carried a gun rather than a knife), making it possible that there are several mechanisms by which these distortions occur.
An influential technique for studying these distortions and their sources has been the misinformation paradigm (Loftus et al. 1978). Here, participants witness an event such as a crime and are later exposed to misinformation about the crime. Participants frequently report the misinformation at time of questioning as part of the original event. What does this imply about how false memories originate, and how do encoding processes contribute? For example, if the crime is not encoded well, but the misinformation is—will this make it likely that the misinformation is later recovered and misattributed to the original event (Loftus and Hoffman 1989)? Do both the content and context of a memory have to be successfully encoded for a true memory to be recovered?
Here, we investigated activity during the two encoding phases of the misinformation paradigm using fMRI (see Fig. 1). Our study is based on the finding of numerous previous studies that reported activity at time of encoding in medial temporal lobe (MTL) and prefrontal cortex (PFC) regions that predicted subsequent recognition memory accuracy—so-called Dm (differences due to memory) effects (e.g., Brewer et al. 1998; Wagner et al. 1998; Fernandez et al. 1999, 2002; Eldridge et al. 2000; Kirchhoff et al. 2000; Otten et al. 2001; Paller and Wagner 2002; Strange et al. 2002; Davachi et al. 2003; Stark and Okado 2003; Gonsalves et al. 2004; Jackson and Schacter 2004; Kirwan and Stark 2004). These studies have at times supported a division of labor within the MTL according to traditional lines between the hippocampus and the adjacent cortex, but sometimes have not (Squire et al. 2004). Here, we attempt to shed light on this issue by investigating false memories that clearly involve errors in either content or contextual components.
Figure 1.
One of eight vignettes used in Session 1 of the behavioral misinformation paradigm. Each vignette was made of 50 still images. The Original Event phase was presented first in the scanner. After a delay, the Misinformation phase was presented in the scanner. All 12 critical items change in the Misinformation phase. The control items remain the same. Both critical and control items are later tested. The generic items remain the same and are not tested.
In this study, we applied a Dm style of analysis to determine whether activity during the two encoding phases (the Original Event and the Misinformation phases) could be used to predict whether the original event or misinformation was later reported during a recognition test administered outside the scanner, and to better understand what neural processes, specifically in the MTL, are involved in true and false memories.
Results
Behavioral results
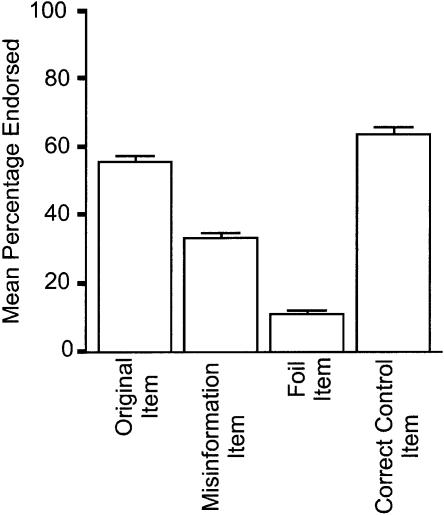
In the scanner, participants watched eight separate vignettes (Original Event phase), and shortly afterward watched the same eight vignettes, but unknowingly with changes made to 12 critical items per vignette (Misinformation phase). Two days later, participants took a three-alternative forced-choice recognition test (Original Event item, Misinformation item, or Foil item) outside the scanner to assess their memory for what participants saw in the Original Event. Accuracy on the recognition memory task is shown in Figure 2. Participants endorsed misinformation items significantly more often than foil items (t(19) = 13.0, P < 0.01), suggesting that this paradigm reliably created false memories— recognition of the items shown in the Misinformation phase when asked about the Original Event phase.
Figure 2.
Endorsement rates during the recognition memory task for the critical items (left three bars) and hit rate for the control items (right bar). Error bars, SEM.
To assess the quality of these false memories and to eliminate simple guesses from our analyses, participants then took a source memory test, in which they indicated from which source they remembered their recognition test answers. Data from the source test (Table 1) showed 68% of the trials were classified as true memories (responding that they saw this item in the first phase or that they noticed a conflict of items across phases) when the item from the Original Event phase was endorsed on the recognition test. When the item from the Misinformation phase was recognized, 47% of the trials were classified as false memories (responding that they saw it in either the first phase or in both phases.)
Table 1.
Source memory test data
| Recognition Test Response | ||||||
|---|---|---|---|---|---|---|
| Source Test Options
|
Original | MI | Foll | Correct Control | Incorrect Control | |
| Saw 1 | 49% | 27% | 26% | 24% | 12% | |
| Saw 2 | 2% | 7% | 5% | 3% | 2% | |
| Saw Both | 14% | 20% | 14% | 39% | 12% | |
| Conflict | 19% | 14% | 11% | 8% | 8% | |
| Guess | 16% | 32% | 45% | 25% | 66% | |
Distribution of responses broken down by response in the recognition memory test.
fMRI results: MTL analysis
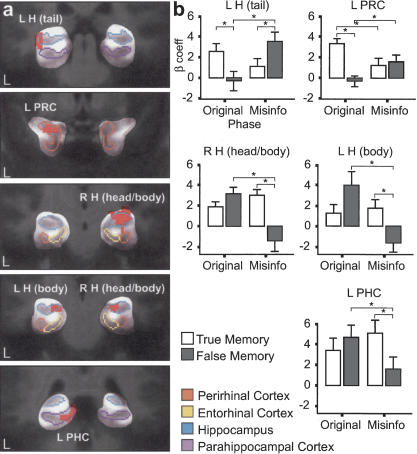
On the MTL, the ROI-AL (Regions Of Interest-based ALignment) technique initially described by Stark and Okado (2003) was performed to increase statistical power and precision of analyses within the MTL. This analysis was distinct from the whole-brain analysis. A voxel-wise three-factor ANOVA was first conducted on the MTL data, where the ROI-AL technique had been applied to identify regions that showed any interactions among type of phase (Original Event or Misinformation) and type of subsequent memory (true or false) within the MTL. Results of this analysis identified five areas within the MTL that showed significant interactions between these two factors (Fig. 3a). These regions consisted of the following: the tail of the left hippocampus (approximate coordinates: x = -33, y = 31, z = -3), the body of the left hippocampus (-22, -23, -8), the head and body of the right hippocampus (32, -17, -7), the temporopolar portion of the left perirhinal cortex (24, 1, -18), and the left parahippocampal cortex (-9, -35, -1). These areas were then treated as functionally defined ROIs, and all voxels within each ROI were collapsed for each participant for further analysis (an α threshold of P < 0.05 was used in all comparisons).
Figure 3.
FMRI results from the MTL ROI-AL analysis. Regions that showed significant interactions between type of phase (Original Event or Misinformation) and type of subsequent memory (True or False). (a) Activity is shown in coronal sections, cropped to show the MTL. Individual components of the MTL are coded by color based on location; blue, hippocampus (H); purple, parahippocampal cortex (PHC); orange, perirhinal cortex (PRC); and yellow, entorhinal cortex. (b) The bar graphs show mean fMRI responses (sum of β coefficients) across participants. Error bars, SEM. Activity for subsequently true memories is shown in white, and activity for subsequently false memories is shown in gray. (*) Comparisons within and across phases that are statistically reliable. The L H (tail) and L PRC show the first interaction pattern (Dm effect) discussed in the Results section. The R H (head/body), L H (body), and L PHC show the second source encoding pattern discussed in the Results section.
Interestingly, two contrasting patterns of activity were observed in this MTL analysis. First, the left hippocampus tail and left perirhinal cortex showed similar interaction patterns [F(1,19) = 13.9, P < 0.01 and F(1,19) = 22.6, P < 0.001, respectively] that were consistent with activity related to item-encoding success, or Dm effects (Fig. 3a,b). During encoding of the Original Event, there was significantly more activity when participants subsequently remembered the item shown in this phase (a true memory), compared with when participants subsequently did not remember the item. That is, there was more encoding activity when an item was later remembered compared with when it was forgotten (or another item was remembered instead). During the Misinformation phase, this same Dm effect was observed. There was more activity for subsequent false memories than for subsequent true memories. This difference in activity was significant in the left hippocampus tail, but not in the left perirhinal cortex. Again, there was more encoding activity for items subsequently remembered (Misinformation items) compared with items subsequently not remembered (Original Event items).
In addition, a comparison of activity across the two encoding phases can be made if one assumes that our baseline task (perceptual identification of an “X” or “T” in a white noise mask) is associated with similar levels of activity in the Original Event and Misinformation phases. Again, the activity is consistent with a Dm effect. In the left hippocampus tail, activity for subsequently true memories was greater during the Original Event phase than during the Misinformation phase, and activity for subsequently false memories was significantly greater during the Misinformation phase than during the Original Event phase. In the left perirhinal cortex, the same pattern of results was observed. Thus, the relative activity during the two encoding phases was predictive of which version of the item would later be remembered.
The three other MTL areas identified, right hippocampus head and body, left hippocampus body, and left parahippocampal cortex, showed a different and nearly opposite pattern from the one described above [F(1,19) = 24.4, P < 0.001; F(1,19) = 14.4, P < 0.01; and F(1,19) = 22.5, P < 0.001, respectively) (Fig. 3a,b). Here, during the Original Event phase, there was a trend for subsequently false memory activity to be greater than subsequently true memory activity, but these differences were not significant in all three regions. During the Misinformation phase, activity for subsequently true memories was significantly greater than activity for subsequently false memories in all three regions. Comparing across phases, activity for subsequently true memories was similar (and not significantly different) for the Original Event phase and Misinformation phase, and activity for subsequently false memories was significantly greater during the Original Event phase than during the Misinformation phase in all three regions (Fig. 3b). This pattern is suggestive of encoding source or contextual aspects of an event when considering that in order to misattribute an item from the Misinformation phase to the Original Event phase to have a false memory, source information should be strongly encoded from the Original Event phase and weakly encoded from the Misinformation phase. Notably, within the MTL and even within the left hippocampus, two contrasting patterns of activity were observed.
fMRI results: Whole-brain analysis
The voxel-wise three-factor ANOVA on the whole-brain data identified 18 regions that showed an interaction among type of phase (Original Event or Misinformation) and type of subsequent memory (true or false) (Table 2). These areas were again treated as functionally defined ROIs for analysis of the interaction. One of these regions was the same left perirhinal cortex region identified in the MTL ROI-AL analysis and showed the same pattern as it had in the ROI-AL analysis. The other MTL regions did not exhibit reliable interactions in this analysis, demonstrating the increased power available with the more accurate and more tightly constrained ROI-AL technique.
Table 2.
MRI results from the whole-brain analysis
| Contrast: True–False | Contrast: Original–MI | ||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Coordinates x, y, z | Original | MI | True | False | Volume |
| Right | |||||||
| Mid Fr G/Sup Fr G | 10/9 | 22, 56, 16 | – | + | – | + | 1109 |
| Ant Cin/Med Fr G | 32/10 | 7, 54, 7 | – | + | + | 1062 | |
| Cin G | 23/24 | 1, –16, 33 | – | + | + | 1000 | |
| Sup Fr G | 8/6 | 6, 48, 41 | – | + | – | + | 984 |
| Med Fr G/Sup Fr G | 10/9 | 6, 61, 26 | – | + | + | 688 | |
| Med Fr G/Cin G | 21, –4, 44 | – | + | – | + | 625 | |
| Thal | 10, –14, 11 | – | + | – | 625 | ||
| Supramarg G/Ang G/Inf Par L | 40 | 49, –51, 36 | – | + | – | 516 | |
| Sup Fr G | 6 | 22, 28, 52 | – | + | – | + | 406 |
| Caud/Thal | 1, –1, 14 | – | + | – | + | 375 | |
| Sup Fr G | 8/6 | 2, 16, 54 | + | – | 359 | ||
| Left | |||||||
| Sup Fr G | 9 | –24, 46, 31 | + | – | + | 734 | |
| Sup Fr G | 8 | –16, 33, 49 | + | + | 734 | ||
| Med Fr G | 9 | –1, 41, 32 | + | – | 750 | ||
| Sup Fr G/Med Fr G | 6 | –3, 1, 52 | + | – | 516 | ||
| Ant Cin/Med Fr G | 32/24/10 | –6, 44, 9 | – | + | + | 375 | |
| Med Fr G # | 10 | –1, 58, 19 | – | + | + | + | 594 |
| Perirhlnal C # * | –29, 1, –18 | + | + | – | 328 | ||
Listed are brain regions that showed a significant interaction between type of phase (Original Event or Misinformation) and type of subsequent memory type (true or false). The regions are divided into Left/Right hemispheres and listed in order of pattern, then volume. The True–False contrast shows significant activity for true memories > activity for false memories (+) and significant activity for false memories > activity for true memories (–) for the Original Event phase and Misinformation phase (within phase comparison). The Orig–MI contrast shows activity during Original Event phase > activity for Misinformation phase (+) and activity during Misinformation phase > activity during Original Event phase (–) for subsequently true and subsequently false memories (across phase comparison). Two regions that showed a slightly different pattern are denoted by #, in which for subsequently true memories, activity was greater during the Original Event phase than during the Misinformation phase and by *, in which for subsequently false memories, activity was greater during the Misinformation phase than during the Original Event phase.
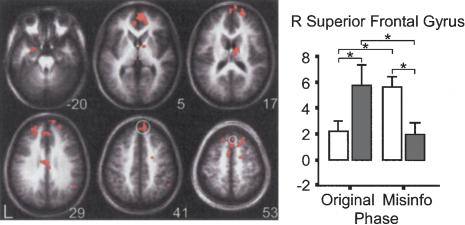
The other 17 regions showed the second interaction pattern discussed in the MTL ROI-AL analysis results (all F's > 16.9, P's < 0.01; see Table 1) (Fig. 4), in which during the Original Event phase, activity for subsequently false memories was greater than activity for subsequently true memories (for the majority of the regions, this difference was significant), and during the Misinformation phase, activity for subsequently true memories was greater than activity for subsequently false memories (for all 17 regions, this difference was significant). Comparing across phases, all 17 regions, except for the left medial frontal gyrus (BA10), showed a trend in which there was greater activity for subsequently true memories during the Misinformation phase than during the Original Event phase. Also, there was a trend in which there was greater activity for subsequently false memories during the Original Event phase than during the Misinformation phase. In the left medial frontal gyrus (BA10), activity for subsequently true memories was significantly greater during the Original Event phase than during the Misinformation phase, and activity for subsequently false memories was significantly greater during the Original Event phase than during the Misinformation phase.
Figure 4.
Sample of whole-brain results. All 18 regions identified in the ANOVA are shown in the axial montage image. The z-coordinate indicates the approximate location of the slice in Talairach coordinates. The bar graph shows the mean fMRI responses across participants for one of the representative regions identified in the ANOVA, the right superior frontal gyrus, highlighted in white on the montage image. The graph shows the second source encoding pattern that is observed in all of the other 18 regions identified except for the left medial frontal gyrus and left perirhinal cortex (see Results section).
In addition, it is worth noting that we observed a hemispheric trend in the whole-brain results in the form of significantly greater subsequent false memory activity compared with subsequent true memory activity during the Original Event phase more frequently in the right than left hemisphere (Table 2). Except for the right superior frontal gyrus, left anterior cingulate, and left medial frontal gyrus, all regions identified in the whole-brain analysis demonstrated this hemispheric pattern. This potentially suggests laterality differences in encoding of source information. Laterality differences have been noted in the PFC regarding encoding/retrieval, successful retrieval/amount of effort during retrieval, and verbal stimuli/pictorial stimuli (Buckner 1996; Nyberg et al. 1996; Buckner et al. 1998a,b; Cabeza and Nyberg 2000; Fletcher and Henson 2001), suggesting that there may be a trend toward hemispheric differences regarding source encoding.
Discussion
Several neuroimaging studies have investigated false memories (for review, see Schacter and Slotnick 2004) but many concentrated on retrieval differences between true and false memories (Schacter et al. 1996, 1997; Fabiani et al. 2000; Cabeza et al. 2001; Okado and Stark 2003) rather than encoding contributions to false memories (but, see Gonsalves and Paller 2000; Gonsalves et al. 2004). The present study highlights the critical role of encoding processes in false memory creation driven by the misinformation effect.
Neural activity during encoding of the Original Event and Misinformation predicted whether true or false information was later reported. While previous neuroimaging memory studies demonstrated that encoding activity predicts what is subsequently remembered (for review, see Paller and Wagner 2002), the present study showed that this Dm effect applies to both true and false memories, particularly for activity in the left tail of the hippocampus and left perirhinal cortex. However, a second pattern of activity consistent with encoding source or contextual aspects was observed in the right hippocampus head and body, left hippocampus body, and left parahippocampal cortex. Thus, while differentiation of function was observed in the MTL, it was not along a dissociation between the hippocampus and adjacent cortex (as the left hippocampus showed both item and source encoding of the same event).
The traditional Dm effect (greater activity for subsequently remembered items than for forgotten items) was observed in the left hippocampus tail and left perirhinal cortex. When encoding activity was greater during the Original Event phase, the Original Event items (true memories) were subsequently recollected. When encoding activity was greater during the Misinformation phase, the Misinformation items (false memories) were subsequently recollected. Thus, in these two regions, activity was correlated with successful encoding of an item later remembered, whether it was from the Original Event phase or from the Misinformation phase. Gonsalves et al. (2004) used a reality monitoring paradigm to examine whether activity during an encoding phase (either viewing or imagining pictures) would later predict whether these pictures would be correctly or incorrectly endorsed as previously viewed. Consistent with our data, they reported a traditional Dm effect in the left hippocampus (remembered vs. forgotten difference for accurate memories). Our results are also in line with previous reports of successful predictive encoding demonstrating that activity in large portions of the MTL (and regions outside of the MTL, such as the PFC) was predictive of subsequently remembered and forgotten stimuli (e.g., Brewer et al. 1998; Wagner et al. 1998; Fernandez et al. 1999, 2002; Eldridge et al. 2000; Kirchhoff et al. 2000; Otten et al. 2001; Davachi and Wagner 2002; Strange et al. 2002; Davachi et al. 2003; Stark and Okado 2003; Jackson and Schacter 2004; Kirwan and Stark 2004). Gonsalves et al. (2004) further showed encoding activity predictive of later false memories in the anterior cingulate, right inferior parietal cortex, and precuneus. In their study and in reality monitoring paradigms in general, vividness of visual imagery is a clear predictor of whether participants will judge a previously imagined item as having been previously viewed, lending a clear interpretation of activity in regions such as these that have been highly associated with visual imagery (Johnson et al. 1977; Gonsalves and Paller 2000; Okado and Stark 2003). The present study did not find these regions to be especially involved in encoding false memories, but uses a paradigm that is not explicitly driven by visual imagery at time of encoding.
In the present study, we suggest that the Dm effect observed in the left hippocampal tail and the left perirhinal cortex is not simply an overall predictive encoding effect. Rather, it may be related to encoding of the item that will be tested on the recognition test, while at the same time not necessarily reflecting the encoding of the source of that information (which may include contextual details of the particular critical slide or the episodic information of phase the slide occurred in). To be labeled a false memory, participants not only choose that item in the recognition test, but they must also attribute it to either the Original Event phase or to both the Original Event and the Misinformation phases in the second source test. Thus, at time of retrieval, the memory for the item itself is drawn from the Misinformation phase and then combined with source components from the Original Event phase. Encoding of the critical item itself is consistent with trials in which activity for subsequently false memories is greater during the Misinformation phase than activity for subsequently true memories (within phase) or greater than activity for subsequently false memories during the Original Event phase (across phase). On the other hand, to be labeled a True Memory, participants not only choose that item in the recognition test, but also accurately attribute the item to the Original Event phase, supporting previous predictive encoding findings. Thus, these results not only replicate and extend the Dm effect to both true and false memories, but they also suggest that the left hippocampus tail and left perirhinal cortex are involved in encoding these critical items themselves.
Numerous studies have explored whether particular components of the MTL are uniquely involved in episodic memory (for review, see Squire et al. 2004). One fMRI study in particular investigated item versus source encoding of verbal stimuli that involved an “image” or “read” task at the time of presentation (Davachi et al. 2003). In the MTL, they found that activity in the left perirhinal cortex predicted later item recognition and not later source recollection. However, activity in the bilateral hippocampus body and left parahippocampal cortex predicted later source recollection and not later item recognition. Our results are largely consistent with their findings, in that the left perirhinal cortex was associated with item encoding, and right hippocampus head and body, left hippocampus body, and left parahippocampal cortex were associated with source encoding (see below). However, in our study, the left hippocampus tail demonstrated activity suggestive of item rather than source encoding. Thus, two different encoding patterns were observed within the left hippocampus.
This source-encoding activity observed in the MTL (right hippocampus head and body, left hippocampus body, and left parahippocampal cortex) was also prevalent predominantly in bilateral and medial PFC in the whole-brain analysis. Greater activity was observed for subsequently false memories than subsequently true memories during the Original Event phase, and during the Misinformation phase, greater activity for subsequently true memories than subsequently false memories. The misinformation effect is often suggested by behavioral studies to be largely driven by source misattributions, in which the source of the misinformation is confused with the source of the original event (for review, see Mitchell and Johnson 2000). When a person witnesses a crime, an ideal situation that enables the misinformation that is later acquired to be embedded into the original crime scene may be if the context and source of the original crime scene is encoded strongly and the source of the misinformation encoded weakly (or rapidly lost). Here, the False Memory condition was derived from critical trials in which participants confidently recognized the critical item shown in the Misinformation phase and attributed this memory to the Original Event phase (or to both phases). On the other hand, the True Memory condition was derived from trial types in which participants accurately attributed the critical original item to the Original Event phase and potentially indicated knowledge of a conflict (where participants remember that different critical items were presented across the two phases). In either case, for True Memory trials, participants have item and source information from the Original Event phase and may additionally have such information from the Misinformation phase. Therefore, activity linked to encoding the source or other episodic aspects of subsequent true memories should be fairly strong during both the Original Event and Misinformation phases.
This suggests strong encoding of the source or contextual aspects of the Original Event if Misinformation items are later to be attributed to this phase. On the other hand, there should be less encoding of the source or contextual components of the Misinformation phase, since items from the Misinformation phase are attributed to the Original Event phase. This is the pattern that is observed in the right hippocampus head and body, left hippocampus body, and left parahippocampal cortex. Davachi et al. (2003) reported the same structures exhibiting encoding activity that predicted later source recollection and not later item recognition.
Outside of the MTL, neuropsychological studies have demonstrated that patients with prefrontal damage can be impaired on tests of source memory, while maintaining intact item recognition (e.g., Janowsky et al. 1989a,b; Shimamura et al. 1990; Mitchell and Johnson 2000). For example, Janowsky et al. (1989a) showed that patients with frontal lobe lesions could recall facts, but had difficulty associating facts with the context in which they were learned. Further, neuroimaging studies investigating source and item retrieval reported that source recognition engaged numerous PFC regions (for reviews, see Mitchell and Johnson 2000; Buckner and Wheeler 2001). These studies implicate the necessity and involvement of the frontal lobes in source memory, and the regions observed in these studies overlap extensively with our current findings, further suggesting that this pattern of activity observed in the MTL and PFC reflect source information encoding. We should note that while we are discussing source memory as an all-or-none phenomenon, it is clear from the behavioral studies using this paradigm that the amount of source information encoded and retrieved can vary, and that probing specifically for sources of memories can often reveal further source knowledge (Lindsay and Johnson 1989; Zaragoza and Lane 1994). While the graded nature of source memory clearly highlights an important aspect of memory encoding and retrieval, our data unfortunately constrained us to making this gradation discrete and to only examine the most confident false memories with the clearest misattribution of source.
Interestingly, it is clear that two distinct encoding patterns emerged from the MTL. One that is suggestive of item encoding, and the other suggestive of source encoding. However, the types of encoding are not consistent with the notion of a clear, binary division of labor between the hippocampus and the adjacent cortex. The hippocampus, for example, was not selectively involved in associative or source memory, and the underlying cortical regions were not selectively involved in nonassociative or item memory. In fact, the left hippocampus in this study appears to be involved in both types of information processing, and activity in the left parahippocampal cortex is consistent with source encoding. These data are therefore more consistent with a graded and more complex division of labor within the MTL (Squire et al. 2004).
So far, we have discussed the second pattern of activity in terms of source or contextual encoding, but alternative interpretations may exist. First, we considered the possibility that this pattern may reflect novel picture encoding (Stern et al. 1996; Suzuki and Eichenbaum 2000; Brown and Aggleton 2001). During the Original Event phase, activity for subsequently true and false memories did not significantly differ, suggesting that encoding activity for novel pictures were similar. During the Misinformation phase, activity for subsequently true memories was similar and did not significantly differ from the encoding activity previously discussed during the Original Event phase, but was significantly greater than activity for subsequently false memories, because the novel critical item was detected for the later true memories. Thus, the novelty-encoding activity during the Misinformation phase resulted in a true memory. On the other hand, the novel critical item was not detected for later false memories, resulting in relatively less novel-encoding activity and a false memory. However, this latter interpretation that the novel critical item was not detected does not explain the fact that participants must detect the novel critical item during the Misinformation phase, because this is what they report on the recognition test. This is the false memory that they endorse.
A related idea is when the Misinformation item is noted to be discrepant from the Original Event item, the critical item is “tagged” (Loftus 1981; Tousignant et al. 1986). Thus, in the Misinformation phase, participants are retrieving information about the Original Event phase, and tag (encode) the Misinformation item when they notice a discrepancy. Consequently, “tagging” should lead to subsequently true memories (and “Conflict” source responses) and “no tagging” should lead to subsequently false memories. This could also be consistent with the pattern of activity observed during the Misinformation phase in the PFC regions and right hippocampus head and body, left hippocampus body, and left parahippocampal cortex. This also highlights the value of experimental designs with sufficient trials to separate the two trial types that made the True Memory condition.
Another related idea and significant caveat pertaining to the activity pattern observed in the Misinformation phase is that incidental retrieval of the Original Event phase may be occurring during the encoding of the Misinformation phase. Participants may be recognizing (and thereby engaging retrieval processes) the presented scene that is identical to the scene presented previously during the Original Event phase with the exception of the critical item. This could account for the increased activity for the True Memory condition during the Misinformation phase compared with the Original Event phase. However, there was no such increase in activity for the False Memory condition in the Misinformation phase compared with the Original Event phase. For the False Memory condition, all regions demonstrating this second interaction pattern showed a decrease in activity during the Misinformation phase compared with the Original Event phase.
There are reports that show that not only do encoding and retrieval tasks engage the same PFC (Buckner et al. 2001) and MTL (Buckner et al. 2001; Stark and Okado 2003) regions, but also that incidental encoding occurs during the retrieval task itself. Thus, it is quite possible that the same regions that are encoding information during the Misinformation phase are also incidentally retrieving information about the Original Event phase during the Misinformation phase (e.g., when participants notice the conflict). The structures highlighted in the MTL analysis to show this pattern have certainly been implicated in memory retrieval (Gabrieli et al. 1997; Eldridge et al. 2000; Stark and Squire 2000, 2001; Cabeza et al. 2001; Yonelinas et al. 2001; Ranganath et al. 2003; Stark and Okado 2003; Kirwan and Stark 2004). Similarly, the PFC is heavily implicated in memory retrieval processes (Buckner 1996; Nyberg et al. 1996; Buckner et al. 1998a,b; Fletcher and Dolan 1999; Cabeza and Nyberg 2000; Cabeza et al. 2001; Rugg et al. 2002). Therefore, it is possible that activity in the Misinformation phase was associated with memory retrieval processes as well. Future studies that have sufficient power to analyze these “conflict” trials will be necessary to address this possibility.
While numerous behavioral studies have explored the misinformation effect (for review, see Loftus et al. 1995), this study was the first to explore the underlying mechanisms of this phenomenon with neuroimaging techniques. We observed that the interaction of encoding processes in the MTL and PFC are critical for true and false memory creation in this paradigm, as activity during the two encoding phases predicted whether the Original Event information or the inaccurate misinformation was reported. This study also highlighted that components of the MTL are not selectively involved in one type of information processing, and even within the same structure of the MTL, different forms of encoding can occur. Together, these data contribute to our understanding of how false memories are created and how the normal memory system operates.
Materials and Methods
Participants
Twenty fluent English speakers (nine male, 11 female) were recruited from the Johns Hopkins University community. The participants were between the ages of 18 and 34 yr, and were right-handed. All participants were naive to the experimental materials and hypotheses, gave written informed consent to participate conforming to local IRB procedures, and were paid for their time.
Materials
Eight unique vignettes, each consisting of 50 color digital slide images were developed. For every vignette, 12 of the 50 slides were critical slides. Critical slides were slides that contained an item that changed across the Original Event and Misinformation phases, and thus served as the misinformation of the events. There were two different sets of critical slides for each vignette, which were counterbalanced across participants. The presentation order of the vignettes was randomized across participants. All slide images were edited to a size of 300 × 300 pixels.
The recognition test consisted of detailed questions regarding what was presented in the Original Event phase. For all eight vignettes, there were a total of 18 questions, 12 critical questions (pertaining to critical, changed slides), and six control questions (pertaining to consistent slides). An example of a critical question was “Where was the man hiding after he stole the girl's wallet and crossed the street?” Each critical question had three options as follows: (1) the detail presented in the Original Event phase (Behind a Door), (2) the detail presented in the Misinformation phase (Behind a Tree), and (3) a foil option (Behind a Car). Control questions were similar in detail to critical questions. An example of a control question was “What kind of store was to the left of the video store?” Each control question had three options as follows: (1) the detail presented in both phases (Hair Salon), (2) a foil option (Music Store), and (3) a foil option (Clothing Store). There was a separate recognition test for all eight vignettes.
The second test phase was a source memory test that followed immediately after completing all eight recognition tests. This test asked from what presentation source participants remembered the answers they indicated on the previous recognition test. There were five options as follows: (1) saw in the first set of presentations, (2) saw in the second set of presentations, (3) saw in both sets of presentations, (4) conflict, and (5) guess. There was a source memory test for every recognition test question for all eight vignettes. All memory tests were administered on paper and self-paced.
Procedure
In Session 1, there were two phases, both of which took place inside the fMRI scanner (Fig. 1). In the first (Original Event) phase, participants watched eight vignettes depicting different events. They were informed that the purpose of the study was to judge whether memory for events is better with one presentation of the event or two presentations. Each slide was presented one at a time for 3500 msec with a 500-msec intertrial interval (ITI). The content of the vignettes ranged from a girl having her wallet stolen by a seemingly helpful man, to a student waiting for a class and interacting with several friends. After one vignette was presented (50 slides), there was a short delay before the presentation of the next vignette. This served as the Original Event phase.
In addition, there were 12 null trials per vignette (96 total) in this phase. Null trials were white mask images with either a blue letter “X” or blue letter “T” randomly placed on the mask (the task was perceptually quite difficult). Participants were asked to press the left button when they saw an “X” and the right button when they saw a “T” using the button box provided. Null trials were presented for 3500 msec with a 500-msec ITI. All of the null trials were randomly intermixed within each event.
After a delay, in which anatomical images of participants' brains were acquired, the second (Misinformation) phase was presented. Here, participants were unknowingly exposed to misinformation about each of the events by watching what they believed were the same vignettes depicting the same eight events, but 12 of the slides (critical slides) within each vignette were slightly altered (Manning and Loftus 1996). For example, in the event mentioned above, in which a man steals a girl's wallet, in the Original Event phase, the man hides behind a door, and in the Misinformation phase, the man hides behind a tree. This served as the Misinformation phase. The presentation style was the same as the Original Event phase. There were also 12 null trials per vignette (96 total) in this phase, randomly intermixed within each vignette.
Due to the constraints of fMRI analysis, we did not use the typical misinformation paradigm, in which the Original Event phase is presented in pictorial form and the Misinformation phase is presented in written narrative form. The two different modalities of the stimuli make correlational fMRI analyses difficult, since memory for pictures and words appears to involve different anatomical regions (Kelley et al. 1998; Stark and Squire 2000, 2001; Papanicolaou et al. 2002). Instead, both phases were presented in pictorial form. While less common, this technique has been used in several behavioral studies and has been found to produce reliable (albeit fewer) false memories (Manning and Loftus 1996). In addition, the typical misinformation task was modified to present eight vignettes in each phase rather than the typical single vignette. This was done to increase the number of false memories available for fMRI analysis.
In Session 2, there were two phases, both of which took place outside of the fMRI scanner 48 h later, and tested participants' memory for the events. In the first recognition test phase, participants were asked what they remembered seeing in the original set of events from Session 1. The test was a three-alternative forced-choice (3AFC) recognition test (original item, misinformation item, and novel/foil item) to avoid much of the retrieval cue complication (McCloskey and Zaragoza 1985). All eight events were tested in the order the vignettes were presented, and within each event, every critical and control item was tested. However, the questions within each test were in random order relative to the chronology of events depicted in the sequence (Loftus 1991).
This recognition test phase was followed by a surprise source memory test, which was based on answers participants gave on the recognition test to increase experimenter confidence in the true and false memories (Loftus et al. 1995). Participants indicated the source of their memory for every question they answered previously in the first test phase.
Original Event critical items that were accurately recognized and further endorsed on the source memory test as option “a” (saw in the first set of presentations) or option “d” (conflict) were considered true memories. Misinformation critical items that were inaccurately recognized and further endorsed on the source memory test as option “a” (saw in the first set of presentations) or option “c” (saw in both sets of presentations) were considered false memories.
fMRI data acquisition
Imaging was performed on a Philips Gyroscan 3T MRI scanner equipped with a whole-brain SENSE coil. By exploiting the sensitivity profiles of multiple surface coils, SENSE (SENSitivity Encoding) imaging can undersample k-space with fewer phase-encoding steps, while still yielding full field of view (FOV) images that are free of aliasing. The result is significantly reduced acquisition time and distortion due to magnetic susceptibility (Pruessmann et al. 1999). This reduction in distortion is very important for studying the structures of the medial temporal lobes, as without it, the distortions induced by the sinus cavities can be significant. Thirty-five T2*-weighted triple-oblique functional images were collected per three-dimensional volume using single-shot echoplanar pulse sequence (80 × 80 matrix, TE = 30 msec, flip angle = 70°, in-plane resolution = 4 × 4 mm, thickness = 3 mm plus a 1-mm interslice gap, TR = 2 sec). Slices were triple-obliques, aligned with the principal axis of both the left and right hippocampus (as determined by a series of sagittal localizer MRI scans for each participant). This was done to optimize the signal from the medial temporal lobes and to minimize partial-voluming effects, so that voxels could be clearly constrained to lie within subregions of the medial temporal lobes. A total of 992 volumes were collected. The task began in synchrony with the acquisition of the fifth volume, to allow for T1 stabilization. After the functional scans, a high-resolution structural MRI was acquired (MP-RAGE pulse sequence, 1 mm3 resolution, 150 triple-oblique axial slices in the same orientation as the functional images) for anatomical localization.
fMRI data analysis
Image analysis was performed using Analysis of Functional NeuroImages (Cox 1996). Functional MRI data were first resampled in time using a Fourier algorithm to align all slices to a common time base. Functional images were then resampled in space to coregister the images and reduce the effects of head motion in three dimensions. During this process, six vectors were created that coded for all possible translations and rotations of the brain. fMRI data from all eight runs for both the Original Event phase and Misinformation phase were concatenated.
Following this processing, the behavioral data were coded into four trial types of interest, and a general linear model (GLM) of the fMRI time series data was constructed using these vectors. The behavioral measures of subsequent recognition and source memory were used to back-sort the fMRI encoding events into a True Memory condition (the participant chose the Original Event at recognition and later indicated the source as the first phase or as noticing the conflict), a False Memory condition (the participant chose the Misinformation item at recognition and later indicated the source as the first phase or both phases), a Generic condition (noncritical trials that were consistent across phases and not later tested), and a Guess condition (critical trials in which participants chose the foil item at recognition or critical trials in which participants chose the Original or Misinformation item at recognition, but indicated Guess on the source test). The True Memory and False Memory conditions each have two types of trials collapsed into one, as there were too few trials to analyze each separately. The GLM also included nuisance vectors coding for first and second-order drift in the MR signal and for three-dimensional head motion.
The GLM was constructed using a deconvolution technique (Ward 2002) that estimates the impulse response function within each voxel and performs a multiple linear regression. The sum of the β coefficients for the time points corresponding to the expected sum over the hemodynamic response (∼2–16 sec after stimulus onset) was taken as the model's estimate of the response to each trial type. Initial spatial normalization was done using each participant's structural MRI to transform data according to the common atlas of Talairach and Tournoux (1988). This transformation was applied to the statistical maps of the β coefficients, and in the process the data, were resampled to 2.5 mm3. The spatially normalized statistical maps of the β coefficients were blurred using a Gaussian filter with a full-width half maximum of 4 mm to help account for variations in the functional anatomy across participants.
An analysis restricted to the MTL was performed using the ROI-AL (Regions Of Interest-based ALignment) technique initially described by Stark and Okado (2003) and refined here. The technique begins with the manual segmentation of regions of interest based on anatomical structure. Here, 10 structures in the MTL (bilateral hippocampal region, temporopolar, perirhinal, entorhinal, and parahippocampal cortices) were defined. The temporopolar portion of perirhinal cortex, the more posterior portion of perirhinal cortex, and the entorhinal cortex were defined according to the techniques described by Insausti et al. (1998). As in our previous research, the parahippocampal cortex was further defined bilaterally as the portion of the parahippocampal gyrus caudal to the perirhinal cortex and rostral to the splenium of the corpus callosum. The hippocampal region (the CA fields of the hippocampus, dentate gyrus, and subiculum) was also defined bilaterally. The original version of ROI-AL (Stark and Okado 2003) uses these segmentations to calculate an additional 12-parameter affine transformation matrix to fine-tune the cross-participant alignment of the segmentations (such that hippocampus will align with hippocampus, perirhinal cortex with perirhinal cortex, etc.). In so doing, ROI-AL significantly improves the overlap across participants, increasing statistical power and precision in localization of cross-participant tests.
Here, the ROI-AL technique was modified to include a separate 12-parameter affine transformation matrix for each individual ROI. Thus, 10 transformation matrices were created and 10 versions of each statistical map (and structural image) were created for each participant. The results of the 10 transformations were combined into a single statistical map by weighing each statistical map's contribution to a voxel in the combination by an estimate of the distance from each ROI to that voxel. This distance was estimated by blurring each transformed ROI by a 5-mm FWHM Gaussian kernel. Thus, the binary segmentation became a blurred map, indicating the area of influence this particular ROI was allowed to have over the combined output. Where overlap across blurred ROIs occurred, the voxel in the combination was weighted by the degree of influence (value in the blur) from each contributing ROI. We have found that by extending ROI-AL in this manner, further gains in statistical power and precision are achieved.
In the ROI-AL analysis and in a second whole-brain analysis, a voxel-wise three-factor analysis of variance (ANOVA) was performed on the β coefficients with two fixed factors (type of phase and type of subsequent memory) and a random factor (subject) to identify interactions between the two factors. These ANOVAs were used to define functional regions of interest (ROIs) that demonstrated any significant interaction in activity that indicated a differential effect of encoding phase (Original Event phase and Misinformation phase) and subsequent memory type (true memories and false memories). In the ROI-AL analysis, a voxel-wise threshold of F > 5.5 (P < 0.03) and a spatial extent threshold of 215 mm3 for a corrected α of P ≈ 0.053 (within the MTL alone) was used. In the whole-brain analysis, a voxel-wise threshold of F > 8.1 (P < 0.01) and a spatial extent threshold of 328 mm3 for a corrected α of P < 0.05 was used.
Acknowledgments
We thank LisaCaitlin Perri, Aalap Shah, Monica Lopez-Gonzalez, and the staff of the F.M. Kirby Center for Functional Brain Imaging for their assistance in data collection. Funding source: NSF BCS-0236431.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.87605.
References
- Brewer, J.B., Zhao, Z., Desmond, J.E., Glover, G.H., and Gabrieli, J.D. 1998. Making memories: Brain activity that predicts how well visual experience will be remembered. Science 281: 1185-1187. [DOI] [PubMed] [Google Scholar]
- Brown, M.W. and Aggleton, J.P. 2001. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2: 51-61. [DOI] [PubMed] [Google Scholar]
- Buckner, R.L. 1996. Beyond HERA: Contributions of specific prefrontal brain areas to long-term memory retrieval. Psychonom. Bullet. Rev. 3: 149-158. [DOI] [PubMed] [Google Scholar]
- Buckner, R.L. and Wheeler, M.E. 2001. The cognitive neuroscience of remembering. Nat. Rev. Neurosci. 2: 624-634. [DOI] [PubMed] [Google Scholar]
- Buckner, R.L., Koustaal, W., Schacter, D.L., Wagner, A.D., and Rosen, B.R. 1998a. Functional-anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage 7: 151-162. [DOI] [PubMed] [Google Scholar]
- Buckner, R.L., Koustaal, W., Schacter, D.L., Dale, A.M., Rotte, M., and Rosen, B.R. 1998b. Functional-anatomic study of episodic retrieval. II. Selective averaging of event-related fMRI trials to test the retrieval success hypothesis. Neuroimage 7: 163-175. [DOI] [PubMed] [Google Scholar]
- Buckner, R.L., Wheeler, M.E., and Sheridan, M.A. 2001. Encoding processes during retrieval tasks. J. Cogn. Neurosci. 13: 406-415. [DOI] [PubMed] [Google Scholar]
- Cabeza, R. and Nyberg, L. 2000. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 12: 1-47. [DOI] [PubMed] [Google Scholar]
- Cabeza, R., Rao, S.M., Wagner, A.D., Mayer, A.R., and Schacter, D.L. 2001. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc. Natl. Acad. Sci. 98: 4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R.W. 1996. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29: 162-163. [DOI] [PubMed] [Google Scholar]
- Davachi, L. and Wagner, A.D. 2002. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J. Neurophys. 88: 982-990. [DOI] [PubMed] [Google Scholar]
- Davachi, L., Mitchell, J.P., and Wagner, A.D. 2003. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. 100: 2157-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge, L.L., Knowlton, B.J., Furmanski, C.S., Bookheimer, S.Y., and Engel, S.A. 2000. Remembering episodes: A selective role for the hippocampus during retrieval. Nat. Neurosci. 3: 1149-1152. [DOI] [PubMed] [Google Scholar]
- Fabiani, M., Stadler, M.A., and Wessels, P.M. 2000. True but not false memories produce a sensory signature in human lateralized brain potentials. J. Cogn. Neurosci. 12: 941-949. [DOI] [PubMed] [Google Scholar]
- Fernandez, G., Effern, A., Grunwald, T., Pezer, N., Lehnertz, K., Dumpelmann, M., Van Roost, D., and Elger, C.E. 1999. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science 285: 1582-1585. [DOI] [PubMed] [Google Scholar]
- Fernandez, G., Klaver, P., Fell, J., Grunwald, T., and Elger, C.E. 2002. Human declarative memory formation: Segregating rhinal and hippocampal contributions. Hippocampus 12: 514-519. [DOI] [PubMed] [Google Scholar]
- Fletcher, P.C. and Dolan, R.J. 1999. Right prefrontal cortex responds to item familiarity during a memory encoding task. Memory 7: 703-713. [DOI] [PubMed] [Google Scholar]
- Fletcher, P.C. and Henson, R.N.A. 2001. Frontal lobes and human memory: Insights from functional neuroimaging. Brain 124: 849-881. [DOI] [PubMed] [Google Scholar]
- Gabrieli, J.D., Brewer, J.B., Desmond, J.E., and Glover, G.H. 1997. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 276: 264-266. [DOI] [PubMed] [Google Scholar]
- Gonsalves, B. and Paller, K.A. 2000. Neural events that underlie remembering something that never happened. Nat. Neurosci. 3: 1316-1321. [DOI] [PubMed] [Google Scholar]
- Gonsalves, B., Reber, P.J., Gitelman, D.R., Parrish, T.B., Mesulam, M.M., and Paller, K.A. 2004. Neural evidence that vivid imagining can lead to false remembering. Psychol. Sci. 15: 655-660. [DOI] [PubMed] [Google Scholar]
- Insausti, R., Juottonen, K., Soininen, H., Insausti, A.M., Partanen, K., Vainio, P., Laakso, M.P., and Pitkanen, A. 1998. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am. J. Neuroradiol. 19: 659-671. [PMC free article] [PubMed] [Google Scholar]
- Jackson, O. and Schacter, D.L. 2004. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 21: 456-462. [DOI] [PubMed] [Google Scholar]
- Janowsky, J.S., Shimamura, A.P., and Squire, L.R. 1989a. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia 27: 1043-1056. [DOI] [PubMed] [Google Scholar]
- Janowsky, J.S., Shimamura, A.P., Kritchevsky, M., and Squire, L.R. 1989b. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav. Neurosci. 103: 548-560. [DOI] [PubMed] [Google Scholar]
- Johnson, M.K., Taylor, T.H., and Raye, C.L. 1977. Fact and fantasy: The effects of internally generated events on the apparent frequency of externally generated events. Mem. Cogn. 5: 116-122. [DOI] [PubMed] [Google Scholar]
- Kelley, W.M., Miezin, F.M., McDermott, K.B., Buckner, R.L., Raichle, M.E., Cohen, N.J., Ollinger, J.M., Akbudak, E., Conturo, T.E., Snyder, A.Z., et al. 1998. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 20: 927-936. [DOI] [PubMed] [Google Scholar]
- Kirchhoff, B.A., Wagner, A.D., Maril, A., and Stern, C.E. 2000. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J. Neurosci. 20: 6173-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan, C.B. and Stark, C.E.L. 2004. Medial temporal lobe activitation during encoding and retrieval of novel face-name pairs. Hippocampus 14: 919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, D.S. and Johnson, M.K. 1989. The eyewitness suggestibility effect and memory for source. Mem. Cogn. 17: 349-358. [DOI] [PubMed] [Google Scholar]
- Loftus, E.F. 1981. Mentalmorphosis: Alterations in memory produced by the mental bonding of new information to old. In Attention & performance IX (ed. J.L.A. Baddeley), pp. 417-434. Erlbaum, Hillsdale, NJ.
- ———. 1991. Made in memory: Distortions in recollection after misleading information. Psychol. Learn. Motivat. 27: 187-215. [Google Scholar]
- Loftus, E.F. and Hoffman, H.G. 1989. Misinformation and memory: The creation of false memories. J. Exp. Psychol. Gen. 118: 100-104. [DOI] [PubMed] [Google Scholar]
- Loftus, E.F., Miller, D.G., and Burns, H.J. 1978. Semantic integration of verbal information into a visual memory. J. Exp. Psychol. [Hum. Learn]. 4: 19-31. [PubMed] [Google Scholar]
- Loftus, E.F., Feldman, J., and Dashiell, R. 1995. The reality of illusory memories. In Memory distortion: How minds, brains, and societies reconstruct the past (ed. D.L. Schacter), pp. 47-68. Harvard University Press, Cambridge, MA.
- Manning, C.G. and Loftus, E.F. 1996. Eyewitness testimony and memory distortion. Japan. Psychol. Res. 38: 5-13. [Google Scholar]
- McCloskey, M. and Zaragoza, M. 1985. Misleading postevent information and memory for events: Arguments and evidence against memory impairment hypotheses. J. Exp. Psychol. Gen. 114: 1-16. [DOI] [PubMed] [Google Scholar]
- Mitchell, K.J. and Johnson, M.K. 2000. Source monitoring: Attributing mental experiences. In Oxford handbook of memory (eds. E. Tulving and F.I.M. Craik), pp. 179-195. Oxford University Press, New York.
- Nyberg, L., Cabeza, R., and Tulving, E. 1996. Pet studies of encoding and retrieval: The HERA model. Psychonom. Bull. Rev. 3: 135-148. [DOI] [PubMed] [Google Scholar]
- Okado, Y. and Stark, C.E.L. 2003. Neural processing associated with true and false memory retrieval. Cogn. Affect. Behav. Neurosci. 3: 323-334. [DOI] [PubMed] [Google Scholar]
- Otten, L.J., Henson, R.N.A., and Rugg, M.D. 2001. Depth of processing effects on neural correlates of memory encoding: Relationship between findings across- and within-task comparisons. Brain 124: 399-412. [DOI] [PubMed] [Google Scholar]
- Paller, K.A. and Wagner, A.D. 2002. Observing the transformation of experience into memory. Trends Cogn. Sci. 6: 93-102. [DOI] [PubMed] [Google Scholar]
- Papanicolaou, A.C., Panagiotis, G.S., Castillo, E.M., Breier, J.I., Katz, J.S., and Wright, A.A. 2002. The hippocampus and memory of verbal and pictorial material. Learn. Mem. 9: 99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmann, K., Weiger, M., Scheidegger, N., and Boesiger, P. 1999. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 42: 952-962. [PubMed] [Google Scholar]
- Ranganath, C., Yonelinas, A.P., Cohen, M.X., Dy, C.J., Tom, S.M., and D'Esposito, M.D. 2003. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42: 2-13. [DOI] [PubMed] [Google Scholar]
- Rugg, M.D., Otten, L.J., and Henson, R.N.A. 2002. The neural basis of episodic memory: Evidence from functional neuroimaging. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 357: 1097-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter, D.L. 1999. The seven sins of memory. Am. Psychol. 54: 182-203. [DOI] [PubMed] [Google Scholar]
- Schacter, D.L. and Slotnick, S.D. 2004. The cognitive neuroscience of memory distortion. Neuron 44: 149-160. [DOI] [PubMed] [Google Scholar]
- Schacter, D.L., Reiman, E., Curran, T., Yun, L.S., Bandy, D., McDermott, K.B., and Roediger, H.L. 1996. Neuroanatomical correlates of veridical and illusory recognition memory: Evidence from positron emission tomography. Neuron 17: 267-274. [DOI] [PubMed] [Google Scholar]
- Schacter, D.L., Buckner, R.L., Koutstaal, W., Dale, A.M., and Rosen, B.R. 1997. Late onset of anterior prefrontal activity during true and false recognition: An event-related fMRI study. Neuroimage 6: 259-269. [DOI] [PubMed] [Google Scholar]
- Shimamura, A.P., Janowsky, J.S., and Squire, L.R. 1990. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia 28: 803-813. [DOI] [PubMed] [Google Scholar]
- Squire, L.R., Stark, C.E.L., and Clark, R.E. 2004. The medial temporal lobe. Annu. Rev. Neurosci. 27: 279-306. [DOI] [PubMed] [Google Scholar]
- Stark, C.E.L. and Okado, Y. 2003. Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. J. Neurosci. 23: 6748-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, C.E.L. and Squire, L.R. 2000. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J. Neurosci. 20: 7776-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2001. Simple and associative recognition memory in the hippocampal region. Learn. Mem. 8: 190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, C.E., Corkin, S., Gonzalez, R.G., Guimares, A.R., Baker, J.R., Jennings, P.J., Carr, C.A., Sugiura, R.M., Vadantham, V., and Rosen, B.R. 1996. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc. Natl. Acad. Sci. 93: 8600-8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange, B.A., Otten, L.J., Josephs, O., Rugg, M.D., and Dolan, R.J. 2002. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J. Neurosci. 22: 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, W.A. and Eichenbaum, H. 2000. The neurophysiology of memory. Ann. N.Y. Acad. Sci. 911: 175-191. [DOI] [PubMed] [Google Scholar]
- Talairach, J. and Tournoux, P. 1988. A co-planar stereotaxic atlas of the human brain. Thieme Medical, New York.
- Tousignant, J.P., Hall, D., and Loftus, E.F. 1986. Discrepancy detection and vulnerability to misleading postevent information. Mem. Cogn. 14: 329-338. [DOI] [PubMed] [Google Scholar]
- Wagner, A.D., Schacter, D.L., Rotte, M., Koutstaal, W., Maril, A., Dale, A.M., Rosen, B.R., and Buckner, R.L. 1998. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281: 1188-1191. [DOI] [PubMed] [Google Scholar]
- Ward, B.D. 2002. Deconvolution analysis of FMRI time series data. In Analysis of functional neuroImages (AFNI); http://afni.nimh.nih.gov/afni/doc
- Yonelinas, A., Hopfinger, J., Buonocore, M., Kroll, N., and Baynes, K. 2001. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: An fMRI study. Neuroreport 12: 359-363. [DOI] [PubMed] [Google Scholar]
- Zaragoza, M. and Lane, S.M. 1994. Source misattributions and the suggestibility of eyewitness memory. J. Exper. Psychol.: Learn. Mem. Cogn. 20: 934-945. [DOI] [PubMed] [Google Scholar]