Abstract
The incidence of renal‐related adverse events (AEs) with canagliflozin in patients with type 2 diabetes mellitus from a pooled population of patients in 7 active‐ and placebo‐controlled trials (N = 5598) and in a 104‐week study vs glimepiride (N = 1450) was low and similar in canagliflozin and non‐canagliflozin groups. In the study vs glimepiride, canagliflozin was associated with an initial acute decrease in estimated glomerular filtration rate (eGFR) that attenuated over time, while eGFR declined progressively over 104 weeks with glimepiride. The incidence of renal‐related AEs with canagliflozin was generally stable over time, while the incidence with glimepiride increased over 104 weeks. In the present analysis, based on postmarketing reports from the US Food and Drug Administration Adverse Event Reporting System, a potential signal was identified for acute kidney injury with all approved sodium glucose co‐transporter 2 (SGLT2) inhibitors (ie, canagliflozin, dapagliflozin and empagliflozin). The early onset of acute kidney injury events with SGLT2 inhibitors in postmarketing reports probably reflects the acute changes in eGFR attibutable to the known renal haemodynamic effects of SGLT2 inhibition.
Keywords: SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Sodium glucose co‐transporter 2 (SGLT2) inhibitors are a novel class of drugs for the treatment of hyperglycaemia in adults with type 2 diabetes mellitus (T2DM).1 SGLT2 is responsible for glucose reabsorption in the proximal tubule of the kidney.1 SGLT2 inhibition lowers the renal threshold for glucose in patients with T2DM, thereby increasing urinary glucose excretion, resulting in lowered blood glucose, a mild osmotic diuresis and a net caloric loss.1 In clinical studies, acute, reversible declines in estimated glomerular filtration rate (eGFR) with commensurate increases in serum creatinine have been observed with SGLT2 inhibitors, consistent with the haemodynamic effect of these agents.2 Antihypertensive agents that target the renin‐angiotensin‐aldosterone system (RAAS; ie, angiotensin‐converting enzyme [ACE] inhibitors and angiotensin II receptor blockers) are also associated with acute, reversible declines in renal function in patients with T2DM early in treatment, as measured by decreased eGFR and increased serum creatinine; these changes are the result of reduced intraglomerular pressure via the haemodynamic effect of RAAS inhibition.3 Treatment with these agents, however, is also associated with reduced proteinuria and reduced risk of end‐stage renal disease.3
The US Food and Drug Administration (FDA) recently revised the labels of the SGLT2 inhibitors, canagliflozin, dapagliflozin and empagliflozin to strengthen existing renal safety warnings by including information on an increased risk of acute kidney injury and recommendations to minimize this risk.4, 5 Based on data from the FDA Adverse Event Reporting System (FAERS) from March 29, 2013 to October 19, 2015, 101 cases of acute kidney injury were reported in 73 and 28 patients treated with canagliflozin and dapagliflozin, respectively.4 Among the 101 cases, 51 reported concomitant ACE inhibitor use, 26 reported concomitant diuretic use, and 6 reported concomitant non‐steroidal anti‐inflammatory use. In 45 of the 101 cases, a change in serum creatinine or eGFR was reported at the time of diagnosis; in 58 cases, the onset of acute kidney injury occurred within 1 month of drug initiation. Of the 101 cases, 78 resulted in drug discontinuation; of these, 56 patients reported improvement after discontinuation, 11 patients did not recover, 4 patients died, and 3 patients recovered with sequelae upon discontinuation. Warnings for acute kidney injury based on postmarketing reports also appear in the labels for other antihyperglycaemic agents, including the dipeptidyl peptidase‐4 inhibitor sitagliptin6 and the glucagon‐like peptide‐1 receptor agonist exenatide.7
To better describe this risk, the present brief report shows an analysis of renal‐related adverse events (AEs) with canagliflozin in patients with T2DM from clinical trials and FAERS postmarketing data with SGLT2 inhibitors marketed in the USA.
2. METHODS
Renal‐related AEs in the canagliflozin clinical development programme were identified based on a predefined list of preferred terms (Table S1, Supporting Information). “Acute kidney injury” was not a preferred term in the Medical Dictionary for Regulatory Activities (MedDRA) until version 18.0, which was released in 2015. Analyses for the present manuscript were performed based on earlier versions of MedDRA; however, investigator‐reported AEs of acute kidney injury would probably have been captured with other dictionary terms (eg, blood creatinine increased, GFR decreased, renal failure acute).
The incidence of renal‐related AEs was assessed in a pooled population that included data from patients with T2DM (N = 5598) enrolled in 7 placebo‐ and active‐controlled studies who were randomized to receive canagliflozin 100 or 300 mg or non‐canagliflozin (ie, placebo, sitagliptin or glimepiride) once daily as monotherapy or in combination with various background antihyperglycaemic agents for up to 104 weeks (Table S2, Supporting Information).8 Data from the ongoing CANagliflozin cardioVascular Assessment Study (CANVAS) in patients with a history/risk of cardiovascular disease and the study in patients with chronic kidney disease were not included in this analysis.8 While also included in the pooled analysis above, additional analyses of renal‐related AEs were performed on data from a randomized, double‐blind, active‐controlled, 104‐week study of canagliflozin 100 and 300 mg vs glimepiride as add‐on to metformin in patients with T2DM (N = 1450),9 including the time to first renal‐related AE and the incidence of renal‐related AEs over time.
Postmarketing reports of acute kidney injury with canagliflozin, dapagliflozin and empagliflozin were obtained from FAERS in April 2016 and included all reports up to December 2015. Associations between drugs and events were analysed through an established signal detection algorithm accounting for sample variability. The empirical Bayes geometric mean (EBGM) score (ie, sample size – adjusted ratio of the observed and expected number of AEs) and the corresponding 90% confidence interval (ie, EB05‐EB95) were calculated based on this model. Associations that met all of the following criteria were considered disproportionally reported: n ≥ 3, EBGM ≥ 2, and EB05 > 1.10, 11
2.1. Ethics approval and consent to participate
All studies included in this analysis were conducted in accordance with the ethical principles that comply with the Declaration of Helsinki and are consistent with good clinical practices and applicable regulatory requirements. Study protocols and amendments were approved by institutional review boards/ethics committees at the participating institutions. All patients provided written informed consent prior to participation.
3. RESULTS
3.1. Clinical trial data
In the pooled population, the incidence of renal‐related AEs was low and similar among groups (2.6%, 2.8% and 2.8% with canagliflozin 100 and 300 mg and non‐canagliflozin, respectively). The most common reported terms were blood creatinine level increased, GFR decreased, and renal impairment. Few patients had serious renal‐related AEs with canagliflozin 100 and 300 mg and non‐canagliflozin (3 [0.2%], 1 [<0.1%] and 2 [<0.1%] patients, respectively).
In the 104‐week study vs glimepiride, decreases in eGFR were observed at week 4 of canagliflozin treatment that attenuated over time; eGFR declined progressively with glimepiride (Figure 1A).9 The proportions of patients with any post‐baseline eGFR value <80 mL/min/1.73 m2 and a decrease of >30% were 6.6%, 8.5% and 8.7% with canagliflozin 100 and 300 mg and glimepiride, respectively; 0.8%, 1.3% and 0.4% of patients, respectively, had a post‐baseline eGFR decrease >50%. Among patients with an eGFR decrease >50% from baseline with either canagliflozin or glimepiride, nearly all had eGFR changes that returned toward baseline values while study drug continued or after study discontinuation. Over the entire 104‐week study, the incidence of renal‐related AEs was low and similar with canagliflozin 100 and 300 mg vs glimepiride (3.1% [15/483], 3.3% [16/485] and 3.3% [16/482], respectively)9; however, within the first 6 months of the study, a numerically higher percentage of patients on canagliflozin 100 and 300 mg experienced a renal‐related AE (primarily blood creatinine increased and GFR decreased) compared with glimepiride (0.6% [3/483], 0.8% [4/485] and 0.2% [1/482], respectively; Figure 1B). Among patients with renal‐related AEs in the first 6 months of treatment, the onset of events occurred earlier with canagliflozin than glimepiride. The mean (median; range) time to onset of the first renal‐related AE was 42.7 (30.0; 27.0‐71.0) and 43.5 (29.5; 29.0‐86.0) days with canagliflozin 100 and 300 mg, respectively; 1 patient in the glimepiride group had a renal‐related AE in the first 6 months, on day 85. The relatively early onset of renal‐related AEs with canagliflozin corresponds to the early decrease in eGFR observed with canagliflozin in Figure 1A. Few patients discontinued as a result of renal‐related AEs over 104 weeks: 1 patient with canagliflozin 100 mg (blood creatinine increased), 7 patients with canagliflozin 300 mg (n = 1, blood creatinine increased; n = 4, GFR decreased; n = 2, renal failure) and 1 patient with glimepiride (GFR decreased).
Figure 1.
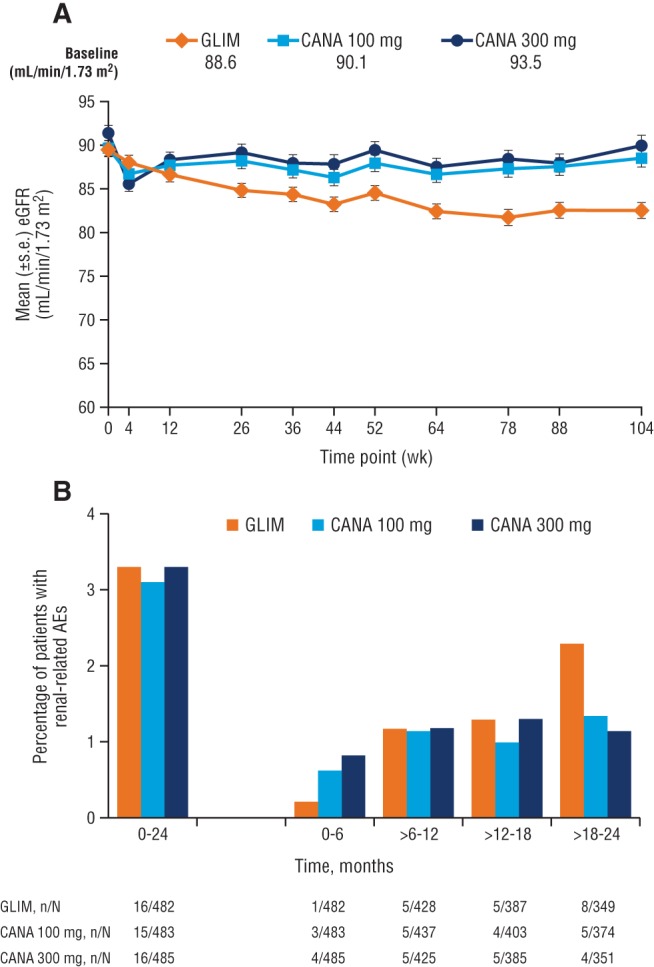
Time course of A, changes in eGFR and B, incidence of renal‐related AEs with canagliflozin and glimepiride over 104 weeks. GLIM, glimepiride; CANA, canagliflozin; s.e., standard error. Panel A reprinted from American Diabetes Association, Diabetes Care , Copyright © 2015. Copyright and all rights reserved. Material from this publication has been used with the permission of the American Diabetes Association.
3.2. Postmarketing data
Analysis of FAERS reports up to the end of 2015 identified 125 cases of acute kidney injury with canagliflozin, 50 with dapagliflozin and 15 with empagliflozin. There were 3 cases with canagliflozin plus metformin, 6 with dapagliflozin plus metformin extended release and 2 with empagliflozin plus linagliptin. The EBGM (EB05‐EB95) was 2.8 (2.4‐3.3), 2.3 (1.8‐2.8) and 2.5 (1.6‐3.8) with canagliflozin, dapagliflozin and empagliflozin, respectively (Table 1). As n > 3, EBGM ≥ 2 and EB05 > 1, a potential signal for acute kidney injury was detected with all 3 SGLT2 inhibitors, as well as dapagliflozin plus metformin extended release.
Table 1.
Summary of postmarketing reports of acute kidney injury for SGLT2 inhibitors up to December 2015
| Drug name | Reports, n | EBGM (EB05‐EB95) |
|---|---|---|
| Monotherapy | ||
| Canagliflozin 1 | 125 | 2.8 (2.4‐3.3) |
| Dapagliflozin 1 | 50 | 2.3 (1.8‐2.8) |
| Empagliflozin 1 | 15 | 2.5 (1.6‐3.8) |
| Combination therapy | ||
| Canagliflozin plus metformin | 3 | 1.5 (0.6‐3.4) |
| Dapagliflozin plus metformin XR 1 | 6 | 3.9 (2.0‐7.4) |
| Empagliflozin plus linagliptin | 2 | 1.3 (0.4‐3.2) |
Abbreviation: XR, extended release.
Disproportionately reported: n ≥ 3, EBGM ≥ 2, and EB05 > 1.
4. DISCUSSION
The recent FDA revision to the labels for the SGLT2 inhibitors canagliflozin, dapagliflozin and empagliflozin suggests an increased risk of acute kidney injury with these therapies based on spontaneous postmarketing reporting to the FDA.4, 5 The present assessment of postmarketing safety reports from FAERS suggests a potential signal for acute kidney injury with all approved SGLT2 inhibitors. While informative to identify potential safety signals in a broader patient population than in clinical trials, analyses of postmarketing reports are limited because of the lack of complete information surrounding these events, such as potential contributing factors, overall drug exposure and concomitant medication use. Additional limitations include potential underreporting and lack of information regarding the number of patients taking the drug. The greater number of postmarketing reports of acute kidney injury with canagliflozin compared with the other agents in this class may reflect the greater length of time that canagliflozin has been on the market.
The incidence of renal‐related AEs in the pooling of 7 studies from the canagliflozin clinical development programme was low and similar with canagliflozin and non‐canagliflozin, with the incidence of these AEs consistent with canagliflozin over 2 years. Renal‐related AEs occurred earlier after treatment initiation with canagliflozin compared with glimepiride. These results are consistent with an analysis of renal‐related AEs with canagliflozin in a pooled population from four 26‐week, placebo‐controlled studies12 and with results from the EMPA‐REG OUTCOME study of the SGLT2 inhibitor in patients with T2DM and established cardiovascular disease, where an increased incidence of acute kidney injury was observed with empagliflozin compared with placebo in the first 90 days of treatment, but the incidence was lower with empagliflozin vs placebo over the duration of the study.13 The early onset of renal‐related AEs with canagliflozin in clinical trials and AEs of acute kidney injury reported to the FDA4 probably reflect the acute initial changes in eGFR as a result of renal haemodynamic effects with canagliflozin and other SGLT2 inhibitors.2 This decline in eGFR is reversible, as eGFR values return to baseline after discontinuation of canagliflozin, suggesting no permanent renal damage.14 Additionally, there are no preclinical and clinical data indicating permanent renal damage or toxicity.2 This profile is consistent with the renal effects of RAAS inhibitors, which are associated with acute changes in renal function, with drug initiation and renoprotective effects over longer treatment duration.15 It has been hypothesized that the haemodynamic effects with SGLT2 inhibition may attenuate glomerular hyperfiltration and albuminuria, providing a similar renoprotective effect for patients with T2DM.1, 14 In a post hoc analysis of renal outcomes in the EMPA‐REG OUTCOME trial, empagliflozin was associated with a slower progression of kidney disease and lower rates of clinically relevant renal events compared with placebo in patients with T2DM and established cardiovascular disease.16 Consistently, in a post hoc analysis of the 104‐week add‐on to metformin vs glimepiride study, canagliflozin was associated with a lower rate of eGFR decline vs glimepiride, further suggesting that canagliflozin may slow the progression of kidney function decline in patients with T2DM.17 Evaluating patients’ volume status before initiating SGLT2 inhibitors and maintaining adequate fluid intake during treatment may minimize the risk of acute kidney injury.
Further research is needed to better understand the incidence of acute kidney injury with SGLT2 inhibitors and the overall renal safety and potential benefits of these agents. The ongoing, prospective, dedicated renal outcome studies Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE; ClinicalTrials.gov Identifier: NCT02065791) and CANagliflozin cardioVascular Assessment Study ‐ renal outcomes (CANVAS‐R; NCT01989754) will provide definitive evidence on the renal safety and renoprotective effects of canagliflozin in patients with T2DM and chronic kidney disease or a history/risk of cardiovascular disease, respectively.
Supporting information
Table S1. Preferred terms used in the analysis of selected renal‐related AEs.
Table S2. Study design and patient population.
ACKNOWLEDGMENTS
Medical writing support was provided by Felicia Gray, PhD, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Conflict of interest
All authors are full‐time employees of Janssen Research & Development, LLC.
Author contributions
M. D., W. C. and N. R. contributed to the design and conduct of the study; the acquisition, analysis and interpretation of data; and drafted, reviewed, approved and wrote the manuscript. Y. Y., D. B., D. S. and J. X. contributed to the analysis and interpretation of the data and drafted, reviewed and approved the manuscript.
Desai M, Yavin Y, Balis D, Sun D, Xie J, Canovatchel W and Rosenthal N. Renal safety of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes mellitus, Diabetes Obes Metab, 2017. doi: 10.1111/dom.12876
Funding information This analysis was supported by Janssen Research & Development, LLC. Medical writing support was provided by Felicia Gray, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
REFERENCES
- 1. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 2. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation. 2016;134(10):752‐772. [DOI] [PubMed] [Google Scholar]
- 3. Remuzzi G, Ruggenenti P, Perico N. Chronic renal diseases: renoprotective benefits of renin–angiotensin system inhibition. Ann Intern Med. 2002;136(8):604‐615. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration . Canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR): drug safety communication – strengthened kidney warnings. http://www.fda.gov/Safety/Medwatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm506554.htm. Accessed November 16, 2016.
- 5.JARDIANCE® (empagliflozin) tablets, for oral use [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015. [Google Scholar]
- 6.JANUVIA™ (sitagliptin) tablets [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2012. [Google Scholar]
- 7.BYETTA® (exenatide) injection [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2010. [Google Scholar]
- 8. Qiu R, Balis D, Xie J, Davies MJ, Desai M, Meininger G. Longer‐term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin. 2017;33(3):553‐562. [DOI] [PubMed] [Google Scholar]
- 9. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double‐blind, phase 3 study. Diabetes Care. 2015;38(3):355‐364. [DOI] [PubMed] [Google Scholar]
- 10. Harpaz R, DuMouchel W, LePendu P, Bauer‐Mehren A, Ryan P, Shah N. Performance of pharmacovigilance signal detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93(6):539‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deshpande G, Gogolak V, Weiss Smith S. Data mining in drug safety. Review of published threshold criteria for defining signals of disproportionate reporting. Pharm Med. 2010;24(1):37‐42. [Google Scholar]
- 12. Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with type 2 diabetes: pooled analysis of phase 3 study results. Postgrad Med. 2014;126(3):16‐34. [DOI] [PubMed] [Google Scholar]
- 13. US Food and Drug Administration . FDA Briefing Document: Endocrine and Metabolic Drug Advisory Committee Meeting; June 28, 2016; Silver Spring, MD. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM508422.pdf. Accessed November 16, 2016.
- 14. Perkovic V, Jardine M, Vijapurkar U, Meininger G. Renal effects of canagliflozin in type 2 diabetes mellitus. Curr Med Res Opin. 2015;31(12):2219‐2231. [DOI] [PubMed] [Google Scholar]
- 15. Bakris GL, Weir MR. Angiotensin‐converting enzyme inhibitor‐associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160(5):685‐693. [DOI] [PubMed] [Google Scholar]
- 16. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 17. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independent of glycemic effects. J Am Soc Nephrol. 2017;28(1):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Preferred terms used in the analysis of selected renal‐related AEs.
Table S2. Study design and patient population.


