Abstract
Bone infection is a common and serious complication in the orthopedics field, which often leads to excessive bone destruction and non‐union. Osteoclast is the only type of cells which have the function of bone resorption. Its over activation is closely related to excessive bone loss. Staphylococcus aureus (S. aureus) is a major pathogen causing bone infection, which can produce a large number of strong pathogenic substances staphylococcal protein A (SPA). However, few studies were reported about the effects of SPA on osteoclastogenesis. In our study, we observed that S. aureus activated osteoclasts and promoted bone loss in bone infection specimens. Then, we investigated the effects of SPA on RANKL‐induced osteoclastogenesis in vitro, the results revealed that SPA promoted osteoclastic differentiation and fusion, and enhanced osteoclastic bone resorption. In addition, we also showed that SPA upregulated the expression of NFATc1 and c‐FOS through the activation of MAPK signaling to promote osteoclastogenesis. Our findings might help us better understand the pathogenic role of S. aureus in bone infection and develop new therapeutic strategies for infectious bone diseases.
Keywords: bone resorption, MAP Kinase, osteoclast, staphylococcal protein A
1. INTRODUCTION
Bone infection is one of the most common and serious complications in fracture surgery. According to the International Association for the study of Internal Fixation (AO) reports, the infection rate associated with open fractures was about 3–40% (Moriarty, Schlegel, Perren, & Richards, 2010). Bone infection often leads to a series of serious consequences such as antimicrobial resistance, amputation, and even death (Onaizi & Leong, 2011). At the same time, Bone infection usually leads to excessive bone destruction, resulting in bone non‐union, and delayed fracture healing, which is the biggest clinical problem for patients and doctors (Kim et al., 2013). Staphylococcus aureus is a major pathogen in bone infection, accounting for 30% of the pathogens in bone infection (Trampuz & Zimmerli, 2006), which can produce a variety of virulence factors such as toxins, enzymes, and so on (Cakir Aktas, Erturan, Karatuna, & Karahasan Yagci, 2013; Tuchscherr et al., 2011). Meanwhile, many pathogenic components including SPA, lipoteichoic acid (LTA), and peptidoglycans are widely present in bacterial wall (Rodriguez‐Carballo, Gamez, & Ventura, 2016). In bone infection, the normal process of bone remodeling could be changed by acute and chronic inflammatory, which leads to the disorder of bone metabolism. Therefore, it is great significance to clarify the role of S. aureus infection in bone remodeling for the treatment of such diseases.
Staphylococcal protein A which widely presents in bacterial wall is one of the most important pathogenic components. Recently, it was found that SPA can be freely secreted into the extracellular environment and can widely bind to host defense proteins to alter the host immune response (Kobayashi & DeLeo, 2013). SPA could also inhibit osteoblast activity, prohibit osteogenesis through a variety of ways, which is an important cause of bone non‐union (Heilmann, 2011; Jauregui, Mansell, Jepson, & Jenkinson, 2013). However, there are few reports about the effects of SPA on osteoclastogenesis and its molecular mechanism.
Osteoclasts are derived from hematopoietic stem cell lineage, which is the only polykaryocytes with bone resorption function in the human skeletal system (Berglund, 2011; Rachner, Khosla, & Hofbauer, 2011). It is well known that macrophage colony stimulating factor (M‐CSF) and Receptor activator of nuclear factor (NF)‐κB ligand (RANKL) are two of the most important cytokines in the process of osteoclast differentiation (Teitelbaum & Ross, 2003). During RANKL induced osteoclastogenesis, Mitogen‐activated protein (MAP) kinases play an important role in regulating the proliferation, differentiation, and bone resorption function of osteoclast. MAP kinases, including ERK, P38, and JNK, which can lead to the activation of transcription factor NFATc1, and eventually promotes osteoclast formation and leads to excessive bone loss (Intini, Katsuragi, Kirkwood, & Yang, 2014). In our research, MAP kinases could be obviously activated by SPA in osteoclast differentiation.
In the present study, we found that SPA could promote osteoclast formation. At the same time, we reported that SPA may regulate osteoclast formation by activating MAP kinase.
2. MATERIALS AND METHODS
2.1. Reagents
RAW264.7 cells were purchased from the American Type Culture Collection (Rockville, MD). Fetal bovine serum (FBS) and Alpha minimal essential Medium (α‐MEM) were obtained from Gibco (life technologies, CA). Penicillin‐streptomycin solution was obtained from Hyclone (Thermo Scientific, MA). Recombinant Mouse M‐CS F and Recombinant Mouse RANKL were obtained from R&D Systems (Minneapolis, MN). Cell Counting Kit‐8 was obtained from Dojindo Molecular Technologies (Dojindo, Japan). TRAP stain kit was obtained from Sigma–Aldrich (St. Louis, MO). Actin Cytoskeleton and Focal Adhesion Staining Kit was purchased from Millipore (Darmstadt, Germany). Antibodies against P38, p‐P38, ERK, p‐ERK, JNK, p‐JNK, and β‐actin were purchased from Cell Signaling (Danvers, MA). ERK, JNK, and P38 MAP kinase inhibitors were purchased from Medchemexpress (Princeton, NJ). Bovine cortical bone slices for Pit formation assay were obtained from Boineslices.com (Jelling, Denmark). Antibodies against NFATC1 and c‐FOS were purchased from Abcam (Cambridge). Staphylococcal protein A was purchased from Sigma–Aldrich.
2.2. Clinical specimens and histological analysis
Three patients diagnosed with traumatic osteomyelitis infected by S. aureus were selected in this study. We also selected the other three patients suffered fracture as control group for the study. Considering the difference of bone mass in different ages, the age of patients ranged from 18 to 30 years old. All samples with the size of 1 × 1 cm were obtained from the fracture site during surgery. This research was approved by Ethics Committee of Daping hospital and performed according to relevant laws.
For histological analysis, samples were fixed in 4% paraformaldehyde for 24 hr. Samples were then decalcified by 10% EDTA solution for 1 month, and the solution was changed every twice days. Samples were prepared into 8 µm thickness bone slices after paraffin embedded, and standard TRAP and HE stain was performed, respectively.
2.3. Cell viability assay in vitro
RAW264.7 cells were cultured in DMEM containing 1% penicillin–streptomycin solution and 10% FBS at 37°C in 5% CO2 condition. RAW264.7 cells were seeded into 96‐well plates at a density of 3 × 103 per well. After adherence, cells were incubated with different dosages of SPA in the presence of 50 ng/ml RANKL and 50 ng/ml M‐CSF for 24 and 72 hr, respectively. Cell Counting Kit‐8 was used to assess cell viability according to the manufacturers’ directions. The absorbency of cells was measured using a 96‐well plate reader at 450 nm.
2.4. Cell apoptosis assay
RAW264.7 cells were cultured in the presence of 50 ng/ml RANKL and 50 ng/ml M‐CSF with different dosages of SPA (0, 0.1, 1, 10, 100 µg/ml) for 24 and 72 hr, respectively. Apoptosis rate of RAW264.7 cells was evaluated by V‐FITC Annexin apoptosis assay kit (Life Technologies) according to the manufacturers’ instructions. In brief, cells were stained with Annexin‐V‐FITC and PI about 15 min away from light. The apoptosis rate was then measured at 488 nm by flow cytometry.
2.5. Tartrate‐resistant acid phosphatase (TRAP) assay
RAW264.7 cells were cultured with 50 ng/ml RANKL and 50 ng/ml M‐CSF with SPA treatment (0, 0. 1, 1, 10, 100 µg/ml). TRAP stain was conducted as previously described (Dou et al., 2016). Briefly, after cultivation for 72 hr, cells were washed with PBS and fixed with 4% paraformaldehyde for 5 min, and then stained with TRAP staining solution for 1 hr at 37°C protected from light according to the protocol. Multinucleated TRAP‐positive cells containing three or more nuclei were considered as osteoclast.
2.6. Actin cytoskeleton and focal adhesion (FAK) staining
As TRAP staining, RAW264.7 cells were cultured in 96‐well plates (5 × 103 per well) with 50 ng/ml RANKL and 50 ng/ml M‐CSF with various concentration SPA treatment for 3 days. Cells were washed with PBS and fixed with 4% paraformaldehyde for 20 min, then permeabilized with 0.1% Triton X–100 for 5 min. Cells were next blocked with 1% BSA in 1× PBS and incubated in primary antibody solution for 1 hr at room temperature. After washing three times with 1× wash buffer, secondary antibody TRITC conjugated Phalloidin were diluted in PBS with ratio of 1:500, and then were incubated with cells for 45 min at room temperature. Finally, Nuclei counterstaining was performed by DAPI for 5 min. Fluorescence images can be visualized with a fluorescence microscope.
2.7. Bone resorption assay in vitro
RAW264.7 cells were seeded into 48‐well plates covered with bovine bone slices at a density of 1 × 104 cells/well and cultured overnight. Cells were cultured in DMEM containing 50 ng/ml RANKL and 50 ng/ml M‐CSF with different dosages of SPA for 5 days and medium was changed every 2 days. Bone resorption pits were stained with toluidine blue to assess bone resorption activity of osteoclast. Resorption areas were observed under a microscope and were analyzed by using ImageJ software (Dou et al., 2014).
2.8. Real‐time qPCR
Total RNA was extracted from cells by using Trizol reagent (Life Technologies). The cDNA was synthesized using reverse transcriptase and oligo‐dT primer. Then the cDNA samples and specific primers including NFATc1, c‐FOS, TRAP, CTSK, MMP9, CTR, OC‐STAMP, DC‐STAMP, and IRF9 were used for PCR amplification. GAPDH were used as internal controls. The specific primers are listed in Table 1.
Table 1.
Primer sequences for qPCR
| Genes | Forward | Reverse | Tm (°C) |
|---|---|---|---|
| TRAP | 5′‐CACTCCCACCCTGAGATTTGT‐3′ | 5′‐CATCGTCTGCACGGTTCTG‐3′ | 61.1 |
| Ctsk | 5′‐ GAAGAAGACTCACCAGAAGCAG‐3′ | 5′‐TCCAGGTTATGGGCAGAGATT‐3′ | 60.3 |
| MMP9 | 5′‐CTGGACAGCCAGACACTAAAG‐3′ | 5′‐CTCGCGGCAAGTCTTCAGAG‐3′ | 60 |
| Ctr | 5′‐CGCATCCGCTTGAATGTG‐3′ | 5′‐TCTGTCTTTCCCCAGGAAATGA‐3′ | 62 |
| OC‐STAMP | 5′‐GGGCTACTGGCATTGCTCTTAGT‐3′ | 5′‐CCAGAACCTTATATGAGGCGTCA‐3′ | 61.6 |
| DC‐STAMP | 5′‐CTAGCTGGCTGGACTTCATCC‐3′ | 5′‐TCATGCTGTCTAGGAGACCTC‐3′ | 62.2 |
| IRF9 | 5′‐GCCGAGTGGTGGGTAAGAC‐3′ | 5′‐GCAAAGGCGCTGAACAAAGAG‐3′ | 62 |
| NFATc1 | 5′‐GACCCGGAGTTCGACTTCG‐3′ | 5′‐TGACACTAGGGGACACATAACTG‐3′ | 61.4 |
| c‐FOS | 5′‐CGGGTTTCAACGCCGACTA‐3′ | 5′‐TTGGCACTAGAGACGGACAGA‐3′ | 61.5 |
| GAPDH | 5′‐AAATGGTGAAGGTCGGTGTG‐3′ | 5′‐ TGAAGGGGTCGTTGATGG‐3′ | 60.6 |
2.9. Western blots
Cells were lysed in PMSF buffer to extract total protein. The samples were transferred to the PVDF membrane using the wet transfer method. After blocking in TBST solution containing 5% skim milk for 2 hr, the bands were incubated with primary antibodies against ERK, JNK, P38 p‐ERK, p‐JNK, p‐P38, c‐FOS, NFATc1, and β‐actin overnight at 4°C. The bands were washed and incubated with secondary antibody for 1 hr at room temperature. β‐actin was performed as an internal control.
2.10. Statistical analysis
All experiments were performed at least three times. All data are expressed as mean ± SD. One‐way ANOVA was used to evaluated the significance of difference between two groups of data, with *p < 0.05, **p < 0.01 being regarded as significant.
3. RESULTS
3.1. S. aureus activated osteoclast and promoted bone loss in clinical specimens
The sections were stained with TRAP and HE stain solution and were then observed under the light microscope. Compared with infection group, the thickness of bone trabecula was thinner in non‐infection group (Figure 1A). Meanwhile, the results of TRAP staining showed that OC surface/bone surface ratio was significantly increased (Figure 1B). All results revealed that S. aureus activated osteoclast and its function.
Figure 1.
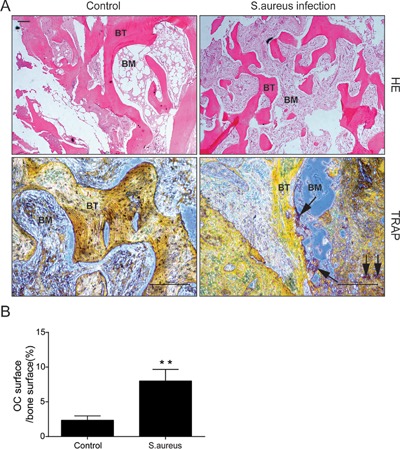
HE stain and TRAP stain of clinical samples. (A) Representative images of HE stain (4×) and TRAP stain (10×); Arrows show osteoclasts around bone trabecula; BM, bone marrow; BT, bone trabecula. Scalebars, 200 μm. (B) Quantitative analysis of OC suface/bone surface ratio
3.2. SPA toxicity evaluation and effects on cell apoptosis
RAW264.7 cells were incubated with different dosages of SPA with or without RANKL and M‐CSF for 24 and 72 hr, respectively. CCK‐8 assay was used to evaluate the effects of SPA on cell proliferation. The results revealed that SPA concentration at 1,000 µg/ml significantly inhibited cell proliferation (Figure 2A). In addition, the effect of SPA on cell apoptosis during RANKL induced osteoclastogenesis was assessed by Annexin‐V/PI Staining. The results showed that SPA had no effect on both early and late apoptosis rate (Supplementary Figure S1).
Figure 2.
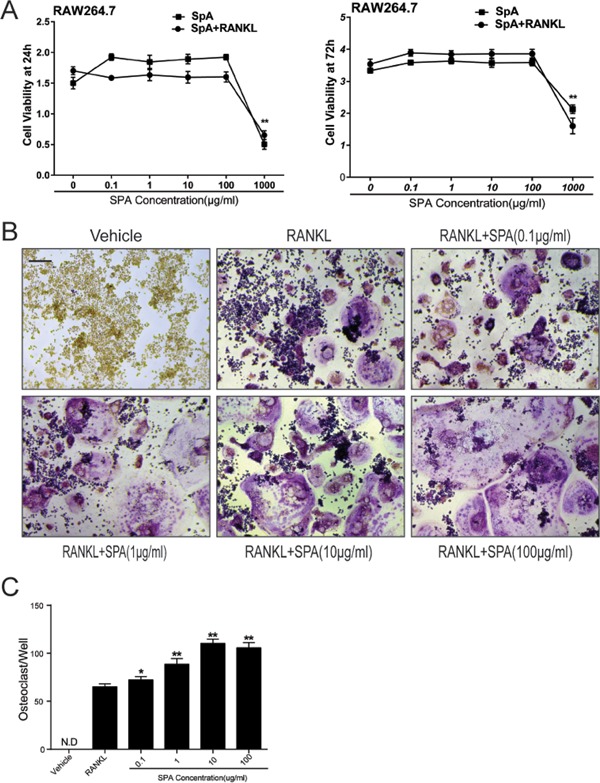
SPA toxicity evaluation and SPA promotes RANKL‐induced osteoclast differentiation. (A) CCK8 analysis of cell viability of RAW264.7 cells treated with different dosages of SPA for 24 and 72 hr with or without RANKL (50 ng/ml) and M‐CSF (50 ng/ml). (B) Representative images of RAW264.7 cells stained for Tartrate‐Resistant Acid Phosphatase (TRAP) (red) treated with RANKL (50 ng/ml) and M‐CSF (50 ng/ml) for 72 hr. According to different concentration of SPA treatment (0, 0. 1, 1, 10, 100 μg/ml), a control group and five experimental groups were set up. Scale bar represents 200 μm. (C) Quantification of osteoclasts (nuclei ≥ 3) in each well (96‐well plate). The data in the figures represent mean ± SD. Significant differences between the treatment and control groups are indicated as *(p < 0.05) or **(p < 0.01)
3.3. SPA promotes RANKL‐induced osteoclast differentiation in vitro
According to the results of CCK8 assay, SPA dosages at 0.1, 1, 10, and 100 µg/ml were selected for further study. RAW264.7 cells were cultured with different dosages of SPA in the presence of RANKL and M‐CSF for 72 hr. TRAP stain was performed in order to observe osteoclast differentiation (Figure 2B). The number of osteoclasts (nuclei ≥ 3) were counted. The results of statistical analysis showed that the number of osteoclasts in the experimental group increased significantly compared with the control group (Figure 2C). Interestingly, the number of osteoclasts were not significantly increased when the concentration of SPA was 100 µg/ml compared with SPA concentration at 10 µg/ml, but the images showed that the morphology of osteoclasts was larger than other groups. Consistently, qPCR results also revealed that osteoclastogenesis associated marker genes including CTSK, TRAP, CTR, and DC‐STAMP were significantly increased when SPA concentration was 10 or 100 µg/ml (Figure 3). All the results revealed that SPA promoted RANKL‐induced osteoclast differentiation in vitro.
Figure 3.
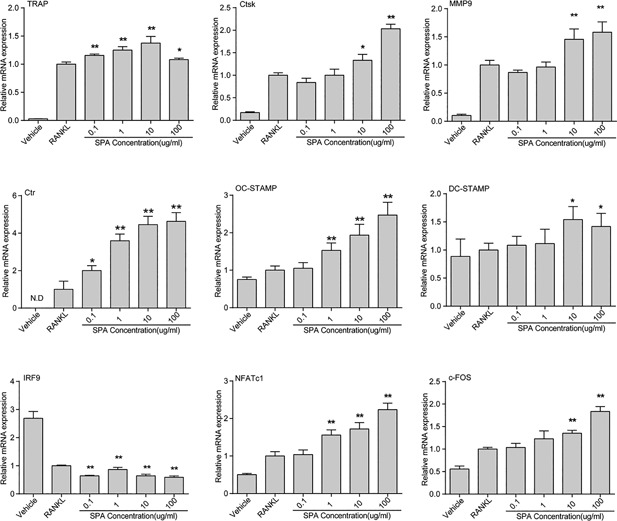
Relative mRNA expression during SPA treatment RAW264.7 cells were seeded (2 × 104 per well) into 24‐well plates and were cultured with different dosages of SPA for 24 and 72 hr with RANKL (50 ng/ml) and M‐CSF (50 ng/ml). TRAP, Ctsk, MMP9, Ctr, OC‐STAMP, DC‐STAMP, and IRF9 was detected at 72 hr. NFATc1 and c‐FOS was analyzed at 24 hr. The data in the figures represent mean ± SD. Significant differences between the treatment and control groups are indicated as *(p < 0.05) or **(p < 0.01)
3.4. SPA promoted RANKL‐induced osteoclast fusion in vitro
To investigate the effects of SPA on osteoclast fusion, we observed osteoclast cytoskeleton by FAK staining, and the number of osteoclasts and average nuclei were counted (Figure 4A). Quantification analysis showed that the number of osteoclasts and average nuclei of osteoclasts was significantly increased by SPA treatments (Figure 4B). As the results of TRAP stain, the number of osteoclasts was not increased when SPA concentration was 100 µg/ml compared with 10 µg/ml, but average nuclei number was increased significantly. Consequently, SPA could increase RANKL‐induced osteoclast formation.
Figure 4.
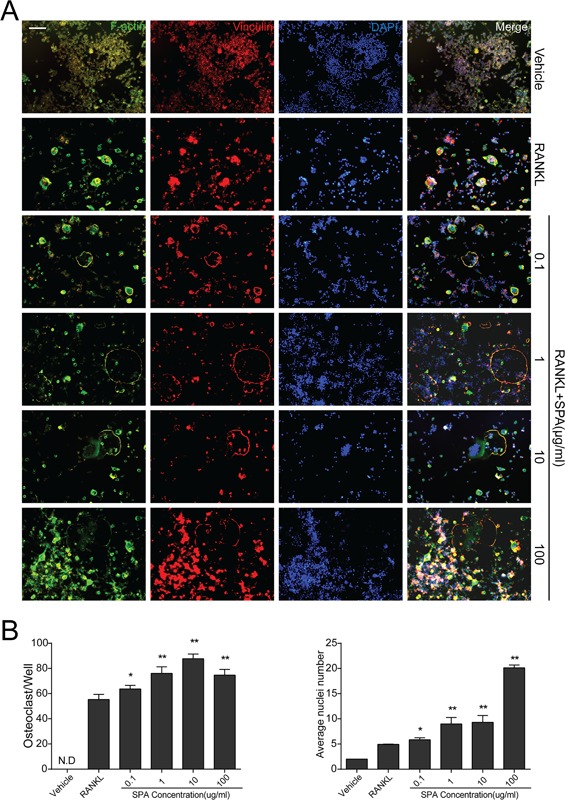
SPA promotes RANKL‐induced osteoclast fusion. (A) Representative images of focal and adhesion staining (FAK) of RAW264.7 cells treated with different concentrations of SPA treatments for 72 hr with RANKL (50 ng/ml) and M‐CSF (50 ng/ml). Experimental groups were the same as before. Scale bar represents 100 μm. (B) Quantification of osteoclasts (nuclei ≥ 3) and average nuclei number in each group (96‐well plate). The data in the figures represent mean ± SD. Significant differences between the treatment and control groups are indicated as *(p < 0.05) or **(p < 0.01)
3.5. SPA enhances RANKL‐induced osteoclast bone resorption activity in vitro
Bone resorption assay was carried on to evaluate the effects of SPA on osteoclast bone resorption activity. RAW264.7 cells were cultured in 48‐well plates covered with bovine bone slices RANKL, M‐CSF, and different dosages of SPA for 5 days. Bone resorption areas were analyzed to assess bone resorption activity of osteoclast (Figure 5A). Statistical results showed that bone resorption areas were significantly increased by SPA treatment (Figure 5B). The results showed that SPA could enhance osteoclast bone resorption activity.
Figure 5.
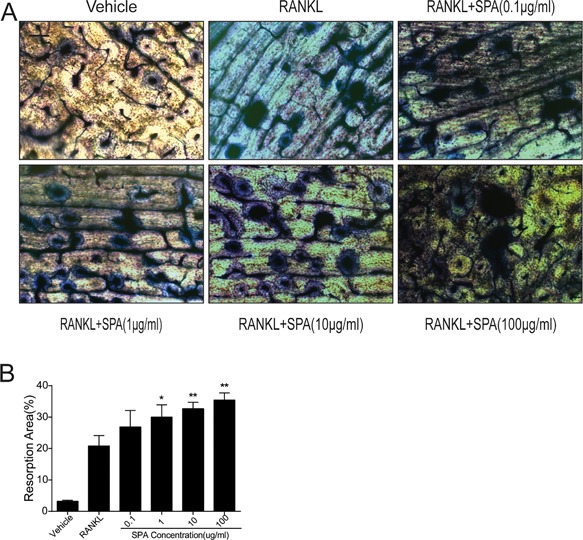
SPA promotes the bone resorption function of osteoclasts. (A) Representative images of RAW264.7 cells cultured on bovine slices treated with RANKL (50 ng/ml) and M‐CSF (50 ng/ml) for 5 days with different dosages of SPA (48‐well plate). Then, bovine slices were stained by toluidine blue. Scale bar represents 400 μm. (B) Quantification of bone resorption area on the bone slices
3.6. SPA promoted RANKL‐induced osteoclastogenesis by upregulating NFATc1 and c‐FOS through MAPK pathways
To explain the mechanism of SPA on osteoclast differentiation, we detected the expression of ERK, JNK, P38, and its phosphorylation levels p‐ERK, p‐JNK, and p‐P38 in MAPK pathways by Western blots. The results showed that the expression of p‐ERK, p‐JNK, and p‐P38 was increased compared with its non‐phosphorylation levels by SPA treatment (Figure 6A). At the same time, the expression of NFATc1 and c‐FOS which were downstream of MAPK signaling were tested (Qiao et al., 2016), and the results showed that the expression was significantly increased (Figure 6B–D).The expression of NFATc1 and c‐FOS were not increased when phosphorylation inhibitors of ERK, JNK, and P38 MAPK were administered (Figure 6E). We also detected the expression of NFATc1 and c‐FOS by qPCR (Figure 3), and the results showed that both of them were significantly increased which were consistent with the results of Western blots. In addition, IRF9, as a c‐FOS inhibitor gene, was detected by qPCR. The results showed that the expression was significantly decreased.
Figure 6.
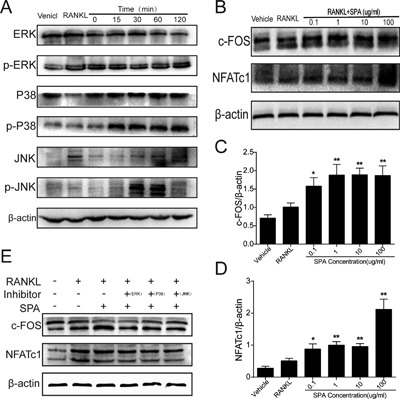
Relative proteins expression during SPA treatment (A) RAW264.7 cells were stimulated with 100 μg/ml SPA in presence of RANKL (50 ng/ml) and M‐CSF (50 ng/ml) for various time periods. Representative blots of phosphorylated or nonphosphorylated forms of MAPKs including ERK, JNK, and p38 are shown. (B) RAW264.7 cells were treated with different concentration of SPA (0, 0. 1, 1, 10, 100 μg/ml) in presence of RANKL (50 ng/ml) and M‐CSF (50 ng/ml) for 72 hr. Western blots were performed to analyze the protein expression of NFATc1 and c‐FOS. (C) and (D) Quantification of NFATc1 and c‐FOS protein levels normalized by β‐actin. (E) The protein expression of NFATc1 and c‐FOS when phosphorylation inhibitors of ERK, JNK, and P38 MAPK were administered. The data in the figures represent mean ± SD. Significant differences between the treatment and control groups are indicated as *(p < 0.05) or **(p < 0.01)
4. DISCUSSION
Most infectious bone diseases such as osteomyelitis, Orthopedic Device Related Infections (ODRI), and Periprothetic Joint Infections (PJI)are mainly caused by S. aureus (Cassat et al., 2013; Kahl, Becker, & Loffler, 2016; Vaudaux et al., 2012). In addition to the typical inflammation symptoms, the most characteristic of these diseases is accompanied by excessive bone destruction and bone non‐union (Olson & Horswill, 2013). Previous reports demonstrated that the femoral bone mass was lost about 10–20% in rat models which were infected by S. aureus (Mendoza Bertelli et al., 2016). In case of trauma or surgery, S. aureus could break through the host barrier for local colonization, is extremely easy to produce bacterial biofilm. Once the biofilm formed, it is able to protect S. aureus from being eliminated by the host immune defense and antibiotics, resulting in consecutively infection (Conlon et al., 2013). The products of S. aureus such as SPA could continue to stimulate osteoblast and osteoclast, leading to changing of the normal process in bone remodeling (Claro et al., 2011; Jin et al., 2013; Widaa, Claro, Foster, O'Brien, & Kerrigan, 2012). In our study, we found that SPA promotes osteoclast differentiation and fusion, and also enhances osteoclastic bone resorption activity.
Bone resorption is an important aspect of bone remodeling, which is mainly controlled by osteoclast. S. aureus and virulence factors can alter the biological behavior of osteoclast and the process of bone resorption. The results from our research support that the biological behavior of osteoclast is changed by SPA treatment. First, we collected clinical samples to observe the effects of S. aureus on bone remodeling, histological analysis indicated that osteoclast was over activated. Second, we investigated the effects of SPA on RANKL‐induced osteoclastogenesis in vitro. Osteoclasts number, average nuclei number and osteoclast resorption activity was significantly increased, the expression of specific genes including CTSK, TRAP, CTR, and DC‐STAMP were also significantly increased. All results demonstrated that SPA treatment can directly change the biological behavior of osteoclast to promote osteoclastogenesis. Our findings are consistent with previous reports that Staphylococcus aureus can promote osteoclast formation (Cavagis et al., 2014; Wei, Kitaura, Zhou, Ross, & Teitelbaum, 2005).
In addition, we found that MAP kinases including ERK, JNK, and P38 was notably activated by SPA treatment. In physiological conditions, the MAP kinases can regulate cell growth, differentiation and many kinds of inflammatory reactions (Rodriguez‐Carballo et al., 2016). ERK signaling is involved in the cell proliferation and differentiation, and the expression of p‐ERK can induce the osteoclast proliferation and differentiation. JNK signaling and p38 signaling play signaling a key role in the process of osteoclast formation, both of them can regulate downstream transcription factor NFATc1 which can promote osteoclast differentiation (Shang et al., 2016). Moreover, we found that the expression of IRF9 is reduced significantly with SPA treatments in the mRNA expression level. IRF9, as a c‐FOS inhibitor gene, can opposite regulate the expression of c‐FOS, and ultimately promote NFATc1 activation resulting in osteoclast formation (Takayanagi, 2003).
As we know, bone formation is another important aspect of bone remodeling, which is mainly controlled by osteoblast. Numerous studies have demonstrated that S. aureus and its virulence factors can decrease osteoblast activity and induce apoptosis through directly invading osteoblast or binding to surface receptors (Chen et al., 2014; Josse et al., 2016). Recently, it has been reported that SPA can accelerate apoptosis of osteoblast, suppress osteoblast proliferation, and inhibit the process of bone formation and mineralization (Kahl et al., 2016). Meanwhile, SPA may stimulate immune cells to produce a variety of inflammatory cytokines such as IFN‐γ, IL‐1, IL‐6, and TNF‐α which could synergize with RANKL to promote osteoclastogenesis (Huang, Drissi, O'Keefe, & Schwarz, 2003). Consequently, SPA may play an important role in bone non‐union and excessive bone destruction caused by bone infection. Nevertheless, S. aureus can produce a variety of virulence factors and its effect on bone remolding is very complex, which suggests that we need further research to understand the mechanism of bone infection.
In conclusion, our study demonstrated that SPA treatment promotes osteoclast differentiation and fusion, and increases bone resorption activity in vitro. We showed that SPA promotes osteoclastogenesis through MAPK signaling activation (Figure 7). The results could be helpful to better understand the pathogenicity of S. aureus in bone infection, and it is meaningful to cure such diseases.
Figure 7.
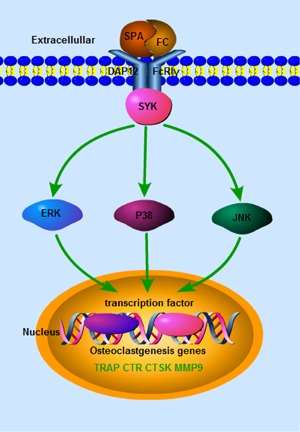
Schematic representation of the proposed action mechanism. SPA promotes Osteoclastogenesis by upregulating NFATc1 and c‐FOS through MAPK signaling
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Figures S1.
ACKNOWLEDGMENTS
This work was supported by Medical Research Funding of PLA of China (AWS14C003), Special Funds for Social Undertaking and Livelihood Security Projects of Chongqing (CSTC2016SHMSZX130068).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTIONS
Yuan Wang, Ce Dou, Shiwu Dong, Jun Fei contributed to design of the study. Yuan Wang, Ce Dou, Xin Liu, Chuan Liu contributed to acquisition of data. Yuan Wang Xin Liu, Jun Fei were involved in collection of Clinical specimens. Yuan Wang, Zhen Cao contributed to statistical analysis. Yuan Wang, Ce Dou, Shiwu Dong, Jun Fei wrote the manuscript. All authors approved the final version of manuscript.
Wang Y, Liu X, Dou C, et al. Staphylococcal protein A promotes osteoclastogenesis through MAPK signaling during bone infection. J Cell Physiol. 2017; 232: 2396–2406. https://doi.org/10.1002/jcp.25774
Contributor Information
Shiwu Dong, Email: dongshiwu@tmmu.edu.cn.
Jun Fei, Email: feijundoctor@sohu.com.
REFERENCES
- Berglund, J. (2011). Orthopaedics: Structural support. Nature, 480(7377), S56–S57. [DOI] [PubMed] [Google Scholar]
- Cakir Aktas, N. , Erturan, Z. , Karatuna, O. , & Karahasan Yagci, A. (2013). Panton‐Valentine leukocidin and biofilm production of Staphylococcus aureus isolated from respiratory tract. Journal of Infection in Developing Countries, 7(11), 888–891. [DOI] [PubMed] [Google Scholar]
- Cassat, J. E. , Hammer, N. D. , Campbell, J. P. , Benson, M. A. , Perrien, D. S. , Mrak, L. N. , … Skaar, E. P. (2013). A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host & Microbe, 13(6), 759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagis, A. , Takamori, E. , Granjeiro, J. , Oliveira, R. , Ferreira, C. , Peppelenbosch, M. , & Zambuzzi, W. (2014). TNFalpha contributes for attenuating both Y397FAK and Y416Src phosphorylations in osteoblasts. Oral Diseases, 20(8), 780–786. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Hou, T. , Luo, F. , Wu, X. , Xie, Z. , & Xu, J. (2014). Involvement of toll‐like receptor 2 and pro‐apoptotic signaling pathways in bone remodeling in osteomyelitis. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 34(6), 1890–1900. [DOI] [PubMed] [Google Scholar]
- Claro, T. , Widaa, A. , O'Seaghdha, M. , Miajlovic, H. , Foster, T. J. , O'Brien, F. J. , & Kerrigan, S. W. (2011). Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS ONE, 6(4), e18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon, B. P. , Nakayasu, E. S. , Fleck, L. E. , LaFleur, M. D. , Isabella, V. M. , Coleman, K. , … Lewis, K. (2013). Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature, 503(7476), 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, C. , Li, J. , Kang, F. , Cao, Z. , Yang, X. , Jiang, H. , … Dong, S. (2016). Dual Effect of Cyanidin on RANKL‐Induced Differentiation and Fusion of Osteoclasts. Journal of Cellular Physiology, 231(3), 558–567. [DOI] [PubMed] [Google Scholar]
- Dou, C. , Zhang, C. , Kang, F. , Yang, X. , Jiang, H. , Bai, Y. , … Dong, S. (2014). MiR‐7b directly targets DC‐STAMP causing suppression of NFATc1 and c‐Fos signaling during osteoclast fusion and differentiation. Biochimica Et Biophysica acta, 1839(11), 1084–1096. [DOI] [PubMed] [Google Scholar]
- Heilmann, C. (2011). Adhesion mechanisms of staphylococci. Advances in Experimental Medicine and Biology, 715, 105–123. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Drissi, M. H. , O'Keefe, R. J. , & Schwarz, E. M. (2003). A rapid multiparameter approach to study factors that regulate osteoclastogenesis: Demonstration of the combinatorial dominant effects of TNF‐alpha and TGF‐ss in RANKL‐mediated osteoclastogenesis. Calcified Tissue International, 73(6), 584–593. [DOI] [PubMed] [Google Scholar]
- Intini, G. , Katsuragi, Y. , Kirkwood, K. L. , & Yang, S. (2014). Alveolar bone loss: Mechanisms, potential therapeutic targets, and interventions. Advances in Dental Research, 26(1), 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui, C. E. , Mansell, J. P. , Jepson, M. A. , & Jenkinson, H. F. (2013). Differential interactions of Streptococcus gordonii and Staphylococcus aureus with cultured osteoblasts. Molecular Oral Microbiology, 28(4), 250–266. [DOI] [PubMed] [Google Scholar]
- Jin, T. , Zhu, Y. L. , Li, J. , Shi, J. , He, X. Q. , Ding, J. , & Xu, Y. Q. (2013). Staphylococcal protein A, Panton‐Valentine leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 32(2), 322–333. [DOI] [PubMed] [Google Scholar]
- Josse, J. , Guillaume, C. , Bour, C. , Lemaire, F. , Mongaret, C. , Draux, F. , … Gangloff, S. C. (2016). Impact of the maturation of human primary bone‐Forming cells on their behavior in acute or persistent staphylococcus aureus infection models. Frontiers in Cellular and Infection Microbiology, 6, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl, B. C. , Becker, K. , & Loffler, B. (2016). Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clinical Microbiology Reviews, 29(2), 401–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Yang, J. , Park, O. J. , Kang, S. S. , Kim, W. S. , Kurokawa, K. , … Han, S. H. (2013). Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 28(11), 2381–2391. [DOI] [PubMed] [Google Scholar]
- Kobayashi, S. D. , & DeLeo, F. R. (2013). Staphylococcus aureus protein A promotes immune suppression. mBio, 4(5), e00764–e00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza Bertelli, A. , Delpino, M. V. , Lattar, S. , Giai, C. , Llana, M. N. , Sanjuan, N. , … Gomez, M. I. (2016). Staphylococcus aureus protein A enhances osteoclastogenesis via TNFR1 and EGFR signaling. Biochimica Et Biophysica Acta, 1862(10), 1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty, T. F. , Schlegel, U. , Perren, S. , & Richards, R. G. (2010). Infection in fracture fixation: Can we influence infection rates through implant design? Journal of Materials Science Materials in Medicine, 21(3), 1031–1035. [DOI] [PubMed] [Google Scholar]
- Olson, M. E. , & Horswill, A. R. (2013). Staphylococcus aureus osteomyelitis: Bad to the bone. Cell Host & Microbe, 13(6), 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaizi, S. A. , & Leong, S. S. (2011). Tethering antimicrobial peptides: Current status and potential challenges. Biotechnology Advances, 29(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Qiao, H. , Wang, T. Y. , Yu, Z. F. , Han, X. G. , Liu, X. Q. , Wang, Y. G. , … Tang, T. T. (2016). Structural simulation of adenosine phosphate via plumbagin and zoledronic acid competitively targets JNK/Erk to synergistically attenuate osteoclastogenesis in a breast cancer model. Cell Death & Disease, 7, e2094. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rachner, T. D. , Khosla, S. , & Hofbauer, L. C. (2011). Osteoporosis: Now and the future. Lancet, 377(9773), 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Carballo, E. , Gamez, B. , & Ventura, F. (2016). P38 MAPK signaling in osteoblast differentiation. Frontiers in Cell and Developmental Biology, 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, W. , Zhao, L. J. , Dong, X. L. , Zhao, Z. M. , Li, J. , Zhang, B. B. , & Cai, H. (2016). Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c‐Fos/NFATc1 signaling pathways. Molecular Medicine Reports, 14(4), 3620–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi, H. (2003). Signaling mechanism in the regulation of osteoclast differentiation by the immune system. Seikagaku The Journal of Japanese Biochemical Society, 75(12), 1535–1540. [PubMed] [Google Scholar]
- Teitelbaum, S. L. , & Ross, F. P. (2003). Genetic regulation of osteoclast development and function. Nature Reviews Genetics, 4(8), 638–649. [DOI] [PubMed] [Google Scholar]
- Trampuz, A. , & Zimmerli, W. (2006). Diagnosis and treatment of infections associated with fracture‐fixation devices. Injury, 37(Suppl 2), S59–S66. [DOI] [PubMed] [Google Scholar]
- Tuchscherr, L. , Medina, E. , Hussain, M. , Volker, W. , Heitmann, V. , Niemann, S. , … Loffler, B. (2011). Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Molecular Medicine, 3(3), 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux, P. , Ferry, T. , Uckay, I. , Francois, P. , Schrenzel, J. , Harbarth, S. , & Renzoni, A. (2012). Prevalence of isolates with reduced glycopeptide susceptibility in orthopedic device‐related infections due to methicillin‐resistant Staphylococcus aureus. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology, 31(12), 3367–3374. [DOI] [PubMed] [Google Scholar]
- Wei, S. , Kitaura, H. , Zhou, P. , Ross, F. P. , & Teitelbaum, S. L. (2005). IL‐1 mediates TNF‐induced osteoclastogenesis. The Journal of Clinical Investigation, 115(2), 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaa, A. , Claro, T. , Foster, T. J. , O'Brien, F. J. , & Kerrigan, S. W. (2012). Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS ONE, 7(7), e40586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Figures S1.


