Abstract
Aims
To investigate efficacy and safety of the sodium–glucose co‐transporter 2 (SGLT2) inhibitor canagliflozin administered as add‐on therapy to the dipeptidyl peptidase‐4 (DPP‐4) inhibitor teneligliptin in patients with type 2 diabetes mellitus (T2DM).
Materials and methods
We conducted a multicentre, randomized, double‐blind, placebo‐controlled, phase 3 clinical trial in Japanese patients with T2DM who had inadequate glycaemic control with teneligliptin. Patients were randomized to receive teneligliptin 20 mg plus either canagliflozin 100 mg (T + C, n = 70) or placebo (T + P, n = 68) once daily. The primary endpoint was the change in glycated haemoglobin (HbA1c) from baseline to week 24. Other endpoints included changes in fasting plasma glucose, body weight, proinsulin/C‐peptide ratio, homeostatic model assessment 2‐%B and adverse events. Patients also underwent mixed‐meal tolerance tests.
Results
The difference between the T + C and T + P groups for HbA1c change from baseline to week 24 was −0.88% (least‐squares mean, P < .001). Fasting plasma glucose, body weight and the proinsulin/C‐peptide ratio were significantly lower in the T + C group than in the T + P group. Homeostatic model assessment 2‐%B improved with T + C compared with T + P. The T + C group exhibited a decrease in the 2‐hour postprandial plasma glucose and plasma glucose area under the curve (AUC)0‐2h in a mixed‐meal tolerance test. No significant between‐group differences were observed for C‐peptide AUC0 ‐2h or glucagon AUC0 ‐2h after meals. Incidences of adverse events were 60.0% and 47.1% in the T + C and T + P groups, respectively. No hypoglycaemia was observed.
Conclusions
Canagliflozin administered as add‐on therapy to teneligliptin was effective and well tolerated in Japanese T2DM patients.
Keywords: canagliflozin, co‐transporter 2 inhibitors, dipeptidyl peptidase‐4 inhibitors, sodium glucose type 2 diabetes mellitus, teneligliptin
1. INTRODUCTION
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors promote insulin secretion in a blood glucose‐dependent manner by increasing active glucagon‐like peptide‐1 (GLP‐1) levels, thereby decreasing glucose levels without causing hypoglycaemia. In Japan, several DPP‐4 inhibitors, including teneligliptin,1, 2 are available and are used in a wide range of patients with type 2 diabetes mellitus (T2DM). Recently, it was reported that DPP‐4 inhibitors are more potent in lowering glycated haemoglobin (HbA1c) levels in Asian populations with T2DM, including Japanese patients, compared with Western (Caucasian) populations.3, 4 The difference in efficacy of DPP‐4 inhibitors between these populations may be related to differences in the pathophysiology of T2DM. For example, Caucasian patients are more likely to develop insulin resistance associated with obesity which then leads to T2DM, whereas Japanese patients more often exhibit a decrease in insulin secretion capacity that leads to the development of T2DM.5, 6, 7, 8 The safety of DPP‐4 inhibitors, particularly the absence of hypoglycaemia, together with the difference in T2DM pathophysiology, possibly contributed to the rapid increase in prescriptions for DPP‐4 inhibitors for T2DM in Japan since their launch in 2009.9, 10, 11
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors, novel antidiabetic agents, have a selective inhibitory effect on SGLT2, a protein expressed in the proximal renal tubules. They enhance urinary glucose excretion, which consequently reduces hyperglycaemia. The efficacy and safety of SGLT2 inhibitors, either as monotherapy or in combination with other drugs, in Japanese patients with T2DM has been reported in several studies.12, 13, 14, 15, 16, 17, 18, 19 Because a large proportion of patients with T2DM in Japan are taking a DPP‐4 inhibitor, SGLT2 inhibitors are often prescribed as add‐on therapy to DPP‐4 inhibitors in clinical practice.20 SGLT2 inhibitors block glucose reabsorption in the kidney, and their use in combination with a DPP‐4 inhibitor brings together distinct mechanisms of action for reducing glucose levels.21 Thus, combination therapy with a DPP‐4 inhibitor and SGLT2 inhibitor would be expected to achieve a potent plasma glucose‐lowering effect.
Previous clinical trials investigating the use of SGLT2 inhibitors as add‐on therapy to DPP‐4 inhibitors in Japanese patients with T2DM and inadequate glycaemic control have been limited to open‐label studies, for example, that by Inagaki et al.14 To date, there have been no randomized, controlled trials examining the efficacy of an SGLT2 inhibitor added on to a DPP‐4 inhibitor in Asian patients. Therefore, to evaluate the efficacy and safety of SGLT2 inhibitors as add‐on therapy to DPP‐4 inhibitors, we conducted a randomized, double‐blind, placebo‐controlled clinical trial in which Japanese patients with T2DM and inadequate glycaemic control with teneligliptin monotherapy along with diet and exercise were randomized to either the SGLT2 inhibitor canagliflozin or placebo once daily as an add‐on to teneligliptin.
2. MATERIALS AND METHODS
2.1. Ethical statement
The Institutional Review Boards at all participating institutions approved the study after reviewing its ethical, scientific, medical and pharmaceutical validity. The study was conducted in accordance with the ethical principles of the Helsinki Declaration, the Japanese “Law for Ensuring the Quality, Efficacy, and Safety of Drugs and Medical Devices,” Good Clinical Practice and the study protocol. The participating institutions are listed online (Appendix S1). The trial was registered at ClinicalTrials.gov (NCT02354235).
2.2. Patients
Informed consent was obtained from all patients prior to enrolment in the trial. Japanese patients with T2DM, aged 20 to 75 years, who had undergone a diet and exercise regimen and had received teneligliptin 20 mg monotherapy once daily for at least 8 weeks prior to initiation of the run‐in period were enrolled. Patients using antidiabetic drugs other than teneligliptin were also eligible, providing the other antidiabetic drug was withdrawn for an 8‐week washout period; that is, they used only teneligliptin for at least 8 weeks before the run‐in period (Figure S1, Supporting Information). Other inclusion criteria were HbA1c ≥ 7.0% and < 10.5% at initiation of the run‐in period and by week 2 of the run‐in period, with a difference of ≤0.5% in HbA1c between those time points, and fasting plasma glucose (FPG) of ≤270 mg/dL at initiation of the run‐in period. Exclusion criteria and withdrawal criteria are given online (Tables S1 and S2, Appendix S1, respectively).
2.3. Study design, treatments and blinding
The study design is shown in Figure S1, Appendix S1. The study was a multicentre, randomized, double‐blind, placebo‐controlled trial. During the 4‐week run‐in period, all patients were administered teneligliptin 20 mg orally and placebo once daily before breakfast. At the start of the treatment period, all patients continued to take teneligliptin 20 mg and were randomized 1:1 in a double‐blind manner by a permuted block method to receive either placebo (T + P) or canagliflozin (T + C) 100 mg (the approved dose in Japan), administered orally once daily before breakfast. After the treatment period, patients were observed for an additional 2 weeks (post‐treatment observation period), during which they continued to receive teneligliptin 20 mg orally once daily before breakfast.
At the end of the 4‐week run‐in period (hereafter referred to as baseline) and at the end of the treatment period, patients underwent a mixed‐meal tolerance test after a ≥10‐hour fast (water was permitted). After basal blood sampling, patients consumed (within 15 minutes) a standard test meal (energy content, 500 kcal; carbohydrate, 60%; lipid, 25%; protein, 15%). Blood samples were obtained at 0.5, 1 and 2 hours after beginning the meal.
2.4. Efficacy outcomes
The primary endpoint was change in HbA1c from baseline to the end of the treatment period. Secondary endpoints included the following: change in HbA1c from baseline at each measurement time point; change in FPG from baseline to each measurement time point and to the end of the treatment period; the proportion of patients achieving HbA1c < 7.0%; the proportion of patients achieving HbA1c < 8.0%; the absolute and percentage changes in body weight; changes in fasting glucagon, proinsulin/C‐peptide ratio, total adiponectin and high‐molecular weight (HMW) adiponectin; change in homeostatic model assessment (HOMA) 2‐%B; 2‐hour postprandial plasma glucose; plasma glucose, C‐peptide and glucagon areas under the curve from 0 to 2 hours (AUC0‐2h) (preprandial to postprandial) and change in C‐peptide AUC0‐2h/plasma glucose AUC 0‐2h ratio.
2.5. Safety
Safety endpoints were adverse events (AEs), hypoglycaemia, laboratory values (haematology, blood biochemistry and urinalysis), electrocardiogram and vital signs. AEs and drug‐related AEs were classified according to MedDRA (J. version 18.1) System Organ Class and Preferred Term. For each event, the number of patients and the incidence were calculated. Hypoglycaemia was classified according to the criteria summarized in Appendix S1.
2.6. Determination of sample size
For the change in HbA1c from baseline to the end of the treatment period, with the assumption that the mean difference to be detected in the T + C group compared with the T + P group was −0.50%, and considering that a decrease of >0.3% is a clinically significant change in HbA1c22 with the SD estimated to be 0.8% based on a previous study,13 55 patients per group were required (t test) to ensure a power of 90% with a 2‐sided significance level of 0.05. Therefore, taking into consideration the safety evaluation and the number of withdrawals, the target sample size was determined to be 140 patients (70 patients per group).
2.7. Statistical analysis
All statistical analyses were performed using Windows SAS (v.9.2 or later version). A 2‐sided test was used, with the significance level set at α = .05. Data that were not measured or were immeasurable because of sample issues were handled as missing data. The missing value was imputed with the last available value, using the last observation carried forward (LOCF) approach.
Efficacy was analysed using the full analysis set. For measurements at the end of the treatment period, descriptive statistics, change from baseline to end of treatment period for each group, 95% confidence interval (CI) of the mean for each group, between‐group difference (T + C − T + P group) and 95% CI of the difference were calculated. The impact of the baseline measurement on changes in each efficacy endpoint was determined by analysis of covariance using the baseline measurement as the covariate. For the primary endpoint, the least square mean (LS mean) and standard error (SE) of the LS mean were calculated for each group. The point estimate of the between‐group difference in LS mean (T + C group − T + P group) as well as the SE, 95% CI and P value were also calculated.
For each secondary endpoint, the change (percent change) from each measurement time point to end of the treatment period (except for HbA1c and evaluation parameters of the mixed‐meal tolerance test) was analysed in the same manner as the primary endpoint. The proportions of patients achieving HbA1c < 7.0% and HbA1c < 8.0% in each group at end of the treatment period were calculated, along with the between‐group difference (T + C group − T + P group) and P value (Fisher's exact test).
Safety analysis was performed on the safety analysis set, which included all randomized patients except those who did not receive any dose of canagliflozin or placebo in combination with teneligliptin during the treatment period or patients for whom no safety data were collected after randomization.
3. RESULTS
3.1. Patients
The dispositions of patients included in each analysis set are shown in Figure S2, Appendix S1. Of the 185 patients who provided informed consent, 177 patients enrolled in the study and received teneligliptin 20 mg and placebo once daily during the 4‐week run‐in period. A total of 47 patients discontinued prior to the treatment period. The remaining 138 patients were randomized to receive placebo (T + P group, n = 68) or canagliflozin 100 mg (T + C group, n = 70) for the treatment period. All patients were included in the full analysis set and the safety analysis set. Seven patients in the T + P group and 3 in the T + C group withdrew from the study during the treatment period; reasons for discontinuation were patient request (n = 3), determination of ineligibility by the investigator because of AEs (n = 3) and development of myocardial infarction, congestive cardiac failure, unstable angina or cerebrovascular disorders (n = 1). Sixty‐one patients in the T + P group and 67 in the T + C group completed the treatment period.
Demographic and other baseline characteristics are shown in Table 1. Age, body mass index (BMI) and baseline HbA1c and FPG values were comparable between groups.
Table 1.
Patient demographics
| T + P (n = 68) | T + C (n = 70) | |
|---|---|---|
| Sex, n (%) | ||
| Male | 53 (77.9) | 54 (77.1) |
| Female | 15 (22.1) | 16 (22.9) |
| Age (years), mean (SD) | 56.0 (9.5) | 58.4 (8.9) |
| Diabetes duration (years), mean (SD) | 6.50 (3.89) | 8.34 (7.74) |
| Body weight (kg), mean (SD) | 73.26 (12.91) | 71.33 (15.94) |
| BMI (kg/m2), mean (SD) | 26.44 (3.87) | 25.53 (4.21) |
| Diabetes complications, n (%) | 27 (39.7) | 33 (47.1) |
| Retinopathy | 8 (11.8) | 15 (21.4) |
| Neuropathy | 6 (8.8) | 13 (18.6) |
| Nephropathy | 17 (25.0) | 15 (21.4) |
| Nondiabetic complications, n (%) | ||
| Hypertension | 32 (47.1) | 33 (47.1) |
| Hyperlipidaemia | 47 (69.1) | 45 (64.3) |
| HbA1c (%), mean (SD) | 7.87 (0.83) | 8.18 (0.90) |
| FPG (mg/dL), mean (SD)1 | 166.3 (33.9) | 173.9 (30.4) |
| eGFR (mL/min/1.73 m2), mean (SD) | 83.9 (17.1) | 84.7 (15.6) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; SD, standard deviation; T + C, teneligliptin plus canagliflozin; T + P, teneligliptin plus placebo.
FPG: 1 mg/dL = 0.0555 mmol/L.
3.2. Efficacy
Changes in HbA1c from baseline to week 24 and changes over time in both groups are shown in Table 2 and Figure 1, respectively. The LS mean ± SE change in HbA1c from baseline to week 24 (LOCF) was −0.10% ± 0.10% in the T + P group and −0.97% ± 0.10% in the T + C group, with a significant between‐group difference of −0.88% (P < .001) (Table 2). HbA1c in the T + C group rapidly decreased from week 4 to week 12 and then remained low to week 24. By contrast, in the T + P group, HbA1c decreased only slightly to week 24 (Figure 1). In addition, the T + C group showed a significant decrease in HbA1c at each time point compared with the T + P group (P < .001 for all time points).
Table 2.
Effects of canagliflozin vs placebo added to teneligliptin on primary and secondary endpoints at 24 weeks
| T + P (n = 68) | T + C (n = 70) | ||
|---|---|---|---|
| HbA1c (%) | n | 68 | 70 |
| Baseline | Mean (SD) | 7.87 (0.83) | 8.18 (0.90) |
| Change from baseline | LS mean (SE) | −0.10 (0.10) | −0.97 (0.10) |
| Difference vs placebo | LS mean (95% CI) | −0.88 (−1.15, −0.60) | |
| P value | <.001 | ||
| FPG1 (mg/dL) | n | 67 | 69 |
| Baseline | Mean (SD) | 167.0 (33.6) | 173.9 (30.6) |
| Change from baseline | LS mean (SE) | 3.9 (3.5) | −34.9 (3.4) |
| Difference vs placebo | LS mean (95% CI) | −38.8 (−48.5, −29.2) | |
| P value | <.001 | ||
| Body weight (kg) | n | 67 | 69 |
| Baseline | Mean (SD) | 73.35 (12.98) | 71.68 (15.79) |
| Change from baseline | LS mean (SE) | −0.78 (0.23) | −2.29 (0.22) |
| Difference vs placebo | LS mean (95% CI) | −1.51 (−2.15, −0.88) | |
| P value | <.001 | ||
| Percent change from baseline | LS mean (SE) | −0.99 (0.31) | −3.32 (0.31) |
| Difference (%) vs placebo | LS mean (95% CI) | −2.33 (−3.20, −1.45) | |
| P value | <.001 | ||
| Fasting proinsulin/C‐peptide | n | 67 | 69 |
| Baseline | Mean (SD) | 0.0166 (0.0080) | 0.0176 (0.0089) |
| Change from baseline | LS mean (SE) | 0.0002 (0.0005) | −0.0029 (0.0005) |
| Difference vs placebo | LS mean (95% CI) | −0.0031 (−0.0045, −0.0016) | |
| P value | < .001 | ||
| HOMA2‐%B | n | 67 | 69 |
| Baseline | Mean (SD) | 38.42 (15.47) | 34.20 (14.77) |
| Change from baseline | LS mean (SE) | −2.04 (1.41) | 10.83 (1.38) |
| Difference vs placebo | LS mean (95% CI) | 12.87 (8.95, 16.79) | |
| P value | <.001 | ||
| Fasting glucagon2 (pg/mL) | 67 | 69 | |
| Baseline | Mean (SD) | 121.4 (19.4) | 116.7 (21.3) |
| Change from baseline | LS mean (SE) | 8.5 (2.1) | 5.7 (2.1) |
| Difference vs placebo | LS mean (95% CI) | −2.7 (−8.6, 3.1) | |
| P value | .355 | ||
| Fasting total adiponectin (µg/mL) | n | 61 | 66 |
| Baseline | Mean (SD) | 3.488 (1.686) | 3.594 (1.714) |
| Change from baseline | LS mean (SE) | −0.234 (0.107) | 0.150 (0.103) |
| Difference vs placebo | LS mean (95% CI) | 0.384 (0.090, 0.677) | |
| P value | .011 | ||
| Fasting HMW adiponectin (µg/mL) | n | 61 | 66 |
| Baseline | Mean (SD) | 1.1135 (0.8935) | 1.2381 (0.9480) |
| Change from baseline | LS mean (SE) | 0.0628 (0.0697) | 0.2612 (0.0670) |
| Difference vs placebo | LS mean (95% CI) | 0.1984 (0.0068, 0.3899) | |
| P value | .043 |
LS mean by analysis of covariance (factor: treatment group; covariate: baseline value)
Abbreviations: CI, confidence interval; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HMW, high‐molecular weight; HOMA, homeostatic model assessment; LS mean, least squares mean; n, number of patients with values at baseline or week 24 (last observation carried forward); SD, standard deviation; SE, standard error; T + C, teneligliptin plus canagliflozin; T + P, teneligliptin plus placebo.
FPG conversion factor: 1 mg/dL = 0.0555 mmol/L.
Fasting glucagon conversion factor: 1 pg/mL = 1 ng/L.
Figure 1.
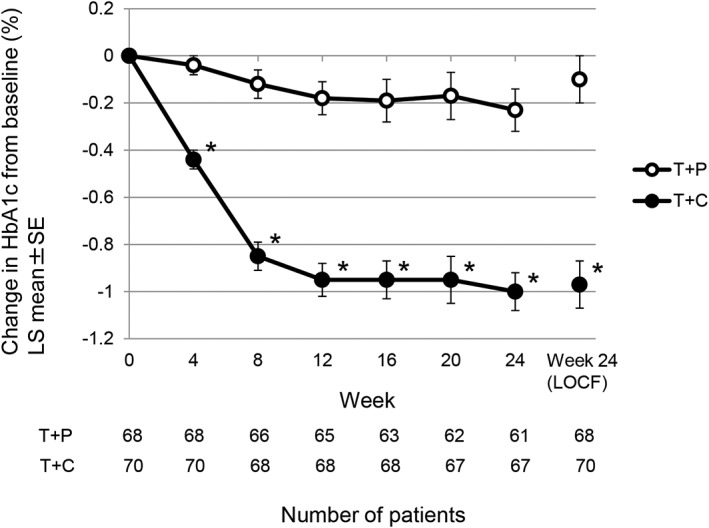
Time course of change in HbA1c level from baseline in the 24‐week treatment period. Values are reported as LS means ± standard error. LS means by analysis of covariance (factor: treatment group; covariate: baseline value). *P < .001 for T + C vs T + P at all time points. HbA1c, glycated haemoglobin; LOCF, last observation carried forward; LS mean, least squares mean; T + C, teneligliptin + canagliflozin; T + P, teneligliptin + placebo
The proportion of patients achieving HbA1c < 7.0% at week 24 was 19.12% in the T + P group and 40.91% in the T + C group. The proportion of patients achieving HbA1c < 8.0% was 30.43% in the T + P group and 80.00% in the T + C group. There was a significant difference in these proportions between the T + C group and the T + P group for both HbA1c targets (P = .008 for HbA1c < 7.0% and P < .001 for HbA1c < 8.0%).
Changes in secondary endpoints from baseline are shown in Table 2. A significant difference in FPG, compared with the T + P group, was observed in the T + C group (−38.8 mg/dL, P < .001). Significant differences between the 2 groups with regard to absolute and percent change in body weight were also seen (−1.51 kg and −2.33%, respectively; P < .001 for both).
Changes in fasting proinsulin/C‐peptide ratio and HOMA2‐%B, as markers of β‐cell function, were significantly greater in the T + C group compared with the T + P group (P < .001 for both). There was no significant difference between the 2 groups for change in fasting glucagon. Compared with the T + P group, the T + C group showed significant increases from baseline to week 24 in both fasting total adiponectin and fasting HMW adiponectin (P = .011 and P = .043, respectively).
Changes in the time courses of plasma glucose, C‐peptide and glucagon after the mixed‐meal tolerance test at baseline and at week 24 are shown in Figure 2. Compared with the T + P group, the T + C group showed improvements in plasma glucose levels at 0.5, 1 and 2 hours after the meal at week 24 (Figure 2A). The time courses of C‐peptide (Figure 2B) and glucagon (Figure 2C) in the mixed‐meal tolerance test were similar for the T + P and T + C groups at baseline and at week 24. Values for each parameter of the meal tolerance test are shown in Table S3, Appendix S1. In the T + C group, the 2‐hour postprandial plasma glucose decreased by 60.1 ± 4.9 mg/dL from baseline to week 24; this reduction was significantly greater than that seen in the T + P group (9.2 ± 5.1 mg/dL), with a difference of −50.9 mg/dL (P < .001). The T + C group also showed larger decreases in plasma glucose AUC0‐2h (−105.9 ± 7.6 h·mg/dL) from baseline to week 24 compared with the T + P group (−5.6 ± 8.0 h·mg/dL), with a difference of −100.3 h·mg/dL (P < .001).
Figure 2.
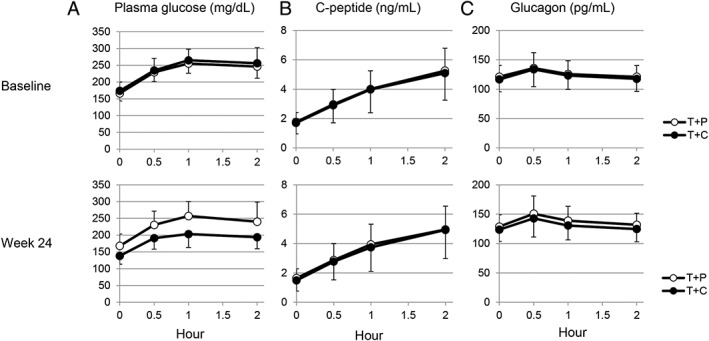
Effects of T + C vs T + P on the time course of plasma glucose (A), C‐peptide (B) and glucagon (C) during the mixed‐meal tolerance test. Values are reported as means + standard deviation (T + P) or mean – standard deviation (T + C). Numbers of patients: 68 (T + P) and 70 (T + C) at baseline, and 61 (T + P) and 67 (T + C) at week 24. T + C, teneligliptin + canagliflozin; T + P, teneligliptin + placebo
There was no significant difference in change from baseline in C‐peptide or glucagon AUC0‐2h at week 24 between the 2 groups. However, change in the C‐peptide AUC0‐2h/plasma glucose AUC0‐2h ratio from baseline to week 24 was significantly greater in the T + C group compared with the T + P group (P < .001).
3.3. Safety
During the treatment and post‐treatment observation periods, AEs occurred in 47.1% and 60.0% and drug‐related AEs occurred in 11.8% and 10.0% of patients in the T + P and T + C groups, respectively. Serious AEs occurred in 2.9% and 1.4% of patients in the T + P and T + C groups, respectively. Serious drug‐related AEs occurred in 1 patient in the T + P group. AEs leading to discontinuation occurred in 2 patients in each group, and drug‐related AEs leading to discontinuation occurred in 1 patient in each group. There were no AEs leading to death. AEs are shown in Table 3.
Table 3.
Adverse events
| T + P (n = 68) | T + C (n = 70) | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| AEs | 32 | (47.1) | 42 | (60.0) |
| Drug‐related AEs | 8 | (11.8) | 7 | (10.0) |
| Serious AEs | 2 | (2.9) | 1 | (1.4) |
| Serious drug‐related AEs | 1 | (1.5) | 0 | (0) |
| AEs leading to discontinuation | 2 | (2.9) | 2 | (2.9) |
| Drug‐related AEs leading to discontinuation | 1 | (1.5) | 1 | (1.4) |
| AEs of special interest | ||||
| Hypoglycaemia | 0 | (0) | 0 | (0) |
| Urinary tract infection | 1 | (1.5) | 0 | (0) |
| Fracture | 0 | (0) | 1 | (1.4) |
| Blood ketone bodies increased | 2 | (2.9) | 2 | (2.9) |
| Cardiovascular‐related events | 2 | (2.9) | 1 | (1.4) |
| Skin and subcutaneous tissue disorders | 2 | (2.9) | 7 | (10.0) |
| Gastrointestinal disorders | 1 | (1.5) | 10 | (14.3) |
| Hepatic function impairment | 2 | (2.9) | 0 | (0) |
Abbreviations: AE, adverse event; T + C, teneligliptin plus canagliflozin; T + P, teneligliptin plus placebo.
No hypoglycaemia was observed in either group. A urinary tract infection‐related AE and a fracture were reported in 1 patient in the T + P group and 1 patient in the T + C group, respectively. Increased blood ketone bodies occurred in 2.9% of patients in both the T + P and T + C groups. All of these cases were mild in severity and no cases of ketoacidosis were observed. Cardiovascular‐related AEs occurred in 2.9% and 1.4% of patients in the T + P and T + C groups, respectively. Skin disorder‐related AEs occurred in 2.9% and 10.0% of patients in the T + P and T + C groups, respectively. One patient in the T + C group developed a rash of moderate severity and discontinued canagliflozin; all other events were mild in severity. AEs related to gastrointestinal disorders occurred in 1.5% and 14.3% of patients in the T + P and T + C groups, respectively. All these events were mild in severity. Two patients in the T + P group developed impaired hepatic function.
Genital infection, osmotic diuresis‐related AEs, dehydration and pyelonephritis, which are associated with use of canagliflozin,23, 24 were not observed in the T + C group. Furthermore, no intestinal obstruction or interstitial pneumonia, which are associated with use of teneligliptin,25, 26, 27 occurred in the T + C group. Transient increases in total ketone bodies were observed in both groups; however, no patients experienced ketone body increase‐related symptoms leading to discontinuation. There were no noteworthy changes in other laboratory values, electrocardiogram findings or vital signs (data not shown).
4. DISCUSSION
This randomized, placebo‐controlled trial investigated the efficacy and safety of canagliflozin as add‐on therapy in Japanese patients who had inadequate glycaemic control with teneligliptin monotherapy. Add‐on canagliflozin to teneligliptin was associated with greater reductions in HbA1c, FPG and postprandial glucose compared with placebo. These results were consistent with those of prior studies of SGLT2 inhibitors added on to a DPP‐4 inhibitor.14, 16
Some patients treated with a DPP‐4 inhibitor require additional antidiabetic agents to achieve and maintain favourable glycaemic control. As we expected, canagliflozin added on to teneligliptin improved glycaemic control and was associated with a reduction in body weight.
The HbA1c‐lowering effect of DPP‐4 inhibitors is influenced by BMI, with reduced efficacy in obese patients,28, 29, 30, 31 because obesity decreases peripheral insulin sensitivity. Conversely, canagliflozin significantly lowers blood glucose and reduces body weight, regardless of BMI.32 It is possible that the greater efficacy of teneligliptin and canagliflozin in lowering HbA1c may be explained in part by the reduction in body weight associated with canagliflozin, which would facilitate the blood glucose‐lowering effect of teneligliptin. In addition, canagliflozin has been reported to increase total GLP‐1 levels.33 This suggests that the combination of teneligliptin and canagliflozin may boost active GLP‐1 levels through the combined effect of canagliflozin on GLP‐1 secretion and DPP‐4 inhibition by teneligliptin.
Both total adiponectin and HMW adiponectin, which were measured as exploratory endpoints, significantly increased in the T + C group compared with the T + P group. This increase in adiponectin may be the result of reduction in body weight in the T + C group.
C‐peptide is secreted with insulin at a ratio of 1:1. C‐peptide was selected as a marker of insulin secretion because canagliflozin might influence insulin clearance but does not affect the kinetics of C‐peptide secretion.34 In the T + C group, the proinsulin/C‐peptide ratio decreased significantly from baseline to week 24 compared with the T + P group. Decreases in the proinsulin/C‐peptide ratio with canagliflozin as monotherapy or as combination therapy (including concomitant use with a DPP‐4 inhibitor) were previously reported in studies conducted in Japanese patients with T2DM.13, 14 Furthermore, we observed an improvement in HOMA2‐%B in the T + C group, suggesting an improvement in pancreatic β‐cell function, which confirms the findings of previous studies of canagliflozin monotherapy or in combination with a DPP‐4 inhibitor.14
In the mixed‐meal tolerance test, a reduction in postprandial plasma glucose occurred in the T + C group. Although there was no difference in the C‐peptide AUC0‐2h between groups, the C‐peptide AUC0‐2h/plasma glucose AUC0‐2h ratio was increased. These results suggest that canagliflozin may improve β‐cell function in the setting of postprandial hyperglycaemia. Taken together, these findings suggest that canagliflozin, which has insulin‐independent glucose‐lowering activity, may reduce glucose toxicity and the burden on β‐cells, leading to an improvement in β‐cell function.
In the current study, no significant differences in fasting glucagon or postprandial glucagon levels were observed between the T + C and T + P groups. Regarding the time course of glucagon after a meal, a previous study reported increased glucagon secretion as an acute effect of canagliflozin (300 mg) compared with placebo.35 Dapagliflozin was shown previously to increase fasting and postprandial glucagon levels,36, 37 while empagliflozin increased glucagon responses after meal ingestion, leading to an increase in endogenous glucose production,38 DPP‐4 inhibitors have been shown to suppress glucagon responses,39 and teneligliptin was also reported to lower postprandial glucagon levels.40 Interestingly, a combination of saxagliptin and dapagliflozin prevented the dapagliflozin‐mediated increase in postprandial glucagon levels.37 For these reasons, the lack of effect of canagliflozin on fasting and postprandial glucagon compared with placebo in this study may be attributable to the suppressive effect of teneligliptin or the use of a low dose of canagliflozin (100 mg).
With regard to safety, the incidence of AEs was higher in the T + C group than in the T + P group. Although the incidence of AEs related to gastrointestinal disorders and skin disorders was higher in the T + C group, only 1 gastrointestinal event and 3 skin disorders were considered to be drug‐related in the T + C group. Examination of the details of AEs related to gastrointestinal or skin disorders revealed no specific individual AEs with a disproportionately high incidence. AEs commonly associated with SGLT2 inhibitors, such as genital infection or osmotic diuresis, were not observed in this study. Furthermore, no hypoglycaemia was observed in either group and there were no new safety signals that have not already been reported for canagliflozin or teneligliptin.
This study had some limitations. First, it was conducted in Japanese patients only. As there are differences in diet as well as T2DM pathophysiology between Japanese and Western populations, including insulin secretion capacity, the study findings may not be extrapolated to other ethnic groups without consideration of these points. In addition, because the present study had a 24‐week treatment period, the long‐term safety and efficacy of canagliflozin as an add‐on therapy to teneligliptin is unknown and needs to be evaluated. Finally, the sample size of this study might be considered small, but the study was appropriately powered based on a sample size calculation, and was designed in accordance with local and international recommendations for clinical trials of antidiabetic drugs.
In conclusion, canagliflozin as add‐on therapy to teneligliptin significantly improved HbA1c and postprandial plasma glucose in this randomized clinical trial in Japanese patients with T2DM, suggesting that the treatment led to an improvement in β‐cell function and relief from burden on β‐cells. We observed no deviations from the known safety profiles of both teneligliptin and canagliflozin in this combination treatment study. These results confirm the efficacy and tolerability of canagliflozin add‐on therapy to teneligliptin in Japanese T2DM patients.
Supporting information
Appendix S1.
ACKNOWLEDGEMENTS
The authors would like to thank J. Ludovic Croxford, PhD, and Stacey C. Tobin, PhD, of Edanz Group Japan K.K. for providing medical writing services. Preparation of the manuscript was funded by Mitsubishi Tanabe Pharma Corporation.
Conflict of interest
T. K. has received consulting fees and/or speakers’ bureau fees from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K. and Takeda Pharmaceutical Co., Ltd.; received research support from Daiichi Sankyo Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; received scholarship grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; belongs to endowed departments by MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd. and Takeda Pharmaceutical Co., Ltd. N. I. has received consulting fees and/or speakers’ bureau fees from Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co. and Takeda Pharmaceutical Co., Ltd.; received research support from Eli Lilly Japan K.K., MSD K.K., and Mitsubishi Tanabe Pharma Corporation; received scholarship grants from Astellas Pharma Inc., AstraZeneca K.K., Bristol‐Myers K.K., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Japan Diabetes Foundation, Japan Tobacco Inc., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd. and Taisho Pharmaceutical Co., Ltd. K. K., K. N., G. K., N. M., N. N., Y. W., H. I. and M. G. are employees of Mitsubishi Tanabe Pharma Corporation.
Author contributions
T. K., N. I. and K. K. were the medical advisors for this study and contributed to the study design. K. N., G. K. and N. M. contributed to the study design and performed data collection. N. N. was involved in data analysis. Y. W., H. I. and M.G. contributed to the writing of the manuscript. All authors contributed to interpretation of data and review of the manuscript, and approved this manuscript for submission.
Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, Nakanishi N, Iijima H, Watanabe Y and Gouda M. Efficacy and safety of canagliflozin as add‐on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: Results of a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2017. https://doi.org/10.1111/dom.12898
Funding information This study was funded by Mitsubishi Tanabe Pharma Corporation.
REFERENCES
- 1. Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc). 2013;49:615‐629. [DOI] [PubMed] [Google Scholar]
- 2. Morishita R, Nakagami H. Teneligliptin: expectations for its pleiotropic action. Expert Opin Pharmacother. 2015;16:417‐426. [DOI] [PubMed] [Google Scholar]
- 3. Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: meta‐analysis. Ann Pharmacother. 2012;46:1453‐1469. [DOI] [PubMed] [Google Scholar]
- 4. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia. 2013;56:696‐708. [DOI] [PubMed] [Google Scholar]
- 5. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66(suppl 1):S37‐S43. [DOI] [PubMed] [Google Scholar]
- 6. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129‐2140. [DOI] [PubMed] [Google Scholar]
- 7. Ahuja V, Kadowaki T, Evans RW, et al. Comparison of HOMA‐IR, HOMA‐β% and disposition index between US white men and Japanese men in Japan: the ERA JUMP study. Diabetologia. 2015;58:265‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadowaki T, Miyake Y, Hagura R, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia. 1984;26:44‐49. [DOI] [PubMed] [Google Scholar]
- 9. Kohro T, Yamazaki T, Sato H, et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54:93‐97. [DOI] [PubMed] [Google Scholar]
- 10. Oishi M, Yamazaki K, Okuguchi F, et al. Changes in oral antidiabetic prescriptions and improved glycemic control during the years 2002–2011 in Japan (JDDM32). J Diabetes Investig. 2014;5:581‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(suppl 1):102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, 12‐week study. Diabetes Obes Metab. 2013;15:1136‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24‐week, randomized, double‐blind, placebo‐controlled, Phase III study. Expert Opin Pharmacother. 2014;15:1501‐1515. [DOI] [PubMed] [Google Scholar]
- 14. Inagaki N, Kondo K, Yoshinari T, Kuki H. Efficacy and safety of canagliflozin alone or as add‐on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: a 52‐week open‐label study. J Diabetes Investig. 2015;6:210‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and safety of canagliflozin in combination with insulin: a double‐blind, randomized, placebo‐controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Araki E, Tanizawa Y, Tanaka Y, et al. Long‐term treatment with empagliflozin as add‐on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:665‐674. [DOI] [PubMed] [Google Scholar]
- 17. Kaku K, Kiyosue A, Inoue S, et al. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014;16:1102‐1110. [DOI] [PubMed] [Google Scholar]
- 18. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol. 2014;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaku K, Inoue S, Matsuoka O, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2013;15:432‐440. [DOI] [PubMed] [Google Scholar]
- 20. Iizuka T, Iemitsu K, Takihata M, et al. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes: interim outcome of the ASSIGN‐K study. J Clin Med Res. 2016;8:116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma MD. Potential for combination of dipeptidyl peptidase‐4 inhibitors and sodium‐glucose co‐transporter‐2 inhibitors for the treatment of type 2 diabetes. Diabetes Obes Metab. 2015;17:616‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Center for Drug Evaluation and Research, Food and Drug Administration. Guidance for industry diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm071624.pdf. Accessed November 23, 2016.
- 23. Ministry of Health, Labour and Welfare, Japan. Pharmaceuticals and medical devices safety information no. 320. https://www.pmda.go.jp/files/000203635.pdf#search='canagliflozin+pyelonephritis+PMDA'. January 2015:20‐26. Accessed June 27, 2016.
- 24. Ministry of Health, Labour and Welfare, Japan. Pharmaceuticals and medical devices safety information no. 327. https://www.pmda.go.jp/files/000208624.pdf#search='canagliflozin+pyelonephritis+PMDA'. October 2015:21‐28. Accessed June 27, 2016.
- 25. Pharmaceuticals and Medical Devices Agency . Summary of investigation results. https://www.pmda.go.jp/files/000153316.pdf#search='teneligliptin+interstitial+pneumonia'. October 21 2014. Accessed June 27, 2016.
- 26. Kishimoto M. Teneligliptin: a DPP‐4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:187‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ministry of Health, Labour and Welfare, Japan. Pharmaceuticals and medical devices safety information no. 318. https://www.pmda.go.jp/files/000197892.pdf#search='teneligliptin++intestinal+obstruction'. November 2014:32‐36. Accessed June 27, 2016.
- 28. Kim SA, Shim WH, Lee EH, et al. Predictive clinical parameters for the therapeutic efficacy of sitagliptin in Korean type 2 diabetes mellitus. Diabetes Metab J. 2011;35:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maeda H, Kubota A, Tanaka Y, Terauchi Y, Matsuba I, ASSET‐K Study Group . The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e20‐e22. [DOI] [PubMed] [Google Scholar]
- 30. Maeda H, Kubota A, Kanamori A, et al. Long‐term efficacy and safety of sitagliptin in the treatment of Japanese type 2 diabetes (ASSET‐K1) to a target of HbA1c < 7%. J Endocrinol Invest. 2013;36:568‐573. [DOI] [PubMed] [Google Scholar]
- 31. Nomiyama T, Akehi Y, Takenoshita H, et al. Contributing factors related to efficacy of the dipeptidyl peptidase‐4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e27‐e28. [DOI] [PubMed] [Google Scholar]
- 32. Inagaki N, Goda M, Yokota S, Maruyama N, Iijima H. Safety and efficacy of canagliflozin in Japanese patients with type 2 diabetes mellitus: post hoc subgroup analyses according to body mass index in a 52‐week open‐label study. Expert Opin Pharmacother. 2015;16:1577‐1591. [DOI] [PubMed] [Google Scholar]
- 33. Kinoshita S, Kondo K. Evaluation of pharmacokinetic and pharmacodynamic interactions of canagliflozin and teneligliptin in Japanese healthy male volunteers. Expert Opin Drug Metab Toxicol. 2015;11:7‐14. [DOI] [PubMed] [Google Scholar]
- 34. Polidori D, Sha S, Heise T, et al. Effect of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, on C‐peptide kinetics. Clin Pharmacol Drug Dev. 2015;4:12‐17. [DOI] [PubMed] [Google Scholar]
- 35. Stein P, Berg JK, Morrow L, et al. Canagliflozin, a sodium glucose co‐transporter 2 inhibitor, reduces post‐meal glucose excursion in patients with type 2 diabetes by a non‐renal mechanism: results of a randomized trial. Metabolism. 2014;63:1296‐1303. [DOI] [PubMed] [Google Scholar]
- 36. Merovci A, Solis‐Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add‐on to metformin therapy. Endocr Pract. 2014;20:1187‐1197. [DOI] [PubMed] [Google Scholar]
- 38. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single‐dose administration in type 2 diabetic patients. Clin Endocrinol Metab. 2007;92:1249‐1255. [DOI] [PubMed] [Google Scholar]
- 40. Eto T, Inoue S, Kadowaki T. Effects of once‐daily teneligliptin on 24‐h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2012;14:1040‐1046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


