Abstract
Background
Continuous treatment is recommended for patients with moderate‐to‐severe psoriasis; however, treatment may need to be interrupted in routine clinical practice.
Objective
To assess outcomes in patients continuously treated with ixekizumab versus those who interrupted therapy and were subsequently retreated with ixekizumab (IXE).
Methods
This analysis used data pooled from two phase 3 trials, UNCOVER‐1 and UNCOVER‐2. Patients were randomized to placebo (PBO), IXE every 4 (Q4W) or IXE every 2 weeks (Q2W) for 12 weeks. Patients with a static Physician's Global Assessment (sPGA) 0, 1 at Week 12 were rerandomized to IXEQ4W, IXE every 12 weeks (not presented) or PBO. We examined outcomes in patients who were continuously treated (IXEQ2W/IXEQ4W; IXEQ4W/IXEQ4W) or withdrawn (IXEQ2W/PBO; IXEQ4W/PBO), and in patients who were withdrawn and retreated with IXEQ4W for 24 weeks after disease relapse (sPGA ≥3).
Results
A total of 1226 treated patients achieved an sPGA 0, 1 at Week 12 and entered the maintenance phase; of these patients, 402 and 416 were rerandomized to PBO and IXEQ4W, respectively. Among patients interrupting treatment, 157 (82.2%) of IXEQ4W/PBO and 176 (83.4%) of IXEQ2W/PBO had an sPGA ≥3 by Week 60; median time to relapse was approximately 20 weeks irrespective of induction dose. At Week 60, continuously treated patients maintained high levels of PASI and sPGA responses (90.0% PASI 75 IXEQ2W/IXEQ4W; 81.9% sPGA 0, 1 IXEQ2W/IXEQ4W, non‐responder imputation). After 24 weeks of retreatment with IXEQ4W (IXEQ2W/PBO/IXEQ4W and IXEQ4W/PBO/IXEQ4W), 87.0% (107 of 123) and 95.1% (97 of 102) (observed), respectively, of patients recaptured PASI 75 and 70.7% (104 of 147) and 82.3% (107 of 130) (observed) recaptured an sPGA 0, 1. Overall, adverse events in continuously treated and retreated patients were comparable.
Conclusion
High levels of response were sustained with continuous ixekizumab treatment through 60 weeks. Most patients who were withdrawn experienced disease relapse, and most of those patients recaptured response after 24 weeks of retreatment.
Introduction
The treatment of psoriasis, an incurable disease, has focused on long‐term reduction in the severity and extent of disease. Hence, continuous therapy has been recommended for optimal long‐term management of moderate‐to‐severe psoriasis.1, 2 However, there are reasons why treatment interruption followed by retreatment with the same drug is required.3 These may include infection, pregnancy and issues such as compliance, drug holidays and loss of insurance coverage.1, 2, 3, 4 The duration of remission following treatment withdrawal is variable,2, 5 perhaps because of mechanism of action and pharmacokinetics of specific drugs, the natural course of the disease and the definition of relapse.
When oral and biologic systemic treatments are interrupted, psoriasis plaques invariably reappear, and reduced efficacy is often observed upon retreatment.6, 7, 8, 9 Although loss of response or suboptimal ability to recapture response to biologics has been suggested to be attributed to development of neutralizing antidrug antibodies (nADA), this does not explain lack of recapture in the subgroup of patients treated with small molecules, which are unlikely to induce nADA.6 In this regard, the fluctuating nature of the disease and optimal strategies for timing of interruption and retreatment have been discussed.6, 9, 10 Thus, it is important to understand the probability and timing of disease recurrence and return of symptoms. It is also critical to know whether retreatment leads to recapture of clinical response.2
Ixekizumab is a high‐affinity monoclonal antibody that selectively targets interleukin (IL)‐17A.11 It has been studied in three randomized, placebo‐controlled, double‐blind clinical trials (UNCOVER‐1, UNCOVER‐2 and UNCOVER‐3)12, 13 and has demonstrated high skin clearance of plaque psoriasis at Week 12, with response maintenance for 60 weeks in a majority of patients in all three trials.12, 13 We report a pooled analysis of two phase 3 ixekizumab trials (UNCOVER‐1 and UNCOVER‐2) with treatment and interruption/retreatment periods; this analysis evaluates safety and efficacy outcomes among patients who were continuously treated, withdrawn from therapy, or withdrawn and retreated after experiencing disease worsening.
Methods
Study design and patients
UNCOVER‐1 (NCT01474512) and UNCOVER‐2 (NCT01597245) are multicentre, phase 3, randomized, double‐blind, placebo‐controlled trials.12, 13 Patients ≥18 years with chronic plaque psoriasis ≥6 months prior to randomization were eligible.12, 13 At screening and baseline, eligible patients had a static Physician's Global Assessment (sPGA) score ≥3; Psoriasis Area and Severity Index (PASI) ≥12; and ≥10% affected body surface area. Trials were compliant with applicable guidelines. Ethical review boards approved protocols and informed consent forms. Signed informed consent was obtained prior to study‐related procedures.
Study design and treatment
In UNCOVER‐1, patients were randomized 1 : 1 : 1 to receive subcutaneous ixekizumab 80 mg every 2 weeks (IXEQ2W), ixekizumab 80 mg every 4 weeks (IXEQ4W) (each with a 160 mg starting dose) or placebo (PBO) for 12 weeks (Fig. 1).12 Randomization was stratified by geographic region (North America or other), previous non‐biologic systemic therapy (inadequate response to, intolerance to or contraindication to <3 or ≥3 conventional systemic therapies) and weight category (<100 kg or ≥100 kg). In UNCOVER‐2, patients were randomized 2 : 2 : 2 : 1 to receive IXEQ2W, IXEQ4W (each with a 160 mg starting dose), etanercept 50 mg twice weekly or placebo for 12 weeks (Fig. 1).13 Randomization was stratified by centre.
Figure 1.
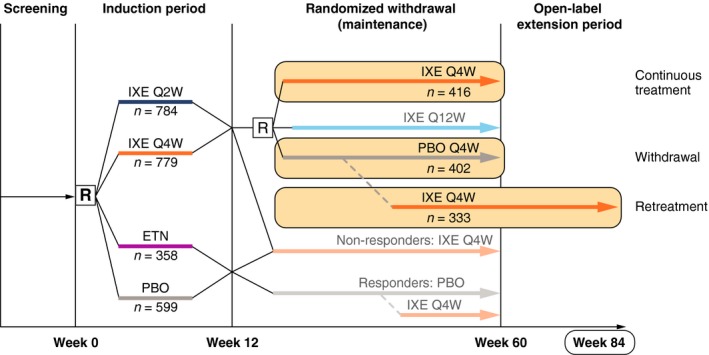
Study Diagram. The study diagram is shown. In UNCOVER‐1 and UNCOVER‐2, the 12‐week induction period was followed by a 48‐week randomized withdrawal (maintenance) period (weeks 12–60), whereby ixekizumab responders at Week 12 (sPGA = 0 or 1) were rerandomized to placebo (withdrawal group), IXEQ4W (continuously treated groups) or ixekizumab 80 mg every 12 weeks (IXEQ12W; not included in analyses). Patients in the withdrawal group who experienced disease worsening (sPGA ≥3 [relapse]) during the maintenance period received open‐label IXEQ4W (retreatment group). Although the maintenance period ended at Week 60, additional data from the long‐term extension period were used up to and including Week 84 to allow for 24 weeks of retreatment. At the time of this database lock, all patients completed Week 60. Not all retreated patients had the opportunity to receive 24 weeks of retreatment. ETN, etanercept; IXE Q2W, ixekizumab 80 mg every 2 weeks; IXE Q4W, ixekizumab 80 mg every 4 weeks; IXEQ12W, ixekizumab 80 mg every 12 weeks; n, number in group; PBO, placebo; R, randomize.
In both trials, the 12‐week induction period was followed by a 48‐week randomized withdrawal (maintenance) period (weeks 12–60), whereby ixekizumab responders at Week 12 (sPGA = 0 or 1) were rerandomized (1 : 1 : 1) to receive subcutaneous placebo, IXEQ4W or ixekizumab 80 mg every 12 weeks (IXEQ12W)12 (Fig. 1). At Week 60, all patients receiving IXEQ4W entered the long‐term extension period and continued to receive IXEQ4W. Visits occurred every 4 weeks during the maintenance period and every 12 weeks during the long‐term extension period. Patients who were rerandomized to IXEQ12W were not included in these analyses. Patients in any group who experienced disease worsening defined by an sPGA ≥3 (relapse) during the maintenance period received open‐label IXEQ4W.
Relapse and retreatment criteria were chosen in consultation with, and agreed to by, regulatory authorities. Cut‐offs were chosen to clearly identify changes while minimizing exposure to placebo.
Objective, populations and outcomes
The objective was to evaluate outcomes in patients who were continuously treated with ixekizumab (Week 12 responders who were rerandomized to IXEQ4W) or withdrawn from treatment (Week 12 responders who were rerandomized to placebo) during the 48‐week maintenance period. Additionally, we evaluated safety and efficacy outcomes in patients who were retreated with IXEQ4W after experiencing disease worsening during withdrawal.
In each population, mean PASI and Itch Numeric Rating Scale (NRS) were evaluated. Additionally, the proportions of patients with ≥75%, ≥90% and 100% improvement in PASI over baseline (PASI 75, PASI 90, PASI 100) or sPGA 0, and sPGA 0, 1 were evaluated. The PASI is a physician‐reported measure combining lesion severity and affected area into a score ranging from 0 (no disease) to 72 (maximal disease).14 The physician‐reported sPGA rated lesions as follows: 0 = clear, 1 = minimal, 2 = mild, 3 = moderate; 4 = severe, 5 = very severe. The Itch NRS is a patient‐reported outcome whereby patients rate their itch on a numeric scale of 0 (no itch) to 10 (worst itch imaginable) in a 24‐h period.15
Among continuously treated patients and withdrawn patients, response rates were summarized through the maintenance period (weeks 12–60). In addition, mean PASI and Itch NRS were calculated for these groups for the induction period and maintenance periods (weeks 0–60). Patients in the continuously treated group who experienced disease worsening continued to receive IXEQ4W (open label) per protocol, and, therefore, data for these patients were analysed in the continuously treated group through Week 60 irrespective of having prior sPGA ≥3. Patients in the withdrawal group who experienced disease worsening at one visit received open‐label IXEQ4W. These patients were considered non‐responders at all subsequent visits; data for these patients were not analysed in the withdrawal group after the time of relapse.
Among patients who were withdrawn and retreated, the time of sPGA ≥3 was considered retreatment Week 0. Response rates were summarized over time from retreatment Week 0 through retreatment Week 24. sPGA 0,1 response rates were calculated for all patients who experienced disease worsening (sPGA ≥3). PASI 75, PASI 90 and PASI 100 response rates were calculated for patients who had sPGA ≥3 and did not have a PASI 75 at the time of sPGA ≥3; the baseline PASI was used in the calculations. Mean PASI and Itch NRS were calculated for 24 weeks of retreatment. Although the maintenance period ended at Week 60, additional data from the long‐term extension period were used up to and including Week 84. This allowed analysis of 24 weeks of retreatment for some additional patients who had an sPGA ≥3 after Week 36. At the time of this database lock, all patients completed Week 60. Not all retreated patients had the opportunity to receive 24 weeks of retreatment.
Adverse events (AEs) were classified based on the Medical Dictionary for Regulatory Activities version 18.0. A treatment‐emergent AE (TEAE) was defined as an event that first occurred or worsened in severity in the maintenance period; all events starting or worsening in the relapse period were counted in the retreatment period and were not included in the maintenance period. Exposure‐adjusted incidence rates (IRs) for TEAEs report the number of unique patients with a specific event per 100 patient‐years of exposure; this calculation uses the sum of all patients' time (in 100 years) of exposure during the treatment period. For each patient, the entire time during the treatment period was counted as exposure time. An event was only counted once if it occurred multiple times in each patient.
Statistical analyses
All patients were analysed per assigned treatment in UNCOVER‐1 and UNCOVER‐2, including the re‐assignment of IXEQ4W to relapsed patients. Categorical efficacy measures were compared using Cochran–Mantel–Haenszel (CMH) tests stratified by study. Categorical variables were analysed using observed data as well as using non‐responder imputation (NRI), which imputes missing values as non‐responders for both the continuously treated and interrupted patients, for Week 60 comparisons. Continuous PASI measures were analysed using an analysis of covariance model on observed values with main effects for treatment and study, and baseline as a covariate. The median time to relapse is the simple arithmetic median time of relapse for each patient from the start of maintenance to first relapse. Safety analyses were conducted on Week 12 sPGA 0, 1 responders entering the maintenance phase. Adverse events are displayed as counts, percentages and IRs for each group.
The sPGA 0, sPGA 0,1, PASI 75, PASI 90 and PASI 100 response rates and the associated 95% confidence intervals (CI) were computed for treatment groups using the Wilson score method without continuity correction and displayed across the maintenance and retreatment periods. For sPGA and PASI outcomes, a comparison of response rates at Week 60 was performed to compare continuous treatment to interrupted treatment (IXEQ4W/IXEQ4W versus IXEQ4W/PBO and IXEQ2W/IXEQ4W versus IXEQ2W/PBO) using a CMH test stratified by study.
Serum samples
Serum samples collected through Week 60 were analysed for antidrug antibodies (ADA) to ixekizumab. Samples were collected at weeks 0, 4, 8 and 12, then every 12 weeks to Week 60 and then every 24 weeks thereafter. Antidrug antibodies were detected using a competitive ligand‐binding format.16
Results
Demographics and disposition
Overall, 1226 ixekizumab‐treated patients in UNCOVER‐1 and UNCOVER‐2 achieved an sPGA of 0 or 1 by Week 12 and entered the maintenance phase. These patients were rerandomized to placebo (n = 402), IXEQ4W (n = 416) or IXEQ12W (n = 408) (IXEQ12W group was excluded from this analysis) during maintenance (Fig. 1). Table 1 shows patient disposition for rerandomized patients during maintenance. Patient disposition diagrams were published.12 Treatment arms were well balanced in terms of demographic and disease characteristics (Table 2).
Table 1.
Patient disposition from study treatment: maintenance period (UNCOVER‐1 and UNCOVER‐2)
| IXEQ2W/PBO N = 211 | IXEQ2W/IXEQ4W N = 221 | IXEQ4W/PBO N = 191 | IXEQ4W/IXEQ4W N = 195 | |
|---|---|---|---|---|
| Completed Period, n (%) | 23 (10.9) | 184 (83.3) | 18 (9.4) | 143 (73.3) |
| Relapsed, n (%) | 176 (83.4) | 27 (12.2) | 157 (82.2) | 36 (18.5) |
| Discontinued, n (%) | 11 (5.2) | 11 (5.0) | 16 (8.4) | 17 (8.7) |
| Adverse event | 2 (0.9) | 6 (2.7) | 6 (3.1) | 7 (3.6) |
| Subject decision | 3 (1.4) | 2 (0.9) | 6 (3.1) | 4 (2.1) |
| Lost to follow‐up | 3 (1.4) | 1 (0.5) | 3 (1.6) | 3 (1.5) |
| Death | 0 | 1 (0.5) | 0 | 1 (0.5) |
| Investigator decision | 0 | 1 (0.5) | 0 | 1 (0.5) |
| Lack of efficacy | 1 (0.5) | 0 | 0 | 1 (0.5) |
| Protocol violation | 1 (0.5) | 0 | 1 (0.5) | 0 |
IXEQ2W, ixekizumab 80 mg every 2 weeks; IXEQ4W, ixekizumab 80 mg every 4 weeks; N, population size; n, number in group; PBO, placebo.
Table 2.
Baseline demographics: maintenance period (UNCOVER‐1 and UNCOVER‐2)
| Characteristic | IXEQ2W/PBO N = 211 | IXEQ2W/IXEQ4W N = 221 | IXEQ4W/PBO N = 191 | IXEQ4W/IXEQ4W N = 195 |
|---|---|---|---|---|
| Age mean (SD), years | 44.5 (12.6) | 43.5 (13.0) | 44.0 (13.2) | 44.1 (13.2) |
| Male, n (%) | 140 (66.4) | 151 (68.3) | 124 (64.9) | 136 (69.7) |
| Race, n (%)a | ||||
| Asian | 8 (3.8) | 8 (3.6) | 9 (4.7) | 9 (4.6) |
| Black or African American | 3 (1.4) | 3 (1.4) | 7 (3.7) | 2 (1.0) |
| White | 198 (93.8) | 208 (94.1) | 172 (90.5) | 184 (94.4) |
| Other or multiple | 2 (0.9) | 2 (0.9) | 2 (1.0) | 0 |
| BMI mean, kg/m2 (SD)a | 30.1 (6.1) | 30.2 (7.0) | 30.1 (6.8) | 30.1 (6.5) |
| Duration of psoriasis symptoms mean, years (SD) | 19.7 (12.2) | 18.4 (12.1) | 18.4 (10.7) | 20.4 (13.1) |
| BSA involved, % (SD) | 26.7 (17.6) | 27.1 (16.5) | 26.0 (15.2) | 25.8 (14.8) |
| Itch NRS score, mean (SD)b | 6.8 (2.5) | 6.5 (2.6) | 6.8 (2.6) | 6.6 (2.7) |
| PASI score, mean (SD) | 19.8 (7.9) | 19.4 (7.3) | 19.4 (6.2) | 19.3 (6.8) |
| sPGA score, mean (SD) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 3.6 (0.6) |
BSA, body surface area; BMI, body mass index; IXEQ2W, ixekizumab 80 mg every 2 weeks; IXEQ4W, ixekizumab 80 mg every 4 weeks; N, population size; n, number in group; NRS, Numeric Rating Scale; PASI, Psoriasis Area and Severity Index; PBO, placebo, sPGA, static Physician's Global Assessment; SD, standard deviation.
n = 190 for IXEQ4W/PBO.
n = 193 for IXEQ4W/IXEQ4W.
Efficacy
Withdrawal and continuous treatment
At Week 60 using NRI, 81.9% of patients with IXEQ2W induction dose and randomized to IXEQ4W during maintenance had an sPGA 0, 1 compared to 7.6% of patients who were randomized to placebo during maintenance (P < 0.001; continuous vs. withdrawal of treatment); 74.4% of patients with IXEQ4W induction dose and randomized to IXEQ4W continuous treatment had an sPGA 0, 1 compared to 6.3% of patients randomized to placebo during maintenance (P < 0.001; continuous vs. withdrawal of treatment) (Fig. 2a,b). High response rates were also attained or maintained through Week 60 for PASI 75, PASI 90 and PASI 100 for both IXEQ2W/IXEQ4W and IXEQ4W/IXEQ4W groups compared to the withdrawal group (Fig. 2).
Figure 2.
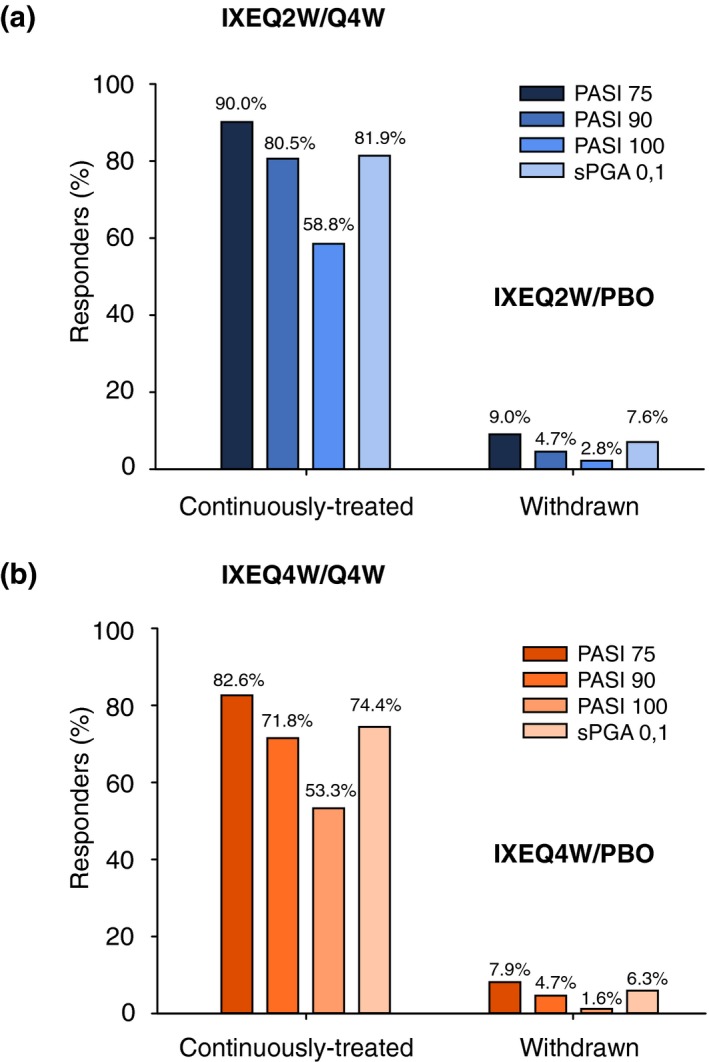
Response at Week 60 in Patients Continuously Treated and Withdrawn from Ixekizumab. Per cent responders using non‐responder imputation is shown. (a) IXEQ2W; (b) IXEQ4W. The numbers above the bars are the per cent responders. IXE, ixekizumab; PASI 75, 75% reduction in the Psoriasis Area and Severity Index; PASI 90, 90% reduction in the Psoriasis Area and Severity Index; PASI 100, 100% reduction in the Psoriasis Area and Severity Index; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks; sPGA, static Physician's Global Assessment.
Among patients who were withdrawn from therapy, 176 (83.4%) of IXEQ2W/PBO and 157 (82.2%) of IXEQ4W/PBO had disease worsening (sPGA ≥3) by Week 60; median time to relapse (sPGA ≥3) was 20.4 weeks for IXEQ2W/PBO and 20.1 weeks for IXEQ4W/PBO. In addition, 149 of 176 (IXEQ2W/PBO) and 124 of 157 (IXEQ4W/PBO) patients had disease worsening with a loss of the initial PASI 75 at the time of disease worsening.
Among patients who interrupted ixekizumab treatment, mean (standard deviation [SD]) PASI increased gradually from Week 12 (0.9 [1.25] IXEQ2W/PBO; 0.9 [1.24] IXEQ4W/PBO) to relapse (9.2 [5.00] IXEQ2W/PBO; 7.9 [4.34] IXEQ4W/PBO) (Fig. 3a). In contrast, low mean [SD] PASI was sustained from Week 12 (1.1 [1.44] IXEQ2W/IXEQ4W; 1.2 [1.66] IXEQ4W/IXEQ4W) through Week 60 (1.0 [2.46] IXEQ2W/IXEQ4W; 1.2 [2.31] IXEQ4W/IXEQ4W) among patients continuing ixekizumab treatment.
Figure 3.

Mean PASI and Itch NRS Score Over Time. Data, including the induction period, are from sPGA 0,1 responders at Week 12. (a) PASI; (b) Itch NRS. The number of patients in each group at each time is shown at the bottom of the graph. NRS, Numeric Rating Scale; IXEQ2W, 80 mg ixekizumab every 2 weeks; IXEQ4W, 80 mg ixekizumab every 4 weeks; PASI, Psoriasis Area and Severity Index; PBO, placebo; IXE Q2W, ixekizumab 80 mg every 2 weeks; IXE Q4W, ixekizumab 80 mg every 4 weeks; sPGA, static Physician's Global Assessment; Q2W, every 2 weeks; Q4W, every 4 weeks.
Mean Itch NRS scores followed the same trends as PASI (Fig. 3b). In patients interrupted from treatment, mean [SD] Itch NRS scores increased from withdrawal at Week 12 (1.0 [1.37] IXEQ2W/PBO; 1.0 [1.30] IXEQ4W/PBO) to relapse (5.0 [2.75] IXEQ2W/PBO; 4.5 [2.80] IXEQ4W/PBO). In continuously treated patients, however, mean [SD] Itch NRS scores remained mostly constant and low from Week 12 (1.1 [1.46] IXEQ2W/IXEQ4W; 1.0 [1.47] IXEQ4W/IXEQ4W) through Week 60 (0.8 [1.47] IXEQ2W/IXEQ4W; 1.1 [1.91] IXEQ4W/IXEQ4W).
Retreatment period
Among patients who had disease worsening (sPGA ≥3), sPGA 0, 1 responses were recaptured in 70.7% of IXEQ2W/PBO/IXEQ4W‐ (Fig. 4a) and 82.3% of IXEQ4W/PBO/IXEQ4W‐treated patients after 24 weeks of retreatment. Likewise, among those who had disease worsening (sPGA ≥3) and lost the initial PASI 75 response, 87.0% and 95.1% of patients from the IXEQ2W and IXEQ4W induction arms, respectively, recaptured PASI 75 after 24 weeks of retreatment with IXEQ4W (Fig. 4b). Furthermore, 63.4% and 78.4% of these patients, respectively, achieved PASI 90, and 31.7% and 45.1% of these patients, respectively, achieved PASI 100 after 24 weeks of retreatment. Comparable results were observed at Week 12 (Fig. 4). Median time to recapture of PASI 75 and itch was 4.0 weeks for both IXEQ2W and IXEQ4W arms.
Figure 4.
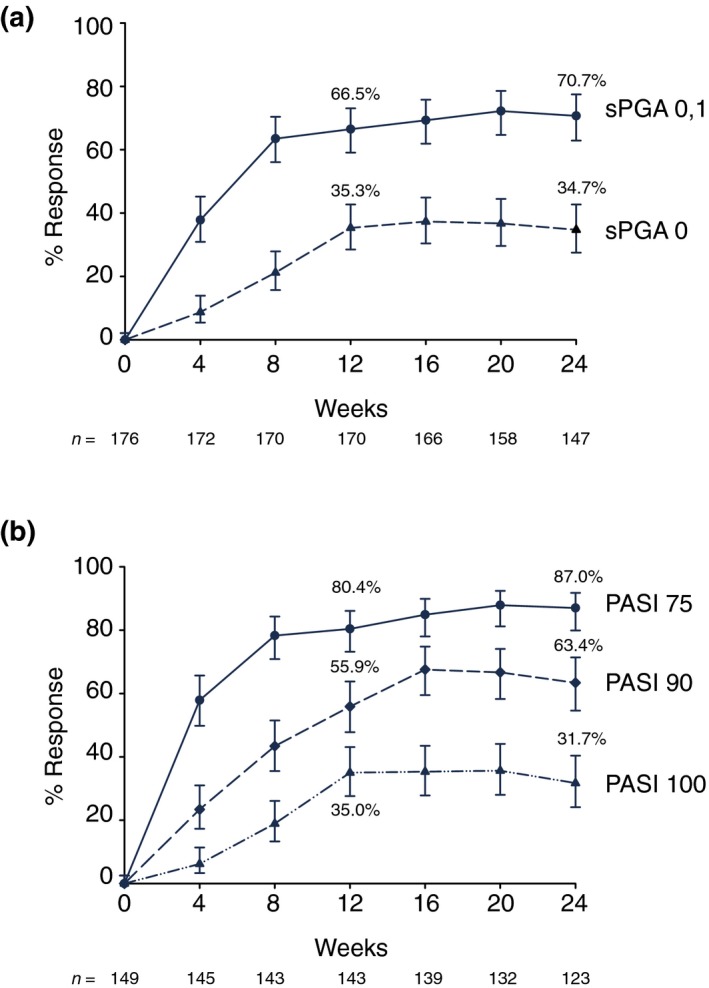
Efficacy in Relapsed and Retreated Patients. (a) sPGA; (b) PASI. Patients were treated with ixekizumab 80 mg Q2W. At Week 12, responders (sPGA 0, 1) were rerandomized to placebo. At relapse, patients were retreated with ixekizumab 80 mg Q4W. Relapse Week 0 includes all relapse patients regardless of which week the relapse occurred. The PASI summary includes only PASI 75 non‐responders at relapse Week 0. The x‐axis shows retreatment week. Percent responders (observed) with 95% CIs are shown. The upper and lower error bars represent the high and low values, respectively, of the 95% CI. CI, confidence interval; Q2W, every 2 weeks; Q4W, every 4 weeks; PASI 75, 75% reduction in the Psoriasis Area and Severity Index; PASI 90, 90% reduction in the Psoriasis Area and Severity Index; PASI 100, 100% reduction in the Psoriasis Area and Severity Index; PBO, placebo; sPGA, static Physician's Global Assessment.
With respect to mean PASI, relapsed patients regained clinical improvements in psoriasis as evidenced by reductions in observed mean PASI after 12 and 24 weeks of retreatment relative to relapse (Week 0 of retreatment) (Fig. 3a; observed mean [SD] 24 weeks: IXEQ2W/PBO/IXEQ4W: 2.0 [3.22], IXEQ4W/PBO/IXEQ4W: 1.1 [1.78]). Likewise, after 12 and 24 weeks of retreatment, relapsed patients regained improvements in their mean [SD] Itch NRS scores (Fig. 3b; observed mean [SD] 24 weeks: IXEQ2W/PBO/IXEQ4W: 1.8 [2.30]; IXEQ4W/PBO/ IXEQ4W: 1.3 [1.97]). Generally, itch reduction showed the same pattern as PASI improvements (Fig. 3b).
Safety
Generally, the safety profiles of the continuously treated and retreated group were comparable (Table 3); no unexpected types of safety events were observed upon retreatment, compared with initial treatment. The most common TEAEs were nasopharyngitis, upper respiratory infection and sinusitis in continuously treated and retreated patients. Most TEAEs were mild or moderate. Two serious AEs (SAEs) occurred in more than one patient in a treatment arm: fall (two patients each in continuously treated and retreated groups) and osteoarthritis (two patients in continuously treated group); other SAEs were reported in one patient per arm.
Table 3.
Patients with adverse eventsa
| Continuously treated (AE occurring in maintenance regardless of relapse status) | Withdrawal to relapse (AE before relapse or occurring in maintenance period for non‐relapsers) | Withdrawn, relapsed and retreated (AEs occurring during retreatment) | ||||
|---|---|---|---|---|---|---|
| IXEQ2W/Q4W N = 221 | IXEQ4W/Q4W N = 195 | IXEQ2W/PBO N = 211 | IXEQ4W/PBO N = 191 | IXEQ2W/PBO N = 176 | IXEQ4W/PBO N = 157 | |
| Total person‐years | 189.2 | 158.1 | 101.5 | 86.8 | 74.2 | 62.2 |
| TEAE, n (%) [IR] | 175 (79.2) [92.5] | 156 (80.0) [98.7] | 58 (27.5) [57.2] | 70 (36.6) [80.6] | 85 (48.3) [114.5] | 78 (49.7) [125.3] |
| Mild | 84 (38.0) [44.4] | 55 (28.2) [34.8] | 28 (13.3) [27.6] | 42 (22.0) [48.4] | 36 (20.5) [48.5] | 46 (29.3) [73.9] |
| Moderate | 74 (33.5) [39.1] | 85 (43.6) [53.8] | 24 (11.4) [23.7] | 25 (13.1) [28.8] | 38 (21.6) [51.2] | 30 (19.1) [48.2] |
| Severe | 17 (7.7) [9.0] | 16 (8.2) [10.1] | 6 (2.8) [5.9] | 3 (1.6) [3.5] | 11 (6.3) [14.8] | 2 (1.3) [3.2] |
| Common TEAEs b , n (%) [IR] | — | — | — | — | — | — |
| Nasopharyngitis | 38 (17.2) [20.1] | 44 (22.6) [27.8] | 4 (1.9) [3.9] | 9 (4.7) [10.4] | 15 (8.5) [20.2] | 20 (12.7) [32.1] |
| Upper respiratory infection | 22 (10.0) [11.6] | 21 (10.8) [13.3] | 4 (1.9) [3.9] | 4 (2.1) [4.6] | 12 (6.8) [16.2] | 13 (8.3) [20.9] |
| Sinusitis | 11 (5.0) [5.8] | 7 (3.6) [4.4] | 2 (0.9) [2.0] | 1 (0.5) [1.2] | 3 (1.7) [4.0] | 4 (2.5) [6.4] |
| Arthralgia | 13 (5.9) [6.9] | 8 (4.1) [5.1] | 6 (2.8) [5.9] | 6 (3.1) [6.9] | 2 (1.1) [2.7] | 0 (0) [0] |
| Injection‐site reactionc | 14 (6.3) [7.4] | 13 (6.7) [8.2] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 2 (1.3) [3.2] |
| Headache | 9 (4.1) [4.8] | 12 (6.2) [7.6] | 2 (0.9) [2.0] | 1 (0.5) [1.2] | 6 (3.4) [8.1] | 3 (1.9) [4.8] |
| SAE, n (%) [IR] | 10 (4.5) [5.3] | 17 (8.7) [10.8] | 6 (2.8) [5.9] | 4 (2.1) [4.6] | 6 (3.4) [8.1] | 1 (0.6) [1.6] |
| Common SAEs, n (%) [IR] d | — | — | — | — | — | — |
| Fall | 2 (0.9) [1.1] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 2 (1.1) [2.7] | 0 (0) [0] |
| Osteoarthritis | 0 (0) [0] | 2 (1.0) [1.3] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] |
| Discontinuations of study drug due to AE (includes death), n (%) [IR] | 8 (3.6) [4.2] | 8 (4.1) [5.1] | 2 (0.9) [2.0] | 6 (3.1) [6.9] | 0 (0) [0] | 0 (0) [0] |
| TEAEs of special interest, n (%) [IR] | — | — | — | — | — | — |
| Infection | 131 (59.3) [69.2] | 111 (56.9) [70.2] | 17 (8.1) [16.8] | 23 (12.0) [26.5] | 55 (31.3) [74.1] | 54 (34.4) [86.8] |
| Injection‐site reactionse | 21 (9.5) [11.1] | 16 (8.2) [10.1] | 2 (0.9) [2.0] | 0 (0) [0] | 2 (1.1) [2.7] | 4 (2.5) [6.4] |
| Cerebro‐cardiovascular events | 1 (0.5) [0.5] | 3 (1.5) [1.9] | 0 (0) [0] | 1 (0.5) [1.2] | 1 (0.6) [1.3] | 0 (0) [0] |
| Malignancies | 2 (0.9) [1.1] | 0 (0) [0] | 0 (0) [0] | 1 (0.5) [1.2] | 0 (0) [0] | 0 (0) [0] |
| Depression | 1 (0.5) [0.5] | 5 (2.6) [3.2] | 1 (0.5) [1.0] | 1 (0.5) [1.2] | 1 (0.6) [1.3] | 0 (0) [0] |
| Crohn's disease | 1 (0.5) [0.5] | 0 (0) [0] | 3 (1.4) [3.0] | 0 (0) [0] | 1 (0.6) [1.3] | 0 (0) [0] |
| Ulcerative colitis | 0 (0) [0] | 1 (0.5) [0.6] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] | 0 (0) [0] |
AE, adverse event; IR, exposure‐adjusted incidence rate; IXEQ2W, ixekizumab 80 mg every 2 weeks; IXEQ4W, ixekizumab 80 mg every 4 weeks; N, population size; n, number in group; PBO, placebo; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Patients with multiple occurrences of these categories are counted once for each category.
Occurring in ≥5% of patients in any arm in any group.
Preferred term.
Occurring in ≥2 patients in any arm in any group.
A composite of several injection‐site reaction‐related preferred terms.
Treatment‐emergent antidrug antibodies were detected in 42 of 220 (19.1%) of tested continuously treated patients during the entire 60‐week treatment period on the recommended dose of IXEQ2W/IXEQ4W, and in 39 of 173 (22.5%) of patients who withdrew from the recommended dose (IXEQ2W) and restarted treatment upon relapse (sPGA ≥3). Neutralizing antibodies were detected in two patients during retreatment in this group and in no continuously treated patients. There were no meaningful differences in % PASI improvement upon retreatment in patients with or without treatment‐emergent ADA (Fig. 5).
Figure 5.
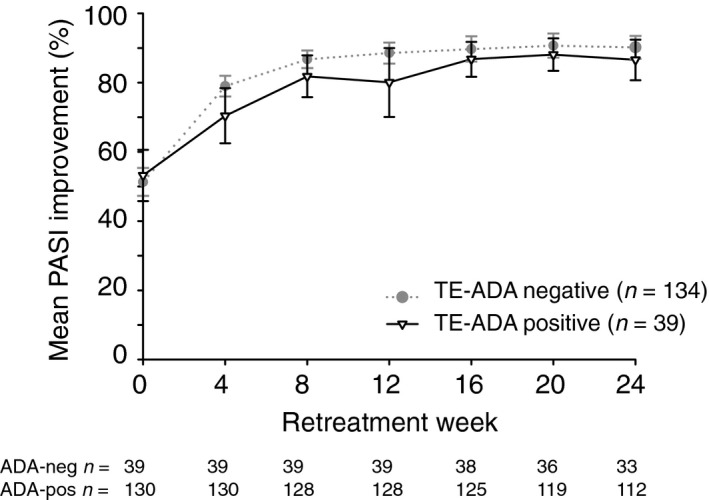
PASI Response Over Time by Treatment‐emergent Antidrug Antibody Status. Date are from LOCF responders at Week 12 (sPGA 0, 1) who relapsed (sPGA ≥3) during maintenance period and received IXEQ4W retreatment (UNCOVER‐1 and UNCOVER‐2 pooled data set). TE‐ADA positive = either in the induction period or in the retreatment period; TE‐ADA negative = in both the induction period and the retreatment period. The IXEQ2W/PBO + IXEQ4W group is shown. The upper and lower error bars represent the high and low values, respectively, of the 95% CI. IXE Q2W, 80 mg of ixekizumab every 2 weeks; IXE Q4W, 80 mg of ixekizumab every 4 weeks; LOCF, last observation carried forward; PASI, Psoriasis Area and Severity Index; PBO, placebo; sPGA, static Physician's Global Assessment; TE‐ADA, treatment‐emergent antidrug antibody.
Discussion
Continuous treatment with ixekizumab provided better and higher maintenance of efficacy up to 60 weeks in patients with moderate‐to‐severe psoriasis compared to treatment interruption. The low and stable mean PASI in continuously treated patients suggested that continuous therapy is the optimal strategy for sustained skin clearance and relapse avoidance. Continuous ixekizumab treatment enabled patients to have consistent itch reduction, as Itch NRS scores were consistently low during maintenance. When treatment was interrupted, efficacy waned, resulting in higher PASI and itch scores with a median time to relapse of approximately 5 months. Retreatment of patients with ixekizumab after interruption of ixekizumab therapy resulted in substantial improvements with recapture of initial responses for most patients.
In the pooled analysis of UNCOVER‐1 and UNCOVER‐2 reported here among patients receiving the recommended induction dosing regimen who were withdrawn and relapsed, 66.5% and 70.7% (observed) of patients recaptured an sPGA 0,1 at 12 and 24 weeks of retreatment, respectively. It is important to highlight that the data presented here are only partially represented in the analysis of retreatment efficacy within the US Prescribing Instructions.17 In the latter analysis, the recapture rate was assessed based on the proportion of patients who recaptured an sPGA 0,1 at any time point within 12 weeks after receiving at least one dose of retreatment, and included data to Week 60. Thus, some patients who relapsed and were retreated after Week 48 (5%) did not receive 12 weeks of retreatment, leading to an underestimation of the recapture rate. In addition, as observed in this analysis with longer retreatment duration, even more patients recaptured the response.
As reported for other biologics,4, 6, 8, 9 however, not all patients regained the same degree of skin clearance upon re‐initiation of ixekizumab when compared with initial responses to this drug. Different withdrawal times and strategies may result in different clinical outcomes, and thus, further exploration of ixekizumab retreatment is warranted. Similar investigations on retreatment have been conducted for secukinumab, another IL‐17A antagonist. Results have varied depending on the retreatment strategy used. In the SCULPTURE trial, Mrowietz and colleagues used a once‐monthly dosing regimen upon retreatment.8 The authors reported that among patients who relapsed, up to 69% regained PASI 75 responses after retreatment. Upon a second treatment interruption and retreatment phase, only 42.4% of patients achieved PASI 75.18 In a pooled analysis of the ERASURE and FIXTURE secukinumab trials,19 among patients with PASI 75 responses at Week 52 who were randomized to treatment withdrawal and experienced disease worsening, recapture rates were increased up to 95% when patients were retreated using weekly dosing through Week 3, and then Q4W dosing thereafter.19 Notably, in the present study involving UNCOVER‐1 and UNCOVER‐2, the initial recommended treatment regimen of ixekizumab 160 mg loading dose and the induction dose of IXEQ2W were not provided upon ixekizumab re‐initiation. Further study is required to understand the impact of providing a loading dose upon retreatment with ixekizumab.
The study designs for UNCOVER‐1 and UNCOVER‐2 provided rigorous and randomized assessments of the consequences of treatment interruption versus continuous treatment. However, the study is limited in that the treatment interruptions were dictated by design rather than by patient and physician decision as would be the case in clinical practice.
In conclusion, continuous treatment resulted in sustained responses through Week 60, and most patients with an initial response, who were rerandomized to placebo, experienced disease recurrence and increased itching with a median time to relapse of approximately 5 months after stopping drug; most of these patients recaptured their clinical responses within 12 to 24 weeks of retreatment with ixekizumab. The median time to recapture of PASI 75 was 4 weeks. Safety profiles of patients with continuous ixekizumab were comparable with those who interrupted treatment and were retreated, with no increase in ADA. The findings presented here suggest that, if necessary to interrupt therapy during routine clinical practice, retreatment with ixekizumab is safe and effective in most patients.
Acknowledgements
The authors thank Lori Kornberg, PhD, who is a full‐time employee of INC Research (Raleigh, NC), for writing support and Missy McKean‐Matthews, MS who is a full‐time employee of InVentiv Health Clinical (Princeton, NJ), for statistical support.
Conflicts of interest
A. Blauvelt has received honoraria and clinical study fees from Eli Lilly and Company for scientific consulting, speaking and performing clinical study work. He has also served as a scientific adviser and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Genentech, GSK, Janssen, Merck, Novartis, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun, UCB and Valeant.
K.A. Papp serves as a consultant for/is a speaker bureau member of/received clinical research grants from/received honoraria from/is an advisory board member of AbbVie, Amgen, Eli Lilly and Company, Janssen, Merck, Sharp and Dohme, Novartis and Pfizer. He is a consultant for/speaker bureau member/received clinical research grants from/is an advisory board member of Astellas. He is a speaker bureau member/ received clinical research grants from/received honoraria from Galderma. He is a consultant for/received honoraria from/an advisory board member of Baxter. He is a consultant for/received clinical research grants from/received honoraria from/is an advisory board member of Boehringer Ingelheim, Celgene, Merck‐Serono, UCB and Valeant. He is a consultant for/received honoraria from Akros, Cipher, Forward Pharma, Funxional Therapeutics, Lypanosys, Mitsubishi Pharma and Vertex. He is a consultant for/ received clinical research grant from/received honoraria from Kyowa Hakko Kirin and Takeda. He is a consultant for/received clinical research grants from Bristol Myers Squibb, Dermira, and LEO Pharma. He is a consultant 7 for Akesis, AstraZeneca, Artax, Can‐Fite, Ferring Pharmaceuticals, Formycon, Genentech, Genexion, Genzyme, Gilead, Meiji Seika Pharma, Merck Serono, Mylan, Pan Genetics, Regeneron, Johnson and Johnson, and Roche. He is a consultant for/an advisory board member of Sanofi‐Aventis. He received clinical research grants from Allergan, Anacor, Celtic, Dow Pharma,
Medimmune and Stiefel. He is a consultant for/received clinical research grants from Baxalta. He received honoraria from Wyeth. He serves as a scientific officer for AbbVie and Anacor. He is on the steering committee of AbbVie, Celgene, Eli Lilly and Company, Forward Pharma, Janssen, Kyowa Hakko Kirin, Merck Sharp and Dohme, Novartis, Pfizer, Regeneron, and Merck‐Serono.
H. Sofen has been a paid consultant and clinical investigator of Eli Lilly and Company. He is an investigator and consultant for Novartis, Janssen, Celgene, Pfizer, Amgen, Sun Pharma, Merck, AbbVie and Boehringer Ingelheim.
M. Augustin has served as consultant to or paid speaker for 8 clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, GSK, Janssen‐Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB and XenoPort.
G. Yosipovitch serves as consultant and received honoraria as member of scientific advisory board of Eli Lilly and Company, Celgene, TREVI, Sanofi‐Aventis, Creabilis, Chugai, Opko, TREVI, Creabilis, Pfizer. He serves as a clinical investigator for Allergan and Genentech and is funded by GSK/Stiefel and LEO Foundation.
N. Katoh has received honoraria as consultant and/or advisory board member and/or acted as paid speaker and/or participated in clinical trials sponsored by Eli Lilly and Company, Celgene, Maruho, Mitsubishi Tanabe, Novartis and Sanofi.
U. Mrowietz has been an advisor and/or received speaker honoraria and/or received grants and/or participated in clinical trials of the following companies: Abbott/AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, Foamix, Forward Pharma, Janssen, LEO Pharma, Medac, MSD, Miltenyi Biotec, Novartis, Pfizer, VBL, XenoPort.
M. Ohtsuki has received honoraria as consultant and/or advisory board member and/or acted as paid speaker and/or participated in clinical trials sponsored by Eli Lilly and Company, AbbVie, Boehringer Ingelheim, Celgene, Eisai, Janssen, Kyowa‐Kirin, LEO Pharma, Maruho, Novartis, Pfizer and Mitsubishi Tanabe.
Y. Poulin received research grants and advisory board honoraria from Eli Lilly and Company. He received research grants from other sponsors, including AbbVie, Amgen, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor/ Janssen, EMD Serono, Galderma, GSK, LEO Pharma, Merck, Novartis, Pfizer, Takeda, UCB Pharma. He served on advisory boards for AbbVie, Amgen and Janssen and was on the speaker's bureau of AbbVie, Amgen, Celgene, GSK‐Stiefel and Janssen.
K.B. Gordon has received research support from Eli Lilly and Company, AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen. He has received honoraria from Eli Lilly and Company, AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Novartis and Pfizer.
D. Shrom, R. Burge, K. See and L. Mallbris are full‐time employees of Eli Lilly and Company and own stock.
Funding sources
This work was funded by Eli Lilly and Company.
References
- 1. Ramirez‐Fort MK, Levin AA, Au SC, Gottlieb AB. Continuous versus intermittent therapy for moderate‐to‐severe psoriasis. Clin Exp Rheumatol 2013; 31(4 Suppl 78): S63–S70. [PubMed] [Google Scholar]
- 2. Brezinski EA, Armstrong AW. Off‐label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS ONE 2012; 7: e33486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mrowietz U, de Jong EM, Kragballe K et al A consensus report on appropriate treatment optimization and transitioning in the management of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2014; 28: 438–453. [DOI] [PubMed] [Google Scholar]
- 4. Papp K, Crowley J, Ortonne JP et al Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol 2011; 164: 434–441. [DOI] [PubMed] [Google Scholar]
- 5. Langley RG, Gordon KB. Duration of remission of biologic agents for chronic plaque psoriasis. J Drugs Dermatol 2007; 6: 1205–1212. [PubMed] [Google Scholar]
- 6. Bissonnette R, Iversen L, Sofen H et al Tofacitinib withdrawal and retreatment in moderate‐to‐severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015; 172: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 7. Griffiths CE, Luger TA, Brault Y, Germain JM, Mallbris L. Retreatment in patients with psoriasis achieving response with etanercept after relapse due to treatment interruption: results from the CRYSTEL study. J Eur Acad Dermatol Venereol 2015; 29: 468–473. [DOI] [PubMed] [Google Scholar]
- 8. Mrowietz U, Leonardi CL, Girolomoni G et al Secukinumab retreatment‐as‐needed versus fixed‐interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double‐blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015; 73: 27–36. [DOI] [PubMed] [Google Scholar]
- 9. Papp K, Menter A, Poulin Y, Gu Y, Sasso EH. Long‐term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open‐label extension study. J Eur Acad Dermatol Venereol 2013; 27: 634–642. [DOI] [PubMed] [Google Scholar]
- 10. Alinia H, Feldman SR. Oral tofacitinib for psoriasis: what happens with interrupted treatment? Br J Dermatol 2015; 172: 1194–1195. [DOI] [PubMed] [Google Scholar]
- 11. Liu L, Lu J, Allan BW et al Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin‐17A. J Inflamm Res 2016; 9: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. New Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 13. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 14. Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244. [DOI] [PubMed] [Google Scholar]
- 15. Naegeli AN, Flood E, Tucker J, Devlen J, Edson‐Heredia E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol 2015; 54: 715–722. [DOI] [PubMed] [Google Scholar]
- 16. Muram TM, Sloan JH, Chain JS et al A highly sensitive and drug‐tolerant anti‐drug antibody screening assay for ixekizumab using affinity capture elution. J Invest Dermatol 2016; 136: 1513–1515. [DOI] [PubMed] [Google Scholar]
- 17. TALTZ‐ixekizumab injection . Full Prescribing Information [Internet]. Eli Lilly and Company, Indianapolis, IN: March 2016 [cited July 8, 2016]. URL http://uspl.lilly.com/taltz/taltz.html#pi. [Google Scholar]
- 18. Mrowietz U, Papavassilis C, Leonardi C, Toth D, Thurston HJ. Secukinumab retreatment‐as‐needed maintenance regimen: efficacy and safety outcomes from the SCULPTURE study. 72nd Annual Meeting of the American Academy of Dermatology, 2014. J Am Acad Dermatol 2014; 70: AB188 [P8229]. [Google Scholar]
- 19. Blauvelt A, Langley RGB, Szepietowski JC et al Secukinumab withdrawal leads to loss of treatment responses in a majority of subjects with plaque psoriasis with re‐treatment resulting in rapid regain of responses: a pooled analysis of two phase 3 trials. 74th Annual Meeting of the American Academy of Dermatology, 2016. J Am Acad Dermatol 2016; 74: AB273 [P3719]. [Google Scholar]


