Abstract
Aims
To investigate the association of novel oral glucose‐lowering drugs (GLDs), compared with that of insulin, with risk of all‐cause mortality, cardiovascular disease (CVD) and severe hypoglycaemia.
Methods
During 2013 to 2014 all patients with type 2 diabetes in Sweden identified as new users of novel oral GLDs, either dipeptidyl peptidase‐4 (DPP‐4) inhibitors or sodium‐glucose cotransporter‐2 (SGLT2) inhibitors (only dapagliflozin available in Sweden during the study period), with those initiating insulin as a comparison group, in the Prescribed Drug Register were included and followed in the Patient and Cause of Death Registers. The novel GLD group and insulin group were matched 1:1 using propensity score. Cox regression models were used to estimate risks.
Results
Of 37 603 patients, 21 758 were matched 1:1 to novel GLD vs insulin groups, with median follow‐up times of 1.51 years (16 304 patient‐years) and 1.53 years (16 306 patient‐years), respectively. Treatment with novel GLDs was associated with a 44% (hazard ratio [HR] 0.56 [95% confidence interval {CI} 0.49‐0.64]), 15% (HR 0.85 [95% CI 0.73‐0.99]) and 74% (0.26 [95% CI 0.12‐0.57]) lower risk of all‐cause mortality, CVD and hypoglycaemia, respectively, compared with insulin treatment. In separate analyses for the two novel GLDs, dapagliflozin was associated with lower risks of all‐cause mortality and CVD (56% [HR 0.44, 95% CI 0.28‐0.70] and 49% [HR 0.51, 95% CI 0.30‐0.86], respectively), while DPP‐4 inhibitor treatment was associated with lower risk of all‐cause mortality (41% [HR 0.59, 95% CI 0.51‐0.67]), but not with CVD (HR 0.87, 95% CI 0.75‐1.01).
Conclusions
Novel oral GLD treatment was associated with lower risk of all‐cause mortality, CVD and severe hypoglycaemia compared with insulin treatment. Dapagliflozin was associated with a lower risk of both all‐cause mortality and CVD, whereas DPP‐4 inhibitor treatment was only associated with lower risk of all‐cause mortality.
Keywords: cardiovascular disease, dapagliflozin, DPP‐4 inhibitor, hypoglycaemia, pharmaco‐epidemiology, type 2 diabetes
1. INTRODUCTION
International guidelines recommend metformin as the first‐line drug treatment in the majority of patients with type 2 diabetes (T2D).1 After varying time on metformin, most patients with T2D need intensified treatment because of disease progression and insufficient glycaemic control.
The choice of glucose‐lowering drug (GLD) as add‐on to ongoing glucose‐lowering therapy includes a number of pharmacological treatments: insulin, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, sulphonylureas, sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors, thiazolidinediones, acarbose or glucagon‐like peptide‐1 (GLP‐1) receptor agonists. There is no consensus on which drug to choose, however, and the focus is on individualized treatment, mainly with the aim of improving glucose control.2
Although insulin initiation can improve glycaemic control rapidly, the complexity of injection regimens, the need to frequently titrate doses, as well as the risk of weight gain and hypoglycaemia remain problematic for many patients.3, 4, 5 Furthermore, safety concerns have been raised regarding increased risk of cardiovascular disease (CVD) and all‐cause mortality in patients with T2D treated with insulin.6, 7, 8, 9, 10
In contrast to insulin treatment, novel glucose‐lowering agents for the treatment of T2D, including SGLT2 inhibitors and DPP‐4 inhibitors, are alternative options with the advantages of oral administration, low risk of hypoglycaemia and weight gain11, 12, 13, 14 and with recent proof of cardiovascular safety.11, 15, 16, 17 Sweden has a relatively high use of insulin for the treatment of T2D compared with other European countries.18, 19, 20
The aim of the present observational study was to investigate whether new initiation of novel oral GLDs, that is, SGLT‐2 inhibitors and DPP‐4 inhibitors, was associated with change in risk of all‐cause mortality, CVD events, or severe hypoglycaemia compared with new initiation of insulin treatment using Swedish nationwide healthcare registry data.
2. MATERIAL AND METHODS
2.1. Data sources
This observational registry study used data from mandatory Swedish national registries as follows: the Prescribed Drug Register, covering all drug prescriptions filled since 2005 using Anatomical Therapeutic Chemical (ATC) codes; the Cause of Death Register (established 1961); and the National Patient Register, covering all hospitalizations and discharge diagnoses since 1987 and all outpatient hospital visits since 2001. All three registers are held by the Swedish National Board of Health and Welfare (NBHW).
2.2. Study population
All patients who for the first time (new users, treatment‐naïve or as add‐on to existing antidiabetic therapy) filled a prescription for either DPP‐4 inhibitors or SGLT2 inhibitors, hereafter called the “novel GLD” group of treatments, or insulin, during the time period July 1, 2013 to December 31, 2014 were identified. The index date was defined as the date of first filled prescription of either a novel GLD or insulin. Patients with a diagnosis of gestational diabetes (International Classification of Diseases [ICD]‐10 code: O24.4) within 1 year of the index date and patients with type 1 diabetes were excluded. Patients with type 1 diabetes were defined as those with a type 1 diabetes diagnosis (ICD‐10 code: E10) and treated with insulin during their first year of GLD treatment, or those aged <30 years at the start of insulin treatment, or aged <15 years at the start of any diabetes medication. Patients with a possible index date for both drug classes included in the novel group were primarily included in the SGLT2 inhibitor group and secondly in the DPP‐4 inhibitor group. For example, a patient filling a DPP‐4 inhibitor prescription prior to an SGLT2 inhibitor prescription was placed in the SGLT2 inhibitor group. The main analyses were carried out according to an on‐treatment approach, and patients were observed from the index date until index drug discontinuation, defined as treatment gap >6 months between filled prescriptions, death, or December 31, 2014. In addition, intention‐to‐treat (ITT) analyses were performed including the follow‐up time after which the novel GLD or insulin treatment was discontinued.
Individual patient‐level data from the three national registers were linked using personal identification numbers (assigned at birth and mandatory when using the public healthcare system). Data linkage was performed by the NBHW, and the linked database was managed by Statisticon AB, Uppsala, Sweden. Baseline treatments, defined by ATC codes, were defined as any identified filled prescription of the treatment of interest during the year prior to the index date. The study protocol was approved by the Stockholm regional ethics committee (registration number 2013/2206‐31).
2.3. Definition of outcomes
Three endpoints were defined: (1) death from any cause; (2) fatal and non‐fatal CVD: a main diagnosis in the inpatient register of myocardial infarction (I21), ischaemic stroke (I63–I64), unstable angina pectoris (I20.0), heart failure (I50) or cardiovascular death (death with an ICD‐10 code I diagnosis as primary cause of death; (3) severe hypoglycaemia: a main or secondary diagnoses in the inpatient register of hypoglycaemia (E16.0, E16.1, or E16.2) or diabetes with coma (ICD‐10 E10.0, E11.0, E12.0, E13.0, or E14.0), as these codes are typically used for hypoglycaemia requiring third party assistance. (See Supporting Information Table S1 for ICD diagnoses and ATC codes.)
2.4. Statistical analyses
The time from index initiation of novel drug or insulin to a clinical event (all‐cause mortality, fatal or non‐fatal CVD, or severe hypoglycaemia) was visualized using Kaplan–Meier graphs. Patients were censored at treatment discontinuation, death or study period end (ie, for clinical endpoints December 31, 2014 and for all‐cause mortality October 31, 2015).
Propensity score was used to match each patient who initiated a novel drug with a patient who initiated insulin (1:1) using caliper 0.2. The probability of having an initiation of a new novel drug (dependent variable) was estimated using a logistic regression model with group and age, gender, diabetes duration, history of myocardial infarction, unstable angina, angina pectoris, coronary revascularization, heart failure, atrial fibrillation, stroke, transitory ischaemic attack, peripheral artery disease, major organ specific bleeding, bariatric surgery, microvascular complications, severe hypoglycaemia, lower limb amputations, chronic obstructive pulmonary disease, kidney disease, cancer, frailty (defined as ≥3 days of hospitalization during the year prior to index date), all separate GLDs, drugs to prevent or treat CVD (angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, low‐/high ceiling diuretics, aldosterone antagonists, warfarin, statins, low‐dose acetylsalicylic acid, antiplatelet drugs, calcium channel blockers, weight loss drugs) and calendar year of both index date and date of first line initiation as independent variables. Propensity score distribution was used to study overlap between the two matched groups.
Statistical analyses comparing treatments (novel group vs insulin) in the matched cohort were performed using Cox proportional hazards models, where the dependence within the matched pairs was handled using a robust estimation of the variances. The matching was performed using the Match function in the R package Matching.21
In a second model, a series of adjusted Cox proportional hazard models was used on the total unmatched population. Directed acyclic graphs22 were used to minimize the risk of bias and identify the two adjustment models (Supporting Information, Figure S2A and B). Separate adjustment models were determined for risk of fatal and non‐fatal CVD and all‐cause mortality (Figure 2A) and severe hypoglycaemia (Figure 2B).
Figure 2.
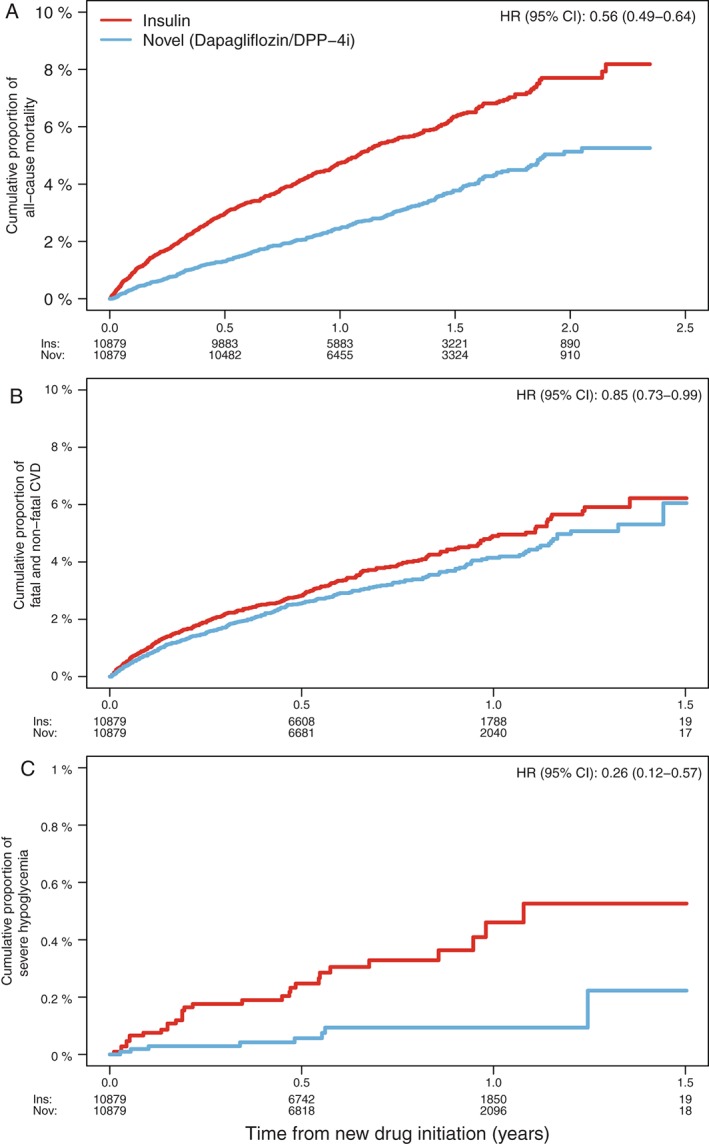
Kaplan–Meier curves and HRs comparing propensity score‐matched novel GLD and insulin groups for A, all‐cause mortality; B, fatal and non‐fatal CVD; and C, severe hypoglycaemia. [Correction added on 28th April, after first online publication: Supplied Figure 2 was previously incorrect and has been amended in this version]
The model for estimating the risk of severe hypoglycaemia was adjusted for age and frailty.23 The model for fatal and non‐fatal CVD was adjusted for age, sex, frailty, prior CVD as defined in Table 1, and use of statins, low‐dose aspirin and antihypertensives. History of CVD was assessed in the National Patient Register from 1987 until index date. The proportional hazard assumptions were assessed by examining Schoenfeld residuals.
Table 1.
Baseline characteristics of patients who were new users of novel GLDs (either dapagliflozin or DPP‐4 inhibitors) compared with new users of insulin
| Unmatched | Propensity score matched 1:1 | ||||
|---|---|---|---|---|---|
| Novel GLD group | Insulin | Novel GLD group | Insulin | P | |
| n = 12 544 | n = 25 059 | n = 10 879 | n = 10 879 | ||
| Age, years (s.d.) | 64.5 (11.9) | 68.3 (14.1) | 65.1 (12.0) | 65.1 (13.5) | .742 |
| Gender (female) | 4978 (40) | 10 626 (42) | 4408 (41) | 4410 (41) | .989 |
| Time since first GLD, years (s.d.) | 4.9 (3.1) | 4.7 (3.6) | 5.0 (3.2) | 5.0 (3.2) | .550 |
| Myocardial infarction | 1152 (9) | 3008 (12) | 1012 (9) | 1017 (9) | .926 |
| CABG | 402 (3) | 1034 (4) | 367 (3) | 370 (3) | .940 |
| PCI | 981 (8) | 2048 (8) | 840 (8) | 849 (8) | .839 |
| Unstable angina | 540 (4) | 1378 (5) | 488 (4) | 471 (4) | .597 |
| Angina pectoris | 1445 (12) | 3606 (14) | 1298 (12) | 1316 (12) | .723 |
| Heart failure | 934 (7) | 3518 (14) | 874 (8) | 906 (8) | .443 |
| Atrial fibrillation | 1222 (10) | 3988 (16) | 1141 (10) | 1155 (11) | .774 |
| Stroke | 1012 (8) | 3658 (15) | 963 (9) | 959 (9) | .943 |
| Hemorrhagic | 156 (1) | 547 (2) | 146 (1) | 137 (1) | .632 |
| Ischemic | 667 (5) | 2570 (10) | 640 (6) | 641 (6) | 1.000 |
| TIA | 301 (2) | 1132 (5) | 286 (3) | 304 (3) | .478 |
| Peripheral artery disease | 572 (5) | 1712 (7) | 527 (5) | 507 (5) | .545 |
| Chronic kidney disease | 306 (2) | 1339 (5) | 295 (3) | 322 (3) | .288 |
| Microvascular disease | 2326 (19) | 6691 (27) | 2225 (20) | 2229 (20) | .960 |
| Severe hypoglycemia | 27 (0) | 124 (0) | 25 (0) | 20 (0) | .551 |
| Cancer | 1897 (15) | 6018 (24) | 1780 (16) | 1791 (16) | .855 |
| COPD | 458 (4) | 1537 (6) | 430 (4) | 433 (4) | .945 |
| Lower limb amputations | 26 (0) | 182 (1) | 26 (0) | 26 (0) | 1.000 |
| Antihypertensives | 9683 (77) | 18280 (73) | 8226 (76) | 8101 (74) | .052 |
| Statins | 7916 (63) | 12927 (52) | 6535 (60) | 6589 (61) | .463 |
| Low dose aspirin | 4014 (32) | 8925 (36) | 3552 (33) | 3560 (33) | .919 |
| β‐blockers | 5306 (42) | 11293 (45) | 4596 (42) | 4663 (43) | .365 |
| Metformin | 10584 (84) | 15865 (63) | 8925 (82) | 8957 (82) | .583 |
| Sulphonylurea | 3763 (30) | 7113 (28) | 3405 (31) | 3431 (32) | .715 |
| DPP‐4 inhibitor | 625 (5) | 2248 (9) | 625 (6) | 621 (6) | .930 |
| GLP‐1 receptor agonist | 458 (4) | 1221 (5) | 457 (4) | 475 (4) | .569 |
| Metiglinides | 549 (4) | 1422 (6) | 525 (5) | 544 (5) | .572 |
| Index year | .435 | ||||
| 2013 | 3347 (27) | 8153 (33) | 3121 (29) | 3068 (28) | |
| 2014 | 9197 (73) | 16906 (67) | 7758 (71) | 7811 (72) | |
| Number of GLD classes dispensed within 1 year before index | |||||
| 0 | 1106 (8.82) | 6749 (26.93) | 1104 (10.15) | 1319 (12.12) | |
| 1 | 7522 (59.96) | 11326 (45.20) | 6268 (57.62) | 5797 (53.29) | |
| 2 | 3631 (28.95) | 6286 (25.08) | 3238 (29.76) | 3440 (31.62) | |
| 3+ | 285 (2.27) | 698 (2.79) | 269 (2.47) | 323 (2.97) | |
| First registered year of dispensed GLD | .999 | ||||
| 2005 | 3051 (24) | 7965 (32) | 2980 (27) | 2975 (27) | |
| 2006 | 904 (7) | 1637 (7) | 831 (8) | 825 (8) | |
| 2007 | 889 (7) | 1463 (6) | 758 (7) | 753 (7) | |
| 2008 | 1041 (8) | 1461 (6) | 849 (8) | 883 (8) | |
| 2009 | 1083 (9) | 1512 (6) | 883 (8) | 895 (8) | |
| 2010 | 1084 (9) | 1352 (5) | 835 (8) | 824 (8) | |
| 2011 | 1113 (9) | 1247 (5) | 803 (7) | 813 (7) | |
| 2012 | 1027 (8) | 1149 (5) | 755 (7) | 745 (7) | |
| 2013 | 1249 (10) | 2775 (11) | 1088 (10) | 1062 (10) | |
| 2014 | 1103 (9) | 4498 (18) | 1097 (10) | 1104 (10) | |
All numbers in parenthesis are percentage if not stated otherwise.
Abbreviations: CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease. PCI, percutaneous coronary intervention; s.d., standard deviation; TIA, transient ischaemic attack.
To estimate the impact of separate drug classes in the novel group, separate matches of SGLT2 inhibitor to insulin and DPP‐4 inhibitors to insulin were performed using the propensity score method and Cox proportional hazards models, as described above. To test the impact of the primary group selection process in a sensitivity analysis, a second method was used, where patients were assigned to the treatment groups based on the first of the three treatments (SGLT2 inhibitor, DPP‐4 inhibitors or insulin) that was initiated.
P values < .05 were taken to indicate statistical significance, and all analyses were conducted using R statistical software (R version 3.2.3).24
3. RESULTS
3.1. Unmatched patient characteristics and treatments
During the observation period, 37 603 patients initiated new therapy with novel GLDs or insulin; 33.4% and 66.6%, respectively (Table 1 and Figure 1). The SGLT2 inhibitor group consisted of dapagliflozin only (no other SGLT2 inhibitor was found in the Prescribed Drug Register during the study period, therefore, this subgroup is hereafter referred to as dapagliflozin) and the DPP‐4 inhibitors group of sitagliptin (94%), saxagliptin (4%), vildagliptin (2%) and linagliptin (0%); and the insulin group consisted of intermediate‐acting (53%), premixed (23%), long‐acting (12%) and short‐acting (12%; Supporting Information, Table S2).
Figure 1.
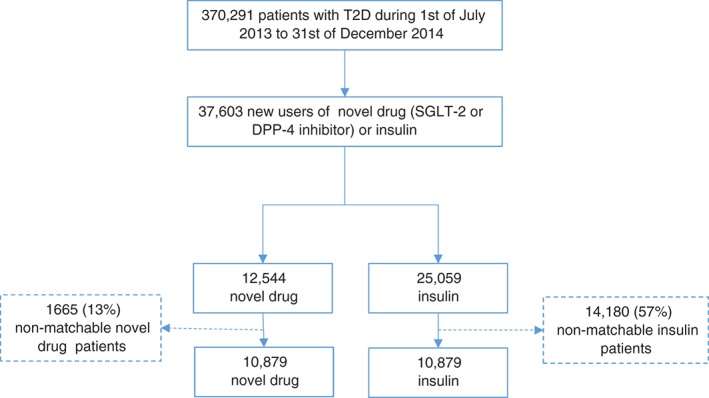
Patient flow chart.
Before matching, patients in the novel GLD group were younger (64.5 vs 68.3 years), less frequently women (40% vs 42%), had a longer time from first GLD (4.9 vs 4.7 years), less microvascular disease (19% vs 27%), and lower cardiovascular burden (previous myocardial infarction, heart failure, stroke) than patients in the insulin group (Table 1). The novel GLD group received more treatment with statins and antihypertensives, but less often low‐dose aspirin and β‐blockers, compared with the insulin group (Table 1). Use of other GLDs did not differ regarding sulphonylurea therapy (30% vs 28%) or GLP‐1 receptor agonist therapy, while metformin was more often used in the novel GLD group (84% vs 63%).
3.2. Propensity score‐matched analyses
After 1:1 propensity score matching, 21 758 patients initiated on either novel drug or insulin were identified (Figure 1). Only 11% of the patients had no GLD treatment during the year before index and the majority of patients filled prescriptions of ≥2 GLDs. The novel GLD and insulin groups were similar with regard to all baseline variables (Table 1) and showed a 92% propensity score distribution overlap (Supporting Information, Figure S1A). CVD prevalence for the whole cohort at baseline was 33% (Supporting Information, Table S3). The median follow‐up times were 1.51 years (16 304 patient‐years) and 1.53 years (16 306 patient‐years) for the novel GLD and insulin groups, respectively. The matched novel GLD group consisted of 19% and 81% new users of dapagliflozin and DPP‐4 inhibitors, respectively. The matched DPP‐4 inhibitor group consisted of sitagliptin (n = 8261; 94%), saxagliptin (n = 398; 5%), vildagliptin (n = 142; 2%), linagliptin (n = 1; 0%). The insulins were intermediate‐acting (63%), premixed (18%), long‐acting (12%) and short‐acting (8%).
In the novel GLD group, crude numbers (incidence per 100 patient‐years) of all‐cause death, fatal and non‐fatal CVD, and severe hypoglycaemia were 330 (2.56), 302 (4.66) and 8 (0.12), respectively, detailed data not shown. The corresponding results for the insulin group were 554 (4.57), 350 (5.49) and 30 (0.46). As illustrated by the Kaplan–Meier curves (Figure 2A‐C), the increased incidences in both groups were proportional to each other, with a continuous increased separation between the curves with increasing follow‐up time.
Compared with the insulin group, the novel group was significantly associated with 44%, 15% and 74% decreased risk of all‐cause mortality, fatal and non‐fatal CVD, and severe hypoglycaemia, respectively (details of hazard ratios [HRs] and 95% confidence intervals [CIs] are shown in Table 2). The ITT analyses showed similar risk estimates to the on‐treatment analyses.
Table 2.
Hazard ratios in new users of novel drugs (either dapagliflozin or DPP‐4 inhibitors) vs insulin using propensity‐matched patients (1:1)
| Number of patients | All‐cause mortality | Fatal/non‐fatal CVD | Severe hypoglycemia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| Propensity‐matched (novel vs insulin) | 21 578 | 0.561 | (0.49‐0.64) | <.001 | 0.851 | (0.73‐0.99) | .037 | 0.261 | (0.12‐0.57) | .001 |
| ITT | 21 578 | 0.581 | (0.52‐0.65) | <.001 | 0.841 | (0.73‐0.97) | .014 | 0.321 | (0.16‐0.65) | .002 |
| Multivariate adjusted (novel vs insulin) | 37 603 | 0.482 | (0.43‐0.54) | <.001 | 0.792 | (0.70‐0.90) | <.001 | 0.233 | (0.11‐0.48) | <.001 |
| ITT | 37 603 | 0.502 | (0.46‐0.56) | <.001 | 0.782 | (0.70‐0.88) | <.001 | 0.313 | (0.17‐0.58) | <.001 |
Multivariate adjusted Cox proportional hazard analyses are presented to test the results with a different statistical method.
Matched by propensity scores based on age, sex, frailty (≥3 days in hospital within 1 year prior to index), comorbidity and treatment.
Adjusted for age, sex, frailty, prior CVD, and use of statins, low‐dose aspirin, and antihypertensives.
Adjusted for age and frailty.
In the subgroup of patients with established CVD at baseline, novel GLD treatment was associated with lower risk of all‐cause mortality compared with insulin treatment, whereas no risk differences were observed regarding CVD (Supporting Information, Table S3). In the separate analyses of the drug classes in the novel group, dapagliflozin was significantly associated with lower CVD risk (HR 0.47 [95% CI 0.24‐0.93]), whereas DPP‐4 inhibitors did not differ from insulin (HR 1.00 [95% CI 0.84‐1.19]; Supporting Information, Table S3).
In the larger cohort of patients without CVD at baseline, novel GLD treatment was associated with lower risks of all three outcomes. In the separate analyses, both dapagliflozin and DPP‐4 inhibitors vs insulin had significantly lower risk associations with all‐cause mortality. The association with CVD did not reach significance for dapagliflozin, whereas for DPP‐4 inhibitors it was significant.
3.3. Multivariate‐adjusted survival analyses
When applying multivariate adjustment to the full cohort of 37 603 patients, novel GLD treatment and insulin treatment were associated with similar lower risks of all‐cause mortality, fatal and non‐fatal CVD, and severe hypoglycaemia compared with the propensity matched model (Table 2). The ITT analyses yielded similar risk estimates to the on‐treatment analyses.
3.4. Separate comparisons of dapagliflozin vs insulin and DPP‐4 inhibitors vs insulin
After propensity score matching (1:2); 6141 patients who initiated either dapagliflozin (n = 2047) or insulin (n = 4094) were identified, while 1:1 matching of DPP‐4 inhibitors resulted in 20 558 patients initiating either a DPP‐4 inhibitor (n = 10 279) or insulin (n = 10 279). No significant baseline differences were observed between the compared groups (Supporting Information, Table S4) and the propensity score distribution overlaps were 93% in both cases (Supporting Information, Figure S1B and C). Baseline CVD prevalence rates were 24% and 33% in the dapagliflozin and DPP‐4 inhibitor groups, respectively (Supporting Information, Table S4).
The median follow‐up times were 1.51 years (6182 patient‐years) and 1.40 years (2866 patient‐years) for the dapagliflozin and insulin groups, respectively, while the median follow‐up times were identical for the DPP‐4 inhibitors and insulin groups: 1.53 years (15 727 patient‐years) for each group.
The crude numbers (incidence per 100 patient‐years) of all‐cause mortality, fatal and non‐fatal CVD, and severe hypoglycaemia events for the propensity score‐matched dapagliflozin vs insulin analyses were 22 (0.98) vs 106 (2.19), 18 (1.68) vs 79 (3.27), and 1 (0.09) vs 5 (0.20), respectively (detailed data not shown). The increased incidence rates in both groups were proportional to each other, with a continuous increased separation between the curves with follow‐up time (Figure 3A‐F). Compared with insulin, dapagliflozin was associated with 56% (HR 0.44 [95% CI 0.28–0.70]) and 49% (HR 0.51 [95% CI 0.30‐0.86]) lower risks of all‐cause mortality and fatal and non‐fatal CVD, respectively (Table 3). Correspondingly, DPP‐4 inhibitor use was associated with an all‐cause mortality risk reduction of 41% (HR 0.59 [95% CI 0.51‐0.67]), but did not reach a statistically significant association with fatal and non‐fatal CVD (HR 0.87 [95% CI 0.75‐1.01]).
Figure 3.
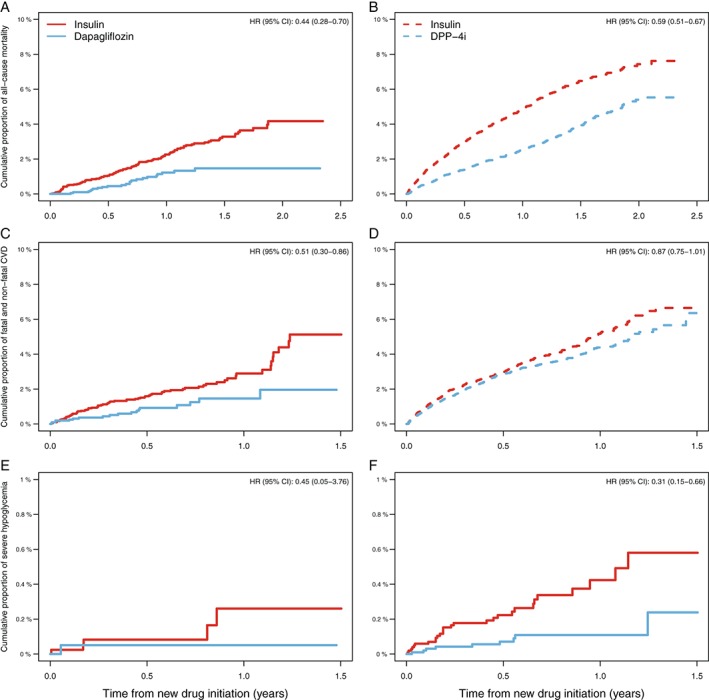
Kaplan–Meier curves and HRs comparing propensity score‐matched dapagliflozin and DPP‐4 inhibitors vs insulin groups for A and B, all‐cause mortality; C and D, fatal and non‐fatal CVD; and E and F, severe hypoglycaemia.
Table 3.
Hazard ratios in new users of dapagliflozin and DPP‐4 inhibitors individually, vs insulin, using propensity‐matched patients
| Number of patients | All‐cause mortality | Fatal/non‐fatal CVD | Severe hypoglycemia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| Propensity matched 1 | ||||||||||
| Dapagliflozin vs insulin | 6139 | 0.44 | (0.28‐0.70) | <.001 | 0.51 | (0.30‐0.86) | .011 | 0.45 | (0.05‐3.76) | .464 |
| DPP‐4 inhibitor vs insulin | 20 558 | 0.59 | (0.51‐0.67) | <.001 | 0.87 | (0.75‐1.01) | .076 | 0.31 | (0.15‐0.66) | .002 |
| ITT (dapagliflozin) | 6139 | 0.38 | (0.26‐0.57) | <.001 | 0.51 | (0.31‐0.84) | .008 | 0.90 | (0.17‐4.65) | .895 |
| ITT (DPP‐4 inhibitor) | 20 558 | 0.59 | (0.53‐0.65) | <.001 | 0.88 | (0.76‐1.01) | .063 | 0.39 | (0.20‐0.75) | .005 |
| Multivariate adjusted | ||||||||||
| Dapagliflozin vs insulin | 28 090 | 0.292 | (0.19‐0.44) | <.001 | 0.412 | (0.26‐0.66) | <.001 | 0.213 | (0.03‐1.52) | .123 |
| DPP‐4 inhibitor vs insulin | 34 730 | 0.472 | (0.41‐0.52) | <.001 | 0.812 | (0.71‐0.91) | .001 | 0.253 | (0.13‐0.51) | <.001 |
| ITT (SGLT2 inhibitor) | 28 090 | 0.262 | (0.18‐0.38) | <.001 | 0.402 | (0.26‐0.63) | <.001 | 0.393 | (0.10‐1.61) | .193 |
| ITT (DPP‐4 inhibitor) | 34 730 | 0.492 | (0.45‐0.54) | <.001 | 0.792 | (0.71‐0.89) | <0.001 | 0.343 | (0.19‐0.62) | <.001 |
Multivariate adjusted Cox proportional hazard analyses are presented to test the results with a different statistical method.
Matched by propensity scores based on age, sex, frailty (3 or more days in hospital within 1 year prior to index), comorbidity and treatment.
Adjusted for age, sex, frailty, prior CVD, and use of statins, low‐dose aspirin, and antihypertensives.
Adjusted for age and frailty.
Risk of severe hypoglycaemia was 69% lower in the DPP‐4 inhibitor group (HR 0.31 [95% 0.15‐0.66]) compared with the insulin group. Lower numbers of severe hypoglycaemia were also observed in the dapagliflozin group (HR 0.45 [95% CI 0.05‐3.76]); however, because there were very few hypoglycaemic events it did not reach statistical significance (Table 3).
3.5. Sensitivity analysis
When assessing new users of dapagliflozin, DPP‐4 inhibitors or insulin, whichever came first to define the novel and insulin group, the number of patients who were able to be matched decreased to 17 848 and the groups were similar at baseline (data not shown). Compared with the insulin group, the novel GLD group was significantly associated with 42%, 17% and 72% decreased risks of all‐cause mortality, fatal and non‐fatal CVD, and severe hypoglycaemia, respectively (details of HRs and 95% CIs are shown in Supporting Information, Table S5). The ITT analyses showed similar risk estimates to the on‐treatment analyses.
4. DISCUSSION
In a T2D population with 33% prevalent CVD and a relatively short GLD treatment history, we have shown that initiation of novel oral GLDs, specifically, dapagliflozin or DPP‐4 inhibitors, was associated with 44%, 15% and 74% decreased risks of all‐cause mortality, fatal and non‐fatal CVD, and severe hypoglycaemia, respectively, compared with new initiation of insulin. When assessing the separate contribution in the novel GLD group compared with insulin, dapagliflozin was associated with 56% and 49% lower risks of all‐cause mortality and fatal and non‐fatal CVD, respectively. DPP‐4 inhibitor treatment was associated with a 41% lower risk of all‐cause mortality, but the estimated HR for fatal and non‐fatal CVD was closer to 1, and not statistically significant when compared with insulin. Risk of severe hypoglycaemia was 69% lower in the DPP‐4 inhibitor group compared with the insulin group. Lower numbers of severe hypoglycaemic events were also observed in the dapagliflozin group; however, because there were few such events, this did not reach statistical significance.
In the recent randomized ORIGIN trial, an assessment of insulin glargine's ability to reduce CVD events by normalizing fasting glucose levels was performed, demonstrating a neutral effect on CVD outcome; however, the extrapolation of the ORIGIN results to a real clinical T2D setting can be questioned.25 Compared with the present study, the ORIGIN trial population was characterized by high CVD morbidity (eg, an almost 4 times higher baseline prevalence of myocardial infarction than in the present study), despite mild hyperglycaemia and, importantly, in the comparator group neither DPP‐4 inhibitors nor SGLT2 inhibitors were used. Furthermore, insulin glargine was associated with an increased risk of CVD in the subgroup of patients without history of CVD, which could support the present findings in a population where 2/3 patients had no previous CVD history.
Concerns have been raised about insulin treatment, as it may be associated with mechanisms negatively affecting risk of CVD.26, 27 To the best of our knowledge, no randomized study using CVD endpoints has compared patients treated with insulin with either DPP‐4 inhibitor and/or SGLT2 inhibitor treatment. In the absence of outcome results from randomized clinical trials, observational studies comparing insulin with alternative per oral GLDs are the best available evidence.6, 9, 28, 29
Our group recently showed that insulin compared with DPP‐4 inhibitor treatment initiated after metformin monotherapy was associated with 41%, 29% and 76% lower risk of all‐cause mortality, CVD and severe hypoglycaemia.29 In the present study, the corresponding risk reductions associated with the novel oral drug treatments vs insulin were very similar; however, the criterion of at least 6‐month metformin monotherapy treatment before the index date in our previous study, resulted in a cohort which was captured at a later stage in the disease progression, and accordingly patients were older, had a longer period of GLD treatment, and more macro‐ and microvascular disease at baseline compared with the present study. This may support the hypothesis that initiation of DPP‐4 inhibitors is associated with a risk reduction in all‐cause mortality and CVD even if used at different stages in the progression of T2D, compared with new initiation of insulin treatment.
Two recent observational studies from Sweden and the USA have compared insulin treatment directly with sulphonylurea after metformin monotherapy.6, 9 These studies showed that patients with T2D treated with sulphonylurea had significantly lower all‐cause mortality risk reduction (17% and 29%, respectively) compared with insulin‐treated patients with T2D. The strengths of the present study were the additional adjustments of both essential laboratory data and diabetes duration. In the present study we show higher risk reduction of all‐cause mortality (44%) in patients with T2D treated with novel GLDs than in those treated with insulin, in contrast to the above‐mentioned studies that used sulphonylurea as the comparator.6, 9 One reason for this finding may be that novel GLDs, which were used in the present study, have been demonstrated to be safe15, 16, 30 and even protective17 with regard to CVD, whereas sulphonylureas, although limited to observational data, have been associated with higher risks in comparison to DPP‐4 inhibitors.6, 23, 31, 32, 33, 34, 35 Hence, the choice of comparator to insulin could be of high importance and the use of newer GLDs is suggested to be a more appropriate comparator because of increased clinical use and new guidelines stating sulphonylureas should be used with caution.5
We found lowered risk of CVD with dapagliflozin compared with insulin, whereas DPP‐4 inhibitors did not show any such association, with the estimated HR being closer to 1 and not significant. This finding was even more apparent in the subpopulation with established CVD at baseline, where the association remained significantly low for dapagliflozin but was completely abolished for DPP‐4 inhibitors. The difference in CVD risk for dapagliflozin could be explained by a class effect, supported by a recent meta‐analysis by Wu et al.,36 in addition to the results from a recent large cardiovascular outcome trial by Zinman et al.17 The neutral CVD risk association with DPP‐4 inhibitors in the present study is consistent with several clinical outcomes trials documenting cardiovascular safety.11, 15, 16, 30 Thus, the difference in risk associations between dapagliflozin and DPP‐4 inhibitors supports the robustness of our findings using an observational study design with data from a real clinical setting.
In the present study, we found a markedly lowered risk (74% lower) of severe hypoglycaemia in the novel‐treated group compared with the insulin‐treated group. In addition, assessing dapagliflozin and DPP‐4 inhibitors separately resulted in similar numerical reductions in severe hypoglycaemia vs insulin treatment. It is well known that insulin treatment is associated with an increased risk of hypoglycaemia,37, 38 in concordance with the present study. Hypoglycaemia has been reported to be associated with CVD and mortality through different pathways23, 37, 39, 40 and could partly explain the increased risks of CVD and mortality seen in the present study. This is in contrast to GLD treatment with DPP‐4 inhibitors and SGLT2 inhibitor drugs, where hypoglycaemia is rare.16, 17
Not only hypoglycaemia but also weight gain might have contributed to the higher risk of all‐cause death and CVD.41 Although patients with T2D starting insulin treatment gain weight,42 it can only be speculated whether this may have contributed to the higher CVD risk observed in the present study. Nevertheless, drugs such as DPP‐4 inhibitors and dapagliflozin are weight‐neutral and weight‐reducing, respectively, which are effects that have been proposed to be protective in terms of CVD risks, and these effects might explain the results seen in this study.41
In summary, despite insulin's clear advantages of blood glucose control and prevention of microvascular disease, there is increasing evidence that other complications may limit its benefits in the treatment of patients with T2D. During the last few years, newer GLDs with less risk of hypoglycaemia and CVD have become available, and may be therapeutic alternatives with a lower risk of serious adverse effects.17, 43
The present results represent, to our knowledge, the first evidence of the cardiovascular benefit of an SGLT2 inhibitor (here, dapagliflozin), in a real clinical setting, supporting a class effect by complementing what has previously been shown, both in a randomized controlled cardiovascular outcome trial and a meta‐analysis.19, 36 The beneficial associations of lowered CVD and mortality risks in the present study should also be interpreted in the context of a T2D population with a lower CVD burden at baseline compared with the clinical randomized trial.17
Notwithstanding these reassuring results, some concerns have been raised regarding adverse events from the novel drugs, such as heart failure44 and pancreatitis for the DPP‐4 inhibitors,45 and sporadic cases of ketoacidosis for SGLT2 inhibitors.46 The relationship of DPP‐4 inhibitors with increased risks of hospitalization for heart failure has not yet been resolved; however, the results of another observational study and a meta‐analysis are not consistent with this finding.31, 44
A strength of the present work is the population‐based, nationwide and unselected real‐world study design, which provides a high external validity and large population, allowing propensity score‐matched analyses. In addition, this is a study with full register coverage for hospitalizations, filled drug prescriptions, and cause of death in a country with an established and complete public healthcare system. Diagnoses in the Swedish Patient Registry have been reported to have high validity,47 and few patients are lost to follow‐up. For the multivariate adjusted analyses directed acyclic graphs used to create the optimal adjustments of hazard models should provide minimal bias.22 The frequent and early use of insulin in Sweden increased the possibility of finding well‐matched patients initiated on DPP‐4 inhibitors or SGLT2 inhibitor, for example, using caliper 0.2 when propensity score‐matching. The reason for the high use of insulin in Sweden18, 20 compared with other European countries19 is not clearly understood, but an explanation could be the Swedish national guidelines48 that state treatment with medium‐ to long‐acting insulin is the highest priority after metformin in pharmacological treatment of T2D. Sensitivity analyses were performed to test the primary method of defining the novel and insulin groups and the results remained robust.
Observational studies such as the present one also have limitations. The present study has no information on laboratory measurements, lifestyle variables, primary healthcare data, or socio‐economic data, and consequently there may be confounding factors that remain; however, the close matching with an extensive number of essential variables and relatively high HRs should minimize the risk of unknown confounding. In addition, we had no information on diabetes duration or body weight, although, by matching for index year and the year of first‐line treatment initiation, a robust proxy for time since diagnosis was used.
In Sweden, no other SGLT2 inhibitor type besides dapagliflozin was used during the study period and we were therefore not able to address potential agent‐specific or class effects. We also had no information on emigration, which could result in loss to follow‐up. However, the on‐treatment analyses used should minimize the effects of patients moving out of Sweden. Furthermore, our assessment of severe hypoglycaemia was crude, including only events leading to hospital admission. It was not possible to evaluate other hypoglycaemic events in this register‐based study. Another limitation is that patients with a recorded hypoglycaemic event had to survive until this occasion and, if anything, this would underestimate the total mortality rate in these patients compared with those without a hypoglycaemic event. Further, the numbers of hypoglycaemia in the dapagliflozin group were small, limiting interpretation in this group.
In conclusion, the present observational study in a general T2D population shows that the novel oral GLDs, dapagliflozin or DPP‐4 inhibitors were associated with lower risk of all‐cause mortality, CVD and severe hypoglycaemia when compared to insulin. When analysed separately, dapagliflozin was associated with a lower risk of both all‐cause mortality and fatal/non‐fatal CVD, whereas DPP‐4 inhibitor treatment was only associated with lower risk of all‐cause mortality. Prospective randomized trials are needed to further elucidate these findings.
Supporting information
Table S1. International Classification of Diseases [ICD] code 9/10 diagnoses and ATC codes used to define comorbidities and treatments.
Table S2. Type of drugs within the propensity 1:1 matched cohort new users of novel drugs (either dapagliflozin or DPP‐4i, dipeptidyl peptidase‐4 inhibitors).
Table S3. Hazard ratios (HRs) in new users of novel (either dapagliflozin or dipeptidyl peptidase‐4 inhibitors [DPP‐4i]) vs. insulin using propensity‐matched patients (1:1), dapagliflozin vs insulin (1:2) and DPP‐4i (1:1) for patients with or without cardiovascular disease (CVD) using on‐treatment approach.
Table S4. Baseline table for new users of dapagliflozin and DPP‐4i (dipeptidyl peptidase‐4 inhibitors) separately vs. insulin using propensity score matched patients.
Table S5. Hazard ratios (HRs) in new users, whichever came first in time, of novel (either dapagliflozin or dipeptidyl peptidase‐4 inhibitors [DPP‐4i]) vs. insulin using propensity‐matched patients (1:1)
Figure S1. A, Propensity score distribution for new users of insulin and novel drugs after matching. B, Propensity score distribution for new users of insulin and dapagliflozin after matching. C, Propensity score distribution for new users of insulin and DPP‐4i after matching.
Figure S2. A, Directed acyclic graphs to define minimal sufficient adjustment sets for estimating the effect of insulin on cardiovascular disease (CVD) (myocardial infarction, stroke, and/or peripheral artery disease): {age, sex, fragile, low‐dose aspirin, antihypertensives, statins, previous CVD}. B, Directed acyclic graphs to define minimal sufficient adjustments sets for estimating the effect of insulin on severe hypoglycemia: {age, fragility}
ACKNOWLEDGMENTS
We are grateful to Urban Olsson and Hilja Brorsson at Statisticon for support with data merging and management of the database, and to Susanna Jerström and Helena Goike at AstraZeneca for logistic support and valuable comments on the manuscript. Thomas Nyström is the guarantor of the manuscript.
Conflict of interest
J. W. E. has received honoraria or research grants from AstraZeneca, NovoNordisk, Bristol‐Myers‐Squibb, Sanofi and MSD. J. B. holds a full‐time position at AstraZeneca as an epidemiologist. D. N. has received consultancy fees from Novo Nordisk, Astra Zeneca and Eli Lilly. M. T. is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, for which AstraZeneca Nordic‐Baltic is a client. T. N. has received unrestricted grants from AztraZeneca and NovoNordisk, and is on the national advisory board of NovoNordisk, Sanofi‐Aventis, Eli Lilly and Boehringer Ingelheim. A. N. has received honoraria from MSD, Astra Zeneca, Eli Lilly and Boerhinger Ingelheim.
Author contributions
All authors participated in the study design. M. T. performed the data collection and statistical analyses after discussion with all authors. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility for the decision to submit for publication.
Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A and Eriksson JW. Novel oral glucose‐lowering drugs are associated with lower risk of all‐cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes, Diabetes Obes Metab, 2017. https://doi.org/10.1111/dom.12889
Funding information This study was sponsored by AstraZeneca AB.
REFERENCES
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140‐149. [DOI] [PubMed] [Google Scholar]
- 3. Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799‐812. [DOI] [PubMed] [Google Scholar]
- 4. Davis TM, Clifford RM, Davis WA, Fremantle Diabetes Study . Effect of insulin therapy on quality of life in type 2 diabetes mellitus: The Fremantle Diabetes Study. Diabetes Res Clin Pract. 2001;52(1):63‐71. [DOI] [PubMed] [Google Scholar]
- 5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(1):84‐113. [DOI] [PubMed] [Google Scholar]
- 6. Ekstrom N, Svensson AM, Miftaraj M, et al. Cardiovascular safety of glucose‐lowering agents as add‐on medication to metformin treatment in type 2 diabetes: report from the Swedish National Diabetes Register. Diabetes Obes Metab. 2016;18(10):990‐998. [DOI] [PubMed] [Google Scholar]
- 7. Mellbin LG, Malmberg K, Norhammar A, Wedel H, Ryden L, DIGAMI 2 Investigators . Prognostic implications of glucose‐lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended follow‐up of the Diabetes Mellitus Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Study. Diabetologia. 2011;54(6):1308‐1317. [DOI] [PubMed] [Google Scholar]
- 8. Ritsinger V, Saleh N, Lagerqvist B, Norhammar A. High event rate after a first percutaneous coronary intervention in patients with diabetes mellitus: results from the Swedish coronary angiography and angioplasty registry. Circ Cardiovasc Interv. 2015;8(6):e002328. [DOI] [PubMed] [Google Scholar]
- 9. Roumie CL, Greevy RA, Grijalva CG, et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all‐cause mortality among patients with diabetes. JAMA. 2014;311(22):2288‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saleh N, Petursson P, Lagerqvist B, et al. Long‐term mortality in patients with type 2 diabetes undergoing coronary angiography: the impact of glucose‐lowering treatment. Diabetologia. 2012;55(8):2109‐2117. [DOI] [PubMed] [Google Scholar]
- 11. Gallwitz B, Rosenstock J, Rauch T, et al. 2‐year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double‐blind, non‐inferiority trial. Lancet. 2012;380(9840):475‐483. [DOI] [PubMed] [Google Scholar]
- 12. Goke B, Gallwitz B, Eriksson J, Hellqvist A, Gause‐Nilsson I, D1680C00001 Investigators . Saxagliptin is non‐inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52‐week randomised controlled trial. Int J Clin Pract. 2010;64(12):1619‐1631. [DOI] [PubMed] [Google Scholar]
- 13. Seck T, Nauck M, Sheng D, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2‐year study. Int J Clin Pract. 2010;64(5):562‐576. [DOI] [PubMed] [Google Scholar]
- 14. Wilding J, Bailey C, Rigney U, Blak B, Beekman W, Emmas C. Glycated hemoglobin, body weight and blood pressure in type 2 diabetes patients initiating dapagliflozin treatment in primary care: a retrospective study. Diabetes Ther. 2016;7(4):695‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232‐242. [DOI] [PubMed] [Google Scholar]
- 16. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 18. Bodegard J, Nathanson D, Nystrom T, Thuresson M, Norhammar A, Eriksson JW. Second‐line Treatment with Sulfonylurea Compared to DPP4 Inhibitors Demonstrated Associations with Earlier Treatment Intensification with Insulin. Stockholm: EASD; 2015. http://www.easdvirtualmeeting.org/resources/second‐line‐treatment‐with‐sulfonylurea‐compared‐to‐dpp4‐inhibitors‐demonstrated‐associations‐with‐earlier‐treatment‐intensification‐with‐insulin–3. Accessed September 2015. [Google Scholar]
- 19. Heintjes E, Overbeek J, Blin P, et al. Type 2 diabetes treatment patterns across Europe [Poster]. 2014. http://www.pharmo.nl/uploads/pdf/Posters/T2DM%20treatment%20patterns%20across%20Europe_ISPOR2014_20141030.pdf. Accessed November 2014. [DOI] [PubMed]
- 20. Persson F, Bodegard J, Birkeland K, et al. HbA1c and second line glucose lowering drug initiation in Denmark, Norway and Sweden: an observational study comparing T2DM management in primary care. 52nd EASD Annual Meeting; September 13 2016; Munich, Germany. [Google Scholar]
- 21. Sekhon J. Multivariate and propensity score matching software with automated balance optimization. J Stat Softw. 2011;7(42):1‐52. [Google Scholar]
- 22. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eriksson JW, Bodegard J, Nathanson D, Thuresson M, Nystrom T, Norhammar A. Sulphonylurea compared to DPP‐4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all‐cause mortality. Diabetes Res Clin Pract. 2016;117:39‐47. [DOI] [PubMed] [Google Scholar]
- 24. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 25. ORIGIN Trial Investigators , Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319‐328. [DOI] [PubMed] [Google Scholar]
- 26. Antoniades C, Tousoulis D, Marinou K, et al. Effects of insulin dependence on inflammatory process, thrombotic mechanisms and endothelial function, in patients with type 2 diabetes mellitus and coronary atherosclerosis. Clin Cardiol. 2007;30(6):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Angiolillo DJ, Bernardo E, Ramirez C, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48(2):298‐304. [DOI] [PubMed] [Google Scholar]
- 28. Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes‐related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013;98(2):668‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Second line initiation of insulin compared with DPP‐4 inhibitors after metformin monotherapy is associated with increased risk of all‐cause mortality, cardiovascular events, and severe hypoglycemia. Diabetes Res Clin Pract. 2017;123:199‐208. [DOI] [PubMed] [Google Scholar]
- 30. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 31. Fu AZ, Johnston SS, Ghannam A, et al. Association between hospitalization for heart failure and dipeptidyl peptidase‐4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care. 2016;39(5):726‐734. [DOI] [PubMed] [Google Scholar]
- 32. Mogensen UM, Andersson C, Fosbol EL, et al. Cardiovascular safety of combination therapies with incretin‐based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus–a retrospective nationwide study. Diabetes Obes Metab. 2014;16(10):1001‐1008. [DOI] [PubMed] [Google Scholar]
- 33. Morgan CL, Mukherjee J, Jenkins‐Jones S, Holden SE, Currie CJ. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP‐4 inhibitors: association with major adverse cardiovascular events and all‐cause mortality. Diabetes Obes Metab. 2014;16(10):977‐983. [DOI] [PubMed] [Google Scholar]
- 34. Ou SM, Shih CJ, Chao PW, et al. Effects on clinical outcomes of adding dipeptidyl peptidase‐4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163(9):663‐672. [DOI] [PubMed] [Google Scholar]
- 35. Seong JM, Choi NK, Shin JY, et al. Differential cardiovascular outcomes after dipeptidyl peptidase‐4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population‐based cohort study. PLoS One. 2015;10(5):e0124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4(5):411‐419. [DOI] [PubMed] [Google Scholar]
- 37.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 39.Control Group, Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288‐2298. [DOI] [PubMed] [Google Scholar]
- 40. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410‐1418. [DOI] [PubMed] [Google Scholar]
- 41. Bodegard J, Sundström J, Svennblad B, Östgren CJ, Nilsson PM, Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary‐care patients. Diabetes Metab. 2013;39(4):306‐313. [DOI] [PubMed] [Google Scholar]
- 42. Jansen HJ, Vervoort GM, de Haan AF, Netten PM, de Grauw WJ, Tack CJ. Diabetes‐related distress, insulin dose, and age contribute to insulin‐associated weight gain in patients with type 2 diabetes: results of a prospective study. Diabetes Care. 2014;37(10):2710‐2717. [DOI] [PubMed] [Google Scholar]
- 43. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schernthaner G, Cahn A, Raz I. Is the use of DPP‐4 inhibitors associated with an increased risk for heart failure? Lessons from EXAMINE, SAVOR‐TIMI 53, and TECOS. Diabetes Care. 2016;39(suppl 2):S210‐S218. [DOI] [PubMed] [Google Scholar]
- 45. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 Diabetes. Diabetes Care. 2017;40(2):284‐286. [DOI] [PubMed] [Google Scholar]
- 46. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849‐2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swedish national guidelines for diabetes care. 2015, p. 120 http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19803/2015‐4‐12.pdf. Accessed November 5 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases [ICD] code 9/10 diagnoses and ATC codes used to define comorbidities and treatments.
Table S2. Type of drugs within the propensity 1:1 matched cohort new users of novel drugs (either dapagliflozin or DPP‐4i, dipeptidyl peptidase‐4 inhibitors).
Table S3. Hazard ratios (HRs) in new users of novel (either dapagliflozin or dipeptidyl peptidase‐4 inhibitors [DPP‐4i]) vs. insulin using propensity‐matched patients (1:1), dapagliflozin vs insulin (1:2) and DPP‐4i (1:1) for patients with or without cardiovascular disease (CVD) using on‐treatment approach.
Table S4. Baseline table for new users of dapagliflozin and DPP‐4i (dipeptidyl peptidase‐4 inhibitors) separately vs. insulin using propensity score matched patients.
Table S5. Hazard ratios (HRs) in new users, whichever came first in time, of novel (either dapagliflozin or dipeptidyl peptidase‐4 inhibitors [DPP‐4i]) vs. insulin using propensity‐matched patients (1:1)
Figure S1. A, Propensity score distribution for new users of insulin and novel drugs after matching. B, Propensity score distribution for new users of insulin and dapagliflozin after matching. C, Propensity score distribution for new users of insulin and DPP‐4i after matching.
Figure S2. A, Directed acyclic graphs to define minimal sufficient adjustment sets for estimating the effect of insulin on cardiovascular disease (CVD) (myocardial infarction, stroke, and/or peripheral artery disease): {age, sex, fragile, low‐dose aspirin, antihypertensives, statins, previous CVD}. B, Directed acyclic graphs to define minimal sufficient adjustments sets for estimating the effect of insulin on severe hypoglycemia: {age, fragility}


