Abstract
The production of biobutanol is hindered by the product's toxicity to the bacteria, which limits the productivity of the process. In situ product recovery of butanol can improve the productivity by removing the source of inhibition. This paper reviews in situ product recovery techniques applied to the acetone butanol ethanol fermentation in a stirred tank reactor. Methods of in situ recovery include gas stripping, vacuum fermentation, pervaporation, liquid–liquid extraction, perstraction, and adsorption, all of which have been investigated for the acetone, butanol, and ethanol fermentation. All techniques have shown an improvement in substrate utilization, yield, productivity or both. Different fermentation modes favored different techniques. For batch processing gas stripping and pervaporation were most favorable, but in fed‐batch fermentations gas stripping and adsorption were most promising. During continuous processing perstraction appeared to offer the best improvement. The use of hybrid techniques can increase the final product concentration beyond that of single‐stage techniques. Therefore, the selection of an in situ product recovery technique would require comparable information on the energy demand and economics of the process. © 2017 American Institute of Chemical Engineers Biotechnol. Prog., 33:563–579, 2017
Keywords: In situ product recovery, ABE Fermentation, n‐Butanol
Introduction
Butanol is a commodity chemical used in a wide range of industries. It can be produced through biological or petrochemical routes. The original process route, started in 1913,1 was via the acetone, butanol, and ethanol (“ABE”) fermentation, but this lost favor to the petrochemical production process. Since the oil crisis in 1973 and the consequent dramatic rise in oil prices, alternative routes for petrochemical derivatives have been sought, sparking a renewal of interest in the ABE fermentation.2, 3
Fermentation originally ceased being the main production route for butanol when it became economically uncompetitive with the petrochemical production of butanol. The main reasons for this were high substrate cost, low solvent yield (approximately 2 wt%), and high energy requirement for butanol recovery by distillation.2, 4 The low yield and high energy requirement are closely related. One reason for the low yield is that the bacteria are inhibited by the butanol produced. This is a natural inherent limitation in the microorganism used for production. The low butanol tolerance necessitates low substrate concentrations, so as to maximize substrate consumption with minimal substrate loss. The low ABE concentration in the fermenter means that there is a high energy demand involved in the traditional distillation separation from a batch fermentation.5 This is compounded by the complex separation of butanol from water due to the azeotrope forming at 55.5 wt% butanol at 101.3 kPa.6
To overcome these problems, various strategies have been investigated, including: strain modifications to improve butanol tolerance,2, 4 in vitro production using immobilized enzymes,7 and fermentation process developments to remove the butanol from the fermentation broth as it is produced have been developed.2 Via such product removal techniques, the butanol concentration in the fermentation broth will be maintained below inhibitory levels, leading to increased productivity and overall titers. This would allow the process to be operated as a fed‐batch, or even continuous, fermentation.8 Relieving product toxicity will also have a significant impact on the economics of the fermentation. Increased productivity could allow a reduction in fermenter size, therefore a reduction in capital expenditure. In situ product recovery (ISPR) should increase the concentration of ABE for downstream processing, thereby reducing the energy demand and operational expenditure of the plant.
This paper provides a review of applied ISPR to ABE fermentations. The primary focus has been free cell (not immobilized or biofilm based) fermentations in a stirred tank reactor (STR), to allow for comparison of the various ISPR techniques. Other reactor configurations, such as immobilized bioreactors, have been considered if STR fermentations have not been performed. The techniques that have been experimentally combined with the ABE fermentation are gas stripping, vacuum fermentations, pervaporation, liquid–liquid extraction, perstraction, and adsorption.
In Situ Product Recovery
The aim of ISPR techniques is to remove the product from the vicinity of the cell as soon as it is formed,9 this should lead to increased productivity and overall titers for inhibited fermentations and reduced waste water treatment costs.10 There have been several comprehensive reviews covering ISPR for a wide range of fermentations and products. Van Hecke et al.11 have provided the most recent review considering developments in ISPR between 2003 and 2013. They highlight that more research is required to prove scalability, long‐term robustness, and stability of the ISPR technology, decreased energy consumption and to maximize the product recovery11.
Since 2012, there has been a dramatic increase in the number of reviews focusing on the ISPR from the ABE fermentation. Abdehagh et al.12 focus on the separation ability of the technique, rather than improvements in production. This study concluded that pervaporation and adsorption show the most promise for ISPR. Huang et al.13 provide an overview of gas stripping, vacuum/flash separations, liquid–liquid extraction, membrane techniques, and adsorption, with a focus on novel separating agents such as ionic liquids and composite membranes. Xue et al.14 qualitatively compares ISPR techniques to conventional distillation, concluding that no ISPR technique will be able to concentrate the products to reagent grade. The most recent review was by Staggs and Nielsen,8 which focused on the mode of application of the ISPR technique, i.e., direct contact or recirculation in an external contactor. To complement these reviews, this review's focus is on the effect of each technique on the fermentation.
To compare the ISPR techniques, the amount of substrate utilized, productivity, and yield have been used. The substrate utilized is the total amount of substrate consumed during the fermentation. The productivity is defined here as the mass (g) of ABE produced per liter of reactor volume per hour. The yield is the mass (g) of ABE produced per mass (g) of substrate used.15 The % substrate utilized, productivity, or yield increase is the percentage difference between the substrate utilized, productivity or yield for the integrated in situ recovery fermentation and the non‐integrated (control) fermentation. These parameters have been selected as they are generally considered as the main parameters of comparison in experimental based literature, particularly productivity and yield. Substrate utilization was selected to demonstrate the improvements in the fermentation, particularly fermentation longevity due to reduced toxicity. Measurement of substrate can be considered more reliable compared to product concentration, which can be highly inaccurate based on product separation methods. The concentrated product is not always directly measured, sometimes being inferred from model solution data or assuming the yield is the same as the control fermentation 16. Also, the final concentration varies based on volume used for calculation, i.e., fermentation volume (which is variable during the fermentation) through to the condensate concentration post separation, particularly with evaporative techniques. Productivity and yield provide a standardized measure of the fermentation performance. By comparing the % increase compared to the control fermentation the effects of various differences in experimental methods should be negated, allowing trends relating to the impact of the ISPR technique on the fermentation to be observed.
This review differs from the previous reviews by taking a quantitative approach to the comparing the experimental data of the ISPR techniques and their impact on the fermentation. This review also considers the final concentration from each ISPR technique that will enter the downstream distillation process, where possible.
Gas stripping
Gas stripping is a separation technique that involves the removal of solvents via dissolution into a gas passing through the fermentation broth. This technique was studied by a range of authors from the mid 1980s (e.g., Ennis et al.17), through to the present day (e.g., Xue et al.18). Numerous publications cover all operation modes and a range of bioreactor configurations.19, 20, 21, 22
Gas stripping for ABE fermentations involves the recycling of the fermentation gases (carbon dioxide and hydrogen), or application of other anaerobic gases such as oxygen‐free nitrogen,17, 23, 24 through the fermenter via a condenser to remove the ABE from the gas stream.25 As it can be performed in situ without the need for expensive equipment and plant modifications, gas stripping is considered a simple technique.26 Based on data from Ezeji et al.,22 the concentration in the gas stream is very dilute at approximately 1.7 mg/L, meaning that large condensing duties will be required, which will significantly increase operating costs. Additionally, the compressor duty to supply gas at flow rates of 2–3 vvm of a plant‐scale reactor is energy intensive.17
A wide range of studies have been performed for gas stripping, with Ezeji's body of work5, 22, 27, 28, 29 being the most comprehensive. A general conclusion to be drawn from this data is that the productivity of the fermentation is improved through the application of gas stripping. The productivities in Table 1 show an increase moving from batch to fed‐batch. It must be noted that the productivity increase seen by Maddox et al.,30 357%, is due to the low productivity of the control fermentation (0.07 g ABE/Lh). This demonstrates that relieving product inhibition has a significant positive effect on the fermentation. This removal of product toxicity has allowed for more substrate to be consumed, with more than a 100% increase in substrate utilization possible.
Table 1.
Free Cell ABE Fermentation Performance with in situ Product Recovery by Gas Stripping in an STR
| Mode | Microorganism | Substrate (Concentration for ISPR) | % Substrate Increase for ISPR (vs. control) | ABE Productivity for ISPR (g ABE/L.h) | % Productivity Increase (vs. Control) | Yield for ISPR (g ABE/g Substrate) | % Yield Increase (vs Control) | Gasa | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Batch | Clostridium acetobutylicum P262b | Lactose (58 g/L) | 101% | 0.31 | 41% | 0.27 | −31% | N2 | 17 |
| C. acetobutylicum P262 b | Whey Permeate/Lactose (199 g/L) | 542% | 0.32 | 357% | 0.35 | 35% | CO2+H2 | 30 | |
| Clostridium beijerinckii BA101 | Glucose (162 g/L) | 263% | 0.6 | 107% | 0.47 | 21% | CO2+H2 | 22 | |
| C. beijerinckii BA101 | Liquefied Corn Starch (LCS) (55 g/L) | 23% | 0.31 | 107% | 0.43 | 5% | CO2+H2 | 29 | |
| C. beijerinckii BA101 | Saccharified Liquefied Corn Starch (SLCS) (64 g/L) | 41% | 0.4 | 74% | 0.41 | 2% | CO2+H2 | 29 | |
| C. beijerinckii CC101 | Wood Pulp Hydrolysate (33 g/L) | 36% | 0.17 | 55% | 0.39 | 18% | CO2+H2 | 31 | |
| C. beijerinckii NRRL B593 c | Glucose/Xylose (60 g/L) | 77% | 0.29 | 81% | 0.32 d | ‐ | CO2+H2 | 16 | |
| Fed‐ Batch | C. beijerinckii BA101 | Glucose (500 g/L) | 1001% | 1.16 | 300% | 0.47 | 21% | CO2+H2 | 5 |
| C. beijerinckii BA101 | Saccharified Liquefied Corn Starch (SLCS) (226 g/L) | 395% | 0.59 | 157% | 0.36 | −10% | CO2+H2 | 29 | |
| C. acetobutylicum P262 b | Whey Permeate (183 g/L) | 576% | 0.26 | 271% | 0.38 | 19% | CO2+H2 | 25 | |
| Continuous | C. beijerinckii BA101 | Glucose (1125 g/L) e | 2278% | 0.92 | 229% | 0.41 | 5% | CO2+H2 | 27 |
| C. beijerinckii NRRL B593 c | Glucose/Xylose (52 g/L) e | 56% | 0.93 | 40% | 0.30 c | ‐ | N2 | 16 | |
| C. beijerinckii NRRL B593 c | Glucose/Xylose (41 g/L) e | 88% | 1.3 | 65% | 0.30 c | ‐ | N2 | 16 |
CO2 and H2 represent recycling of the gases produced during fermentation.
C. acetobutylicum P262 has since been reclassified as Clostridium saccharobutylicum P262.32
C. beijerinckii NRRL B593 produces isopropanol instead of acetone.16
Yield has been assumed equal to the yield of the batch for calculations of the productivity.16
The dilution rate was 0.003 h−1, 0.06h−1, and 0.11 h−1, respectively.
Interestingly, there is a decrease in productivity when moving to a continuous fermentation compared to the fed‐batch fermentation, but it should be noted that this comparison is based upon only one strain C. beijerinckii BA101. In the continuous fermentations performed by Ezeji et al.,27 this decrease in productivity cannot be related to the decrease in yield (0.92 g/L.h and 0.41 g/g for continuous27 compared to 1.16 g/L.h and 0.47 for fed‐batch5), because if the yield was the same as in the fed‐batch fermentation the productivity would still be lower. Low productivity is a result of the cyclic fermentation profile, switching between the acidogenic and solventogenic phase, which means that a true steady state is not attained. The reduced yield is due to the removal of some of the nutrients from the process, in the reactor bleed, meaning 100% sugar utilization was not possible.27
There are some results in Table 1 which show the yield of the gas stripping process being greater than the theoretical yield of the bacteria. In the work by Ezeji et al.,5, 22, 27 the increased yield, 0.41–0.47, is contributed to the consumption of other carbon sources present in the complex medium used, such as sodium acetate. It is also suspected that less substrate is used for biomass maintenance, therefore a greater product yield is possible.5 No reason was provided for the higher than expected yield in the case of Ezeji et al.29 In some cases, a decrease in yield compared to the non‐integrated fermentation is seen, for example Ennis et al.17 observed a 31% decrease in yield; but this is probably related to inefficient condensing capability, meaning that not all solvents are captured and are consequently not accounted for when calculating the yield, as seen by Groot et al.24 and Ezeji et al.5 who take into account solvent losses when calculating the overall yield. This inability to capture all the solvents has a knock‐on effect, meaning that the productivities cannot be assumed to be accurate, adding further uncertainty to any comparison between operating modes. de Vrije et al.16 overcame the loss of products by assuming the same yield as the control fermentation, 0.30–0.32 g/g, and used this to calculate the productivity. This calculation method is likely to provide an inaccurate result as it is assumed that the ISPR technique has no negative or positive effect on the microorganism's performance.
Gas stripping has limitations due to the low ABE concentration in fermentation broth, large quantities of water removed and high gas flow rates required.33 Xue et al.33 proposed that operating at higher butanol concentrations, 8 g butanol/L compared to 5 g butanol/L, would increase the concentration of product in the vapor and reduce the energy for separation. The downside to this is 8 g butanol/L is often inhibitory to the bacteria. Xue et al. 33 tested this idea in an immobilized fermentation using an intermittent gas flow rate. The gas flow only operated while the butanol concentration in the broth was greater than 8 g butanol/L. This gas stripping regime saw a 33% increase in productivity compared to the control, while the yield remained constant. Stripping at a higher concentration saw a condensate concentration of 195.9 g ABE/L, compared to 76.8 g ABE/L achieved by Ezeji et al.29 in a fed‐batch free cell fermentation with saccharified liquefied cornstarch with the butanol maintained no higher than 5 g butanol/L.
Hybrid Gas Stripping
Since 2013 there has been a flurry of investigations into hybrid separations. A major focus of the hybrid separation processes has been improving the efficiency of gas stripping. This has included investigations into multi‐stage gas stripping processes, increasing the temperature at which gas stripping is performed and hybrid gas‐stripping pervaporation processes,16, 18, 19, 34, 35, 36 with the aim of reducing the energy requirements for further separation of the condensate. Oudshoorn et al.37 estimated the selectivity of gas stripping for butanol to be 4–22, which is low compared to distillation with an estimated selectivity of 72, thereby the recovered solution is not very concentrated. It has been widely noted that to achieve significant decreases in the energy for downstream purification, two‐phase separation needs to be observed in the recovered ABE solution.19, 21, 31 To achieve this phase separation, it has been suggested that the butanol concentration in the fermenter should be greater than 8 g/L, but concentrations this high start to impact on the fermentation performance.19, 34
To achieve this higher concentration, Xue et al.36 proposed a two‐stage gas stripping process. The aqueous phase condensate from the first stripping stage, 153 g ABE/L, being subjected to gas stripping to achieve a more concentrated solution, 447 g ABE/L. When combined with the organic phase from the first stripping stage the final product solution was 532 g ABE/L.36 The first stage reduced inhibition in the fermenter, while the second stage increased the concentration of condensate. Xue et al.34 proceeded to further optimize the process and achieved a final product concentration of 671 g ABE/L, predicting a 50% decrease in operational energy to 7–15 MJ/kg butanol.14de Vrije et al.16 discussed the use of increasing the temperature while gas stripping to improve the selectivity of the process. de Vrije et al. 16 utilized the bacteria's natural sporulation cycle for a repeated batch process. The broth was heated to 70°C at the end of a batch to remove the products via enhanced gas stripping and heat shock the spores to restart the fermentation with fresh media added. The final condensate concentration, nor total product formation was not stated so this cannot be compared to the two‐stage process proposed by Xue et al.34 Chen et al.19 also investigated the use of a higher stripping temperature, but combined the fermentation with an immobilized cell bioreactor. Immobilization of the cells allowed the fermentation medium to be heated to 70°C without impacting the viability of the bacteria. This saw condensate concentrations of 703 g butanol/L in the organic phase and 78 g butanol/L in the aqueous phase. The combined concentration was 150 g butanol/L 19, indicating that a two‐stage stripping system will offer better performance.
Gas stripping has also been combined with pervaporation, using a carbon nanotube filled polydimethylsiloxane (CNT‐PDMS) membrane.18 Gas stripping was first performed on the fermentation broth to relieve ABE toxicity. Pervaporation was then performed on the aqueous phase portion of the condensate to further increase the final product concentration. This method produced a final product concentration of 623 g ABE/L,18 which is slightly lower than that achieved using the two‐stage gas stripping process (671 g ABE/L).34 Xue et al.18 predict that the energy for the pervaporation step will be as low as 4 kJ/kg butanol due to the starting solution containing 80 g/L butanol. The overall two‐stage gas stripping‐pervaporation process would require ∼20 MJ/kg butanol. Compared to two‐stage gas stripping, a hybrid gas stripping‐pervaporation process is more complex, producing a lower product concentration and requires more energy for this stage of the process.
These hybrid techniques apply a second/enhanced separation stage to the fermentation. Other than de Vrije et al.,16 have all focused on immobilized fermentations. It would be useful to see the potential impact these hybrid techniques (if possible) could have when combined with free cell fermentations. Liu et al.38 and van der Merwe et al.39 proposed flowsheets with alternative product concentration techniques to distillation for the ABE fermentation. It would be advantageous to complete a similar analysis for the various hybrid options to help decide which further concentration techniques would be best suited for an industrial process.
Vacuum fermentation
Vacuum fermentations have a reduced pressure in the fermenter, causing the ABE to “boil off” at fermentation temperature. Vacuum fermentations were first used in the ethanol industry to selectively remove ethanol from fermentation broths. The use of a vacuum for an ABE fermentation should be more straightforward than for an ethanol fermentation, as the Clostridium sp. used are strict anaerobes.40 The viability of vacuum fermentations was experimentally tested by Mariano et al.40, 41, 42
Mariano et al 40 demonstrated that it is possible to recover ABE from fermentation broths under vacuum on a laboratory scale with no adverse effects on the bacteria. The system was initially characterized using a model ABE solution, with concentrations ranging from 5–15 g butanol/L, but this was found to be unrepresentative of real fermentation broths in which the gas created by the bacteria expands under reduced pressure, stripping the solvents from the broth. This effectively creates a hybrid gas stripping‐vacuum system. Mariano et al.41 reported that under constant vacuum conditions, the rate of removal of butanol was approximately 10 times higher than that found by Ezeji et al.22 using gas stripping, which could reduce the butanol concentration by up to 68.5%.41 Performing the fermentation under vacuum was able to achieve butanol concentrations of less than 1 g/L in the fermentation broth.40 More recently, vacuum fermentation was also proven to be effective with combining with simultaneous saccharification, fermentation, and recovery.43 Qureshi et al.43 successfully demonstrated the combined process using 86 g/L corn stover as a feedstock, in simultaneous saccharification, fermentation, and recovery. The ability to utilize lignocellulosic feedstocks as well as combining feedstock treatment with the fermentation and recovery should also see a reduction in capital and operational costs.
Two vacuum modes have been investigated: constant and cyclic. Cyclic vacuum fermentations were found to be considerably more competitive in terms of energy demand than traditional distillation. The cyclic vacuum process allows the concentration of butanol to build up, then reduces the concentration rapidly by applying a vacuum for 2 h, repeating this process throughout the fermentation.42 This is the only variation in operation that has been investigated. Currently, all trials have been on batch fermentations (Table 2) with a maximum applied vacuum time of 30 h,40 so whether vacuum fermentation can be extended for improved productivity is unknown. Whether this extended time at reduced pressure would impact microbial performance is also unknown.40, 41, 42
Table 2.
Free Cell ABE Fermentation Performance with in situ Product Recovery by Vaccum in an STR
| Mode | Microorganism | Substrate (Concentration for ISPR) | % Substrate Increase for ISPR (vs. control) | ABE Productivity for ISPR (g ABE/L.h) | % Productivity Increase (vs. control) | Yield (g ABE/g Substrate) | % Yield Increase (vs. control) | Operating Temperature (°C) | Vacuum Range (mmHg) | Vacuum Operating Mode | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch | C. beijerinckii P260 | Glucose (62 g/L) | 38% | 0.34 | 31% | 0.24 | −31% | 37 | 711–737 | Intermittent | 40 |
| C. beijerinckii NCIMB 8052 | Glucose (66 g/L) | 56% | 0.37 | 54% | 0.34 | −8% | 35 | 711–737 | Intermittent | 42 | |
| C. beijerinckii P260 | Glucose (58 g/L) | 29% | 0.28 | 8% | 0.22 | −37% | 37 | 711–737 | Continuous | 10 | |
| C. beijerinckii NCIMB 8052 | Glucose (65 g/L) | 54% | 0.43 | 79% | 0.29 | −22% | 35 | 711–737 | Continuous | 41 | |
| C. beijerinckii P260 | Corn Stover (83 g/L) | 7% | 0.34 | 55% | 0.39 | 30% | 35 | 584 | Continuous | 43 |
All of the literature focusing on vacuum fermentations for the ABE process is a product of the same researchers.40, 41, 42, 43 In each of these studies, inefficient condensation resulted in low ABE capture with ABE condensate concentration of 16–49 g/L, lower than the concentration required for spontaneous phase separation.40 Consequently, the yield has been underestimated as demonstrated by the negative yield increases seen in Table 2. Qureshi et al.43 accounted for losses of ABE in the system, based on previous work, and therefore achieved a positive yield increase of 30%. This appears to be a common flaw in evaporative techniques, in particular vacuum fermentation and gas stripping.5, 43 Another potential problem with the use of vacuum fermentations, highlighted by Mariano et al.,40, 42 was that a small concentration of acids (up to 0.4 g/L) was detected in the condensate. As the fermentation utilizes acids as intermediates, acid removal is undesirable during ISPR as it will reduce the yield of the process.
Mariano et al.42 assessed the energy requirement for the addition of a vacuum to the fermentation. They showed that the use of a vacuum reduced the downstream distillation energy requirement by 11.2 MJ/kg butanol for a continuous vacuum and 15 MJ/kg butanol for intermittent vacuum. When combined with the energy required for the vacuum fermentation, the total energy requirement became 32.4 and 22.0 MJ/kg butanol for continuous and intermittent vacuum, respectively. For a comparable batch process without ISPR, the energy requirement was 26.8 MJ/kg butanol. The use of a continuous vacuum will see an increase in the plant energy demand, defeating one of the main purposes of adding ISPR. The use of an intermittent vacuum sees an 18% decrease in energy. Mariano et al.44 have demonstrated that as the butanol concentration in the first distillation column increases, the energy requirements rapidly decrease. This can be inferred as the reason the intermittent vacuum fermentation requiring less energy than the continuous vacuum fermentation, where the condensate concentrations were 51.5 g ABE/L and 33 g ABE/L, respectively 42.
Generally, the conclusions over application of vacuum to ABE fermentations are not definitive, as there is not enough data (Table 2). The application of a vacuum can increase the substrate utilization and productivity of the fermentation, but without an efficient product capture step the benefits are not observed, as significant product is lost. The decreased yield would have a negative effect on the process economics, impacting the amount of feedstock required.
Pervaporation
Pervaporation utilizes a membrane between the fermentation broth and the gaseous phase,45 a simplified schematic is shown in Figure 1. Pervaporation renders the flowsheet more complex, as generally, an external unit is required to perform the separation. It is possible for pervaporation to be performed within the bioreactor,46, 47 but this is an unusual configuration.
Figure 1.
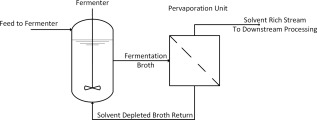
Diagram of a fermentation with in situ product recovery by pervaporation.
Pervaporation has been shown to increase the substrate utilization, productivity, and yield of ABE fermentations (see Table 3). It is the most widely researched area in relation to ISPR and the ABE fermentation. Within this body of research, there is a greater focus on membrane performance than integrated fermentation performance.48 Of the work performed where the ABE fermentation is coupled to a pervaporation system, no definitive conclusion can be drawn on fermentation or pervaporation operating conditions.
Table 3.
Free Cell ABE Fermentation Performance with in situ Product Recovery by Pervaporation in an STR
| Mode | Microorganism | Substrate (Concentration for ISPR) | % Substrate Increase for ISPR (vs. Control) | Productivity for ISPR (g ABE/Lh) | % Productivity Increase (vs. Control) | Yield for ISPR (g ABE/g Substrate) | % Yield Increase (vs. Control) | Membrane | Driving Force | Driving Force Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch | C. beijerinckii BA101 | Glucose (60 g/L) | 5% | 0.5 | 47% | 0.42 | 42% | Silicone tubing | Air | 2–18 L/min | 51 |
| C. acetobutylicum XY16 | Glucose (60 g/L) | 15% | 0.3 | 50% | 0.37 | 29% | PDMS/ceramic composite | Vacuum | <400Pa | 59 | |
| C. saccharo‐butylicum P262 | Whey Permeate/Lactose (211 g/L) | 424% | 0.43 | 207% | 0.37 | 19% | Silicone | N2 | 40–42 L/h | 67 | |
| C. acetobutylicum DP217 | Cassava (70 g/L) | 0% | 0.51 | 21% | 0.36 | 9% | Silicalite‐PDMS/PAN | Vacuum | 280 Pa | 58 | |
| Fed‐Batch | C. acetobutylicum ATCC824 | Glucose (199 g/L) | 138% | 0.66 | 47% | 0.27 | −18% | PDMS | Vacuum | 200 Pa | 52 |
| C. acetobutylicum ATCC824 | Glucose (276 g/L) | 231% | 0.94 | 109% | 0.28 | −15% | SDS | Vacuum | 200 Pa | 52 | |
| C. acetobutylicum XY16 | Glucose (200 g/L) | 285% | 0.21 | 5% | 0.28 | −3% | PDMS/ceramic composite | Vacuum | <400Pa | 59 | |
| C. acetobutylicum XY16 | Glucose (200 g/L) | 285% | 0.25 | 25% | 0.3 | 3% | PDMS/ceramic composite | Vacuum | <400Pa | 59 | |
| C. beijerinckii BA101 | Glucose (384 g/L) | 568% | 0.98 | 180% | 0.43 | −2% | Silicone | Air | 2–18 L/min | 68 | |
| C. acetobutylicum ATCC824 | Glucose (445 g/L) | 950% | 0.18 | 33% | 0.35 | 21% | Silicalite‐Silicone | Vacuum | 266‐666 Pa | 57 | |
| C. acetobutylicum DP217 | Cassava (736 g/L) | 951% | 0.76 | 80% | 0.38 | 15% | Silicalite‐PDMS/PAN | Vacuum | 280 Pa | 58 | |
| C. acetobutylicum P262 a | Whey Permeate (123 g/L) | 355% | 0.14 | 100% | 0.34 | 6% | Poly‐propylene | N2 | 10–20 L/min | 25 | |
| C. acetobutylicum ATCC 55025 | Glucose (172 g/L) | 144% | 0.46 | 15% | 0.32 | 3% | Zeolite‐PDMS | Vacuum | <1 kPa | 60 | |
| Cont‐inuous | C. acetobutylicum ATCC824 | Glucose (100g/L)/Xylose (50 g/L) b | 201% | 0.65 | 126% | 0.30 | 67% | PDMS (Pervatech) | Vacuum | 960 Pa | 53 |
C. acetobutylicum P262 has since been reclassified as C. saccharobutylicum P262.32
The overall dilution rate was 0.0017 h−1.
For pervaporation, the major decision to be made is the choice of membrane, as the ideal membrane should selectively allow the transfer of ABE while retaining butyric acid, acetic acid, water, and nutrients. The membrane also needs to be minimally fouling, so that it is not blocked by cells adhering to the membrane surface. Table 3 shows that a range of organophilic membranes have been tested, all of which show an improvement in the productivity of the fermentation. While silicone (including polydimethylsiloxane, PDMS) has been the most investigated membrane, as it is commercially available, inexpensive, and offers easy manipulation for the development of laboratory‐scale pervaporation units,45, 46, 49, 50, 51, 52, 53, 54 other membrane choices, including polypropylene,25 oleyl alcohol liquid membrane on a polypropylene support,55 polystyrene‐b‐polydimethylsiloxane‐b‐polystyrene (SDS),52 and PDMS‐supported ionic liquid membranes56 have been investigated. More recently, there has been a move toward the use of composite membranes, utilizing a combination of materials for improved selectivity and flux performance. This has included silicalite‐silicone composite membrane57 silicalite‐PDMS/polyacrylonitrile (PAN) membrane,58 PDMS/ceramic composite,59 zeolite‐mixed PDMS60 and carbon nanotube filled PDMS (CNT‐PDMS).61 A wider range of membranes have been investigated for butanol/water or ABE/water solutions, but this does not necessarily transfer to the performance in conjunction with a fermentation.48
The membrane choice affects the selectivity and diffusion rates of the ABE, which will determine the concentration of the ABE in the permeate. Unfortunately, the selectivity is in competition with the flux. Improvements in flux can be made to pervaporation through the use of higher temperatures, for example, but this would require an additional step of microorganism removal prior to heating the pervaporation feed stream (to 65–78°C), and cooling of the retentate prior to re‐addition to the bioreactor.62, 63 Other factors such as feed concentration and composition, biomass concentration, and sweep gas flow rate also influence the membrane flux.51, 64 Qureshi and Blaschek51 and Gapes et al.65 state that the application of a vacuum on the permeate side increased the flux compared to the application of a sweep gas, which explains why recent research has focused on vacuums.59, 62, 66
The use of a composite membrane has also been proposed as a method of achieving non‐competitive flux and selectivity. Polymers have high flux, are relatively cheap and easy to fabricate into a membrane, but are prone to aging over a long time. Inorganic materials are often highly selective for butanol and have good strength but are expensive. By combining both materials together, the membrane should be more selective with a sufficient flux while not having a prohibitive cost for scale‐up.13 Li et al.58 used a silicalite‐PDMS/PAN membrane and were able to achieve an average permeate concentration of 201 g ABE/L, resulting in spontaneous phase separation. Xue et al.61 used a CNT‐PDMS membrane. They were able to achieve a butanol separation factor of 16.6 and butanol titers over 100 g butanol/L in the permeate, when the membrane consists of 10% carbon nanotubes. This membrane has not been directly applied to the ABE fermentation. Zeolite‐PDMS were also tested by Xue et al.,60 with an 80% zeolite loaded PDMS membrane being combined with a free cell fermentation. The condensate collected contained 253 g ABE/L, which formed an organic phase containing over 600 g butanol/L. The membrane surface was smooth and non‐porous, this reduced fouling by the bacteria as there were no pores or imperfections for the bacteria to stick to.60 The addition of a second material to increase the selectivity and flux significantly improves the compatibility with the ABE process. Combine this with achieving high purity ABE (greater than 250 g ABE/L in the total permeate) means the downstream energy required for product recovery would decrease.58 Currently, these membranes have only been made for research purposes. An economic study is required to understand the impact the use of these novel membranes would have on the process.
As pervaporation is an evaporative ISPR technique, the product is captured through condensation. The inefficiencies of condensation of ABE have already been discussed in the sections concerning gas stripping and vacuum fermentation. In the papers regarding these techniques, the inefficiencies of complete product capture are acknowledged, but this has not been the case for pervaporation. In Table 3, it can be observed, for fed‐batch fermentations, a negative yield increase compared to the control fermentation. The fed‐batch fermentations are not inhibited by ABE or limited by available substrate, as an increase in substrate utilization is observed, yet there is a decrease in yield compared to the control fermentation. This indicates the potential loss of product, possibly through incomplete condensation. Incomplete product capture appears to be an inherent issue where the ABE is transferred to the vapor phase and needs to be considered when designing the fermentation process.
Liquid‐liquid extraction
Liquid‐liquid extraction (LLE) is a common technique in the processing industries. It exploits the differences between relative solubility of a compound in two immiscible components. Typically, the solvent used is an organic liquid which is immiscible in water, therefore when applied to a fermentation broth, the product will preferentially transfer from the aqueous phase into the organic phase.
There are a variety of key parameters that need to be considered, described in Table 4. Davison and Thompson69 also stated that the operability of the LLE system and the method of contacting must be considered, especially for plant or pilot plant operation, which was only considered by Roffler et al.70 One of the biggest challenges with LLE is finding an extractant which can satisfactorily meet all of the key characteristics for the extractant.
Table 4.
Key Characteristics for LLE Extractant
| Key Characteristic | Ref. |
|---|---|
| Non‐toxic to the microorganism | 69, 71 |
| Have a high partition coefficient (high capacity) | 69, 71 |
| Immiscible with water and not emulsion‐forming with aqueous phase | 71 |
| Favorable physical properties, for example a low viscosity and a large density difference compared to water | 71, 72 |
| High chemical stability, particularly at high temperatures (to ease extractant renewal) | 72 |
| Sterilizable | 71 |
| Commercially available at low cost | 71, 72, 73 |
Ishii et al.71 and Roffler et al.74 performed a series of extractive batch fermentations to find a suitable extractant for the ABE fermentation, and both concluded that oleyl alcohol is an acceptable extractant for butanol. It is non‐toxic to the microorganisms and has a good distribution coefficient with an average distribution coefficient for butanol of 4.4.74 Table 5 shows that oleyl alcohol increases both yield and productivity compared to a non‐integrated fermentation, unlike other tested extractants. As a consequence of this, oleyl alcohol is the most widely studied extractant, with Roffler et al.74 performing batch fermentations and fed‐batch fermentations,75 before moving to a scale down industrial fed‐batch system70 (the outcomes of these are shown in Table 5) and an economic assessment of a commercial process of a continuous ABE fermentation with an inline recovery unit using oleyl alcohol.76 The fed‐batch fermentations demonstrate an improvement over integrated batch fermentations, with substrate utilization and productivity increases over 100% being achieved.75 Roffler et al.74, 75 reported final organic phase butanol concentrations of 24–30 g butanol/L. This is a small concentration increase, especially compared to those seen in the condensate for the evaporative techniques. ABE separation from the oleyl alcohol should be less intensive than separation from water, as there is no water‐butanol/water‐ethanol azeotrope formations, but the low concentration will impact the energy requirement.
Table 5.
Free Cell ABE Fermentation Performance with in situ Product Recovery by Liquid‐Liqud Extraction in an STR
| Mode | Microorganism | Substrate (Concentration for ISPR) | % Substrate Increase for ISPR (vs. Control) | Productivity for ISPR (g ABE/Lh) | % Productivity Increase (vs. Control) | Yield for ISPR (g ABE/g Substrate) | % Yield Increase (vs. Control) | Extractant | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Batch | C. acetobutylicum ATCC824 | Glucose (97 g/L) | 18% | 0.69 | 19% | 0.17 | −6% | Kerosene | 74 |
| C. acetobutylicum ATCC824 | Glucose (78 g/L) | −5% | 0.53 | −9% | 0.17 | −6% | 50wt% Dodecanol in kerosene | 74 | |
| C. acetobutylicum ATCC824 | Glucose (89 g/L) | 9% | 0.43 | −26% | 0.16 | −11% | 30wt% Tetradecanol in kerosene | 74 | |
| C. acetobutylicum ATCC824 | Glucose (100 g/L) | 22% | 0.72 | 24% | 0.19 | 6% | Oleyl Alcohol | 74 | |
| C. acetobutylicum ATCC824 | Glucose (100g/L) | 22% | 0.71 | 22% | 0.17 | −6% | 50wt% Oleyl alcohol in decane fraction | 74 | |
| C. acetobutylicum ATCC824 | Glucose (100 g/L) | 22% | 0.74 | 28% | 0.18 | 0% | 50wt% Oleyl alcohol in benzyl benzoate | 74 | |
| Clostridium saccharoper‐butylacetonicum N1‐4 | Potato glucose (75 g/L) | 34% | 0.52 | 2% | 0.38 | 0% | Oleyl Alcohol | 87 | |
| C. saccharoper‐butylacetonicum N1‐4 | Potato glucose (74 g/L) | 32% | 0.55 | 8% | 0.4 | 5% | Methylated crude palm oil (CPOE) | 87 | |
| C. acetobutylicum BCRC10639 (ATCC824) | Glucose (unknown) | 0.27 | 28% | 0.21 | 15% | Biodiesel | 88 | ||
| C. acetobutylicum ATCC824 | Glucose (86 g/L) | 51% | 0.36 | 9% | 0.36 | −8% | Oleyl Alcohol | 86 | |
| C. acetobutylicum ATCC824 | Glucose (117 g/L) | 105% | 0.46 | 39% | 0.37 | −4% | Oleyl Alcohol with gas stripping | 86 | |
| Fed‐Batch | C. acetobutylicum ATCC824 | Glucose (155 g/L) | 91% | 0.9 | 55% | 0.24 | 33% | Oleyl Alcohol | 75 |
| C. acetobutylicum ATCC824 | Glucose (218 g/L) | 169% | 1.5 | 159% | 0.22 | 22% | Oleyl Alcohol | 75 | |
| C. acetobutylicum ATCC824 | Glucose (303 g/L) | 274% | 1.3 | 124% | 0.21 | 17% | Oleyl Alcohol | 75 | |
| C. acetobutylicum ATCC824 | Glucose (86 g/L) | 60% | 0.17 | −23% | 0.23 | −21% | PPG1200 | 83 | |
| C. acetobutylicum BCRC10639 (ATCC824) | Glucose (unknown) | 0.30 | 37% | 0.31 | 65% | Biodiesel | 88 | ||
| C. acetobutylicum P262 a | Whey Permeate (68.6 g/L) | 154% | 0.15 | 114% | 0.35 | 9% | Oleyl Alcohol | 25 | |
| C. acetobutylicum ATCC824 | Glucose (300 g/L) | 270% | 1 | 72% | 0.19 | 6% | Oleyl Alcohol | 70 |
C. acetobutylicum P262 has since been reclassified as C. saccharobutylicum P262.32
Other solvents have been tested with fermentations, such as decanol, dibutylphthalate, 2‐butyl‐1‐octanol, and poly (propylene glycol) 1200.77, 78, 79, 80, 81, 82, 83 Decanol has a high distribution coefficient for butanol, 6.2, but is toxic to the bacteria and dissolves into the fermentation broth.78, 79 Evans and Wang79 investigated a mixture of decanol‐oleyl alcohol as an extractant, where it was observed that a mixture containing 40% of decanol was detrimental to fermentation. On the other hand, Bankar et al.81 did perform a successful continuous fermentation with a 20% decanol, 80% oleyl alcohol mixed extractant with a two‐stage immobilized reactor. A maximum solvent productivity of 2.07 g/L.h was achieved in the second stage reactor at a dilution rate of 0.5 h−1.The downside of this was the final product concentration only reached 25.32 g ABE/L. Dibutylphthalate was used as an extractant by Wayman and Parekh77 but Roffler et al.74 ruled out its use in a fermentation due to the density being very similar to water making the removal of the extractant from the fermentation broth difficult. Barton and Daugulis83 screened 63 organic solvents and decided that poly (propylene glycol) 1200 (PPG) was the best extractant. This was largely due to the high partition coefficient and biocompatibility of the extractant. Unfortunately, PPG did not show the same promise in fed‐batch fermentations as a reduction in both productivity (−23%) and yield (−21%) was seen, Table 5. The authors have associated this with the extraction of acids and intermediates into the PPG as the glucose uptake rate had increased by 60% compared to the control, with only a 26% increase in solvent formation.83 Extraction of acids is not a desirable trait in the extractant, as the acids cannot be assimilated into the desired products. 2‐butyl‐1‐octanol was suggested as an extractant as a result of González‐Peñas et al.84 extractant screening method. It was selected as it was shown to be biocompatible, with an increase in yield over the control experiment. Surprisingly, González‐Peñas et al.84 found 2‐butyl‐1‐octanol to be a better extractant than oleyl alcohol which has been the go‐to extractant for ISPR. 2‐butyl‐1‐octanol produced a yield of 27.43 w/w% compared to 25.5 w/w% for oleyl alcohol. It also had a greater distribution coefficient (6.76) and selectivity (644) for butanol compared to oleyl alcohol (4.57, 295) 84.
With LLE, the achieved yields are very low (Table 5), especially compared with evaporative techniques like gas stripping and pervaporation (Tables 2 and 3). With evaporative techniques, the average yield is 0.35 g ABE/g substrate which is close to the theoretical yield. The average yield for LLE is 0.25 g ABE/g substrate, which is approximately 40% lower than evaporative techniques. This reduction in yield between techniques is likely to be related to the contact of extractant with fermentation broth. Either substrate or acids is being removed from the broth into the extractant or the extractant is having a toxic effect. While these extractants have been selected due to being biocompatible, the sustained contact over the duration of the fermentation could be having a negative impact. In contrast, there is a general improvement in productivity over the control fermentation; therefore, further work would be required to understand why the yield is lower than other techniques.
One of the proposed advantages of ISPR techniques is the increased product titers, reducing the downstream separation energy demand. Unfortunately, the product concentration in the organic phase is rarely stated; rather the total product quantity/concentration based on the fermenter volume is specified. Without this concentration, it is difficult to assess the extraction efficiency in the same manner as evaporative techniques, where the final condensate concentration is stated.
For an economically viable process, it is essential that the extractant is recyclable, meaning that the removal of ABE is straightforward. Unfortunately, removal of ABE from the extractant and regeneration of the extractant have not been discussed much in the literature. Roffler et al.76 developed a steam stripping or distillation system for the removal of ABE from oleyl alcohol as part of their economic assessment. This was successful as oleyl alcohol has a boiling point of 282–349°C, significantly higher than that of butanol. This technique has not been subjected to rigorous testing to understand the process and effects on the oleyl alcohol76. Vacuum distillation followed by flash separation has been suggested as an alternative method of separation, but this has not been proven experimentally.85 This is a very general investigation into the fermentation performance and energy requirements, not providing any indication whether flash separation of ABE from oleyl alcohol would be beneficial to the process. Lu and Li86 have suggested applying gas stripping to the extractant phase inside the fermenter. This regenerates the extractant while it is still in contact with the fermentation broth, removing the need for external distillation. The results of a bottle experiment have proven to be promising as the gas stripped extractant experiment had greater productivity and yield than a standard LLE experiment. The results are shown in Table 5. The ABE concentrations in the condensate from gas stripping stage ranged between 166 and 204 g ABE/L. This is a significant increase in product concentration compared to the butanol concentration in the extractant of 40 g butanol/L oleyl alcohol,86 although it is lower than the concentrations exhibited by the two‐stage gas process (500–700 g ABE/L34, 36). Lu and Li86 did not report any energy requirements for this system and Roffler et al.70 did not report the concentration of ABE after separation from the extractant, making a comparison with distillation or other ISPR techniques difficult.
To circumvent the re‐extraction step, Ishizaki et al.,87 Li et al.,73 and Yen and Wang88 have investigated the use of an extractant that would allow for direct use as a biofuel while in the extracted form. The use of biodiesel (methylated fatty acids, e.g., methylated crude palm oil) was shown to reduce the need for recovery from the extractant,73, 87, 88 as it produces an ABE‐enriched biodiesel, which significantly improves the quality of biodiesel with an increased cetane number and a reduced cold filter plugging point.73 The disadvantage with biodiesel is that it preferentially removes butyric acid from the fermentation, which is required by the bacteria to produce butanol.73 Yen and Wang88 suggest that the rate of butyric acid extraction is lower than the rate of assimilation by the bacteria; therefore, a negative impact was not seen, as confirmed by Table 5, the yield increased by 15% over the control fermentation without extraction by biodiesel. Ishizaki et al.87 demonstrated that the use of methylated crude palm oil is competitive in terms of fermentation characteristics compared to an extractive fermentation using oleyl alcohol, Table 5; with an 8% improvement in productivity and 5% yield improvements compared to 2% and 0% respective improvements with oleyl alcohol. The idea of using biodiesel as an extractant to create a superior biofuel is attractive and could form part of an ABE‐based biorefinery creating multiple products 89. If this were the case, the end use of the ABE produced needs to be considered when choosing an extractant.
Perstraction (membrane extraction)
Perstraction is a development from liquid‐liquid extraction experiments. It works on the same principles of mass transfer of ABE from the aqueous phase to an organic solvent, but the organic solvent and fermentation broth are separated by a membrane. The ABE transfers across the membrane into the organic phase. It is very similar to pervaporation, but has a liquid on the permeate side to provide the “driving force” rather than a gas or vacuum. If the key criteria for LLE, outlined in Table 4, are not achieved, then LLE is not possible with the ABE fermentation. A membrane separating the two process streams can in principle overcome these problems.90
The main technique has been extraction into oleyl alcohol across a silicone membrane, as this has favorable partition characteristics for butanol.25, 90, 91, 92, 93 Qureshi and Maddox92 demonstrated in a batch fermentation that perstraction could easily improve the substrate utilization by 694%, productivity by 163%, and yield by 33%, Table 6. However Grobben et al.93 investigated the use of fatty acid methyl esters from sunflower oil, which would allow for the direct use of the extractant and biobutanol as a biofuel (this is similar to the work by Li et al.73 and Ishizaki et al.87). The use of fatty acid methyl esters did not match the performance by oleyl alcohol with a 40% decrease in productivity compared to the control fermentation and only a 16% increase in substrate utilization, Table 6. This could be due to the lower distribution coefficient for ABE in the fatty acid methyl esters (0.45, 1.1, and 0.05, respectively) compared to oleyl alcohol creating a lower driving force across the membrane. Jeon and Lee90 investigated two other solvents for the use of perstraction; polypropylene glycol and tributyrin. Table 6 shows that while the alternative solvents show improvement over the non‐integrated fermentation, productivity increases of 68% for polypropylene glycol and 42% for tributyrin, oleyl alcohol remains the best extractant for the recovery of ABE. When choosing possible extractants for perstraction, it seems that the same criteria for selecting an extractant for LLE were used in case of any back‐extraction of the solvent into the fermentation broth.25
Table 6.
Free Cell ABE Fermentation Performance with in situ Product Recovery by Perstraction in an STR
| Mode | Microorganism | Substrate (Concentration for ISPR) | % Substrate Increase for ISPR (vs. Control) | Productivity for ISPR (g ABE/Lh) | % Productivity Increase (vs. Control) | Yield for ISPR (g ABE/g Substrate) | % Yield Increase (vs. Control) | Membrane | Extractant | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Batch | C. acetobutylicum P262a | Lactose/Whey Permeate (227 g/L) | 694% | 0.21 | 163% | 0.44 | 33% | Silicone tubing | Oleyl Alcohol | 92 |
| C. saccharoperbutyl‐acetonicum N1‐4 | Potato Glucose (89 g/L) | 50% | 0.32 | −16% | 0.21 | −23% | PTFE | Oleyl Alcohol | 95 | |
| C. saccharoperbutyl‐acetonicum N1‐4 | Potato Glucose (86 g/L) | 45% | 0.39 | 3% | 0.23 | −13% | PTFE | 1‐Dodecanol | 95 | |
| Fed‐Batch | C. acetobutylicum ATCC824 | Corn Mash/Glucose (601 g/L) | 902% | 1.02 | 113% | 0.36 | 23% | Silicone tubing | Oleyl Alcohol | 90 |
| C. acetobutylicum ATCC824 | Corn Mash/Glucose (422 g/L) | 603% | 0.81 | 69% | 0.35 | 21% | Silicone tubing | Polypropylene glycol | 90 | |
| C. acetobutylicum ATCC824 | Corn Mash/Glucose (155 g/L) | 158% | 0.68 | 42% | 0.32 | 9% | Silicone tubing | Tributyrin | 90 | |
| C. acetobutylicum DSM1731 (ATCC8529) | Potato powder (92 g/L) | 26% | 1.00 | 59% | 0.35 | 35% | Poly‐propylene | Oleyl Alcohol/Decanol | 93 | |
| C. acetobutylicum DSM1731 (ATCC8529) | Potato powder (85 g/L) | 16% | 0.38 | −40% | 0.32 | 23% | Polypropylene | Fatty acid methyl esters (MFA) | 93 | |
| C. acetobutylicum P262 a | Whey Permeate (123 g/L) | 355% | 0.24 | 243% | 0.37 | 16% | Silicone tubing | Oleyl Alcohol | 25 | |
| Contin‐uous | C. acetobutylicum ATCC824 | Corn Mash/Glucose (2134 g/L) b | unknown | 2.27 | 305% | 0.33 | 2% | Silicone tubing | Oleyl Alcohol | 91 |
C. acetobutylicum P262 has since been reclassified as C. saccharobutylicum P262.32
Dilution rate was 0.2 h−1.
There have been two examples, Shukla et al.94 and Tanaka et al.,95 where toxic solvents have been used in a perstraction system. Shukla et al.94 chose 2‐ethyl‐1‐hexanol as an extractant. 2‐ethyl‐1‐hexanol is known to be toxic to bacteria, but was considered less toxic than 1‐octanol, and therefore would be an acceptable extractant.96 The results of the toxicity tests of 1‐octanol or 2‐ethyl‐1‐hexanol were not published, so the degree of toxicity under the conditions described is unknown. Shukla et al.94 used a hollow fiber polypropylene membrane and did not report any ill effects from the use of this extractant; although an immobilized C. acetobutylicum was used for the fermentation, this could reduce the chances of the bacteria coming into contact with the solvent at the membrane interface. The only acknowledgement of using a toxic extractant was by Tanaka et al.,95 who chose 1‐dodecanol as an extractant. It was selected based on a high partition coefficient of 5.14, but it is unknown why 1‐dodecanol was chosen over other high‐distribution, toxic extractants such as 1‐octanol with a distribution coefficient of 5.6–7.33.97 The same levels of bacterial growth were seen when using 1‐dodecanol and oleyl alcohol as an extractant for perstraction. While the same levels of growth were seen and there was a slight increase in productivity (3%), there was a 13% decrease in yield compared to the control fermentation. The authors have not commented on this, as the maximum butanol productivity for both oleyl alcohol and 1‐dodecanol, 0.979 g/L.h was 1.25 times higher than the maximum butanol productivity for the control, 0.817 g/L.h.
One of the problems of LLE was the trade‐off between having a high partition coefficient and being non‐toxic to the bacteria. It was thought that the use of a membrane to aid the extractive process would allow for the use of extractants with higher partition coefficients. As seen in Table 6 from the description above most researchers have “played it safe” using extractants known to be non‐toxic to the bacteria. The earlier research appeared to indicate that some extractant is leaching across the membrane into the aqueous phase.98 Groot et al.98 believed that this was related to sorption of the solvent to the membrane. Some tests they performed using hexanol and silicone exhibited toxic effects to the fermentation, although data confirming this is not shown.98 Jeon and Lee90 stated that tributyrin had a partial inhibitory effect over time; therefore the fermentation with perstraction could not be run for as long, only 84 h consuming 154 g/L glucose compared to the oleyl alcohol based fermentation which operated for 209 h consuming 601 g/L glucose, resulting in the conclusion that non‐toxic solvents had to be used for perstraction. If this is true, one of the motivations for research into perstraction has been incorrect meaning there might be very little advantage of using perstraction rather than LLE. In contrast, Qureshi and Maddox92 suspected that back diffusion was a possible reason for the fermentation stopping, but later disregarded it, as it was shown that the fermentation stopped due to nutrient depletion. Combining this with the successful fermentations performed by Shukla et al.94 and Tanaka et al.95 using toxic extractants, there is no conclusive evidence that higher partition coefficient extractants cannot be used.
The majority of research has used silicone tubing as the membrane, Table 6,25, 90, 91, 92, 98, 99 because it is widely available and has acceptable mass transfer characteristics. It is also possible that it was chosen because it is readily available in the laboratory and easy to configure into an appropriate system.25, 90, 91, 92, 98 Jeon and Lee90 chose it as it has high permeability for butanol and acetone, can be autoclaved, has high mechanical strength, is easy to handle, biologically inert, compatible with many organic solvents, and had been used for pervaporation with the ABE fermentation.90 Qureshi and Maddox92 stated similar reasons for using a silicone membrane, including that silicone had been proven not to foul and there was no dead space for bacterial growth on the tubing. There has been very little comparison in terms of other membrane options. Only Groot et al.98 have compared possible membrane options, which were silicone, neoprene, and latex. Based on the mass transfer coefficient, silicone had the highest coefficient for all extractants tested compared to neoprene and latex. For hexanol, the corresponding mass transfer coefficient was 5.2 × 10−7 m/s for silicone, 0.4 × 10−7 m/s for neoprene, and 0.3 × 10−7 m/s for latex; meaning that the membrane choice will have a significant impact on the mass transfer in the system. No comparisons of polypropylene hollow fiber membranes and silicone have been performed. Grobben et al.93 have used an alternative polypropylene membrane. Polypropylene membranes appear comparable to silicone tubing (Table 6), but different bacterial strains and substrates have been used for the fermentation. Shukla et al.94 used a Celgard X20, a hydrophobic microporous hollow fiber membrane. It is suspected that this is also a polypropylene membrane which was commercially available at the time of research. Tanaka et al.95 chose a polytetrafluorothylene (PTFE) membrane as it is more hydrophobic than other membranes that have been used, therefore it should be more selective for ABE. It is evident that membranes for perstraction need to be optimized; as membrane development continues and become more commercially available, it is likely that more sophisticated industrially applicable membranes will become available.92
Comparing the fermentations in Table 6 with oleyl alcohol LLE fermentations in Table 5, not much difference can be seen in fermentation performance. Perstraction appears to enable higher fermentation productivities. Perstraction does show a greater, more consistent increase in yield, between the ISPR and control fermentation compared to the LLE fermentations; achieving an average yield of 0.33 g/g for integrated fermentations. This could be because the membrane reduces transfer of key nutrients and intermediates into the extractant phase. Similar to LLE, the recovery of ABE from the extractant is not considered, nor is the product concentration in the organic phase consistently reported. Qureshi and Maddox92 reported that the butanol concentration never exceeded 10 g butanol/L oleyl alcohol, although the extractant was replaced with fresh extractant five times during the fermentation. The extractant was replaced to limit the product build up in the fermenter, but this concentration is lower than that reported for LLE at 40 g butanol/L oleyl alcohol.86 Unless the extractant concentration can be increased or optimized for better fermentation performance, this lower extractant concentration is likely to increase the energy for distillation. In this scenario, the use of perstraction with non‐toxic extractants will have to be suitably justified to be applied to the ABE fermentation.
Adsorption
Adsorption is the binding of a compound onto the surface of a solid adsorbent or resin. It is the oldest technique investigated for the use of ISPR from ABE fermentations. In 1948, Weizmann et al.100 first investigated butanol adsorption to relieve product inhibition and reduce the energy demand due to distillation.
A wide range of adsorbents have been used in conjunction with the butanol and the ABE fermentation, and this list is continually evolving as new, more complex adsorbents become available. Some of the adsorbents used are activated carbon,100, 101, 102, 103 silicalite or silicalite‐based zeolites,23, 102, 104, 105 and polymeric resins.23, 101, 103, 106, 107 The initial conclusion from Qureshi et al.108 was that silicalite adsorbents improved the fermentation the most as they have the ability to concentrate fermentation broth from 5 g butanol/L to 810 g butanol/L, but more recent work exhibits a tendency toward polymeric resins.106, 109, 110, 111 Over time, the adsorbents used have become increasingly complex with Cousin Saint Remi et al.102 recommending ZIF‐8, a metal organic framework adsorbent from Sigma‐Aldrich as a superior adsorbent to silicalite. The most recent work published on adsorption has moved back to the use of commercially available resins, along with selecting an activated carbon resin Norit ROW 0.8 to be combined with the fermentation broth rather than a polymeric resin such as Dowex Optipore L‐493 and SD‐2.103
The majority of the adsorbents have not been tested while coupled to an ABE fermentation, rather using a model ABE solution instead. Thus, there is a small amount of fermentation data to compare in Table 7. Yang et al.112 are one of the few who have investigated an adsorbent in conjunction with fermentation. They demonstrated that the addition of 30% resin to a fermentation can achieve 130% increase in productivity of the fermentation. When this was adapted to a fed‐batch fermentation with external column, the productivity increased by 233% for a single cycle adsorption, but 323% with multiple adsorption cycles. In the past two years, more research has focused on combining adsorption with the fermentation. Liu et al.113 combined the adsorption using KA‐I resin with an immobilized biofilm reactor. A membrane was used to ensure no biomass came into contact with the adsorbent. Two adsorption modes were investigated, selective for butanol and co‐adsorption of acetone, both these methods observed a reduction in productivity and yield by 37% and 9% for the butanol selective adsorption and 9% and 7% when acetone was co‐adsorbed. Lee et al.114 added the adsorbent directly to the fermenter. Fouling was not observed, but the authors commented on the potential physical interaction between the biomass and adsorbent being detrimental to the fermentation. The reasons suggested for this appear to be tenuous, but the mode of adsorption should be considered to minimize impact on the bacteria. This work demonstrated that in batch fermentations, using a modified C. acetobutylicum ATCC 824, the product concentration could be increased due to the adsorption of products, reducing toxicity. The final broth concentration reached 10 g butanol/L; this is the same as the fermentation with no ISPR, and the same yield was observed in both fermentations.114 Follow‐up work by Lee et al.115 using an ex situ adsorption column, combined with a fed‐batch fermentation using C. beijerinckii NCIMB 8052, saw no detrimental effects to the fermentation. The integrated and non‐integrated fermentation both had the same yield of 0.31, but the integrated fermentation had an increased total product concentration of 26 g ABE/L compared to 18.5 g ABE/L. No productivity was supplied for these fermentations.115 Wiehn et al.116 proposed an expanded bed adsorption process. This allows the fermentation broth to pass through the adsorbent without the need for microbial separation, due to the increased voidage space in the bed. A reduced biomass concentration was observed, compared to the control fermentation, but the overall fermentation metrics appeared positive with increases in both yield and productivity by 14% and 65%, respectively, Table 7. A limited degree of fouling was observed in the 72 h experiment, this could be a bigger issue in longer fermentations.116 Xue et al.103 used an activated carbon resin in both free cell STR fermentations and an immobilized bioreactor. The results of the free cell fermentation are shown in Table 7 with a productivity decrease of 3% and no change in the yield. The immobilized cell fermentation had a productivity increase of 22% and the same yield as the control fermentation. The batch fermentation only used a single cycle adsorbent, whereas the immobilized fermentation had three adsorbent cycles enabling greater product removal, hence a higher productivity. The immobilized fermentation eventually ceased due to a build‐up of acetone to 18 g/L in the fermentation broth, inhibiting the bacteria.103
Table 7.
Free Cell ABE Fermentation Performance with in situ Product Recovery by Adsorption in an STR
| Mode | Microorganism | Substrate (Concentration for ISPR) | % Substrate Increase for ISPR (vs. Control) | Productivity for ISPR (g/Lh) | % Productivity Increase (vs. Control) | Yield for ISPR (g/g) | % Yield Increase (vs. Control) | Adsorption Mode | Adsorbent | g adsorbent/mL liquor | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch | C. acetobutylicum ATCC824 | Glucose (92 g/L) | 22–47% | 0.53 | 33% | 0.32 | 3% | Batch (in situ) | Polyvinyl‐pyridine (PVP) resin | 5% | 112 |
| 0.63 | 58% | 0.32 | 2% | 10% | |||||||
| 0.74 | 85% | 0.31 | 1% | 20% | |||||||
| 0.92 | 130% | 0.32 | 3% | 30% | |||||||
| Fed‐Batch | C. acetobutylicum ATCC824 | Glucose (190 g/L) | 334% | 1.33 | 233% | 0.32 | 2% | Single cycle (ex situ column) | Polyvinyl‐pyridine (PVP) resin | n/a | 117 |
| C. acetobutylicum ATCC824 | Glucose (1199 g/L) | 2636% | 1.69 | 323% | 0.32 | 4% | Cyclic (ex situ column) | Polyvinyl‐pyridine (PVP) resin | n/a | 117 | |
| C. acetobutylicum ATCC824 | Glucose (180 g/L) | 67% | 0.72 | 14% | 0.28 | 65% | Expanded Bed Adsorption (ex situ) | Poly (styrene‐co‐divinylbenzene) (Dowex Optipore L493) | n/a | 116 | |
| C. acetobutylicum JB200 | Glucose (158 g/L) | 267% | 0.34 a | −3% | 0.22 a | 0% | Single cycle (ex situ column) | Activated Carbon (Norit ROW 0.8) | n/a | 103 |
Productivity of butanol production and butanol yield, rather than for total products.
Weizmann et al.,100 in agreement with Yang et al.112 and Lin et al.109 observed that the adsorption is competitive. Butanol is adsorbed in preference to acetone, for example. The order of preference for adsorption was ethanol as the weakest, followed by acetone, then butanol as the strongest.109 Yang et al.112 found that the order was ethanol, acetone, acetic acid, butanol, then butyric acid. This order is undesirable, as the butyric acid displaces the butanol, the desired product to be removed, and hinders the conversion of the butyric acid to butanol. Additionally, Xue et al.103 experienced the increased concentration of other products in the broth causing the fermentation to stop. This also raises questions as to whether any key nutrients are adsorbed during the process, which would be highly undesirable.
A downside of adsorption is that it is inherently a batch process, as the ABE has to bind to the adsorbent and then, once it has reached capacity, desorption has to occur. In many experiments, batch adsorption was performed,101, 105, 107, 112 meaning that once the adsorbent has reached capacity it can no longer relieve product inhibition. This indicates that the ratio of adsorbent to broth needs to be optimized, as the productivity varies with the quantity of adsorbent, Table 7. The alternative is operating a minimum of two external packed bed columns in a cyclic manner, allowing for one column to be adsorbing, while the other is desorbing.23, 108, 110, 117 Operation in this cyclic manner with a fed‐batch fermentation yielded a favorable fermentation productivity, Table 7.103, 117 This operating mode would reduce the adsorbent inventory required per fermentation, although the product removal would have to occur externally from the bioreactor. Ideally, the development of a continuous adsorption process (e.g., simulated moving bed adsorption) would be best suited, to allow the simplest removal of ABE and regeneration of adsorbent.
Once the adsorbent has reached its capacity the ABE needs to be removed and the adsorbent regenerated. The main methods of adsorption are through increasing the temperature,102, 103 displacement with steam114or another solvent, e.g., methanol,109, 112 or through vacuum evaporation.106, 116 The recovered butanol titers ranged from 43 to 167 g/L.103, 114 If the higher concentrations are consistently achievable, then the desorbed titers are similar to that achieved by LLE86, but still lower than the concentrations achieved in gas stripping.36
Comparison of Techniques
Currently, no review has compared all possible ISPR techniques for ABE fermentations. One challenge is that it is difficult to make accurate comparisons between work performed by different groups, as different methods and procedures have been followed. This includes the use of different strains, media, reactor configurations, and mode of operation. To make a valid comparison between the different ISPR techniques, they have principally been compared in terms of their fermentation performance in STRs (see Tables 1−3 and 5−7). The downside of this approach is that not all techniques have been coupled to an ABE fermentation in an STR. Furthermore, there is significant variability in the data extracted within each ISPR technique.
From batch fermentations, it can be observed that every technique tested (gas stripping, vacuum fermentations, pervaporation, liquid–liquid extraction, perstraction, and adsorption) can have a positive effect on the fermentation. This is due to the removal of the butanol inhibition allowing for complete utilization of the substrate and the possibility of prolonged fermentations. This is seen by the significant increase in substrate utilization in Tables 1−3 and 5−7. Only in two batch fermentations was an increase in substrate not observed, Tables 3 and 5. In the case of pervaporation, no additional substrate was fed to the reactor and both the control and integrated fermentation consumed all the substrate supplied.58 For LLE, a decrease in substrate utilization was observed with a 50 wt% dodecanol in kerosene extractant; there was also a decrease in productivity and yield which could be related to toxicity of dodecanol to the bacteria.74, 95 The most prominent ISPR techniques for batch fermentations are gas stripping (see Table 1) and pervaporation (Table 3). It is difficult to compare different adsorption processes, as a major factor in the improvement in productivity is the quantity of adsorbent added to the broth which is not always reported, Table 7.
Where overcoming product inhibition has been successfully demonstrated for any technique, the next step is to perform a fed‐batch fermentation which increases substrate loading and fermentation time giving higher productivity. Increases of substrate between 100 and 900% compared to the control fermentation are common, Tables 1, 3, and 5−7. Fed batch data is only reported for: gas stripping, pervaporation, liquid–liquid extraction, perstraction, and adsorption. Adsorption, Table 7, enables the greatest improvement in productivity compared to standard batch fermentations, though the mechanism of adsorption contact has changed. This is closely followed by gas stripping, but the data in Table 1 has a degree of unreliability, as the productivity was calculated including estimated solvent losses.5 In all cases, a condenser was not sufficient to capture all the solvents produced, so the true productivity of the fermentation is unknown. This is a common problem with evaporative techniques due to the highly volatile nature of acetone and the high dilution of the vapors. Liquid–liquid extraction, however, appears to perform well with relatively repeatable increases in productivity and yield, Table 5.75
In literature, there have been some discrepancies between what is considered a fed‐batch and continuous process. There have been several occurrences where a fed‐batch fermentation has been called continuous in literature.52, 58 The continuous process described by Shin et al.52 and Li et al.58 is the same as the fed‐batch process described by Wu et al.59 and Qureshi and Blaschek.68 It must be recognized that for ISPR, typical process definitions no longer match the process. With a fed‐batch ISPR process, there is a feed in but there is also a product stream out. The product stream is not representative of the fermenter, in the same way it would be for a traditional continuous process, and the fermenter cannot be considered steady state as the concentrations (particularly biomass) change. Although often a concentrated feed is used to maintain a constant fermentation volume.
Continuous fermentations can be performed, but the continuous removal of fermentation broth leads to a lower increase in yield than for fed‐batch fermentations (Table 6). In spite of this reduced yield, greater consistency in improved productivity is seen. The reduction in yield for continuous fermentations is due to substrate removal via the outlet stream being accounted for as substrate consumption. This can have a significant effect on the process economics, as the substrate has the largest contribution to the cost of production.1 The dilution rate of a continuous fermentation controls the growth rate of the bacteria. From the limited data comparing continuous free cell fermentations, the dilution rate does not appear to affect the ISPR. Only gas stripping, pervaporation, and perstraction have been combined with continuous fermentation, with a greater focus on the application of fed‐batch fermentations. Interestingly, perstraction, Table 6, shows the greatest improvement in productivity for continuous fermentations closely followed by gas stripping, Table 1.
In recent years, there has been a growing trend to immobilized fermentations. A good example of this is Xue et al.18, 33, 36, 103 who have combined immobilized bioreactors with gas‐stripping and adsorption. Immobilized reactors have also been considered for combination with pervaporation and LLE.80, 118, 119 Immobilized reactors are not yet a realized commercial technology for the ABE fermentation,39 but in combination with an ISPR could allow for enhanced separation conditions, e.g., high temperatures,19 without being detrimental to the bacteria. Experimental comparisons of immobilized fermentations with ISPR need to be investigated to understand if immobilized fermentations should have an increased focus compared to free cell fermentations.
One of the factors driving the application of ISPR to the ABE fermentation is the potential energy reduction, due to increased ABE concentration going to downstream processing. The energy associated with separation is generally not considered alongside the experimental results. Xue et al.120 suggest that energy reductions will not be observed if the ISPR technique cannot concentrate the ABE to more than 40 wt%. This would allow removal of the beer stripper from product purification, which has the largest energy demand, concentrating the fermentation broth from 2 wt% ABE.120 None of the single‐single stage techniques have suggested they can concentrate the product to this concentration. The hybrid, two‐stage separation processes based upon an initial gas stripping stage are the only techniques to create an ABE solution greater than 40 wt%, with concentrations over 650 g ABE/L possible.34 To date, hybrid systems have only considered two‐stage gas stripping,34 combined gas‐stripping and pervaporation,18 and extractive gas stripping.86 There is the potential for many more hybrid or two‐stage separation systems to be designed. The economics of two‐stage systems will need to be considered and compared to a traditional batch and single‐stage ISPR process. The application of a single stage ISPR and beer stripper is effectively a two‐stage process; therefore the cost of implementations and operation could be a deciding factor for commercial implementation.
The review has compared six ISPR techniques based on available experimental data focusing on free cell fermentations. To mitigate the effects of various experimental methods, the % increase of substrate utilized, productivity, and yield was considered. The experimental data has successfully demonstrated that ISPR has a positive impact on the fermentation. The generation of models to represent the fermentation with integrated ISPR could help speed up developments in ISPR. They can help focus developments to the techniques which would provide the greatest improvements to the process. Experimental work can then be completed to validate the model results and confirm there are no biocompatibility issues. This will reduce the time and expense of testing every ISPR possibility experimentally, and provide a comparable baseline. The comparison of different experimental studies can also be improved through standardization of published experimental results for ISPR processes. This can be done by ensuring that enough data is provided to enable a mass balance of the process to be calculated, hence enabling an easier comparison; see supplementary material for a suggestive list of data to be included.
Conclusion
From the comparison of STR fermentations, it is possible to say that all techniques exhibit improvements in fermentation productivity and that for different operating modes different techniques appear to be superior. For batch fermentations, gas stripping, and pervaporation were favorable, for fed‐batch: adsorption and gas stripping, and continuous perstraction has the greatest improvement. The use of novel two‐stage or hybrid techniques also needs to be considered, particularly their compatibility with free cell STR fermentations. This means that the decision on which technique to apply will be based on additional data such as energy consumption and an economic analysis. These should be considered alongside the fermentation data, and this would help to categorically state which is the best ISPR technique. Future work should include process optimization as part of trying new feedstocks, improved strains, or separating agents (e.g., membranes, adsorbents, and extractants).
Acknowledgements
The author is sponsored by Green Biologics Ltd. and funded by the EPSRC (EP/G037620/1).
Literature Cited
- 1. Jones DT, Woods DR. Acetone‐butanol fermentation revisited. Microbiol Rev. 1986;50:484–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durre P. Fermentative butanol production: bulk chemical and biofuel. Ann N. Y Acad Sci. 2008;1125:353–362. [DOI] [PubMed] [Google Scholar]
- 3. Ni Y, Sun Z. Recent progress on industrial fermentative production of acetone–butanol–ethanol by Clostridium acetobutylicum in China. Appl Microbiol Biotechnol. 2009;83:415–423. [DOI] [PubMed] [Google Scholar]
- 4. Durre P. Fermentative production of butanol–the academic perspective. Curr Opin Biotechnol. 2011;22:331–336. [DOI] [PubMed] [Google Scholar]
- 5. Ezeji TC, Qureshi N, Blaschek HP. Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed‐batch technique and product inhibition by gas stripping. Appl Microbiol Biotechnol. 2004;63:653–658. [DOI] [PubMed] [Google Scholar]
- 6. Mariano A, Filho R. Improvements in biobutanol fermentation and their impacts on distillation energy consumption and wastewater generation. Bioenerg Res. 2012;5:504–514. [Google Scholar]
- 7. Reiße S, Haack M, Garbe D, Sommer B, Steffler F, Carsten J, Bohnen F, Sieber V, Brück T. In vitro bioconversion of pyruvate to n‐butanol with minimized cofactor utilization. Front Bioeng Biotechnol. 2016;4:74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staggs KW, Nielsen DR. Improving n‐butanol production in batch and semi‐continuous processes through integrated product recovery. Process Biochem. 2015;50:1487–1498. [Google Scholar]
- 9. Freeman A, Woodley JM, Lilly MD. In‐situ product removal as a tool for bioprocessing. Nat Biotechnol. 1993;11:1007–1012. [DOI] [PubMed] [Google Scholar]
- 10. Roffler SR, Blanch HW, Wilke CR. In situ recovery of fermentation products. Trends Biotechnol. 1984;2:129–136. [Google Scholar]
- 11. Van Hecke W, Kaur G, De Wever H. Advances in in‐situ product recovery (ISPR) in whole cell biotechnology during the last decade. Biotechnol Adv. 2014;32:1245–1255. [DOI] [PubMed] [Google Scholar]
- 12. Abdehagh N, Tezel FH, Thibault J. Separation techniques in butanol production: challenges and developments. Biomass Bioenergy. 2014; 60:222–246. [Google Scholar]
- 13. Huang HJ, Ramaswamy S, Liu Y. Separation and purification of biobutanol during bioconversion of biomass. Sep Purif Technol. 2014;132:513–540. [Google Scholar]
- 14. Xue C, Zhao JB, Chen LJ, Bai FW, Yang ST, Sun JX. Integrated butanol recovery for an advanced biofuel: current state and prospects. Appl Microbiol Biotechnol. 2014;98:1–12. [DOI] [PubMed] [Google Scholar]
- 15. Qureshi N, Blaschek HP. Recovery of butanol from fermentation broth by gas stripping. Renew Energy. 2001;22:557–564. [Google Scholar]
- 16. de Vrije T, Budde M, van der Wal H, Claassen PAM, López‐Contreras AM. “In situ” removal of isopropanol, butanol and ethanol from fermentation broth by gas stripping. Bioresour Technol. 2013; 137:153–159. [DOI] [PubMed] [Google Scholar]
- 17. Ennis BM, Marshall CT, Maddox IS, Paterson AHJ. Continuous product recovery by in‐situ gas stripping/condensation during solvent production from whey permeate using Clostridium acetobutylicum . Biotechnol Lett. 1986;8:725–730. [Google Scholar]
- 18. Xue C, Liu F, Xu M, Zhao J, Chen L, Ren J, Bai F, Yang ST. A novel in situ gas stripping‐pervaporation process integrated with acetone‐butanol‐ethanol fermentation for hyper n‐butanol production. Biotechnol Bioeng. 2016;113:120–129. [DOI] [PubMed] [Google Scholar]
- 19. Chen Y, Ren H, Liu D, Zhao T, Shi X, Cheng H, Zhao N, Li Z, Li B, Niu H, Zhuang W, Xie J, Chen X, Wu J, Ying H. Enhancement of n‐butanol production by in situ butanol removal using permeating–heating–gas stripping in acetone–butanol–ethanol fermentation. Bioresour Technol. 2014;164:276–284. [DOI] [PubMed] [Google Scholar]
- 20. Chen HZ, Liu ZH, Dai SH. A novel solid state fermentation coupled with gas stripping enhancing the sweet sorghum stalk conversion performance for bioethanol. Biotechnol for Biofuels 2014;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu C, Zhao J, Yang ST, Wei D. Fed‐batch fermentation for n‐butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour Technol. 2012;104:380–387. [DOI] [PubMed] [Google Scholar]
- 22. Ezeji TC, Qureshi N, Blaschek HP. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J Microbiol Biotechnol. 2003;19:595–603. [Google Scholar]
- 23. Ennis BM, Qureshi N, Maddox IS. In‐line toxic product removal during solvent production by continuous fermentation using immobilized Clostridium acetobutylicum . Enzyme Microbial Technol. 1987;9:672–675. [Google Scholar]
- 24. Groot WJ, Lans RGJM, Luyben KCAM. Batch and continuous butanol fermentations with free cells: integration with product recovery by gas‐stripping. Appl Microbiol Biotechnol. 1989;32:305–308. [Google Scholar]
- 25. Qureshi N, Maddox IS, Friedl A. Application of continuous substrate feeding to the ABE fermentation: relief of product inhibition using extraction, perstraction, stripping, and pervaporation. Biotechnol Prog. 1992; 8:382–390. [Google Scholar]
- 26. Qureshi NQ, Blaschek HB. Evaluation of recent advances in butanol fermentation, upstream, and downstream processing. Bioprocess Biosystems Eng. 2001;24:219–226. [Google Scholar]
- 27. Ezeji T, Qureshi N, Blaschek H. Microbial production of a biofuel (acetone–butanol–ethanol) in a continuous bioreactor: impact of bleed and simultaneous product removal. Bioprocess Biosystems Eng. 2013; 36:109–116. [DOI] [PubMed] [Google Scholar]
- 28. Ezeji TC, Karcher PM, Qureshi N, Blaschek HP. Improving performance of a gas stripping‐based recovery system to remove butanol from Clostridium beijerinckii fermentation. Bioprocess Biosystems Eng. 2005;27:207–214. [DOI] [PubMed] [Google Scholar]
- 29. Ezeji T, Qureshi N, Blaschek H. Production of acetone butanol (AB) from liquefied corn starch, a commercial substrate, using Clostridium beijerinckii coupled with product recovery by gas stripping. J Ind Microbiol Biotechnol. 2007;34:771–777. [DOI] [PubMed] [Google Scholar]
- 30. Maddox IS, Qureshi N, Roberts‐Thomson K. Production of acetone‐butanol‐ethanol from concentrated substrate using Clostridium acetobutylicum in an integrated fermentation‐product removal process. Process Biochem. 1995;30:209–215. [Google Scholar]
- 31. Lu C, Dong J, Yang ST. Butanol production from wood pulping hydrolysate in an integrated fermentation–gas stripping process. Bioresour Technol. 2013; 143:467–475. [DOI] [PubMed] [Google Scholar]
- 32. Keis S, Shaheen R, Jones DT. Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol. 2001;51:2095–2103. [DOI] [PubMed] [Google Scholar]
- 33. Xue C, Zhao J, Lu C, Yang ST, Bai F, Tang IC. High‐titer n‐butanol production by Clostridium acetobutylicum JB200 in fed‐batch fermentation with intermittent gas stripping. Biotechnol Bioeng. 2012;109:2746–2756. [DOI] [PubMed] [Google Scholar]
- 34. Xue C, Du GQ, Sun JX, Chen LJ, Gao SS, Yu ML, Yang ST, Bai FW. Characterization of gas stripping and its integration with acetone–butanol–ethanol fermentation for high‐efficient butanol production and recovery. Biochem Eng J. 2014;83:55–61. [Google Scholar]
- 35. Setlhaku M, Heitmann S, Górak A, Wichmann R. Investigation of gas stripping and pervaporation for improved feasibility of two‐stage butanol production process. Bioresour Technol. 2013;136:102–108. [DOI] [PubMed] [Google Scholar]
- 36. Xue C, Zhao J, Liu F, Lu C, Yang ST, Bai FW. Two‐stage in situ gas stripping for enhanced butanol fermentation and energy‐saving product recovery. Bioresour Technol. 2013;135:396–402. [DOI] [PubMed] [Google Scholar]
- 37. Oudshoorn A, van der Wielen LAM, Straathof AJJ. Assessment of options for selective 1‐butanol recovery from aqueous solution. Ind Eng Chem Res. 2009;48:7325–7336. [Google Scholar]
- 38. Liu J, Fan LT, Seib P, Friedler F, Bertok B. Downstream process synthesis for biochemical production of butanol, ethanol, and acetone from grains: generation of optimal and near‐optimal flowsheets with conventional operating units. Biotechnol Prog. 2004;20:1518–1527. [DOI] [PubMed] [Google Scholar]
- 39. van der Merwe AB, Cheng H, Görgens JF, Knoetze JH. Comparison of energy efficiency and economics of process designs for biobutanol production from sugarcane molasses. Fuel 2013;105:451–458. [Google Scholar]
- 40. Mariano AP, Qureshi N, Filho RM, Ezeji TC. Bioproduction of butanol in bioreactors: new insights from simultaneous in situ butanol recovery to eliminate product toxicity. Biotechnol Bioeng. 2011;108:1757–1765. [DOI] [PubMed] [Google Scholar]
- 41. Mariano AP, Qureshi N, Maciel Filho R, Ezeji TC. Assessment of in situ butanol recovery by vacuum during acetone butanol ethanol (ABE) fermentation. J Chem Technol Biotechnol. 2012;87:334–340. [Google Scholar]
- 42. Mariano AP, Filho RM, Ezeji TC. Energy requirements during butanol production and in situ recovery by cyclic vacuum. Renew Energy 2012;47:183–187. [Google Scholar]
- 43. Qureshi N, Singh V, Liu S, Ezeji TC, Saha BC, Cotta MA. Process integration for simultaneous saccharification, fermentation, and recovery (SSFR): production of butanol from corn stover using Clostridium beijerinckii P260. Bioresour Technol. 2014;154:222–228. [DOI] [PubMed] [Google Scholar]
- 44. Mariano AP, Keshtkar MJ, Atala DIP, Maugeri Filho F, Wolf Maciel MR, Maciel Filho R, Stuart P. Energy requirements for butanol recovery using the flash fermentation technology. Energy Fuels 2011;25:2347–2355. [Google Scholar]
- 45. Groot WJ, Qever CE, Kossen NWF. Pervaporation for simultaneous product recovery in the butanol/isopropanol batch fermentation. Biotechnol Lett. 1984;6:709–714. [Google Scholar]
- 46. Larrayoz MA, Puigjaner L. Study of butanol extraction through pervaporation in acetobutylic fermentation. Biotechnol Bioeng 1987;30:692–696. [DOI] [PubMed] [Google Scholar]
- 47. Cho CW, Hwang ST. Continuous membrane fermentor separator for ethanol fermentation. J. Membrane Sci. 1991;57:21–42. [Google Scholar]
- 48. Liu G, Wei W, Jin W. Pervaporation membranes for biobutanol production. ACS Sustainable Chem Eng. 2013;2:546–560. [Google Scholar]
- 49. Groot WJ, Schoutens GH, Beelen PN, Oever CE, Kossen NWF. Increase of substrate conversion by pervaporation in the continuous butanol fermentation. Biotechnol Lett. 1984; 6:789–792. [Google Scholar]
- 50. Qureshi N, Maddox IS. Application of novel technology to the ABE fermentation process. Appl Biochem Biotechnol. 1992;34‐35:441–448. [Google Scholar]
- 51. Qureshi N, Blaschek HP. Production of acetone butanol ethanol (ABE) by a hyper‐producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol Prog. 1999;15:594–602. [DOI] [PubMed] [Google Scholar]
- 52. Shin C, Baer ZC, Chen XC, Ozcam AE, Clark DS, Balsara NP. Block copolymer pervaporation membrane for in situ product removal during acetone–butanol–ethanol fermentation. J. Membrane Sci. 2015;484:57–63. [Google Scholar]
- 53. Van Hecke W, Vandezande P, Dubreuil M, Uyttebroek M, Beckers H, De Wever H. Biobutanol production from C5/C6 carbohydrates integrated with pervaporation: experimental results and conceptual plant design. J Ind Microbiol Biotechnol. 2015;43:1–12. [DOI] [PubMed] [Google Scholar]
- 54. Xue C, Du GQ, Chen LJ, Ren JG, Bai FW. Evaluation of asymmetric polydimethylsiloxane‐polyvinylidene fluoride composite membrane and incorporated with acetone‐butanol‐ethanol fermentation for butanol recovery. J. Biotechnol 2014;188:158–165. [DOI] [PubMed] [Google Scholar]
- 55. Matsumura M, Kataoka H, Sueki M, Araki K. Energy saving effect of pervaporation using oleyl alcohol liquid membrane in butanol purification. Bioprocess Biosystems Eng. 1988; 3:93–100. [Google Scholar]
- 56. Izák P, Schwarz K, Ruth W, Bahl H, Kragl U. Increased productivity of Clostridium acetobutylicum fermentation of acetone, butanol, and ethanol by pervaporation through supported ionic liquid membrane. Appl Microbiol Biotechnol. 2008;78:597–602. [DOI] [PubMed] [Google Scholar]
- 57. Qureshi N, Meagher MM, Huang J, Hutkins RW. Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite–silicone composite membrane from fed‐batch reactor of Clostridium acetobutylicum . J Membrane Sci. 2001;187:93–102. [Google Scholar]
- 58. Li J, Chen X, Qi B, Luo J, Zhang Y, Su Y, Wan Y. Efficient production of acetone–butanol–ethanol (ABE) from cassava by a fermentation–pervaporation coupled process. Bioresour Technol. 2014;169:251–257. [DOI] [PubMed] [Google Scholar]
- 59. Wu H, Chen XP, Liu GP, Jiang M, Guo T, Jin WQ, Wei P, Zhu DW. Acetone–butanol–ethanol (ABE) fermentation using Clostridium acetobutylicum XY16 and in situ recovery by PDMS/ceramic composite membrane. Bioprocess Biosystems Eng. 2012;35:1057–1065. [DOI] [PubMed] [Google Scholar]
- 60. Xue C, Yang D, Du G, Chen L, Ren J, Bai F. Evaluation of hydrophobic micro‐zeolite‐mixed matrix membrane and integrated with acetone–butanol–ethanol fermentation for enhanced butanol production. Biotechnol for Biofuels 2015;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xue C, Du GQ, Chen LJ, Ren JG, Sun JX, Bai FW, Yang ST. A carbon nanotube filled polydimethylsiloxane hybrid membrane for enhanced butanol recovery. Scientific Rep. 2014;4:5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van Hecke W, Vandezande P, Claes S, Vangeel S, Beckers H, Diels L, De Wever H. Integrated bioprocess for long‐term continuous cultivation of Clostridium acetobutylicum coupled to pervaporation with PDMS composite membranes. Bioresour Technol. 2012;111:368–377. [DOI] [PubMed] [Google Scholar]
- 63. Cai D, Zhang T, Zheng J, Chang Z, Wang Z, Qin Py, Tan Tw. Biobutanol from sweet sorghum bagasse hydrolysate by a hybrid pervaporation process. Bioresour Technol. 2013;145:97–102. [DOI] [PubMed] [Google Scholar]
- 64. Qureshi N, Blaschek HP. Butanol recovery from model solution/fermentation broth by pervaporation: evaluation of membrane performance. Biomass Bioenergy. 1999;17:175–184. [Google Scholar]
- 65. Gapes JR, Nimcevic D, Friedl A. Long‐term continuous cultivation of Clostridium beijerinckii in a two‐stage chemostat with on‐line solvent removal. Appl Environ Microbiol. 1996;62:3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Hecke W, Hofmann TD, Wever H. Pervaporative recovery of ABE during continuous cultivation: enhancement of performance. Bioresour Technol. 2013;129:421–429. [DOI] [PubMed] [Google Scholar]
- 67. Qureshi N, Friedl A, Maddox IS. Butanol production from concentrated lactose/whey permeate: use of pervaporation membrane to recover and concentrate product. Appl Microbiol Biotechnol. 2014;98:1–9. [DOI] [PubMed] [Google Scholar]
- 68. Qureshi N, Blaschek HP. Butanol production using Clostridium beijerinckii BA101 hyper‐butanol producing mutant strain and recovery by pervaporation. Appl Biochem Biotechnol. 2000;84‐86:225–235. [DOI] [PubMed] [Google Scholar]
- 69. Davison B, Thompson J. Continuous direct solvent extraction of butanol in a fermenting fluidized‐bed bioreactor with immobilized Clostridium acetobutylicum . Appl Biochem Biotechnol. 1993;39‐40:415–426. [Google Scholar]
- 70. Roffler SR, Blanch HW, Wilke CR. In situ extractive fermentation of acetone and butanol. Biotechnol Bioeng. 1988;31:135–143. [DOI] [PubMed] [Google Scholar]
- 71. Ishii S, Taya M, Kobayashi T. Production of butanol by Clostridium acetobutylicum in extractive fermentation system. J Chem Eng Jap. 1985;18:125–130. [Google Scholar]
- 72. Weilnhammer C, Blass E. Continuous fermentation with product recovery by in‐situ extraction. Chem Eng Technol. 1994;17:365–373. [Google Scholar]
- 73. Li Q, Cai H, Hao B, Zhang C, Yu Z, Zhou S, Chenjuan L. Enhancing clostridial acetone‐butanol‐ethanol (ABE) production and improving fuel properties of ABE‐enriched biodiesel by extractive fermentation with biodiesel. Appl Biochem Biotechnol. 2010;162:2381–2386. [DOI] [PubMed] [Google Scholar]
- 74. Roffler SR, Blanch HW, Wilke CR. In‐situ recovery of butanol during fermentation. Bioprocess Eng. 1987;2:1–12. [Google Scholar]
- 75. Roffler SR, Blanch HW, Wilke CR. In‐situ recovery of butanol during fermentation. Bioprocess Eng. 1987;2:181–190. [Google Scholar]
- 76. Roffler S, Blanch HW, Wilke CR. Extractive fermentation of acetone and butanol: process design and economic evaluation. Biotechnol Prog. 1987;3:131–140. [Google Scholar]
- 77. Wayman M, Parekh R. Production of acetone‐butanol by extractive fermentation using dibutylphthalate as extractant. J. Fermentation Technol. 1987; 65:295–300. [Google Scholar]
- 78. Eckert G, Schügerl K. Continuous acetone‐butanol production with direct product removal. Appl Microbiol Biotechnol. 1987;27:221–228. [Google Scholar]
- 79. Evans PJ, Wang HY. Enhancement of butanol formation by Clostridium acetobutylicum in the presence of decanol‐oleyl alcohol mixed extractants. Appl Environ Microbiol. 1988;54:1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qureshi N, Maddox IS. Continuous production of acetone‐butanol‐ethanol using immobilized cells of Clostridium acetobutylicum and integration with product removal by liquid‐liquid extraction. J Fermentation Bioeng. 1995; 80:185–189. [DOI] [PubMed] [Google Scholar]
- 81. Bankar SB, Survase SA, Singhal RS, Granstrom T. Continuous two stage acetone‐butanol‐ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B 5313. Bioresour Technol. 2012;106:110–116. [DOI] [PubMed] [Google Scholar]
- 82. González‐Peñas H, Lu‐Chau T, Moreira M, Lema J. Assessment of morphological changes of Clostridium acetobutylicum by flow cytometry during acetone/butanol/ethanol extractive fermentation. Biotechnol Lett. 2014;37:1–8. [DOI] [PubMed] [Google Scholar]
- 83. Barton WE, Daugulis A. Evaluation of solvents for extractive butanol fermentation with Clostridium acetobutylicum and the use of poly(propylene glycol) 1200. Appl Microbiol Biotechnol. 1992;36:632–639. [Google Scholar]
- 84. González‐Peñas H, Lu‐Chau TA, Moreira MT, Lema JM. Solvent screening methodology for in situ ABE extractive fermentation. Appl Microbiol Biotechnol. 2014;98:5915–5924. [DOI] [PubMed] [Google Scholar]
- 85. Shi Z, Zhang C, Chen J, Mao Z. Performance evaluation of acetone–butanol continuous flash extractive fermentation process. Bioprocess Biosystems Eng. 2005;27:175–183. [DOI] [PubMed] [Google Scholar]
- 86. Lu KM, Li SY. An integrated in situ extraction‐gas stripping process for Acetone–Butanol–Ethanol (ABE) fermentation. J Taiwan Inst Chem Engineers. 2014;45:2106–2110. [Google Scholar]
- 87. Ishizaki A, Michiwaki S, Crabbe E, Kobayashi G, Sonomoto K, Yoshino S. Extractive acetone‐butanol‐ethanol fermentation using methylated crude palm oil as extractant in batch culture of Clostridium saccharoperbutylacetonicum N1‐4 (ATCC 13564). J Biosci Bioeng. 1999;87:352–356. [DOI] [PubMed] [Google Scholar]
- 88. Yen HW, Wang YC. The enhancement of butanol production by in situ butanol removal using biodiesel extraction in the fermentation of ABE (acetone–butanol–ethanol). Bioresour Technol. 2013;145:224–228. [DOI] [PubMed] [Google Scholar]
- 89. García V, Päkkilä J, Ojamo H, Muurinen E, Keiski RL. Challenges in biobutanol production: how to improve the efficiency? Renew Sustainable Energy Rev. 2011;15:964–980. [Google Scholar]
- 90. Jeon YJ, Lee YY. Membrane‐assisted extractive butanol fermentation. Ann N. Y Acad Sci. 1987;506:536–542. [DOI] [PubMed] [Google Scholar]
- 91. Jeon YJ, Lee YY. In situ product separation in butanol fermentation by membrane‐assisted extraction. Enzyme Microbial Technol. 1989;11:575–582. [Google Scholar]
- 92. Qureshi N, Maddox IS. Reduction in butanol inhibition by perstraction: utilization of concentrated lactose/whey permeate by Clostridium acetobutylicum to enhance butanol fermentation economics. Food Bioprod Process. 2005;83:43–52. [Google Scholar]
- 93. Grobben N, Eggink G, Petrus Cuperus F, Huizing H. Production of acetone, butanol and athanol (ABE) from potato wastes: fermentation with integrated membrane extraction. Appl Microbiol Biotechnol. 1993;39:494–498. [Google Scholar]
- 94. Shukla R, Kang W, Sirkar KK. Acetone–butanol–ethanol (ABE) production in a novel hollow fiber fermentor–extractor. Biotechnol Bioeng. 1989;34:1158–1166. [DOI] [PubMed] [Google Scholar]
- 95. Tanaka S, Tashiro Y, Kobayashi G, Ikegami T, Negishi H, Sakaki K. Membrane‐assisted extractive butanol fermentation by Clostridium saccharoperbutylacetonicum N1‐4 with 1‐dodecanol as the extractant. Bioresour Technol. 2012;116:448–452. [DOI] [PubMed] [Google Scholar]
- 96. Shukla R, Kang W, Sirkar KK. Toxicity of organic solvents toclostridium acetobutylicum for extractive ABE fermentation. Appl Biochem Biotechnol. 1988;18:315–324. [Google Scholar]
- 97. Kim J, Iannotti E, Bajpai R. Extractive recovery of products from fermentation broths. Biotechnol Bioproc E. 1999;4:1–11. [Google Scholar]
- 98. Groot WJ, Soedjak HS, Donck PB, Lans RGJM, Luyben KCAM, Timmer JMK. Butanol recovery from fermentations by liquid‐liquid extraction and membrane solvent extraction. Bioprocess Eng. 1990;5:203–216. [Google Scholar]
- 99. Shah M, Lee YY. Process improvement in acetone‐butanol production from hardwood by simultaneous saccharification and extractive fermentation. Appl Biochem Biotechnol. 1994;45‐46:585–597. [Google Scholar]
- 100. Weizmann C, Bergman E, Sulzbacher M, Pariser E. Studies in selective extraction and adsorption III. The adsorption of acetone, butylalcohol and 2:3 butanediol from dilute solution. J Soc Chem Ind. 1948;67:225–227. [Google Scholar]
- 101. Groot WJ, Luyben KCAM. In situ product recovery by adsorption in the butanol/isopropanol batch fermentation. Appl Microbiol Biotechnol. 1986;25:29–31. [Google Scholar]
- 102. Cousin Saint Remi J, Baron G, Denayer J. Adsorptive separations for the recovery and purification of biobutanol. Adsorption 2012;18:367–373. [Google Scholar]
- 103. Xue C, Liu F, Xu M, Tang IC, Zhao J, Bai F, Yang ST. Butanol production in acetone‐butanol‐ethanol fermentation with in situ product recovery by adsorption. Bioresour Technol. 2016; 219:158–168. [DOI] [PubMed] [Google Scholar]
- 104. Milestone NB, Bibby DM. Concentration of alcohols by adsorption on silicalite. J Chem Technol Biotechnol 1981;31:732–736. [Google Scholar]
- 105. Maddox IS. Use of silicalite for the adsorption of n‐butanol from fermentation liquors. Biotechnol Lett. 1982; 4:759–760. [Google Scholar]
- 106. Nielsen DR, Prather KJ. In situ product recovery of n‐butanol using polymeric resins. Biotechnol Bioeng. 2009;102:811–821. [DOI] [PubMed] [Google Scholar]
- 107. Nielsen L, Larsson M, Holst O, Mattiasson B. Adsorbents for extractive bioconversion applied to the acetone‐butanol fermentation. Appl Microbiol Biotechnol. 1988;28:335–339. [Google Scholar]
- 108. Qureshi N, Hughes S, Maddox I, Cotta M. Energy‐efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess Biosystems Eng. 2005; 27:215–222. [DOI] [PubMed] [Google Scholar]
- 109. Lin X, Wu J, Jin X, Fan J, Li R, Wen Q, Qian W, Liu D, Chen X, Chen Y, Xie J, Bai J, Ying H. Selective separation of biobutanol from acetone–butanol–ethanol fermentation broth by means of sorption methodology based on a novel macroporous resin. Biotechnol Prog. 2012; 28:962–972. [DOI] [PubMed] [Google Scholar]
- 110. Lin X, Wu J, Fan J, Qian W, Zhou X, Qian C, Jin X, Wang L, Bai J, Ying H. Adsorption of butanol from aqueous solution onto a new type of macroporous adsorption resin: studies of adsorption isotherms and kinetics simulation. J Chem Technol Biotechnol 2012;87:924–931. [Google Scholar]
- 111. Eom MH, Kim W, Lee J, Cho JH, Seung D, Park S, Lee JH. Modeling of a biobutanol adsorption process for designing an extractive fermentor. Ind Eng Chem Res. 2012;52:603–611. [Google Scholar]
- 112. Yang X, Tsai GJ, Tsao GT. Enhancement of in situ adsorption on the acetone‐butanol fermentation by Clostridium acetobutylicum . Sep Technol. 1994;4:81–92. [Google Scholar]
- 113. Liu D, Chen Y, Ding FY, Zhao T, Wu JL, Guo T, Ren HF, Li BB, Niu HQ, Cao Z, Lin XQ, Xie JJ, He XJ, Ying HJ. Biobutanol production in a Clostridium acetobutylicum biofilm reactor integrated with simultaneous product recovery by adsorption. Biotechnol Biofuels. 2014;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lee SH, Eom MH, Kim S, Kwon MA, Choi JDR, Kim J, Shin YA, Kim KH. Ex situ product recovery and strain engineering of Clostridium acetobutylicum for enhanced production of butanol. Process Biochem. 2015;50:1683–1691. [Google Scholar]
- 115. Lee SH, Eom MH, Choi JD, Kim S, Kim J, Shin YA, Kim KH. Ex situ product recovery for enhanced butanol production by Clostridium beijerinckii. Bioprocess Biosyst Eng. 2016;39:695–702. [DOI] [PubMed] [Google Scholar]
- 116. Wiehn M, Staggs K, Wang Y, Nielsen DR. In situ butanol recovery from Clostridium acetobutylicum fermentations by expanded bed adsorption. Biotechnol. Prog. 2014;30:68–78. [DOI] [PubMed] [Google Scholar]
- 117. Yang X, Tsao GT. Enhanced acetone‐butanol fermentation using repeated fed‐batch operation coupled with cell recycle by membrane and simultaneous removal of inhibitory products by adsorption. Biotechnol Bioeng. 1995;47:444–450. [DOI] [PubMed] [Google Scholar]
- 118. Friedl A, Qureshi N, Maddox IS. Continuous acetone‐butanol‐ethanol (ABE) fermentation using immobilized cells of Clostridium acetobutylicum in a packed bed reactor and integration with product removal by pervaporation. Biotechnol Bioeng 1991;38:518–527. [DOI] [PubMed] [Google Scholar]
- 119. Bankar SB, Survase SA, Ojamo H, Granström T. The two stage immobilized column reactor with an integrated solvent recovery module for enhanced ABE production. Bioresour Technol. 2013; 140:269–276. [DOI] [PubMed] [Google Scholar]
- 120. Xue C, Zhao XQ, Liu CG, Chen LJ, Bai FW. Prospective and development of butanol as an advanced biofuel. Biotechnol Adv. 2013;31:1575–1584. [DOI] [PubMed] [Google Scholar]


