Abstract
Aim
We examined the effects on gastrointestinal (GI) tolerance of a novel infant formula that combined specific fermented formula (FERM) with short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides (scGOS/lcFOS), with a 9:1 ratio and concentration of 0.8 g/100 mL.
Methods
This prospective, double‐blind, randomised, controlled trial comprised 432 healthy, term infants aged 0–28 days whose parents decided to not start, or discontinued, breastfeeding. Infant formula with scGOS/lcFOS+50%FERM, scGOS/lcFOS+15%FERM, 50%FERM and scGOS/lcFOS were tested. Parents completed standardised seven‐day diaries on GI symptoms, crying, sleeping and stool characteristics each month until the infants were 17 weeks.
Results
All the formulas were well tolerated. At four weeks, the overall incidence of infantile colic was significantly lower (8%) with scGOS/lcFOS+50%FERM than scGOS/lcFOS (20%, p = 0.034) or 50%FERM (20%, p = 0.036). Longitudinal modelling showed that scGOS/lcFOS+50%FERM‐fed infants also displayed a persistently lower daily crying duration and showed a consistent stool‐softening effect than infants who received formula without scGOS/lcFOS.
Conclusion
The combination of fermented formula with scGOS/lcFOS was well tolerated and showed a lower overall crying time, a lower incidence of infantile colic and a stool‐softening effect in healthy term infants. These findings suggest for the first time that a specific infant formula has a preventive effect on infantile colic in formula‐fed infants.
Keywords: Fermented formula, Gastrointestinal tolerance, Infant formula, Infantile colic, Prebiotic
Abbreviations
- FERM
A specific fermented formula called Lactofidus
- FGID
Functional gastrointestinal disorders (FGIDs)
- GI
Gastrointestinal
- scGOS/lcFOS
Short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides
Key notes.
We examined the effects on gastrointestinal tolerance of a novel infant formula that combined specific fermented formula with short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides (scGOS/lcFOS).
The combination of scGOS/lcFOS and 50% fermented formula was particularly well tolerated, with 60% less infantile colic at four weeks of age and lower daily crying duration.
The findings demonstrate the possible preventive effects of infant formula on infantile colic for the first time.
Introduction
Exclusive breastfeeding is the preferred feeding method for all newborn infants and provides complete nutrition to support growth and development in early life 1. A wide range of short‐term and long‐term neuronal, immune, metabolic and gut health benefits have been reported for breastfed infants 2, 3, 4. Gut health in early life has been shown, in this context, to have an impact on fundamental aspects of psychosocial, physical and mental well‐being in later life. As it is not always possible to provide an infant with human milk, any substitutes should provide functional benefits that are as close as possible to those of human milk.
This study presents the results of a randomised, controlled trial of a novel infant formula on gastrointestinal (GI) tolerance, based on the secondary outcome parameters of growth, safety and GI tolerance. The primary outcome parameters of growth and safety have previously been described 5. This novel infant formula combines two well‐defined and established concepts that have each been demonstrated to have beneficial effects on gut health: a specific fermented formula (FERM) called Lactofidus (Danone Nutricia, Steenvoorde, France) and a specific mixture of prebiotic oligosaccharides, namely short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides (scGOS/lcFOS), with a ratio of 9:1 and a concentration of 0.8 g/100 mL 6, 7.
The FERM has previously been reported to alleviate symptoms of GI discomfort in infants that consumed it in a thickened formula 6. Fermentation is a natural process that was initially developed for food preservation and later became a controlled means to equip foods with particular properties, such as specific tastes, textures and health benefits 8. FERM is distinct from infant formulas that are, for example, supplemented with prebiotics or probiotics or contain hydrolysed protein 6, 9, 10, 11.
The novel scGOS/lcFOS infant formula, with a ratio of 9:1 and a concentration of 0.8 g per 100 mL, was developed based on scientific insights from human milk research 12. Formula supplementation with scGOS/lcFOS has been demonstrated to modulate early microbiota development, gut ecophysiology, stool consistency and immune functions in ways that are close to that found in breastfed infants 7, 13, 14, 15.
We hypothesised that the combination of the specific fermented formula with the specific prebiotic mixture, scGOS/lcFOS, could have a complementary and beneficial effect on gut health and function, for example by preventing functional GI disorders and related signs and symptoms in infants. Here, we present the secondary outcomes, namely GI tolerance parameters and crying time, of the novel infant formula concept, using a prospective, double‐blind, randomised, controlled trial design.
Methods
Trial design and registration
This trial was a prospective, double‐blind, randomised, four‐arm parallel group, controlled, multicentre equivalence trial on growth, safety and GI tolerance. It included hospitals and private practices in France, Belgium and Ireland. Each of the 432 infants was randomly assigned to one of the four parallel groups, which contained equal numbers. The study was registered with the Dutch Trial Register on September 17, 2010 (registration number NTR2521), and the approval of the relevant ethics committees in the participating countries was obtained before the start of the study. Baseline measurements were taken on study inclusion, and parents received the assigned infant formula with written preparation instructions and were advised to feed infants on demand.
Study products
The composition of the trial products has previously been described 5. In brief, all study products were iso‐caloric, powdered, cows’ milk‐based infant formulas for bottle‐fed babies aged 0–6 months and each 100 mL contained about 66 kilocalories of energy, 1.35 g of protein, 8.2–8.4 g of carbohydrate, 3.0–3.1 g of lipids, plus vitamins and minerals, according to European Commission Directive 2006/141/EC.
The infant formulas only varied in the amount of formula produced by a specific fermentation process, as well as the presence of scGOS/lcFOS, with a ratio of 9:1 and concentration of 0.8 g/100 mL. The formulas that were tested contained: (i) just scGOS/lcFOS, (ii) just 50%FERM, (iii) scGOS/lcFOS+15%FERM and (iv) scGOS/lcFOS+50%FERM.
The percentages of FERM related to the ratio of fully fermented infant formula mixed with nonfermented infant formula during production: for example, two of the formulas consisted of a blend of fermented and nonfermented formula in a ratio of 15:85 (scGOS/lcFOS+15% FERM), and 50:50 (scGOS/lcFOS+50% FERM). The only formula tested in this trial that did not contain scGOS/lcFOS was 50%FERM.
The rationale behind the prebiotic supplementation of a product with 50% FERM was based on the previously observed beneficial effects of the separate components on GI comfort 7, 10. The product with a combination of prebiotics and 15% FERM was included to assess a potential dose dependency, given the unknown impact the newly developed formulas would have on safety and tolerance 5. All the formulas had a similar taste, smell and colour and were manufactured according to good manufacturing practices by Nutricia, Steenvoorde, France, and were provided by Nutricia Research, the Netherlands. The study products were stored in a secure and limited access storage area at the sites, where they were protected from extremes of light, temperature and humidity.
Study conduction
The parents or guardians of infants likely to meet the eligibility criteria were informed about the study. After obtaining informed consent at the combined screening and baseline visit, the eligible infants were randomly assigned to one of the four study groups. At this first visit, baseline data were collected and the parents were given one of the four study products and diaries. Baseline data included their general experience of the severity of GI symptoms since birth, stool frequency and consistency and the duration and number of crying and sleeping episodes.
Further study visits were conducted at four, eight, 13 and 17 weeks after birth (Fig. 1A). Depending on the age at inclusion, the four‐week visit may have been combined with the screening and baseline visit. Anthropometrical measurements were performed at each visit, and adverse events, the use of medication and nutritional supplements were documented. Parents were asked to record GI symptoms, crying and sleeping, stool characteristics and study product intake in the diary they were given, for the seven‐day period prior to the study visits. The only possible exception was the four‐week visit, depending on age of inclusion. Two weeks after the final visit, a follow‐up phone call took place to record adverse events and the use of medication and nutritional supplements. When we included this noninterventional follow‐up period of two weeks in the study period, the duration for each infant from randomisation to the final phone call was 15–19 weeks, depending on the infant's age at inclusion.
Figure 1.
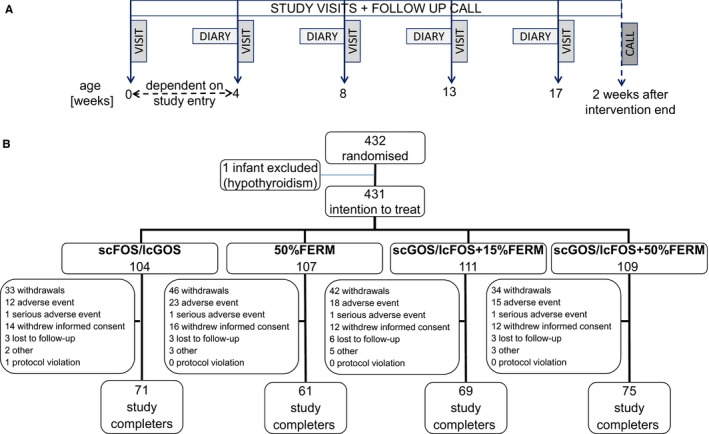
(A) Study visits were conducted at four, eight, 13 and 17 weeks after birth. At four weeks, this visit may have been combined with the screening/baseline visit, depending on the age at inclusion. (B) Randomisation, dropouts and study completers. From the 432 randomised infants, one infant with hypothyroidism was excluded from the intention to treat population (n = 431), which constitutes the primary analysis set for GI and tolerance parameters.
Study sites, inclusion and exclusion criteria, and compliance
Infants were recruited by 24 hospitals and private practices in Belgium (10 sites), Ireland (seven sites) and France (seven sites). According to the study protocol, infants were eligible to take part if they were healthy born at a gestational age of 37–42 weeks and aged 28 days or less at the time of inclusion. They also needed to have a birthweight between the 10th and 90th percentile, according to locally applicable growth charts, and the latter was adjusted during the study to a birthweight between 2500 and 4500 g. The written informed consent of a parent or guardian was also required.
The exclusion criteria were congenital conditions and/or previous or current illnesses that could interfere with the study, a known or increased risk of cows’ milk allergy, soy allergy and/or lactose intolerance, gestational diabetes, participation in another clinical trial and the investigator's uncertainty about the willingness or ability of the parents to comply with the protocol requirements.
Parents or guardians of infants were instructed, whenever possible, not to feed the child other infant formulas than the study product during the study and to complete the diaries that were provided in the seven‐day period prior to the scheduled study visits. The study product compliance, in terms of the duration of intake, was assessed.
In order to encourage mothers to continue breastfeeding as long as possible, the exclusion criterion of breastfeeding more than once a day was replaced by any breastfeeding. We decided that only mothers and parents who autonomously decided to stop breastfeeding should be informed about the study and be allowed to participate with their infant. We also added that parents or guardians should be aged at least 18 years. The inclusion criterion of a parent's or guardian's written informed consent was adapted by a country‐specific amendment that it was sufficient for one of the parents or guardians to sign the consent form.
Outcome measures and data collection
The primary outcome parameter was weight gain in grams per day from inclusion until 17 weeks of age, and the results have been previously reported 5. The secondary outcome parameters were severity of the GI symptoms, namely constipation, diarrhoea, abdominal distension, flatulence, regurgitation, vomiting, diaper dermatitis and arching of the back, the frequency and duration of crying and sleeping, the incidence of infantile colic and the frequency and consistency of stool characteristics.
The primary objective of the study was to test for equivalence of weight gain per day between the control and test products. The power calculation that was needed has been presented elsewhere 5. No separate sample size calculation was performed in respect to the secondary outcomes.
An interim analysis was performed after 106 infants, approximately 40%, of the initially calculated sample size of 280 infants, completed the intervention period to evaluate the assumptions for the sample size calculation and to analyse the safety of the products tested. An independent data monitoring committee evaluated the interim results.
The randomisation sequence was computer generated. Product stratification was applied for the centre and sex of the infant. All details of the randomisation, including block size, were unknown to the investigator, site staff and study staff from Nutricia Research, except for the clinical studies supplies manager of Nutricia Research.
After the eligibility of an infant was assessed and informed consent was obtained, the infant was included in the study. Based on the order in which infants entered the study, and the stratification factor, they were assigned a randomisation number and the correspondingly numbered, opaque, sealed randomisation envelope was opened, revealing the code of the study product – A, B, C or D – that was assigned to the randomisation number beforehand.
Data were collected from the study sites, while respecting the blinding and anonymity of all the infants. All data were entered by double data entry (blind verification) into the clinical trial database, which was ClinTrial, version 4.6 (Oracle USA, Inc., Redwood City, CA 94065, USA). Data remained blinded until the data review meeting was completed, the statistical analysis plan was validated and the database was locked.
Baseline data were based on the parent's recall. After this, the parents were asked to complete seven‐day diaries with daily entries during the week before the next study visit, which were scheduled when the infants were four, eight, 13 and 17 weeks. They were asked to return the diaries at the next visit. The GI symptoms that were evaluated were constipation, diarrhoea, abdominal distension, flatulence, regurgitation, vomiting, diaper dermatitis and arching of the back. Parents were asked to score these from 0 to 3 on a daily basis, when these were absent, mild, moderate or severe, respectively.
The periods when the infant cried or slept were also recorded. Based on the parents’ entries, the total duration of crying and sleeping per day was transferred into the case report form, as well as the number of crying and sleeping episodes per day.
In addition, parents received instruction on how to assess and record the consistency of each stool that was passed on a five‐point scale, as watery (score = 1), soft, pudding like (=2), soft, formed (=3), dry formed (=4) or dry, hard pellets (=5).
Statistical analysis
Of the 432 randomised infants, one infant with hypothyroidism was excluded from the intention to treat population, as this infant was not considered as healthy as first thought and had been erroneously randomised (Fig. 1B). The 431 infants in the study population were defined as the primary set for the analysis of the secondary tolerance parameters: GI symptoms, crying and sleeping, stool characteristics. Only diaries with three or more days of completed entries for each parameter that was analysed were considered for analysis.
From the diary data, namely the daily recorded severity score on GI symptoms, a mean value per symptom was calculated for each infant and age category, at baseline, four, eight, 13 and 17 weeks. The baseline data were based on parental recall. The same parameters and process were used to determine mean values on the frequency and duration of crying and sleeping. Furthermore, the information recorded in the diaries on crying duration was used to calculate the incidence of infantile colic, which was considered to be present, if the infant cried for at least three hours per day for at least three days, based on the adapted ROME III criteria 16. The parent's recall of the mean duration of crying per day was only collected at baseline, but we did not ask how many times the crying exceeded three hours each week. Therefore, the incidence of colic was not calculated at baseline.
A mean value was calculated per infant and age category from the parental recall at baseline and the diary data on stool consistency score and frequency.
Two‐sample t‐tests were used for the continuous data – mean severity scores for GI symptoms, crying and sleeping duration, number of crying and sleeping episodes, mean stool consistency and mean stool frequency, and the Wilcoxon rank‐sum tests were used if there was violation of the normality assumption and/or the presence of outliers. The incidence of GI symptoms and infantile colic was analysed by the chi‐square test, or in case of sparse cell counts, by Fisher's exact test. Statistical tests were performed at each week of age: at baseline, except for infantile colic, and at four, eight, 13 and 17 weeks.
The first test product – scGOS/lcFOS+50%FERM – was compared with both control products 50%FERM and scGOS/lcFOS, and the second test product – scGOS/lcFOS+15%FERM – was only compared with one control product, scGOS/lcFOS. However, using the second test product with 15%FERM also provided insight into the effect of the level of fermentation on GI tolerance against the first test product, scGOS/lcFOS + 50%FERM.
Daily crying time was analysed post hoc using a longitudinal model. For this, the total duration of crying per diary day was calculated for each infant. The time, in terms of the number of days each infant was on the study product, was standardised prior to its use as the independent variable in the regression analysis. It was defined as ti = (days on study product – the sample mean number of days on study product)/standard deviation of days on study product.
The association between infantile colic with GI symptoms was analysed post hoc by combining all the study product arms to investigate the incidence of a GI symptom by the incidence of colic at the ages of four and eight weeks. The incidence of a GI symptom was derived from the severity scores and calculated using the chi‐square test for each infant and age category. A symptom was considered to be present, and more severe than just mild, when the severity score of an infant was more than one. Fisher's exact test was used for sparse cell counts. Furthermore, odds ratios and corresponding 95% confidence intervals were calculated. The numbers of infants with infantile colic was not equally distributed across the study arms due to the intervention, for example at four weeks, it affected 20% of infants in the 50%FERM study arm and scGOS/lcFOS arm, versus 8% of the infants in the scGOS/lcFOS+50%FERM study arm. Therefore, it was not statistically sound to compare the association between functional symptoms with infantile colic for each intervention group due to different formulas and treatment bias and differences in incidence.
No correction for covariates and no correction for multiple testing were applied.
Results
Participant flow, demographics, other infant characteristics
The demographics and characteristics of the intention to treat population when they entered the study have been previously described 5. In brief, 276 of the 432 randomised infants completed the study and the reasons for the study withdrawals are detailed for each study arm (Fig. 1B). There were no statistically significant differences in the number of infants who terminated the study early, the reasons for withdrawal, the overall study compliance and the study product intake between the study arms (data not shown). The primary objective of the trial on growth and safety has previously been reported 5. In brief, all infant formulas resulted in normal growth in healthy infants and there were no differences between formulas in the number or severity of adverse events 5.
Interim analysis
During the interim analysis, the independent data monitoring committee concluded that there was no need to increase the sample size above the number initially calculated and that there were no concerns regarding safety.
Gastrointestinal symptom scores
Over the entire intervention period, and in all the four study arms, the median symptom scores for constipation, diarrhoea, abdominal distension, vomiting, diaper dermatitis and arching of the back were below one, which indicated that they were mild. The highest mean GI symptom scores that were reported by the parents were flatulence and regurgitation. The only statistically significant difference for these two symptoms was found for flatulence at eight weeks of age, where the Wilcoxon rank‐sum test showed that scGOS/lcFOS+50%FERM (median 1.2) displayed a higher average symptom score than scGOS/lcFOS+15%FERM (median 1.0, p = 0.017,) and scGOS/lcFOS (median 1.0, p = 0.01). There were no statistically significant differences found for any other symptom with a symptom score above one.
Stool frequency and consistency
There were no statistically significant differences in the average stool frequency during the study period between the study arms according to the Wilcoxon rank‐sum test (Fig. 2A). On average, scGOS/lcFOS+50%FERM displayed, lower stool consistency scores (p < 0.001) compared to 50%FERM at four, eight, 13 and 17 weeks of age when using the test (Fig. 2B). In addition, scGOS/lcFOS+50%FERM (mean 1.94, p = 0.033) and scGOS/lcFOS (mean 1.95, p = 0.043,) displayed a lower stool consistency than scGOS/lcFOS+15%FERM at the age of eight weeks (Fig. 2B).
Figure 2.
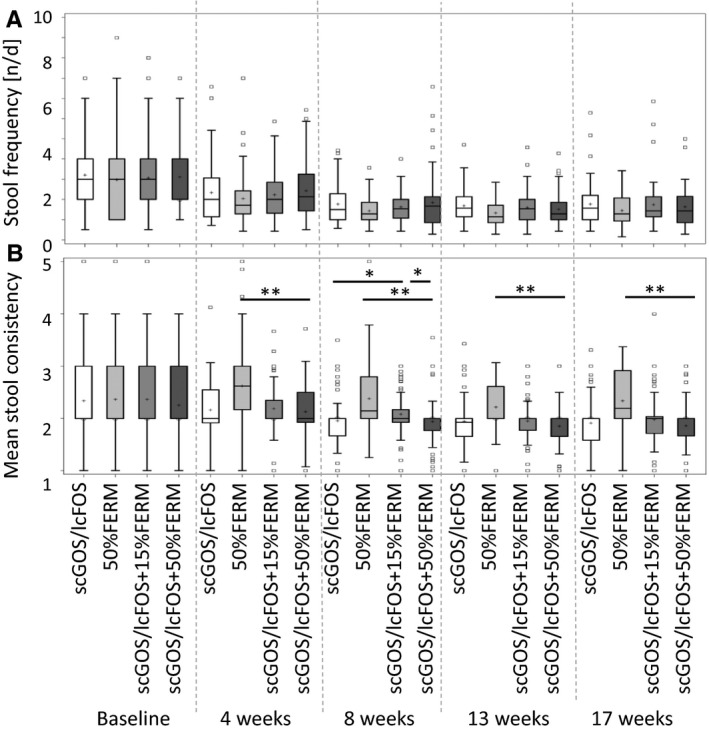
Stool parameters. A mean value was calculated per infant and week from the parental diaries for (A) frequency, (B) consistency (watery = 1, soft, pudding like = 2, soft, formed = 3, dry formed = 4 or dry, hard pellets = 5). (*p ≤ 0.05, **p < 0.001; Wilcoxon rank‐sum test).
Crying time and incidence of infantile colic
T‐tests for each time point failed to show statistically significant differences in the duration of crying between the study arms.
At four weeks of age, the statistical average for the number of crying episodes per day was significantly lower with scGOS/lcFOS+50%FERM than 50%FERM (median 2.64 versus 3.38 crying episodes per day, p = 0.030), according to the Wilcoxon rank‐sum test (Fig. 3A).
Figure 3.
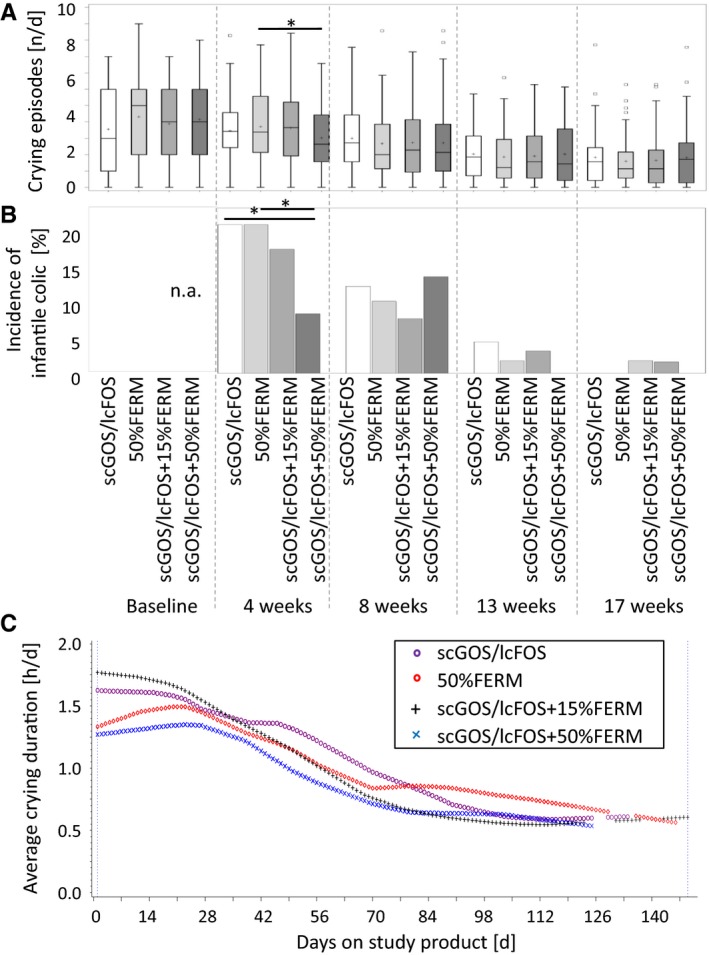
Crying and infantile colic. (A) mean number of crying episodes per day, (B) incidence of infantile colic according to adapted Rome III criteria (≥3 hours of crying per day, for at least three days during one week) given as the percentage of infants that fulfilled these criteria. (*p ≤ 0.05, crying episodes calculated using Wilcoxon rank‐sum test; infantile colic calculated at four and eight weeks using the chi‐square test and 13 and 17 weeks with the Wilcoxon rank‐sum test). (C) Longitudinal modelling of average crying duration per infant in hours per day. There was no statistically significant difference in the median crying duration at baseline.
Across all four study arms, the highest incidence of infantile colic, based on the adapted Rome III criteria – 16.1% of 292 infants – was found at four weeks of age and was lower at later time points, namely 10.6% of 284 infants at eight weeks, 2.2% of 272 infants at 13 weeks and 0.7% of 267 infants at 17 weeks.
At four weeks of age, the chi‐square test showed a statistically significantly lower incidence of infantile colic with scGOS/lcFOS+50%FERM (8% of 75 infants) than 50%FERM (20% of 70 infants, p = 0.036) and scGOS/lcFOS (20% of 75 infants, p = 0.034; Fig. 3B). There were no statistically significant differences at other time points.
During the 17‐week infantile formula study period, scGOS/lcFOS+50%FERM‐fed infants persistently displayed a significantly lower daily crying duration than scGOS/lcFOS‐fed infants (p = 0.037; Fig. 3C). The statistical model provided a statistical inference for differences in profiles covering the entire intervention period. For example, after 56 days on the study product, the mean crying duration for scGOS/lcFOS was 74 minutes and this was 25% lower, at 55 minutes, for scGOS/lcFOS+50%FERM. There was no statistically significant difference in the median crying duration at baseline between the four study arms.
Duration and number of sleeping episodes
There was no indication of a statistically significant difference in the number of sleeping episodes or sleeping duration at any time point for any study group comparison (data not shown).
Results of the post hoc analysis on infantile colic
Irrespectively of the study groups, the GI symptoms that had the highest incidence rates at four and eight weeks of age were flatulence (50% and 42.9% of infants, respectively) and regurgitation (25.7% and 24.3% of infants, respectively). The association between GI symptoms and infantile colic at four and eight weeks of age is reported in Figure 4. At 13 and 17 weeks of age, the incidence of infantile colic was too low for statistical assessment. For example, at four weeks of age, infants with colic had an approximately threefold to sixfold higher chance of also having flatulence, regurgitation, constipation, diarrhoea or vomiting than the infants without colic (Fig. 4).
Figure 4.
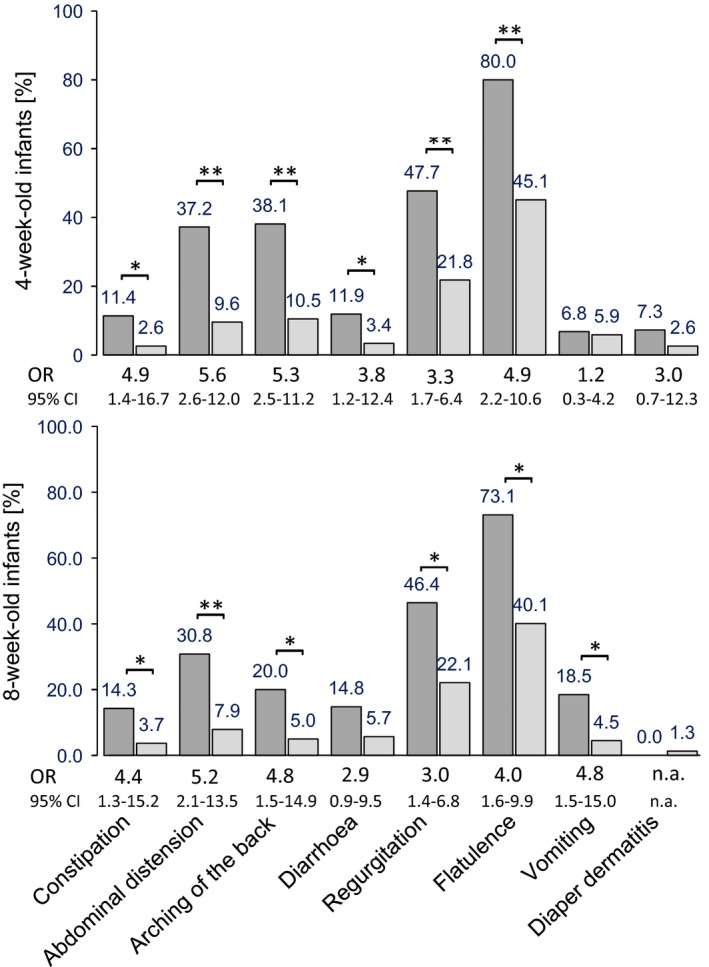
Incidence of gastrointestinal symptoms in infants with and without infantile colic. For example, at four weeks of age, infants with infantile colic had a 4.9 (1.4–16.7) higher chance of also having constipation than infants without colic. OR = odds ratio, CI = confidence interval; **p < 0.001, *p < 0.01, n.a. = not applicable; p values relate to difference in incidence – Fisher's exact test was used for all symptoms except flatulence and diarrhoea at four and eight weeks, and arching of the back at eight weeks, where the chi‐square test was used.
Discussion
Gut health in early life is an integrated concept that includes, and impacts on, the development of the immune, metabolic and neuronal systems. Due to the complex and highly dynamic development of the GI system and microbiota in early life, healthy term infants can experience a number of functional gastrointestinal disorders (FGIDs) and related signs and symptoms early in life, which cannot be explained by obvious structural or biochemical abnormalities 16, 17. It has been reported that at least one of two infants display one or more FGIDs, such as functional constipation or infantile colic, or related signs and symptoms such as hard stools or excessive crying 18, 19. Even though FGIDs are mostly seen as benign in nature, and usually resolve over time, these may constitute early traumatic events that cause appreciable distress to infants and parents, with pending long‐term consequences for the well‐being of both 20, 21, 22, 23, 24, 25, 26, 27. Considering the high incidence and impact of early‐life FGIDs, their prevention would be preferable to their treatment, with the implication that nutritional solutions would be preferable to pharmacological interventions. The evidence for the prevention of FGIDs and related signs and symptoms by nutritional concepts is limited. This study presents evidence in this area, based on the results of the secondary outcome parameters of a four‐arm, randomised controlled trial on growth, safety and GI tolerance of a novel infant formula.
The general strengths of this study include the double‐blind randomised, controlled trial design, the detailed daily diaries on crying time, for example when compared to weekly recall, and other GI tolerance parameters. We concluded that all formulas were well tolerated, based on the low GI symptom scores from all four study arms over the entire intervention period. However, it was shown as a secondary study outcome that the incidence of infantile colic was influenced by the infant formula composition. An incidence of infantile colic of 20% in the intervention groups that received either scGOS/lcFOS or 50% FERM was in the range of the incidence reported for population‐based studies and reviews 18, 19. The formula containing the combination of scGOS/lcFOS with 50% FERM displayed a 60% lower incidence of infantile colic at four weeks of age, compared to those containing either FERM or scGOS/lcFOS. These results suggest a synergistic effect of the FERM and scGOS/lcFOS, and the underlying mode of action may require further investigation.
Given that all the collected data were from healthy term infants, any effect on infants with infantile colic, namely 20% of the study population 18, 19, would be expected to only have a limited effect on the average crying time of the total study group. Nonetheless, longitudinal modelling demonstrated that during the 17‐week intervention, there was a persistently, and significantly, lower daily crying duration in the scGOS/lcFOS+50%FERM‐fed infants than in the scGOS/lcFOS‐fed infants. This suggests that this formula had an effect during the whole intervention period and not just around the age of four weeks. Based on the study design, namely the inclusion of healthy term infants shortly after birth, the lower incidence of infantile colic and the lower crying duration in infants fed the combination of 50% FERM and scGOS/lcFOS, we can conclude that this combination may have had a preventive effect on infantile colic. To the best of our knowledge, there is surprisingly little quantitative data available to substantiate the origin of GI infantile colic. A broad range of nongastrointestinal conditions, ranging from migraine to the maladaptive behaviour of infants, have been suspected to be linked to infantile colic 17. Although the data presented here were derived from a nutritional intervention study, the overall picture that emerged was that parental reported functional GI symptoms were more commonly observed in infants with infantile colic than those without (Fig. 4). As functional GI symptoms can cause considerable discomfort, these could be causally linked to crying or an underlying process that is by itself associated with inconsolable crying. A potential next step would be to confirm the link of functional GI symptoms and infantile through, for example, a prospective observational study.
Hard stools can be commonly observed in infants fed with a formula without prebiotic oligosaccharides, but are rarely seen in breastfed infants 15, 28. For example, infants below one year of age have been reported to constitute the highest rate of constipation‐related emergency department visits in the United States 29. The difference in stool consistency between breastfed and formula‐fed infants has partly been attributed to human milk oligosaccharides, which are present in human milk at levels of approximately 10 g/L 7, 30. In this study, infants who consumed the formula containing scGOS/lcFOS+50%FERM had softer stools than the infants consuming formula with just 50% FERM. Whether this effect also has the potential to prevent constipation needs to be evaluated in future trials.
Conclusion
This study investigated the safety and tolerance of a novel infant formula that combined scGOS/lcFOS with a 9:1 ratio and concentration of 0.8 g/100 mL and a specific FERM, in healthy term infants. Based on the low mean GI symptom scores that were obtained, all of the study formulas appeared to be well tolerated. The combination of scGOS/lcFOS and 50% FERM showed a secondary study outcome of consistent stool softening when it was compared to the formula without scGOS/lcFOS. It also showed a significantly lower incidence of infantile colic at four weeks of age, when compared to formulas that contained either FERM or scGOS/lcFOS. Interestingly, longitudinal modelling of daily crying duration over the 17 weeks of the intervention showed that scGOS/lcFOS+50%FERM‐fed infants also displayed a persistently lower daily crying duration throughout the study period. Overall, this may indicate a synergistic effect of the partly fermented formula combined with scGOS/lcFOS. More studies are needed to further evaluate this effect.
Funding
Nutricia Research, the Netherlands, provided the funding to conduct the study. The funder contributed to the design of the study, interpretation of the findings and writing of the manuscript.
Conflict of interests
TL and HB are employees of Nutricia Research.
Acknowledgements
The authors would like to thank all the families who participated in the study, as well as all the participating paediatricians from Ireland (Drs Perry, Harrington, Murphy, Belton and. O'Doherty), France (Drs Morville, Thiriez, Beley, Simeoni, Savanger and Logre and Professor Hankhard) and Belgium (Drs Franckx, Jespers, Logghe, Van Eldere, Vandeputte, Verlinde, and Vertruyen) and their research staff for their contribution to the study. We would also like to thank Stefanie Schoen, Jannie Ausma and Berend Jan Velthuis from Nutricia Research for their support.
References
- 1. Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, et al. Breast‐feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2009; 49: 112–25. [DOI] [PubMed] [Google Scholar]
- 2. Lamberti LM, Zakarija‐Grkovic I, Fischer Walker CL, Theodoratou E, Nair H, Campbell H, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta‐analysis. BMC Public Health 2013; 13(Suppl 3): S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387: 475–90. [DOI] [PubMed] [Google Scholar]
- 4. Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days ‐ intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 2014; 25: 428–38. [DOI] [PubMed] [Google Scholar]
- 5. Huet F, Abrahamse‐Berkeveld M, Tims S, Simeoni U, Beley G, Savanger C, et al. Partly fermented infant formulae with specific oligosaccharides support adequate infant growth and are well‐tolerated. J Pediatr Gastroenterol Nutr 2016t; 63: e43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roy P, Aubert‐Jacquin C, Avart C, Gontier C. Benefits of a thickened infant formula with lactase activity in the management of benign digestive disorders in newborns. Arch Pediatr 2004; 11: 1546–54. [DOI] [PubMed] [Google Scholar]
- 7. Oozeer R, van Limpt K, Ludwig T, Ben Amor K, Martin R, Wind RD, et al. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human‐milk oligosaccharides. Am J Clin Nutr 2013; 98: 561S–71S. [DOI] [PubMed] [Google Scholar]
- 8. Ross RP, Morgan S, Hill C. Preservation and fermentation: past, present and future. Int J Food Microbiol 2002; 79: 3–16. [DOI] [PubMed] [Google Scholar]
- 9. Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, Hernell O, et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr 2005; 41: 584–99. [DOI] [PubMed] [Google Scholar]
- 10. van de Heijning BJ, Berton A, Bouritius H, Goulet O. Gastrointestinal symptoms in infants are a potential target for fermented infant milk formulae: a review. Nutrients 2014; 6: 3942–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szajewska H, Skorka A, Piescik‐Lech M. Fermented infant formulas without live bacteria: a systematic review. Eur J Pediatr 2015; 174: 1413–20. [DOI] [PubMed] [Google Scholar]
- 12. Moro GE, Mosca F, Miniello V, Fanaro S, Jelinek J, Stahl B, et al. Effects of a new mixture of prebiotics on faecal flora and stools in term infants. Acta Paediatr Suppl 2003; 91: 77–9. [DOI] [PubMed] [Google Scholar]
- 13. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr 2008; 138: 1091–5. [DOI] [PubMed] [Google Scholar]
- 14. Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr 2008; 138: 1818S–28S. [DOI] [PubMed] [Google Scholar]
- 15. Scholtens PA, Goossens DA, Staiano A. Stool characteristics of infants receiving short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides: a review. World J Gastroenterol 2014; 20: 13446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2006; 130: 1519–26. [DOI] [PubMed] [Google Scholar]
- 17. Shamir R, St James‐Roberts I, Di Lorenzo C, Burns AJ, Thapar N, Indrio F, et al. Infant crying, colic, and gastrointestinal discomfort in early childhood: a review of the evidence and most plausible mechanisms. J Pediatr Gastroenterol Nutr 2013; 57(Suppl 1): S1–45. [DOI] [PubMed] [Google Scholar]
- 18. Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Cokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr 2015; 61: 531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iacono G, Merolla R, D'Amico D, Bonci E, Cavataio F, Di Prima L, et al. Gastrointestinal symptoms in infancy: a population‐based prospective study. Dig Liver Dis 2005; 37: 432–8. [DOI] [PubMed] [Google Scholar]
- 20. Forsyth BW, Canny PF. Perceptions of vulnerability 3 1/2 years after problems of feeding and crying behavior in early infancy. Pediatrics 1991; 88: 757–63. [PubMed] [Google Scholar]
- 21. Canivet C, Jakobsson I, Hagander B. Infantile colic. Follow‐up at four years of age: still more “emotional”. Acta Paediatr 2000; 89: 13–7. [DOI] [PubMed] [Google Scholar]
- 22. Brown M, Heine RG, Jordan B. Health and well‐being in school‐age children following persistent crying in infancy. J Pediatr Child Health 2009; 45: 254–62. [DOI] [PubMed] [Google Scholar]
- 23. Bonilla S, Saps M. Early life events predispose the onset of childhood functional gastrointestinal disorders. Rev Gastroenterol Mex 2013; 78: 82–91. [DOI] [PubMed] [Google Scholar]
- 24. Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res 2007; 62: 240–5. [DOI] [PubMed] [Google Scholar]
- 25. Anand KJ, Runeson B, Jacobson B. Gastric suction at birth associated with long‐term risk for functional intestinal disorders in later life. J Pediatr 2004; 144: 449–54. [DOI] [PubMed] [Google Scholar]
- 26. Partty A, Kalliomaki M, Salminen S, Isolauri E. Infant distress and development of functional gastrointestinal disorders in childhood: is there a connection? JAMA Pediatr 2013; 167: 977–8. [DOI] [PubMed] [Google Scholar]
- 27. Indrio F, Di Mauro A, Riezzo G, Cavallo L, Francavilla R. Infantile colic, regurgitation, and constipation: an early traumatic insult in the development of functional gastrointestinal disorders in children? Eur J Pediatr 2015; 174: 841–2. [DOI] [PubMed] [Google Scholar]
- 28. Quinlan PT, Lockton S, Irwin J, Lucas AL. The relationship between stool hardness and stool composition in breast‐ and formula‐fed infants. J Pediatr Gastroenterol Nutr 1995; 20: 81–90. [DOI] [PubMed] [Google Scholar]
- 29. Sommers T, Corban C, Sengupta N, Jones M, Cheng V, Bollom A, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol 2015; 110: 572–9. [DOI] [PubMed] [Google Scholar]
- 30. Stahl B, Thurl S, Zeng J, Karas M, Hillenkamp F, Steup M, et al. Oligosaccharides from human milk as revealed by matrix‐assisted laser desorption/ionization mass spectrometry. Anal Biochem 1994; 223: 218–26. [DOI] [PubMed] [Google Scholar]


