Abstract
Background
Adjuvant chemotherapy after resection of colorectal cancer (CRC) lung metastases may reduce recurrences and improve survival. We investigated the effects of adjuvant chemotherapy after curative resection of lung CRC metastases on prognosis.
Methods
We retrospectively reviewed records of our patients undergoing pulmonary metastasectomy from CRC between January 2000–March 2014. Data were analyzed with Kaplan-Meier survival analysis and Cox proportional hazards models.
Results
One-hundred (56 men; median age, 66 years) of 128 consecutive patients who underwent complete resection for first lung colorectal metastases were analyzed. Postoperative 5-year rates of overall survival (OS) and relapse-free survival (RFS) were 76% and 41%, respectively. Adjuvant chemotherapy strongly affected RFS and OS by multivariable analysis compared to surgery alone (RFS: HR, 0.49; 95% CI, 0.27–0.88; P = 0.016 and OS: HR, 0.35; 95% CI, 0.14–0.81; P = 0.014). Similar effects of adjuvant chemotherapy occurred in subgroups respectively classified according to number of lung metastases and preoperative serum carcinoembryonic antigen (CEA) level.
Conclusions
Adjuvant chemotherapy after curative resection of lung metastases might strongly affect the prognosis of metastatic CRC patients. Even patients with single metastatic lesions and normal preoperative CEA level appeared to receive benefits from such chemotherapy. Narrowing of suitable candidates by predicting the effects of systemic chemotherapy and prospective randomized studies are needed.
Keywords: Pulmonary metastasis, Colorectal cancer, Adjuvant chemotherapy, Pulmonary metastasectomy
Highlights
-
•
Adjuvant chemotherapy after pulmonary metastasectomy might strongly affect prognosis of metastatic CRC patients.
-
•
Even patients with single metastatic lesions and normal preoperative CEA level appeared to receive benefits from such chemotherapy.
-
•
Narrowing of suitable candidates by predicting the effects of systemic chemotherapy and prospective randomized studies are needed.
1. Introduction
During the last decade, chemotherapy regimens containing the 5-fluorouracil (FU)-based drugs oxaliplatin, irinotecan, and bevacizumab showed impressive improvements in survival for patients with both metastatic and resected colorectal cancer (CRC) at pathological stage III [1], [2], [3], [4], [5], [6], [7], [8], [9]. These encouraging results have led many surgeons to believe that surgical resection in combination with pre- or postoperative systemic chemotherapy might improve the prognosis of patients with metastatic CRC and to take an aggressive stance in the management of hepatic and lung metastases. However, most of these recent reports are about hepatic metastases [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Only a few studies have shown a potential survival benefit from postoperative chemotherapy administered after complete removal of lung metastases from CRC [20], [21], [22], [23]. Hence, we investigated whether adjuvant chemotherapy administered after pulmonary metastasectomy provides benefits for patients with lung metastases from CRC and how strongly it affects patient prognosis.
2. Patients and methods
This retrospective cohort study was approved by the Kitasato University Medical Ethics Committee (Approval Number: B16-234). We examined clinical and pathological records of all consecutive patients undergoing lung resections for pulmonary colorectal carcinoma metastases performed from 1 January 2000 to 31 March 2014 in the Department of Thoracic Surgery, Kitasato University Hospital. Inclusion criteria for this study were 1) pathologically proven complete resection, 2) first pulmonary metastasectomy, and 3) curative treatment of extrapulmonary lesions before pulmonary metastasectomy. Preoperative evaluation included that by computed tomographic imaging of the chest, abdomen, or brain or by magnetic resonance imaging. Fluorodeoxyglucose-positron emission tomography was performed in a recent series of patients if liver metastasis, peritoneal dissemination, or other extrapulmonary recurrence was suspected. Almost all of the patients underwent the follow-up and surveillance that are recommended by the Japanese Society for Cancer and the Colon and Rectum. The guideline recommends a physician-performed physical examination, blood tests including a tumor marker every 3 months, and chest/abdominal computed tomography scans every 6 months for the first 3 years and every 6 months thereafter.
There was no definitive indication for post-lung resection chemotherapy during the study period in Japan. Therefore, each doctor judged whether the patients should receive postoperative chemotherapy considering the below-mentioned factors and social background.
We investigated clinical and pathological prognostic factors such as age, sex, number of pulmonary metastases, serum carcinoembryonic antigen (CEA) level before lung resection, primary cite of CRC, disease-free interval between definitive treatment of primary CRC and detection of pulmonary metastases (DFI), liver metastases before lung resection, pathological stage of the resected CRC, adjuvant chemotherapy after resection of CRC, operative procedure for the lung, pathological nodal involvement, pathological tumor size, and adjuvant chemotherapy after the first pulmonary metastasectomy, which was previously shown to have an effect on survival and recurrence in the literature. Furthermore, to better understand the background of the patients, we used a new variable, extrapulmonary lesion before pulmonary metastasectomy, which indicates whether patients have liver metastases including synchronously or metachronously, local recurrence, recurrence in the lymph node of the para-aorta, and positive peritoneal lavage cytology before lung resection. This variable seems to show more precisely which patients were close to having systemic versus local disease just before pulmonary metastasectomy. Patients presenting with synchronous lung diseases when they underwent operations for CRC were assigned a DFI of 0. Pathological tumor size refers to the size of the largest tumor when multiple metastases were present. If the two staged thoracotomies for bilateral metastases were done within 6 months, the day of the second staged resection was calculated as the time of first metastasectomy. The primary endpoint of this study was relapse-free survival (RFS) because the principal objective of this study was to estimate the clinical effect of adjuvant chemotherapy.
2.1. Statistical analysis
Overall survival (OS) was defined as the interval between lung metastasectomy and death from any cause. RFS, the primary endpoint, was also calculated from the date of lung metastasectomy to the date of proven recurrence or death from any cause. Data for patients lost to follow-up were censored on the date the patient was last seen alive without recurrence. Cumulative survival curves were estimated with the Kaplan-Meier method and compared with the log-rank test and the Wilcoxon signed rank test. Multivariable analysis was performed with the Cox proportional hazards regression model to estimate the independent prognostic effect of adjuvant chemotherapy on RFS and OS by adjusting for confounding factors. Variables were selected for multivariable analysis on the basis of the results of the univariate analysis, statistical independence, and clinical significance for the outcome of pulmonary metastasectomy. The variables in the Adjuvant and Surgery alone groups were compared using Fisher exact test or Pearson's chi-square test for categorical variables. A P value of <0.05 was considered significant. All analyses were conducted with JMP version 11.0 (SAS Inc., Cary, NC) and EZR ([Saitama Medical Center, Jichi Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html; Kanda], a graphical user interface for R [The R Foundation for Statistical Computing, Vienna, Austria, Ver. 2.13.0] and a modified version of R commander [Ver. 1.8–4]).
3. Results
3.1. Patient characteristics
During the study period, 128 consecutive patients underwent pulmonary resection for lung metastases from CRC. Excluded were 10 patients with an incomplete resection at their first pulmonary metastasectomy, 17 patients with non-first metastasectomy, and 1 patient with inadequate information on postoperative chemotherapy and follow-up. Finally, 100 patients remained and were analyzed in this study. Patient characteristics are shown in Table 1. New chemotherapy regimens containing oxaliplatin, irinotecan, and molecular-targeted drugs were administered in 29 of the 42 patients undergoing adjuvant chemotherapy after pulmonary resection. Breakdown of the patients by the pathological stage of CRC showed 4 patients in stage I, 28 in stage II, 45 in stage III, and 23 in stage IV. Of the 23 stage IV patients, 6 had synchronous liver metastases, 11 had synchronous lung metastases (the remaining 89 patients had metachronous lung metastases), and 6 had both synchronous lung and liver metastases. Metachronous liver metastases were present in 8 patients. Patients with resectable synchronous lung metastasis underwent pulmonary resection after surgery for the primary lesion and after controlling other metastatic sites. No patients with unresectable multiple lung metastases were converted from systemic chemotherapy to surgery for lung metastases. There were too few cases pathologically proven to have intrapulmonary lymph node metastasis to analyze statistically. At the time of analysis, 25 patients had died of the disease, 2 patients had died of other causes, and 56 patients had experienced recurrence. The median duration of follow-up was 80 months from colorectal operation and 51 months from pulmonary resection for colorectal metastasis. We observed 70% of the patients over 3 years and 43% of the patients over 5 years.
Table 1.
Clinicopathological characteristics of the patients.
| Variable | n (%) |
|---|---|
| Age (years) | 64.7 (±10.0) |
| Sex | |
| M | 56 (56) |
| F | 44 (42) |
| Adjuvant chemotherapy after PM | |
| Yes | 42 (42) |
| No | 58 (58) |
| Number | |
| Single | 72 (72) |
| Multiple | 28 (28) |
| CEA (ng/ml) | |
| ≥ 5 | 24 (24) |
| < 5 | 76 (76) |
| Operative procedures | |
| Lobectomy/segmentectomy | 24 (24) |
| Wedge resection | 76 (76) |
| Nodal involvement (pathological) | |
| Yes | 2 (2) |
| No | 20 (20) |
| Unknown | 78 (78) |
| Tumor size (pathological) | |
| < 20 mm | 68 (68) |
| ≥ 20 mm | 32 (32) |
| Primary site of CRC | |
| Colon | 50 (50) |
| Rectum | 50 (50) |
| Pathological stage of CRC | |
| I/II | 32 (32) |
| III/IV | 68 (68) |
| Adjuvant chemotherapy after RC | |
| Yes | 56 (56) |
| No | 44 (44) |
| Liver metastasis before PM | |
| Yes | 19 (19) |
| No | 81 (81) |
| Extrapulmonary lesion before PM | |
| Yes | 34 (34) |
| No | 66 (66) |
| DFI (years) | |
| ≥ 3 | 22 (22) |
| < 3 | 78 (78) |
CEA: serum carcinoembryonic antigen level before lung resection, CRC: colorectal cancer, DFI: disease-free interval between definitive treatment of primary CRC and detection of pulmonary metastases, OS: overall survival, PM: pulmonary metastasectomy, RC: resection of colorectal cancer, RFS: relapse-free survival.
We investigated the differences in prognostic variables of the patients between the adjuvant chemotherapy group and the surgery alone group (Table 2). There were no significant differences in the distribution of prognostic factors. Furthermore, the number of patients who underwent systemic chemotherapy with new regimens between the perioperative period of CRC and/or just before lung resection was not different in the patient groups with or without adjuvant chemotherapy after pulmonary metastasectomy.
Table 2.
Prognostic variables among patients undergoing and not undergoing adjuvant chemotherapy after pulmonary metastasectomy.
| Variables | Adjuvant (n = 42) | Non-adjuvant (n = 58) | P Value |
|---|---|---|---|
| Age (≥75 years) | 4 (10%) | 12 (21%) | 0.133 |
| Sex (Male) | 25 (60%) | 31 (53%) | 0.546 |
| Number (Multiple) | 15 (36%) | 13 (22%) | 0.144 |
| CEA (ng/ml) (≥5) | 9 (21%) | 15 (26%) | 0.608 |
| Nodal involvement (Yes) | 0 (0%) | 2 (3%) | 0.224 |
| Size (≥2 cm) | 9 (21%) | 23 (39%) | 0.054 |
| Primary site (Rectum) | 22 (52%) | 28 (48%) | 0.685 |
| pStage of CRC (III/IV) | 27 (64%) | 41 (70%) | 0.498 |
| Adjuvant CT after RC | 25 (60%) | 32 (55%) | 0.664 |
| LM before PM (Yes) | 6 (14%) | 13 (22%) | 0.307 |
| EPL before PM (Yes) | 14 (33%) | 20 (35%) | 0.905 |
| DFI (years) (≥3/< 3) | 9 (21%) | 12 (21%) | 0.907 |
3.2. Survival analysis
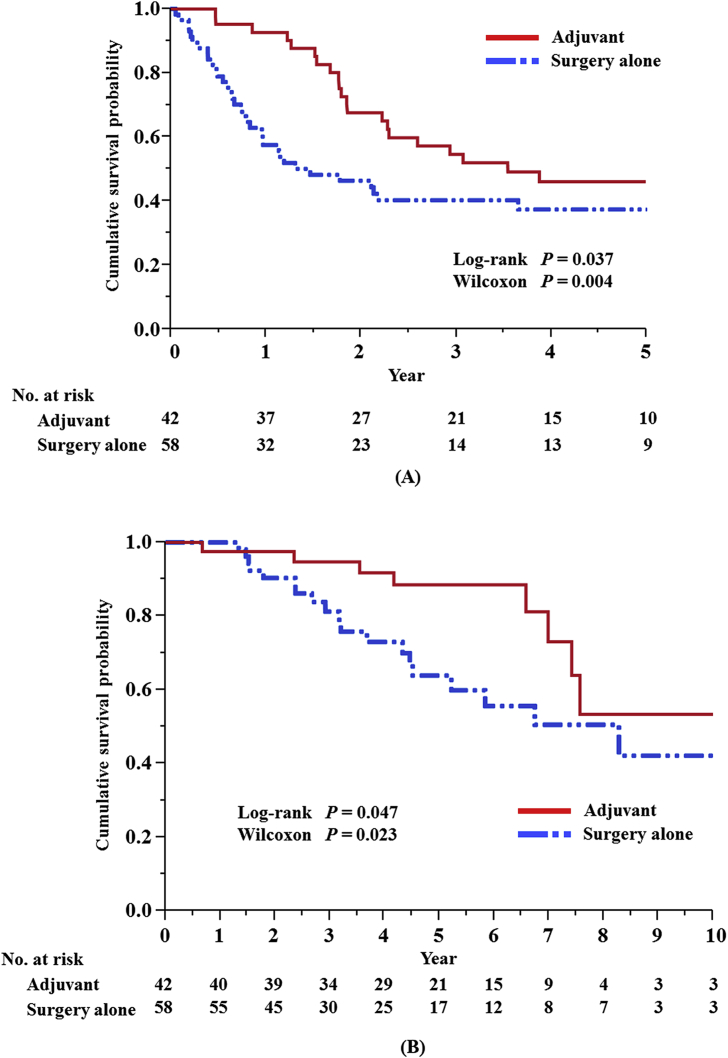
For the 100 patients, rates of 5-year OS and 5-year RFS after pulmonary metastasectomy were 76% and 41%, respectively. The difference in cumulative RFS and OS survival curves of the adjuvant chemotherapy group versus the surgery alone group were statistically significant (RFS: log-rank test, P = 0.037 and Wilcoxon test, P = 0.004; OS: log rank test, P = 0.047 and Wilcoxon test, P = 0.023, respectively) (Fig. 1). As a result of the above-mentioned selection of variables, we judged that 8 prognostic factors were essential for multivariable analysis.
Fig. 1.
Relapse-free survival (A) and overall survival (B) after resection of lung colorectal metastases in patients with and without adjuvant chemotherapy.
We selected 8 prognostic factors for multivariable analysis on the basis of the results of the univariate analysis, statistical independence, and clinical significance for the outcome of pulmonary metastasectomy (Table 3). Multivariable analysis for RFS showed that adjuvant chemotherapy (hazard ratio [HR]: 0.49, confidence interval [CI]: 0.85–2.71, P = 0.016), the number of metastases (HR: 2.35, CI: 1.24–4.40, P = 0.010), pre-thoracotomy CEA level (HR: 2.70, CI: 1.48–4.80, P = 0.002), and pathological stage of CRC (HR: 1.85, CI: 1.01–3.54, P = 0.047), significantly affected the prognosis of patients with lung resection for pulmonary metastases from CRC (Table 3). Among these prognostic factors, only the pathological stage of CRC was not significant in the multivariable analysis for OS. Furthermore, stepwise analysis using the Akaike information criterion value, Bayesian information criterion value, and P-value selected the first three prognostic factors (adjuvant chemotherapy, number of metastases, and pre-thoracotomy CEA) mentioned above in the final model.
Table 3.
Prognostic factors by multivariate analysis for RFS and OS.
| Variables | RFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age (≥75/< 75 years) | 1.22 | 0.52–2.62 | 0.634 | 0.89 | 0.19–3.09 | 0.868 |
| Sex (M/F) | 1.51 | 0.85–2.71 | 0.158 | 1.54 | 0.67–3.84 | 0.315 |
| Adjuvant CT (Yes/No) | 0.49 | 0.27–0.88 | 0.016 | 0.35 | 0.14–0.81 | 0.014 |
| Number (Multiple/Single) | 2.35 | 1.24–4.40 | 0.010 | 2.70 | 1.08–6.68 | 0.034 |
| CEA (≥5/< 5 ng/ml) | 2.70 | 1.48–4.80 | 0.002 | 2.38 | 1.03–5.32 | 0.043 |
| pStage of CRC (III, IV/I, II) | 1.85 | 1.01–3.54 | 0.047 | 1.54 | 0.64–4.09 | 0.345 |
| LM before PM (Yes/No) | 1.13 | 0.54–2.18 | 0.739 | 0.68 | 0.19–2.00 | 0.510 |
| DFI (≥3/< 3 years) | 1.28 | 0.66–2.33 | 0.451 | 0.79 | 0.281.91 | 0.617 |
CEA: serum carcinoembryonic antigen level before lung resection, CI: confidence interval, CRC: colorectal cancer, CT: chemotherapy, DFI: disease-free interval between definitive treatment of primary CRC and detection of pulmonary metastases, HR: hazard ratio, LM: liver metastasis, OS: overall survival, PM: pulmonary metastasectomy, pStage: pathological stage of colorectal cancer, RFS: relapse-free survival.
3.3. Subgroup analysis
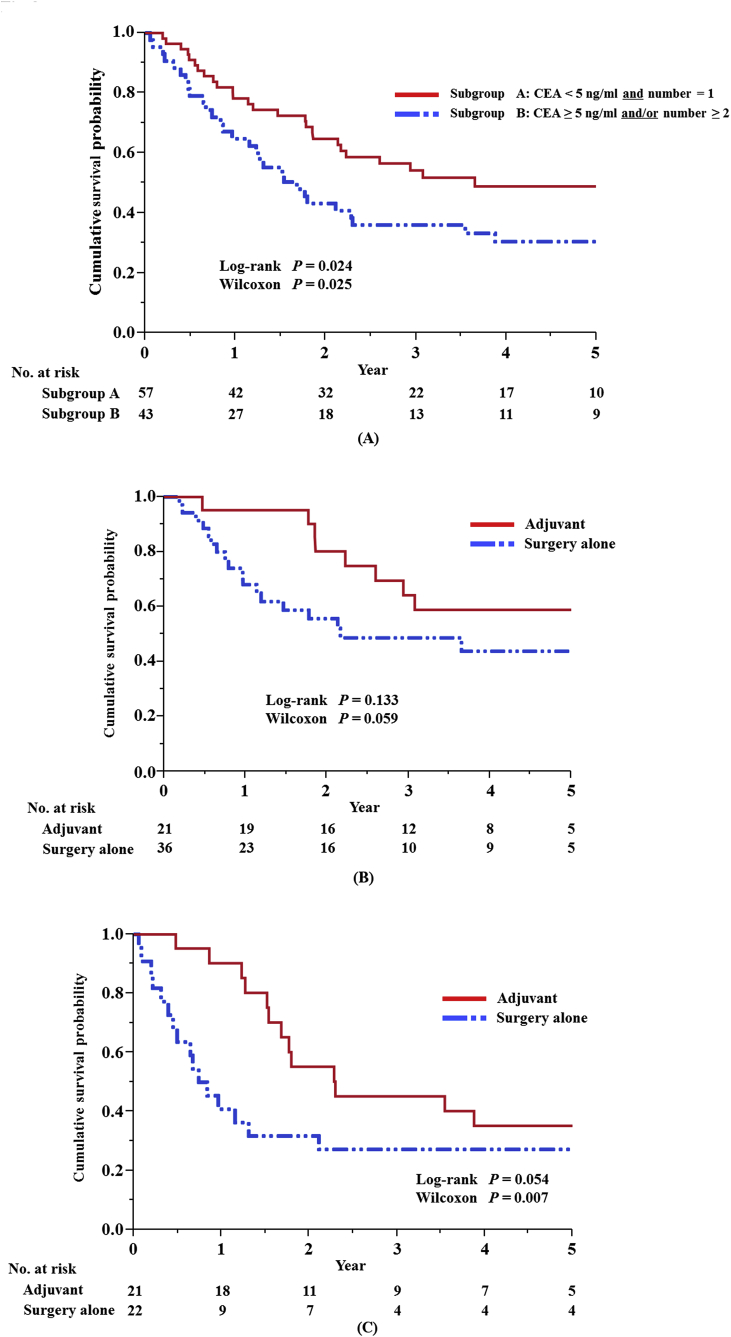
After dividing the patients into two groups based on the two most important prognostic factors, the number of metastases and pre-thoracotomy CEA level (Fig. 2A), we investigated the effects of adjuvant chemotherapy after lung resection in these two subgroups (Fig. 2B and C). Similar effects of adjuvant chemotherapy were observed in both groups. Similar trends were also seen in terms of OS (data not shown).
Fig. 2.
Relapse-free survival (RFS) after resection of lung colorectal metastases. (A) RFS according to the number of metastases and pre-thoracotomy serum carcinoembryonic antigen (CEA) level. (B) RFS in patients with versus without adjuvant chemotherapy in the subgroup of patients with a single metastatic lesion and a normal preoperative CEA level. (C) RFS in patients with versus without adjuvant chemotherapy in the subgroup of patients with more than two metastatic lesions and/or an abnormally high preoperative CEA level.
4. Discussion
The advancements in both systemic chemotherapy and surgical technique and the distinct improvements in the outcomes of CRC patients over the past 15 years have led many surgeons to take a more aggressive stance in the management of metastatic CRC disease. However, the optimal selection of patients for lung resection and how to combine systemic chemotherapy with an aim to increasing curable cases are controversial. Some studies of liver metastases from CRC have explored the benefits of perioperative systemic chemotherapy [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [21], but they remain to be shown.
In terms of lung metastases from CRC, there are only a few papers that suggest the potential of adjuvant chemotherapy after lung resection [20], [21], [22], [23]. Mitry et al. showed the potential benefit of adjuvant chemotherapy after curative resection of either liver or lung CRC metastases in their meta-analysis of two randomized trials that were initiated in the early 1990s (the French FFCD 9002 and EORTC/NCIC trials) [21]. In their paper, differences in disease-free survival (DFS) and OS in the systemic 5-FU-based adjuvant chemotherapy group versus the surgery alone group were not statistically significant (5-year DFS rates were 36.7% and 27.7%, respectively [P = 0.058], and 5-year OS rates were 52.8% and 39.6%, respectively [P = 0.095]). However, the differences in the cumulative survival curves showed potential clinical significance. Unfortunately, the fact that the majority of the patients in their study had resected liver metastases rather than lung metastases make our interpretation of their results more difficult. Nozawa et al. showed the potential benefit of adjuvant administration of a FOLFOX regimen [23]. Unfortunately, they also have the same limitation as that of the Mitry et al. study [21]. Just recently, Park et al. reported results similar to our data [20]. They showed the potential benefit of adjuvant chemotherapy including modern chemotherapy with a DFS of 73% (HR 0.65, P = 0.049).
Our study revealed that adjuvant chemotherapy after pulmonary metastasectomy had a strong effect on the prognosis of patients with lung colorectal metastases on the basis of two variables, the number of metastases and pre-thoracotomy CEA level. We consider this result to be very reliable and reasonable because there appear to be no major differences in the degree of importance of the other prognostic factors except for adjuvant chemotherapy between our study and other previous reports [24], [25], [26], [27]. Further, the number three ranking of the HR of adjuvant chemotherapy on prognosis fits not only our clinical sense but also the data of Park et al. [20] This means that despite the advancements made in systemic chemotherapy, the effect of adjuvant chemotherapy on prognosis has not yet become as important as the effect of the most robust prognostic factors, the number of metastases and pre-thoracotomy CEA level, which respectively manifest the degree of malignancy and the extent of the microscopic spread of the tumor.
In addition to the main analysis, the results of subgroup analysis showed the validity of the effect of adjuvant chemotherapy. Furthermore, the results of subgroup analysis interestingly suggested that even patients with a single metastatic lesion and normal preoperative CEA levels appeared to receive benefits from post-lung resection adjuvant chemotherapy. This means that patients with a single metastatic lesion and a normal preoperative CEA level might have systemic disease. This is also supported by our data showing a frequent rate of recurrence after pulmonary resection in patients with a single CRC metastatic lesion and a normal preoperative CEA level (Fig. 2B).
Of the chemotherapy used in the study patients, 31% did not consist of modern standard chemotherapies containing oxaliplatin, irinotecan, and molecular-targeted drugs. However, this did not have a strong influence on the interpretation of the results of our study.
In regard to the prognostic factors, our results were nearly the same as those of a number of case series and meta-analyses reported previously. This indicates that the number of metastases and pre-thoracotomy CEA level are the most important prognostic factors. Other prognostic factors, such as DFI, pathological stage of CRC, liver metastasis before pulmonary metastasectomy, tumor size, and sex have smaller effects on prognosis than the number of metastases and pre-thoracotomy CEA level have. Although lymph node involvement is also one of the strong prognostic factors after lung resection for pulmonary metastases from CRC, we could not include it in our multivariable analysis because only a few patients had lymph node involvement. The reason might be that our surgical indications for pulmonary metastases of CRC were more conservative than those of institutions in other countries around the world. This might result from the fact that in Japan, lymph node involvement was already thought of as a contraindication in many institutions in the 1990s.
Limitations in our study include all biases caused by the retrospective design and the small sample size. We could not obtain information on performance status, doubling time, and chemotherapy data between the colorectal operation and pulmonary resection, which may have affected the results. Furthermore, this study might have a strong selection bias because the candidates for adjuvant chemotherapy were selected according to the responsible surgeon's judgement. Judging from Table 2, although not statistically significant, the adjuvant chemotherapy group seemed to be slightly younger and to have slightly less lymph node metastasis than the surgery alone group. Moreover, Fig. 1 showed a slight possibility of immortal time bias. Therefore, it cannot be denied that these facts led to a favorable outcome in the adjuvant chemotherapy group. However, judging from the extent of the HR of adjuvant chemotherapy in the multivariable analysis, the results of the subgroup analysis, and recent developments in chemotherapy, we believe that even at the lowest estimation, adjuvant chemotherapy after lung resection has a strong effect on the prognosis of patients with pulmonary metastases from CRC.
In the future, to confirm our results and to realize the true benefits from adjuvant chemotherapy, it will be necessary to narrow down the suitable candidates for adjuvant chemotherapy after lung resection. Some potential solutions might include administration of systemic chemotherapy during the interval between the diagnosis of lung metastases and pulmonary metastasectomy or the discovery of a reliable new biomarker that predicts the effect of systemic chemotherapy.
5. Conclusion
In conclusion, our results showed that adjuvant chemotherapy after curative resection of lung metastases might have a strong effect on the prognosis of patients with metastatic CRC. To confirm our results and to maximize the effect of current chemotherapy, both narrowing down of the suitable candidates by predicting the effect of systemic chemotherapy and the performance of prospective randomized studies are clearly warranted.
Ethical approval
This retrospective study was approved by the Kitasato University Medical Ethics Committee (Approval Number: B16-234).
Sources of funding
There are no sources of funding or research grants received in the course of study.
Author contribution
Kazu Shiomi contributed in (1) the conception and design of the study, (2) drafting and revision the article, (3) supervision of the study, and (4) final approval of the version to be submitted.
Masanori Naito contributed in (1) the conception and design of the study, (2) drafting and revision the article, (4) final approval of the version to be submitted.
Takeo Sato contributed in (1) the conception and design of the study, (2) drafting and revision the article, (4) final approval of the version to be submitted.
Takatoshi Nakamura contributed in (1) the conception and design of the study, (2) drafting and revision the article, (4) final approval of the version to be submitted.
Hiroyasu Nakashima contributed in (1) the conception and design of the study, (2) drafting and revision the article, (4) final approval of the version to be submitted.
Conflicts of interest
There are no conflicts of interest to declare.
Research registration unique identifying number (UIN)
Research registry 2326.
Guarantor
Kazu Shiomi and Masanori Naito.
Adjuvant CT: chemotherapy after pulmonary metastasectomy, CEA: serum carcinoembryonic antigen level before lung resection, CT: chemotherapy, DFI: disease-free interval between definitive treatment of primary CRC and detection of pulmonary metastases, EPL: extrapulmonary lesion, LM: liver metastasis, Nodal involvement: pathological lymph node involvement of lung, PM: pulmonary metastasectomy, pStage: pathological stage of colorectal cancer, RC: resection of colorectal cancer.
Acknowledgements
We thank all of the members of the Department of Thoracic Surgery and Surgery of Kitasato University School of Medicine for helping in the management of this study.
References
- 1.Colucci G., Gebbia V., Paoletti G. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the gruppo oncologico dell'italia meridionale. J. Clin. Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 2.Giantonio B.J., Catalano P.J., Meropol N.J. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A., Sargent D., Goldberg R.M., Schmoll H.J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J. Clin. Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H., Fehrenbacher L., Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Saltz L.B., Clarke S., Díaz-Rubio E. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C., André T., Achille E. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 7.André T., Boni C., Navarro M. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 8.Kuebler J.P., Wieand H.S., O'Connell M.J. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J. Clin. Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 9.Yothers G., O'Connell M.J., Allegra C.J. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J. Clin. Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bismuth H., Adam R., Lévi F. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann. Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. discussion 520–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaunoit T., Alberts S.R., Sargent D.J. Chemotherapy permits resection of metastatic colorectal cancer: experience from Intergroup N9741. Ann. Oncol. 2005;16:425–429. doi: 10.1093/annonc/mdi092. [DOI] [PubMed] [Google Scholar]
- 12.Folprecht G., Gruenberger T., Bechstein W.O. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 13.Giacchetti S., Itzhaki M., Gruia G. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann. Oncol. 1999;10:663–669. doi: 10.1023/a:1008347829017. [DOI] [PubMed] [Google Scholar]
- 14.Masi G., Loupakis F., Pollina L. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann. Surg. 2009;249:420–425. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 15.Pozzo C., Basso M., Cassano A. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann. Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 16.Nordlinger B., Sorbye H., Glimelius B. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 17.Portier G., Elias D., Bouche O. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J. Clin. Oncol. 2006;24:4976. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi A., Hasegawa K., Saiura A. A randomized controlled trial evaluating efficacy of adjuvant oral uracil-tegafur (UFT) with leucovorin after resection of folorectal cancer liver metastases: the UFT/LV study (abstract) J. Clin. Oncol. 2014;32:5. suppl; abstr 3584. [Google Scholar]
- 19.Ychou M., Hohenberger W., Thezenas S. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann. Oncol. 2009;20:1964. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 20.Park H.S., Jung M., Shin S.J. Benefit of adjuvant chemotherapy after curative resection of lung metastasis in colorectal cancer. Ann. Surg. Oncol. 2016;23:928–935. doi: 10.1245/s10434-015-4951-z. [DOI] [PubMed] [Google Scholar]
- 21.Mitry E., Fields A.L., Bleiberg H. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J. Clin. Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 22.Riquet M., Foucault C., Cazes A. Pulmonary resection for metastases of colorectal adenocarcinoma. Ann. Thorac. Surg. 2010;89:375–380. doi: 10.1016/j.athoracsur.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Nozawa H., Kitayama J., Sunami E. FOLFOX as adjuvant chemotherapy after curative resection of distant metastases in patients with colorectal cancer. Oncology. 2011;80:84–91. doi: 10.1159/000328761. [DOI] [PubMed] [Google Scholar]
- 24.Pfannschmidt J., Dienemann H., Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann. Thorac. Surg. 2007;84:324–328. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 25.Pfannschmidt J., Hoffmann H., Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J. Thorac. Oncol. 2010;5(6 Suppl 2):S172–S179. doi: 10.1097/JTO.0b013e3181dca330. [DOI] [PubMed] [Google Scholar]
- 26.Blackmon S.H., Stephens E.H., Correa A.M. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for colorectal cancer. Ann. Thorac. Surg. 2012;94:1802–1809. doi: 10.1016/j.athoracsur.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Salah S., Watanabe K., Welter S. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann. Oncol. 2012;23:2649–2655. doi: 10.1093/annonc/mds100. [DOI] [PubMed] [Google Scholar]