Abstract
Aims
The intrauterine environment is considered to affect immunological development in fetus, leading to an increased risk of developing allergy. In particular, maternal lipopolysaccharides (LPS) administration might regulate the development of allergic disease in offspring. Several studies have shown that being obese relates to a higher prevalence of allergic diseases compared to normal weight. The present study explored the effects of inducing maternal inflammation with LPS before pregnancy on body weight, physical composition including body fat, adipokine production, and pathology of allergic rhinitis in offspring.
Main methods
Female mice received a single intraperitoneal injection of LPS (2 μg/g BW). After 5 days of LPS administration, female mice were mated with males, and experimental allergic rhinitis was induced in female offspring. Immunization and nasal challenge with ovalbumin (OVA) were performed at 7 and 8 weeks of age. Allergic rhinitis-like symptoms, OVA-specific IgE and adipokines in sera, body weight, fat pad weight, and cytokine production by splenocytes in these 9-week-old offspring.
Key findings
Maternal LPS administration results in a significant increase in body weight, visceral fat accumulation, and serum leptin concentration, and the dominance of Th1 in Th balance. Nevertheless, there was no statistical difference in OVA-specific IgE titer and allergic-like symptoms between the groups.
Significance
In conclusion, maternal LPS promoted leptin production and altered Th balance in mice offspring, but not improved allergic symptoms in a mouse model of allergic rhinitis. It might suggest that inflammation during pregnancy plays a role in the adipose tissue function which could diversely influence allergic inflammation in offspring.
Keywords: Immunology, Metabolism, Nutrition, Health Sciences
1. Introduction
The prevalence of allergic diseases has been increasing over the years. This dramatic increase in recent years is attributed to environmental factors such as changes in dietary habits or more hygienic environments, rather than genetic factors. Some research has investigated the prevention of acquiring the susceptibility to allergy and allergenic sensitization/development, but no measures have been established to prevent allergic diseases.
The development of allergic diseases involves a complex interaction between acquired immunity and natural immunity (Holgate and Polosa, 2008). Allergic diseases are characterized as a helper T (Th) 2-cell response, which is dominant in helper T cell response to allergens and it causes an allergic response. It has been reported that infants who develop allergy already have a strong tendency to show Th2 cell-biased immune response and the intrauterine environment is considered to affect immunological development in infants (Warner et al., 2000).
Recent studies have confirmed that the amount of lipopolysaccharide (LPS) composition of cell walls of gram-negative bacteria in the environment relates to a risk of developing allergic diseases (Gereda et al., 2000; Braun-Fahrlander et al., 2002; Bottcher et al., 2003), and exposure to LPS especially during the first year of life could prevent the development of allergic diseases (Riedler et al., 2001). Therefore, during fetal life, changes in the immune system may occur in fetuses, leading to an altered risk of developing an allergy (Warner et al., 2000). In previous animal studies, maternal LPS application before and during pregnancy suppressed the development of allergic diseases in the infant (Gerhold et al., 2006; Blumer et al., 2005; Cao et al., 2010; Kirsten et al., 2011). The mechanism is explained by a shift toward unspecific Th1-type immune response induced by LPS recognition by innate immunity, thus suppressing Th2-mediated allergic disease (Gerhold et al., 2006). However, the studies which demonstrated factors that may affect pathological conditions of allergy other than Th1/Th2 balance are insufficient. Meanwhile, it is known that direct LPS administration as well as obesity affects the immune system through modulation of hormone secretion and the nervous system. Maternal LPS administration might influence metabolic elements, such as body weight, fat accumulation and serum levels of leptin in adult offspring (Nilsson et al., 2001; Reul et al., 1994). These parameters are known to be aberrant in obesity or insulin resistance. Thus, we assume immunological modulation by maternal LPS administration have effects on both immunological phenotype and metabolic status in offspring.
On the other hand, several studies have shown that being obese or overweight relates to a higher prevalence of asthma or atopic disease compared to normal weight (Luder et al., 1998; Mishra, 2004). On the basis of the facts mentioned above, maternal LPS administration may have dual actions on allergic pathology of offspring: inhibitory effects on Th1/Th2 balance and deteriorating allergic pathology in cases of changes of body composition. Therefore, the present study explored the effects of inducing maternal potent inflammation by LPS administration before pregnancy on body weight, physical composition including body fat, adipokine production, and pathology of allergic rhinitis in offspring.
2. Materials and methods
2.1. Mice
DBA/1J mice were purchased from Sankyo Labo Service Corporation, INC (Tokyo, Japan). They were allowed free access to a non-purified MF diet (Oriental Yeast Co. Ltd., Osaka, Japan) and water, and were kept in a light-, temperature-, and humidity-controlled environment throughout the experiment (12–12 h light-dark cycle, temperature 23 ± 1 °C, 55 ± 5% relative humidity). This study was approved by the Institutional Animal Care and Use Committee at Japan Women's University (approval number II 12–8), and the animals were maintained in accordance with the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan.
2.2. Protocol
Female mice received a single intraperitoneal injection of LPS (from E. coli 0111:B4, Sigma-Aldrich Co. LLC., 2 μg/g body weight). The sham group received only phosphate buffered saline (PBS). After 5 days of LPS or PBS administration, female mice were mated with males in cages. The offspring were weaned at 3 weeks and male offspring were removed from the cage. Only female offspring were used for further experiment and were given the same diet as their mothers. The offspring that were born from dam received LPS are termed the maternal LPS group, and the offspring that were born from the sham group were termed the control group. The experimental design is shown in Fig. 1. Body weight and the amount of dietary intake were determined once a week.
Fig. 1.
Experimental design. Female mice received a single intraperitoneal injection of LPS. After 5 days of LPS administration, female mice were mated with males. The offspring were weaned at 3 weeks and male offspring were removed from the cage. Only female offspring were used for further experiment and were given the same diet as their mothers. The offspring that were born from dam and received LPS are termed the maternal LPS group (n = 7), and the offspring that were born from the sham group were termed the control group (n = 7). Female offspring were induced experimental allergic rhinitis. At 7 and 8 weeks old, all female offspring mice were immunized intraperitoneally with ovalbumin (OVA) with aluminum potassium sulfate (Alum). A week after the booster immunization, the mice were intranasally challenged with OVA solution by daily instillation for 6 days.
Female offspring were induced experimental allergic rhinitis. Immunization and nasal challenge with ovalbumin (OVA, grade V, Sigma-Aidrich) were performed according to described protocols (Onishi et al., 2007), with some modifications. At 7 and 8 weeks old, all female offspring mice were immunized intraperitoneally with 100 μg of OVA with 4 mg of aluminum potassium sulfate in 500 μl of total volume. A week after the booster immunization, the mice were intranasally challenged with OVA solution (25 μg/ml solution in PBS, 20 μl/mouse) by daily instillation for 6 days. To evaluate allergic rhinitis-like symptoms, the mice were placed in a cage (one animal/cage) just after the last intranasal instillation with OVA; the number of sneezes and scratches were counted for 5 min under blinded conditions, and were subsequently killed by cervical dislocation on the next day of evaluation of allergic rhinitis-like symptoms. After blood collection, serum was obtained by centrifugation and stored at −30 °C until analysis.
2.3. Measurement of OVA-specific IgE
The amount of OVA-specific IgE antibody in sera was determined by enzyme-linked immunosorbent assay (ELISA) using OVA (100 μg/ml in NaHCO3 buffer, pH9.5) to coat plates. After incubation of sera (sera were used to diluted for 50 times) on coated plates, BD OptEIA™ (Mouse IgE ELISA Set, biotinylated Detection antibody and Streptavidin-HRP, BD Biosciences, San Diego, CA, USA) was used for detection. The enzyme reaction used 3,3',5,5'-tetramethylbenzeidine (MP Biomedicals, LCC, Solon, OH) in sodium-citrate buffer in the presence of 0.01% H2O2. The reaction was stopped by the addition of 2N-HCl, and absorbance was measured at 450 nm and 570 nm. Data are shown as values obtained by subtracting optimal density (OD) of non-coated well from OD of OVA-coated well.
2.4. Splenocyte culture
Spleens were aseptically removed and placed in RPMI 1640 medium (NISSUI, Tokyo, Japan). Single cell suspensions were made by teasing spleen apart with a stainless-steel mesh and filtering through a 250-μm nylon mesh. Cell suspensions were collected in sterile conical tubes and were washed 3 times in RPMI 1600 medium containing 5% heat inactivated fetal calf serum, L-glutamine (2 mmol/L, GIBCO, Grand Island, NY), penicillin (100 U/mL, GIBCO), and streptomycin (100 μg/mL, GIBCO), followed by centrifugation at 1,200 rpm for 10 min at 4 °C. Cells were diluted in medium to 1 × 107 cells/ml. Splenocytes (5 × 105cells/100 μl per well) were cultured in the presence of 100 μg/ml OVA as a specific response, 5 μg/ml concanavalin A (ConA, Difco Laboratories, Detroit, MI), or 10 μg/ml LPS (Difco Laboratories) as a non-specific response at 37 °C in 5% CO2.
2.5. Proliferative response of splenocytes by Alamar Blue assay
After 120 h (with OVA) or 72 h of culture (with mitogen), the proliferative response of the splenocytes was measured by Alamar Blue assay. Alamar Blue solution (Serotec, Oxford, UK) was added to the cultured medium, and cells ware incubated for 4 h at 37 °C in 5% CO2. Fluorescence was measured at 544–590 nm. Fluorescence form each OVA- or mitogen-stimulated well was compared with that from non-stimulated wells to determine lymphocyte proliferation.
2.6. Measurement of cytokine production
Cytokine production was measured in supernatants of ConA- (IL-2, IL-4, IL-10, IFN-γ, and IL-17) or LPS- (IL-6, TNF-α) stimulated splenocytes. Cytokine levels were measured with DuoSet ELISA Development system (R&D systems, Minneapolis, MN, USA). Culture supernatants were collected after incubation for 48 h, centrifuged, and stored at −30 °C until analyses.
2.7. Measurement of adipokines
Serum adiponectin and leptin levels were measured with DuoSet ELISA Development system (R&D Systems).
2.8. Statistical analyses
Data are shown as mean ± standard error. For statistical testing of normality, the Kolmogorov-Smirnov test was used. Normally distributed variables were compared by Student’s t test and non-normally distributed variables by the Mann-Whitney U test. Results were considered statistically significant when P < 0.05. IBM SPSS statistics 22 (IBM Japan, Tokyo, Japan) was used for all the statistical calculations.
3. Results
3.1. Body weight
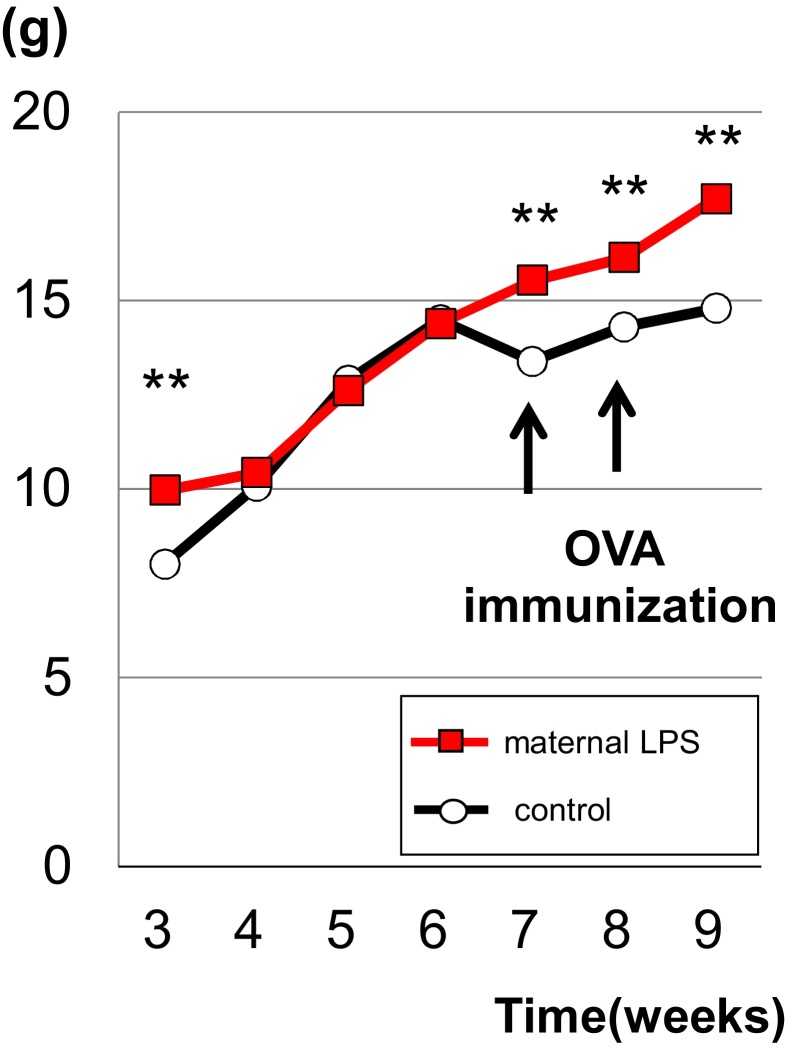
The changes in body weight from weaning to the end of the experiment were recorded (Fig. 2). At weaning, the body weight in the offspring born from LPS-exposed dams (maternal LPS group) was significantly higher than those of the control group. The significant difference between groups disappeared over time, although the LPS group had no weight loss after immunization with OVA, resulting in significantly higher body weight compared to the control group. Following the induction of allergic rhinitis by immunization and intranasal instillation with OVA, body weights were significantly higher in the LPS group up to 10 weeks of age.
Fig. 2.
Time course of changes in body weight of offspring of the control group and the maternal LPS group. Body weight was determined once a week. The difference between the 2 groups of mice was significant at 3 weeks, point of weaning, and after first immunization (7 to 9 weeks) (Values are mean ± SE, **; P < 0.01).
3.2. Organ weights
Among organ weights (spleen, thymus, liver, kidney, subperitoneal and inguinal fat), spleen weight was significantly lower in the maternal LPS group than in the control group. Subperitoneal fat weight measured as an indicator of amount of visceral fat was significantly higher in the LPS group than in the control group, whereas the difference in inguinal fat weight, indicator of subcutaneous fat, was not detected between the groups (Table 1).
Table 1.
Organ weights.
| Control | Maternal LPS | |
|---|---|---|
| Spleen (g) | 0.125 ± 0.023 | 0.081 ± 0.034* |
| Thymus (g) | 0.047 ± 0.008 | 0.046 ± 0.007 |
| Liver (g) | 0.915 ± 0.076 | 0.886 ± 0.052 |
| Kidney (g) | 0.228 ± 0.017 | 0.222 ± 0.014 |
| Subperitoneal fat pad (g) | 0.07 ± 0.035 | 0.131 ± 0.019** |
| Inguinal fat pad (g) | 0.129 ± 0.044 | 0.158 ± 0.07 |
Values are mean ± SE.
P < 0.05.
P < 0.01.
3.3. Serum levels of adipokines and leptin/adiponectin ratio
The serum levels of leptin were significantly higher than those of the control group. The serum leptin/adiponectin ratio (L/A ratio) was significantly higher in the maternal LPS group than in the control group (Fig. 3).
Fig. 3.
Serum levels of adipokines and Leptin/Adiponectin ratio. Serum was obtained on the next day of evaluation of allergic rhinitis-like symptoms. Serum levels of (A) adiponectin, (B) Leptin, and (C) Leptin/Adiponectin ratio (Values are mean ± SE, **; P < 0.01).
3.4. Proliferative response of splenocytes
Proliferation responses of spleen lymphocytes stimulated by mitogens, ConA for T cells and LPS for B cells, were significantly suppressed in the maternal LPS group compared to those in the control group. OVA-stimulated proliferative response was also suppressed in the maternal LPS group (Fig. 4).
Fig. 4.
Proliferative response of solenocytes. The proliferative response of the splenocytes was measured by Alamar Blue assay after 120 h (with OVA) or 72 h of culture (with mitogen). Fluorescence form each OVA- or mitogen-stimulated well was compared with that from non-stimulated wells to determine lymphocyte proliferation. ConA, concanavalin; LPS, lipopolysaccharide; OVA, ovalbumin; SI, stimulation index (Values are mean ± SE,*; P < 0.05, **; P < 0.01).
3.5. Cytokine production by splenocytes and helper T (Th) balance
The maternal LPS group demonstrated a significantly higher level of IFN-ɤ, resulting in a higher ratio of IFN-ɤ/IL-4 than in the control group; it suggests an inhibition in Th2-dominant balance generally observed by inducing allergy. Compared to the control group, the level of IL-10 produced from T cells was significantly higher and IL-17 production from Th17 was comparable. The level of TNF-α was significantly lower in the maternal LPS group (Table 2).
Table 2.
Cytokine production by splenocytes.
| Control | Maternal LPS | |
|---|---|---|
| IFN-γ (ng/ml) | 3.51 ± 0.234 | 4.69 ± 0.324* |
| IL-2 (ng/ml) | 1.39 ± 0.233 | 1.66 ± 0.325 |
| IL-4 (pg/ml) | 49.12 ± 3.353 | 47.88 ± 2.534 |
| IL-6 (pg/ml) | 45.70 ± 4.500 | 58.80 ± 4.700 |
| IL-10 (pg/ml) | 175.63 ± 49.05 | 295.02 ± 35.634* |
| IL-17 (ng/ml) | 0.69 ± 0.070 | 0.64 ± 0.082 |
| TNF-α (pg/ml) | 214.70 ± 6.185 | 142.94 ± 26.727* |
| IFN-γ/IL-4 ratio | 73.00 ± 9.330 | 102.61 ± 9.792* |
Splenocytes (5 × 105cells/100 μl per well) were cultured after 48 h of culture in the presence of 100 μg/ml ovalbmin(OVA), 5 μg/ml concanavalin A (ConA), or 10 μg/ml lipopolysaccharide (LPS) as a non-specific response at 37 °C in 5% CO2. ELISA was used on supernatans of splenocytes stimulated with either LPS(IL-6,TNF-α) or ConA(others). Values are mean ± SE, *; P < 0.05
3.6. OVA-specific IgE titer and allergic-like symptoms
Both OVA-specific IgE titer and allergic rhinitis-like symptoms (expressed as total numbers of sneezing and scratching in 5 min) increased in the maternal LPS group and the control group compared to female offspring without intranasal challenge with OVA (non-allergic group). There was, however, no statistical difference between the maternal LPS group and the control group (Table 3).
Table 3.
OVA-specific IgE titer and allergic rhinitis-like symptms (sneezes and scratches).
| Control | Maternal LPS | Allergy(−) | |
|---|---|---|---|
| OVA specific IgE (OD) | 0.036 ± 0.010 # | 0.026 ± 0.008 # | 0.000 ± 0.000 |
| Sneezes and scratches (times/5 min) | 51.4 ± 12.1 ✝ | 60 ± 12.2 # | 28 ± 2.96 |
OVA-specific IgE antibody titer in sera was determined by enzyme-linked immunosorbent assay (ELISA) using OVA to coat plates. Data are shown as values obtained by subtracting optimal density (OD) of non-coated well from OD of OVA-coated well. Allergy(−), female offspring without intranasal challenge with OVA. Allergic rhinitis-like symptoms were evaluated at the number of sneezes and scratches for 5 min under blinded conditions just after the last intranasal instillation with OVA. #:P < 0.05 vs allergy(−), ✝:P < 0.1 vs allergy(−), OVA, ovalbmin; OD, optical density.
4. Discussion
In the present study, we investigated the effects of maternal inflammation on metabolic and immune function of offspring. The maternal LPS group showed a significant increase of body weight and visceral fat accumulation and a significantly higher serum leptin concentration than the control group.
Leptin is an adipocyte-derived adipokine that regulates food intake and energy expenditure. Because of enlarged fat cells in obesity, recruitment of inflammatory cells into adipose tissue leads to an abnormal production of adipokines (Ito et al., 2007). It is reported that blood levels of leptin correlate with amounts of body fat and an increase in obesity. Infection or inflammatory stimulation also increases leptin production (Grunfeld et al., 1996), and the elevated production of leptin in patients with allergic diseases such as asthma and atopic dermatitis, suggests that leptin is involved in the association between obesity and allergic inflammation (Wong et al., 2007). In the present study, the result demonstrated a higher serum leptin level in the maternal LPS group in which increased body fat and visceral fat accumulation were observed. Although we could not show the direct link between allergic inflammation in offspring and the maternal inflammation induced by LPS, we further need to clarify the mechanism how maternal LPS-induced inflammation lead to both the elevated serum level of leptin and the increased visceral fat accumulation in offspring, which may relate to allergic inflammation.
In our study, the maternal LPS group in which allergy was induced also showed significantly higher levels of IFN-γ, resulting in a higher ratio of IFN-γ/IL-4 than the control group. Despite the fact that proliferation response of spleen lymphocytes was suppressed by ConA, IFN-γ production stimulated by ConA was higher level in the maternal group. This finding suggests that maternal exposure to LPS produced a predominant Th1 state in offspring. Leptin affects differentiation and activation of T cells, and it induces cytokine production such as IFN-ɤ, which may contribute to the importance of Th2 dominant inflammation from the view point of Th balance. On the other hand, leptin also influences eosinophils to promote MCP-1 production. MCP-1 is a chemokine that has been shown to provoke aggregation and release of histamines and prostaglandin D2 from mast cells in type I allergy; therefore, leptin may also be involved in mediating Th2 immune responses (Wong et al., 2007). Previous studies also suggested that leptin delayed the apoptosis of eosinophils, and this finding suggests an involvement of leptin in sustaining chronic inflammation (Wong et al., 2007). It has been shown that the increased leptin production led to a reduction in adipose tissue-infiltrating regulatory T cells in obese individuals (Zeng and Chi, 2013), hence exacerbating local inflammation. Thus, leptin has multimodal effects on Th balance and modification of inflammation. In our study, the Th balance assessed by splenocytes function as an index of generalized immunity, showed Th1 dominant balance, and OVA-stimulated proliferative response was suppressed in the maternal LPS group, however, there was no reduction in OVA-specific IgE titer and clinical symptom of allergy. Although the proliferative response to OVA and Th balance are also some important indicators of the allergic reaction, it is not clarified why clinical symptom was not changed in this study. Leptin may have promoted local inflammation induced by allergy. Since leptin has multimodal effects on allergic inflammation which may be essential for the association between immunological response and adipose tissue, further study is warranted to explore how leptin acts when allergic reaction is induced.
In recent years, the prevalence of allergic diseases is increasing dramatically in developed countries, and changes in environmental factors, but not genetic factors, may be a cause of this condition. The number of obese people is increasing and epidemical studies have showed that an increase in the prevalence of atopic disease or asthma is greater in obese children/adults compared to their peers with normal weight (Luder et al., 1998; Mishra, 2004). In obese people, weight reduction significantly improved the severity and symptoms of asthma (Stenius-Aarniala et al., 2000). Our study demonstrates that body weight at weaning and the end of study and subperitoneal fat weight as an indicator of amounts of visceral fat was significantly higher in the maternal LPS group than in the control. Despite the different timing of administration and dosage in the present study, a study showed that maternal LPS injection during pregnancy resulted in high serum levels of corticosterone and testosterone in the male offspring at 12 weeks old (Nilsson et al., 2001). In addition, a high dose of testosterone subcutaneously administered to female rats within 3 h after birth led to a pronounced insulin resistance, an increased body weight, and changes in body fat distribution (Nilsson et al., 1998). The results obtained in this study also showed higher body weights at weaning and the end of experiment and changes of fat accumulation and adipokine production which may result in induction of insulin resistance in the maternal LPS group. The increased body weight and fat accumulation with LPS challenge during pregnancy may be mediated by testosterone activity.
In accordance with these results, this study also evaluated L/A ratio. Although the direct association between L/A ratio and allegic index was not observed in this study. It is reported that allergic rhinitis patients had higher serum levels of leptin and lower serum levels of adiponectin than control, and both of them were correlated with nasal symptom (Hsueh et al., 2010). It is assumed that high L/A ratio may be one of the factors which modulated allergy condition of offspring. In fact, our study suggested that inflammation during pregnancy may increase the weight of adipose tissue in offspring, and it cause a qualitative functional change in the adipose tissue shown as an imbalanced adipokine production. To prove this assumption, distribution and functional change of adipose tissue in relation to adipokine production should be further investigated.
Previous studies in mice showed that a high-fat diet during pregnancy increased body weight and visceral fat accumulation (Gregorio et al., 2010; Khan et al., 2005) and caused inflammatory changes in the adipose tissue in offspring (Murabayashi et al., 2013). In addition, a high-fat dietary environment in the fetal period impaired antigen-specific IgG production (Odaka et al., 2010). Although maternal inflammation in our study was LPS-induced acute strong inflammation, rather than obesity- or a high-fat diet-induced modest and chronic inflammation, the results of the increased body weight, fat accumulation, and elevated L/A ratio were consistent in offspring.
5. Conclusion
Maternal LPS administration induced an increased body weight, fat accumulation, and elevated L/A ratio in offspring. Leptin has multimodal effects on Th balance and inflammation. It was suggested that further studies are necessary to evaluate the effects of inflammation during pregnancy on interaction of adipose tissue function and allergic inflammation in offspring.
Declarations
Author contribution statement
Atsuko Imai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Keiko Satoi: Analyzed and interpreted the data; Wrote the paper.
Eka Fujimoto: Performed the experiments; Analyzed and interpreted the data.
Kazuto Sato: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
No additional information is available for this paper.
Acknowledgments
We thank Ms. M. Kusumi, Ms. K. Tsukahara for their excellent technical assistance and support.
References
- Blumer N., Herz U., Wegmann M., Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin. Exp. Allergy. 2005;35(3):397–402. doi: 10.1111/j.1365-2222.2005.02184.x. CEA2184 [pii] [DOI] [PubMed] [Google Scholar]
- Bottcher M.F., Bjorksten B., Gustafson S., Voor T., Jenmalm M.C. Endotoxin levels in estonian and swedish house dust and atopy in infancy. Clin. Exp. Allergy. 2003;33(3):295–300. doi: 10.1046/j.1365-2222.2003.01562.x. 1562 [pii] [DOI] [PubMed] [Google Scholar]
- Braun-Fahrlander C., Riedler J., Herz U., Eder W., Waser M., Grize L., Allergy and Endotoxin Study Team Environmental exposure to endotoxin and its relation to asthma in school-age children. New Engl. J. Med. 2002;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- Cao L., Wang J., Zhu Y., Tseu I., Post M. Maternal endotoxin exposure attenuates allergic airway disease in infant rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298(5):L670–L677. doi: 10.1152/ajplung.00399.2009. [DOI] [PubMed] [Google Scholar]
- Gereda J.E., Leung D.Y., Thatayatikom A., Streib J.E., Price M.R., Klinnert M.D., Liu A.H. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355(9216):1680–1683. doi: 10.1016/s0140-6736(00)02239-x. S014067360002239X [pii] [DOI] [PubMed] [Google Scholar]
- Gerhold K., Avagyan A., Seib C., Frei R., Steinle J., Ahrens B., Hamelmann E. Prenatal initiation of endotoxin airway exposure prevents subsequent allergen-induced sensitization and airway inflammation in mice. J. Allergy Clin. Immunol. 2006;118(3):666–673. doi: 10.1016/j.jaci.2006.05.022. S0091-6749(06)01197-3 [pii] [DOI] [PubMed] [Google Scholar]
- Gregorio B.M., Souza-Mello V., Carvalho J.J., Mandarim-de-Lacerda C.A., Aguila M.B. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am. J. Obstetrics Gynecol. 2010;203(5) doi: 10.1016/j.ajog.2010.06.042. e1-495. e8. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Zhao C., Fuller J., Pollack A., Moser A., Friedman J., Feingold K.R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. The Journal of Clin. Investig. 1996;97(9):2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S.T., Polosa R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008;8(3):218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- Hsueh K.C., Lin Y.J., Lin H.C., Lin C.Y. Serum leptin and adiponectin levels correlate with severity of allergic rhinitis. Pediatr. Allergy Immunol. 2010;21(1 (Pt 2)):e155–e159. doi: 10.1111/j.1399-3038.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Ito A., Suganami T., Miyamoto Y., Yoshimasa Y., Takeya M., Kamei Y., Ogawa Y. Role of MAPK phosphatase-1 in the induction of monocyte chemoattractant protein-1 during the course of adipocyte hypertrophy. J. Biol. Chem. 2007;282(35):25445–25452. doi: 10.1074/jbc.M701549200. M701549200 [pii] [DOI] [PubMed] [Google Scholar]
- Khan I.Y., Dekou V., Douglas G., Jensen R., Hanson M.A., Poston L., Taylor P.D. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am. J. Physiol. 2005;288(1):R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- Kirsten T.B., de Oliveira B.P., de Oliveira A.P., Kieling K., de Lima W.T., Palermo-Neto J., Bernardi M.M. Single early prenatal lipopolysaccharide exposure prevents subsequent airway inflammation response in an experimental model of asthma. Life Sci. 2011;89(1–2):15–19. doi: 10.1016/j.lfs.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Luder E., Melnik T.A., DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and hispanic children. J. Pediatr. 1998;132(4):699–703. doi: 10.1016/s0022-3476(98)70363-4. S0022-3476(98)70363-4 [pii] [DOI] [PubMed] [Google Scholar]
- Mishra V. Effect of obesity on asthma among adult indian women. Int. J. Obesity Relat. Metab. Disord. 2004;28(8):1048–1058. doi: 10.1038/sj.ijo.0802700. [DOI] [PubMed] [Google Scholar]
- Murabayashi N., Sugiyama T., Zhang L., Kamimoto Y., Umekawa T., Ma N., Sagawa N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur. J. Obstetrics Gynecol. Reprod. Biol. 2013;169(1):39–44. doi: 10.1016/j.ejogrb.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Nilsson C., Larsson B.M., Jennische E., Eriksson E., Bjorntorp P., York D.A., Holmang A. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology. 2001;142(6):2622–2630. doi: 10.1210/endo.142.6.8191. [DOI] [PubMed] [Google Scholar]
- Nilsson C., Niklasson M., Eriksson E., Bjorntorp P., Holmang A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J. Clin. Investig. 1998;101(1):74–78. doi: 10.1172/JCI1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka Y., Nakano M., Tanaka T., Kaburagi T., Yoshino H., Sato-Mito N., Sato K. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity (Silver Spring, Md.) 2010;18(9):1688–1694. doi: 10.1038/oby.2009.513. [DOI] [PubMed] [Google Scholar]
- Onishi N., Kawamoto S., Ueda K., Yamanaka Y., Katayama A., Suzuki H. Dietary pulverized konjac glucomannan prevents the development of allergic rhinitis-like symptoms and IgE response in mice. Biosci. Biotechnol. Biochem. 2007;71(10):2551–2556. doi: 10.1271/bbb.70378. JST.JSTAGE/bbb/70378 [pii] [DOI] [PubMed] [Google Scholar]
- Reul J.M., Stec I., Wiegers G.J., Labeur M.S., Linthorst A.C., Arzt E., Holsboer F. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. J. Clin. Investig. 1994;93(6):2600–2607. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedler J., Braun-Fahrlander C., Eder W., Schreuer M., Waser M., Maisch S. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. S0140-6736(01)06252-3 [pii] [DOI] [PubMed] [Google Scholar]
- Stenius-Aarniala B., Poussa T., Kvarnstrom J., Gronlund E.L., Ylikahri M., Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320(7238):827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J.A., Jones C.A., Jones A.C., Warner J.O. Prenatal origins of allergic disease. The J. Allergy Clin. Immunol. 2000;105(2 Pt 2):S493–S498. doi: 10.1016/s0091-6749(00)90049-6. a100088[pii] [DOI] [PubMed] [Google Scholar]
- Wong C.K., Cheung P.F., Lam C.W. Leptin-mediated cytokine release and migration of eosinophils: Implications for immunopathophysiology of allergic inflammation. Eur. J. Immunol. 2007;37(8):2337–2348. doi: 10.1002/eji.200636866. [DOI] [PubMed] [Google Scholar]
- Zeng H., Chi H. The interplay between regulatory T cells and metabolism in immune regulation. Oncoimmunology. 2013;2(11):e26586. doi: 10.4161/onci.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]