Abstract
Background
Appropriate utilization of vancomycin is important to attain therapeutic targets while avoiding clinical failure and the development of antimicrobial resistance. Our aim was to observe the use of vancomycin in an intensive care population, with the main focus on achievement of therapeutic serum concentrations (15–20 mg/l) and to evaluate how this was influenced by dose regimens, use of guidelines and therapeutic drug monitoring.
Methods
A prospective observational study was carried out in the intensive care units at two tertiary hospitals in Norway. Data were collected from 83 patients who received vancomycin therapy, half of these received continuous renal replacement therapy. Patients were followed for 72 h after initiation of therapy. Blood samples were drawn for analysis of trough serum concentrations. Urine was collected for calculations of creatinine clearance. Information was gathered from medical records and electronic health records.
Results
Less than 40% of the patients attained therapeutic trough serum concentrations during the first 3 days of therapy. Patients with augmented renal clearance had lower serum trough concentrations despite receiving higher maintenance doses and more loading doses. When trough serum concentrations were outside of therapeutic range, dose adjustments in accordance to therapeutic drug monitoring were made to less than half.
Conclusion
The present study reveals significant challenges in the utilization of vancomycin in critically ill patients. There is a need for clearer guidelines regarding dosing and therapeutic drug monitoring of vancomycin for patient subgroups.
Editorial Comment.
In this observation study of dosing of vancomycin in critically ill patients at two large university hospitals, the authors found that therapeutic trough serum concentrations for the antibiotic were often not reached within 3 days, despite the fact that clinicians increased dose and decreased dosing intervals. The authors suggest that a more structured dosing protocol could help in this setting.
Vancomycin is a glycopeptide antibiotic for use against infections with gram‐positive bacteria. Recently, consumption has increased due to an increase in the incidence of infections by methicillin‐resistant S. aureus (MRSA). Increased use has been associated with higher minimum inhibitory concentration (MIC)‐levels, the so‐called “MIC‐creep”.1, 2, 3 Although the reason for this creep is debatable, it has caused clinical concern in regards to the use of vancomycin.4, 5 Focus on appropriate utilization of the drug has intensified.
Intensive care units (ICU) are especially extensive in their use of antimicrobial agents and the use is increasing.6 Their contribution to the development of antibacterial resistance may therefore be significant. Additionally, due to the severity of the illness of most patients in the ICU, particular care must be taken to avoid therapeutic failure. Despite the production of numerous protocols and guidelines to guide critical care practitioners in giving appropriate therapy, failure to achieve the recommended target trough serum concentrations of 15–20 mg/l for vancomycin is still frequent in the ICU‐population.7 Regular collaboration with infectious disease practitioners and clinical pharmacists has been shown to have positive effects on attaining therapy targets, and decreasing the overuse of antibiotics.8, 9
The aim of the present study was to find the proportion of ICU‐patients whose vancomycin trough serum concentrations reached therapeutic levels during the first 3 days of therapy in two different tertiary hospitals. Both CRRT‐ and non‐CRRT‐patients were included. The study also aimed to find the proportions of trough serum concentrations within resistance‐driving levels, and to observe the use of vancomycin in terms of indication, dose regimens, and therapeutic drug monitoring (TDM).
Methods
The present study was carried out as a part of PharmacoCRRT2012 (NCT01582360 at clinicaltrials.gov). It is a prospective observational study, which was initiated in 2013 at the intensive care units at two tertiary hospitals: Rikshospitalet (RH) and Ullevaal (UUS), which are parts of Oslo University Hospital. UUS is the major trauma center in Norway, RH is the major organ transplant center. The study protocol was approved by the Regional Committees for Medical and Research ethics and the Data Inspectorate Health Authority at Oslo University Hospital on 5 November 2012.
From May 2013 until October 2015, patients were screened to see if they met the following inclusion criteria: Initiation of vancomycin therapy within the last 24 h, age > 18, and expected treatment‐time > 72 h. In accordance with the PharmacoCRRT2012‐protocol, half of the patients included were in need of continuous renal replacement therapy (CRRT), the other half were not. An informed consent from the patient or next of kin was obtained prior to inclusion.
Observation time was 72 h after initiation of vancomycin therapy. Blood samples for analyzes of vancomycin trough concentrations were drawn immediately prior to vancomycin dosing at 24 h (24 h), 48 and 72 h after the initial dose. Vancomycin concentrations were determined using a commercial assay (Cobas C Systems, Roche). For patients not in need of CRRT, urine was collected for calculation of creatinine clearance from initiation of therapy until the next morning, and then for every 24 h at a time, for calculation of creatinine clearance. The following information was gathered from medical records: 90‐day mortality, documentation of initiation and indications for vancomycin therapy, whether or not a dose adjustment was made, vancomycin doses, and clinical laboratory data results. Data were entered into a case report form.
Rybak et al. suggest loading doses of 25–30 mg/kg for critically ill patients in international consensus guidelines.10 We therefore defined loading dose as ≥ 25 mg/kg in this investigation.
Endpoints
The primary endpoints of the study were the proportions of vancomycin trough serum concentrations within therapeutic range (15–20 mg/l) and proportions of resistance‐driving trough serum concentrations (< 10 mg/l).10 Secondary endpoints were as follows: (1) Indication for use. (2) Dose regimens used. (3) Use of guidelines (a): Norwegian Directorate of Health, National guidelines for use of antibiotics in hospitals 2013. (b): Local guidelines on glycopeptide antibiotics (UUS 2010)). (4) Regular support from pharmacists. (5) Therapeutic drug monitoring (TDM).
Groups
Since the patient subpopulation admitted to UUS and RH differs, and the two hospitals also have organizational differences which might influence how antibiotic therapy is given, we chose to compare the two hospitals. We also compared patients in need of CRRT (CRRT‐group) with those who did not receive CRRT (non‐CRRT‐group).
Statistical analysis
Normally distributed data were expressed as means and standard deviation (SD), non‐normally distributed data as medians with interquartile range (IQR). Independent samples t‐test was used when comparing mean values, Pearson Chi‐Square test was used when comparing categorical data. Mann–Whitney Test was used when comparing median values, and Spearman's correlation coefficient was used to calculate the association between two continuous variables. Changes in categorical variables within a group over time were analyzed using a McNemars test. A significance level of 5% was used. Data were analyzed using IBM SPSS Statistics 22.
Results
Patient characteristics
A total of 83 patients were included in the study. Demographic characteristics are presented in Table 1. The groups at UUS and RH were similar in terms of age, sex, SAPS II, 90‐day mortality, body weight, and RIFLE score.
Table 1.
Baseline demographics
| RH (n = 40) | UUS (n = 43) | P value | |||
|---|---|---|---|---|---|
| Missing, n | Missing, n | ||||
| Age, mean (95% CI) | 53.6 (49.6–58.1) | 0 | 49.2 (44.3–54.1) | 0 | 0.184 |
| Male gender, n (%) | 28 (70.0) | 0 | 33 (76.7) | 0 | 0.410 |
| Body weight (kg), mean (95% CI) | 81.1 (75.3–86.9) | 1 | 87.9 (79.3–96.5) | 0 | 0.198 |
| SAPS II, mean (95% CI) | 49.3 (43.7–55.0) | 5 | 43.2 (39.2–47.2) | 2 | 0.070 |
| 90 days mortality, n (%) | 11 (27.5) | 0 | 11 (25.6) | 0 | 0.843 |
| CRRT, n (%) | 16 (40.0) | 20 (46.5) | |||
| Patients with AKI, n (%) according to RIFLE‐criteria | 25 (64.1) | 1 | 24 (55.8) | 0 | 0.445 |
| Diagnosis, n (%) | |||||
| Cardiovascular | 3 (7.5) | 4 (9.3) | 0.771 | ||
| Hematological | 7 (17.5) | 0 (0) | 0.006 | ||
| Neurological | 5 (12.5) | 3 (7.0) | 0.400 | ||
| Pneumonia | 8 (20.0) | 2 (4.7) | 0.037 | ||
| Septicemia | 14 (35.0) | 6 (14.0) | 0.027 | ||
| Postoperative complications | 13 (32.5) | 16 (37.2) | 0.658 | ||
| Trauma | 0 (0) | 19 (44.2) | < 0.001 | ||
| Others | 14 (35.0) | 9 (20.9) | 0.156 | ||
| CRP, mean (95% CI) | 143.4 (113.7–173.2) | 0 | 232.7 (195.3–270.1) | 0 | 0.000 |
| S‐creatinine (μmol/l), mean (95% CI) | |||||
| Total average | 138.4 (91.9–184.9) | 0 | 133.9 (102.8–165.0) | 0 | 0.869 |
| CRRT | 216.8 (109.1–324.6) | 185.8 (130.3–241.3) | 0.570 | ||
| Non‐CRRT | 86.1 (67.2–105.1) | 88.7 (66.2–111.3) | 0.855 | ||
| S‐bilirubin (μmol/l), median, (IQR) | 43.0 (10.0–131.0) | 1 | 15.0 (9.0–34.0) | 0 | 0.035 |
CI, Confidence interval; RH, Rikshospitalet; UUS, Ullevaal Hospital; SAPS, Simplified Acute Physiological Score; CRRT, continuous renal replacement therapy; AKI, acute kidney injury; RIFLE, Risk, Injury, and Failure; and Loss; and End‐stage kidney disease; IQR, inter‐quartile range.
CRP was significantly higher at UUS than RH on all days. The proportion of patients with a diagnosis of sepsis or pneumonia was significantly higher at RH than UUS. Creatinine clearance was significantly higher at UUS than RH during the observation time of the study (Table 2).
Table 2.
Creatinine clearance (non‐CRRT‐patients only) (ml/min)
| Number of hours after initiation of therapy | Rikshospitalet (RH) | Ullevaal (UUS) | P value | ||
|---|---|---|---|---|---|
| Mean (95% CI) | No. of patients | Mean (95% CI) | No. of patients | ||
| 24 h | 88.8 (62.0–115.6) | 16 | 138.3 (114.6–161.9) | 21 | 0.006 |
| 48 h | 90.7 (64.9–116.5) | 18 | 136.7 (113.9–159.5) | 21 | 0.008 |
| 72 h | 96.5 (63.3–129.6) | 18 | 138.6 (117.1–160.0) | 23 | 0.025 |
Primary endpoints
Trough serum concentrations of vancomycin
Trough serum concentrations within therapeutic range
A total of 237 trough serum concentrations were drawn (114 from RH, 123 from UUS), twelve were missing. The proportion of trough serum concentrations within therapeutic range (15–20 mg/l) was 21.0% at 24 h, 16.3% at 48 h, and 32.9% at 72 h (Fig. 1). There were no significant differences between RH and UUS on any of the days. Neither were there significant differences between the CRRT‐ and non‐CRRT‐groups at the two hospitals.
Figure 1.
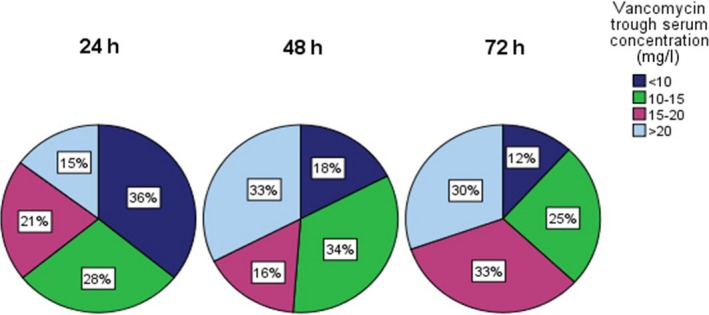
Distribution of vancomycin trough serum concentrations in percentages during the first 72 h after initiation of vancomycin therapy. [Colour figure can be viewed at wileyonlinelibrary.com]
Furthermore, there was no significant change in the proportion of trough serum concentrations within therapeutic range from 24 to 72 h (P = 0.19).
Trough serum concentrations which may contribute to antibiotic resistance
The proportion of patients with resistance‐driving trough serum concentrations (< 10 mg/l) was 35.8% at 24 h, 17.5% at 48 h, and 11.8% at 72 h (Fig. 1). There was no significant difference between UUS and RH on any of the study days. However, there was a significant decrease in the proportion of trough serum concentrations < 10 mg/l from 24 to 72 h (P ≤ 0.001).
Secondary endpoints
Indications for therapy and documentation
Initiation of vancomycin therapy was documented in the medical records by a critical care physician in 90.2% of the treatments. The indication for initiation of therapy was empiric in 66.3% and directed in 26.5% of therapies. In the remaining 7.2%, a clear indication was not documented. In those cases where therapy was directed, S. epidermidis and E. faecium were the most common pathogens (Table 3.)
Table 3.
Identified pathogens in directed vancomycin therapy
| Pathogen | Source of identified pathogen | Number of specimens | % |
|---|---|---|---|
| Enterococci | |||
| E. faecium | 9* | 38 | |
| Abdominal | 4 | ||
| Blood culture | 4 | ||
| Intravascular catheter | 2 | ||
| Pleural drain | 1 | ||
| E. faecalis | 2 | 8 | |
| Wound secretion | 1 | ||
| Blood culture | 1 | ||
| Staphylococci | |||
| MRSA | Blood culture | 1 | 4 |
| S. epidermidis | 9* | 38 | |
| Blood culture | 6 | ||
| Intravascular catheter | 2 | ||
| Dialysis catheter | 1 | ||
| Pleural biopsy (pyothorax) | 1 | ||
| S. hominis | Blood culture | 1 | 4 |
| S. lugdumensis | Blood culture | 1 | 4 |
| S. aureus (suspected MRSA) | Blood culture | 1 | 4 |
| Total | 24 | 100 | |
MRSA, methicillin‐resistant staphylococcus aureus. *The numbers beneath add up to a greater total than the number with the asterix. This is because in some of the patients the pathogen was found in more than one source.
Dose regimens
Loading and maintenance doses
A total of 82 initial doses were registered, one was missing. A total of 28% of the patients received a loading dose (> 25 mg/kg). The percentage of patients who received a loading dose was significantly higher at UUS than at RH (39.5% vs. 15.4%, P = 0.02). When subgrouped into CRRT –and non‐CRRT‐groups, the proportion receiving a loading dose was significantly higher in the non‐CRRT‐group at UUS than at RH (52.2% vs. 17.4%, P = 0.01). There was no significant differences in the use of loading dose between the CRRT‐groups (P = 0.35).
A total of 238 maintenance doses were gathered, eleven were missing. Maintenance doses were significantly higher at UUS than RH on all study days. However, when subgrouped into CRRT‐ and non‐CRRT‐groups, the difference between the hospitals only remained in the non‐CRRT‐group (Table 4).
Table 4.
Maintenance dose at RH and UUS when subgrouped into CRRT‐ and non‐CRRT‐groups (mg/kg)
| No. of hours | Hospital | N | Mean | Std. deviation | 95% CI | P value | |
|---|---|---|---|---|---|---|---|
| Non‐CRRT | 24 h | RH | 23 | 12.7 | 3.9 | 11.0–14.4 | < 0.001 |
| UUS | 22 | 23.0 | 7.8 | 19.5–26.4 | |||
| 48 h | RH | 23 | 13.6 | 5.7 | 11.1–16.1 | 0.007 | |
| UUS | 23 | 20.2 | 9.4 | 16.2–24.3 | |||
| 72 h | RH | 23 | 12.2 | 6.5 | 9.4–15.0 | < 0.001 | |
| UUS | 23 | 21.0 | 8.0 | 17.6–24.5 | |||
| CRRT | 24 h | RH | 15 | 12.8 | 4.2 | 10.5–15.2 | 0.100 |
| UUS | 19 | 16.7 | 8.0 | 12.9–20.6 | |||
| 48 h | RH | 15 | 12.1 | 3.2 | 10.3–13.9 | 0.908 | |
| UUS | 19 | 12.3 | 6.1 | 9.3–15.2 | |||
| 72 h | RH | 15 | 11.7 | 3.8 | 9.6–13.8 | 0.684 | |
| UUS | 18 | 12.4 | 5.7 | 9.6–15.2 |
RH, Rikshospitalet; UUS, Ullevaal Hospital; CRRT, continuous renal replacement therapy.
Dose frequency
A total of 245 average dose frequencies were registered (117 from RH, 128 from UUS), four were missing (Fig. 2). The mean dosing interval was significantly higher at UUS than RH (13.6 h vs. 11.1, P = 0.003). When subgrouped into CRRT‐ and non‐CRRT‐groups, the mean dose interval was significantly higher in the CRRT‐group at UUS than the CRRT‐group at RH, respective values being 16.5 and 12.4 h (P < 0.001). There was no significant difference between the two non‐CRRT‐groups (P = 0.22).
Figure 2.
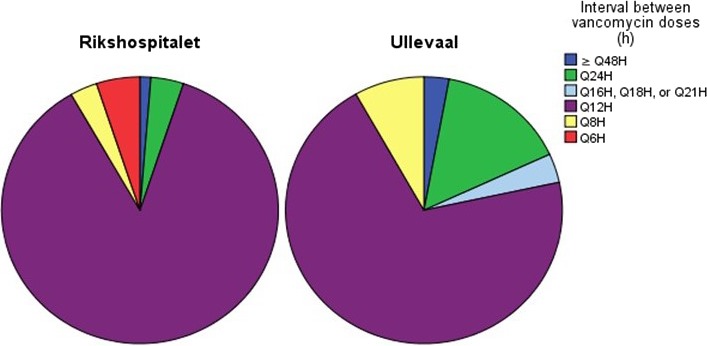
Dosing intervals between vancomycin administration during the first 72 h of vancomycin therapy. [Colour figure can be viewed at wileyonlinelibrary.com]
Effect of creatinine clearance
We gathered 115 creatinine clearance values (non‐CRRT‐patients only), twelve were missing. Creatinine clearance was significantly higher in the patient population at UUS than RH on all study days (Table 2). There was a significant negative correlation between creatinine clearance and trough serum concentrations (r = −0.53, P ≤ 0.001) (Fig. 3).
Figure 3.
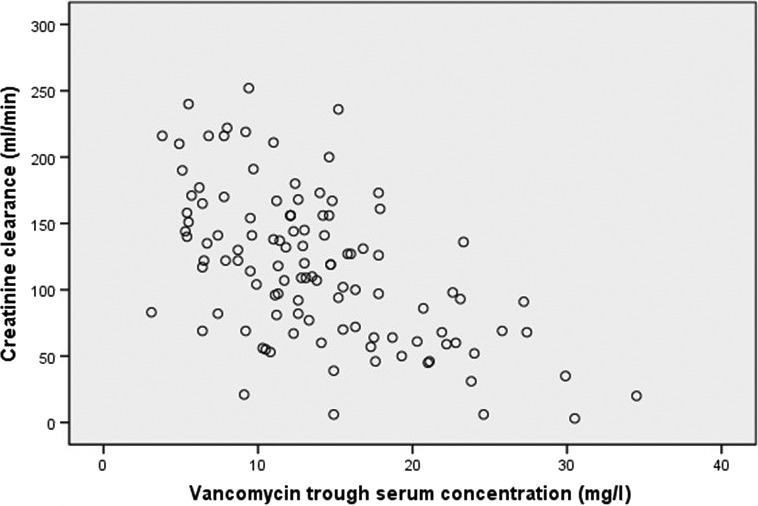
Creatinine clearance vs. vancomycin trough serum concentrations (r = −0.53, P < 0.001).
Clinical guidance in vancomycin therapy
Clinical decision support at RH and UUS are presented in Table 5. A total of 245 vancomycin doses were evaluated to see whether dosage adjustments were made when trough serum concentrations were outside of therapeutic range, four were missing. One hundred and eighty‐two (76.8%) of the trough serum concentrations drawn were outside of therapeutic range (Fig. 4). A change was made to the dose regimen in 48.9% of these. UUS made significantly more changes to dose regimens than RH in response to nontherapeutic trough serum concentrations, the respective values being 62.9% and 37.1% (P = 0.002). The total proportion of dosage adjustments increased significantly from 24 to 48 h (P = 0.001) and from 24 to 72 h (P = 0.02).
Table 5.
Available guidelines and clinical decision support from pharmacists and infectious disease practitioners at Rikshospitalet and Ullevaal university hospitals
| Guidelines for dosing and therapeutic drug monitoring of vancomycin | Routine collaboration with infectious disease practitioner | Routine collaboration with pharmacists | |
|---|---|---|---|
| RH | No | Once weekly | 2.5 days per week |
| UUS | Yes | Once weekly | 5 days per week |
Figure 4.
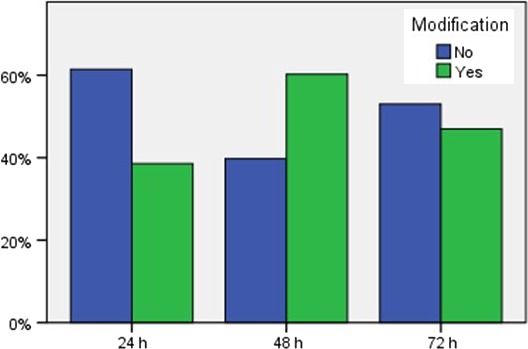
Dosage modification in response to vancomycin trough serum concentrations < 15 mg/l or > 20 mg/l at 24, 48, and 72 h. [Colour figure can be viewed at wileyonlinelibrary.com]
In fear of being too strict when analyzing dose adjustments based on only the range 15–20 mg/l, an additional analysis was done to find the proportion of adjustments made when concentrations were < 10 or > 25 mg/l. An appropriate dosage adjustment was made in 46.2% of these concentrations.
Documentation of TDM
Considerations of vancomycin trough serum concentration and whether or not an adjustment was necessary were documented in 18.9% of the patient journals. Among those cases where an adjustment to therapy was made, considerations were documented in the patient journal in 34 out of 121 (28.1%) cases. In those cases where no dose adjustment was made, considerations were documented in 13 of 125 (10.4%) cases.
Discussion
Optimizing vancomycin therapy in a critical care setting has been the focus of numerous studies over the past few years.6, 10, 11, 12, 13, 14, 15 Guidelines have been produced in an attempt to reduce the occurrence of therapeutic failure and the development of resistant pathogens.10 Despite these measures, the present study demonstrates discouraging results with vancomycin trough serum concentrations of subtherapeutic levels in 70–80% in ICU‐patients during the first 3 days of therapy. This applied to all groups, independent of whether they received CRRT or not. Additionally, our data show that the proportion of vancomycin trough serum concentrations which might contribute to antibiotic resistance (< 10 mg/l) was high 24 h after initiation of therapy, and remained high after three days of therapy.
Our study is not the first to present frequent occurrence of subtherapeutic trough serum concentrations. Blot et al. presented similar results in their multicenter point‐prevalence study.7 Forty‐five percent of the patients did not achieve the minimum threshold value of ≥ 15 mg/l. Wilson and Burns also presented frequent subtherapeutic serum concentrations in patients receiving continuous veno‐venous hemodialysis (therapeutic value defined as ≥ 15 mg/l), the values being 44% of medical ICU‐patients and 46% of surgical ICU‐patients.15
This leads us to the following question: Why are success rates so low?
Less than one‐third of the patients received a loading dose, which is a prerequisite to attain therapeutic concentrations rapidly. There were, however, significant differences between the two hospitals in dosing regimens. Loading doses were administered more frequently and maintenance doses were higher at UUS than at RH. This could partly be explained by daily availability of pharmacists and use of guidelines in the ICU at UUS.7 Despite these differences, success rates were not significantly higher at UUS.
A possible explanation for this is that the patient group at UUS largely consisted of a trauma subgroup. Trauma patients are at a greater risk of augmented renal clearance (ARC),16 and this was reflected in the average creatinine clearance at UUS, which was > 130 ml/min on all days of the study. Campassi et al. demonstrated that patients with ARC had lower plasma concentrations of vancomycin during the first days of therapy despite higher doses, and none of the patients reached therapeutic levels on the first day of therapy.11 Medellin‐Garibay and co‐workers recently (2016) proposed guidelines for vancomycin dosing in trauma patients with the aim of greater achievement of trough concentrations within therapeutic range (15–20 mg/l).12 However, their proposed guidelines largely resemble the doses that were administered to the patients in our study. Based on our data, we can conclude that the guidelines presented by Medellin‐Garibay and co‐workers are not sufficient to attain therapeutic serum concentrations in this population.
Others have proposed that increased loading doses and higher dose frequencies or continuous infusions are necessary in order to achieve higher success rates.10, 13, 14, 17 However, even with continuous infusion, a sufficiently high loading dose is necessary to prevent subtherapeutic concentrations.17 This may be due to an increase in distribution volume for hydrophilic drugs such as vancomycin in critically ill patients. Additionally, vancomycin serum concentrations during the first days of therapy will also depend on creatinine clearance. Low creatinine clearance levels can result in supratherapeutic vancomycin concentrations.18
The low success rates presented in our data were somewhat surprising, as we would have expected subtherapeutic trough serum concentrations to be corrected through the process of therapeutic drug monitoring (TDM). TDM is the traditional clinical method of monitoring vancomycin therapy, and has been so for many years. Ye and co‐workers concluded in their systematic review that TDM “significantly increases the rate of clinical efficacy” in patients treated with vancomycin.19 Unfortunately, this study demonstrates that appropriate dose adjustments were only made to half of patients with trough serum concentrations outside of therapeutic range. Hence, there is room for considerable improvement. Increased pharmaceutical collaboration and implementation of a protocol for vancomycin dose adjustments to aid in TDM might increase the proportion of appropriate dose adjustments. Such a protocol can easily be internalized in modern electronic medical records, and thereby ensure improved follow‐up.
Vancomycin therapy is used for treatment of gram‐positive infections such as Staphylococci and Enterococci. In accordance with data presented by Candeloro et al., vancomycin is more commonly used as empiric treatment rather than directed therapy.20 Only a quarter of the therapies were directed. Both units in this study routinely meet with infectious disease practitioners. Rimawi and co‐workers demonstrated that daily collaboration between infectious disease practitioners and critical care practitioners can “significantly reduce medical ICU antibiotic overuse” without increasing mortality, which in turn can reduce healthcare costs.9
The present study has several limitations. Firstly, we were not always able to gather precise weights of the patients. In those cases where precise weights could not be obtained, estimated weight was used. Secondly, the most precise target for vancomycin therapy is the attainment of an AUC/MIC ≥ 400. However, trough serum concentrations were used as a surrogate marker for AUC/MIC. Last, but not least, due to the nature of this study, the physicians at the units were aware of its occurrence, and may have been affected by this knowledge when making decisions regarding vancomycin therapy.
In conclusion, the present study reveals significant challenges in the utilization of vancomycin in a critically ill patient population. Trough serum concentrations were frequently subtherapeutic. This represents an immediate threat to the patients of therapeutic failure and increases the risk of developing bacteria with reduced antibiotic susceptibility. Patients with augmented renal clearance such as trauma patients are particularly in need of close monitoring to achieve proper vancomycin concentrations. The study also reveals that the proportion of appropriate dose adjustments in accordance with TDM was low. The implementation of a dosing protocol would be likely to contribute to more appropriate dose adjustments. Unfortunately, guidelines for vancomycin dose regimens are still warranted for patient subgroups.13, 17, 18, 21
Bakke V, Sporsem H, Von der Lippe E, Nordøy I, Lao Y, Nyrerød HC, Sandvik L, Hårvig KR, Bugge JF, Helset E. Vancomycin levels are frequently subtherapeutic in critically ill patients: a prospective observational study. Acta Anaesthesiologica Scandinavica 2017.
Conflicts of interest
None of the authors have any conflict of interest to report.
Funding
This study has no funding.
References
- 1. Alvarez R, Lopez Cortes LE, Molina J, Cisneros JM, Pachon J. Vancomycin: optimizing its clinical use. Antimicrob Agents Chemother, 2016;60:2601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0‐24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta‐analysis. PLoS ONE 2016; 11: e0146224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reardon J, Lau TT, Ensom MH. Vancomycin loading doses: a systematic review. Ann Pharmacother 2015; 49: 557–65. [DOI] [PubMed] [Google Scholar]
- 4. Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 2011; 52: 975–81. [DOI] [PubMed] [Google Scholar]
- 5. Steinmetz T, Eliakim‐Raz N, Goldberg E, Leibovici L, Yahav D. Association of vancomycin serum concentrations with efficacy in patients with MRSA infections: a systematic review and meta‐analysis. Clin Microbiol Infect 2015; 21: 665–73. [DOI] [PubMed] [Google Scholar]
- 6. Alvarez‐Lerma F, Grau S. Management of antimicrobial use in the intensive care unit. Drugs 2012; 72: 447–70. [DOI] [PubMed] [Google Scholar]
- 7. Blot S, Koulenti D, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Roberts JA. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care 2014; 18: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Momattin H, Zogheib M, Homoud A, Al‐Tawfiq JA. Safety and outcome of pharmacy‐led vancomycin dosing and monitoring. Chemotherapy 2015; 61: 3–7. [DOI] [PubMed] [Google Scholar]
- 9. Rimawi RH, Mazer MA, Siraj DS, Gooch M, Cook PP. Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome. Crit Care Med 2013;41:2099–107. [DOI] [PubMed] [Google Scholar]
- 10. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health‐System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49: 325–7. [DOI] [PubMed] [Google Scholar]
- 11. Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC, Previgliano L, Rubatto NP, Benites MH, Estenssoro E, Dubin A. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Rev Bras Ter Intensiva 2014; 26: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medellin‐Garibay SE, Ortiz‐Martin B, Rueda‐Naharro A, Garcia B, Romano‐Moreno S, Barcia E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. J Antimicrob Chemother 2016; 71: 471–9. [DOI] [PubMed] [Google Scholar]
- 13. Minkute R, Briedis V, Steponaviciute R, Vitkauskiene A, Maciulaitis R. Augmented renal clearance–an evolving risk factor to consider during the treatment with vancomycin. J Clin Pharm Ther 2013; 38: 462–7. [DOI] [PubMed] [Google Scholar]
- 14. Rosini JM, Laughner J, Levine BJ, Papas MA, Reinhardt JF, Jasani NB. A randomized trial of loading vancomycin in the emergency department. Ann Pharmacother 2015; 49: 6–13. [DOI] [PubMed] [Google Scholar]
- 15. Wilson FP, Berns JS. Vancomycin levels are frequently subtherapeutic during continuous venovenous hemodialysis (CVVHD). Clin Nephrol 2012; 77: 329–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, Boots RJ, Lipman J. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med 2014; 42: 520–7. [DOI] [PubMed] [Google Scholar]
- 17. Saugel B, Nowack MC, Hapfelmeier A, Umgelter A, Schultheiss C, Thies P, Phillip V, Eyer F, Schmid RM, Huber W. Continuous intravenous administration of vancomycin in medical intensive care unit patients. J Crit Care 2013; 28: 9–13. [DOI] [PubMed] [Google Scholar]
- 18. Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS, Schmid RM, Huber W. Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: an observational study in intensive care unit patients. J Crit Care 2014; 29: 351–5. [DOI] [PubMed] [Google Scholar]
- 19. Ye ZK, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta‐analysis. PLoS ONE 2013; 8: e77169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Candeloro CL, Kelly LM, Bohdanowicz E, Martin CM, Bombassaro AM. Antimicrobial use in a critical care unit: a prospective observational study. Int J Pharm Pract 2012; 20: 164–71. [DOI] [PubMed] [Google Scholar]
- 21. Ye ZK, Li C, Zhai SD. Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PLoS ONE 2014; 9: e99044. [DOI] [PMC free article] [PubMed] [Google Scholar]


