Abstract
Aims
To compare adherence (proportion of days covered [PDC]), persistence, and treatment patterns among patients with type 2 diabetes mellitus (T2DM) newly initiating glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs). More specifically, the main objectives were to compare dulaglutide vs exenatide once weekly and dulaglutide vs liraglutide.
Methods
Patients with T2DM newly initiating dulaglutide, albiglutide, exenatide once weekly, exenatide twice daily and liraglutide between November 2014 and April 2015 were hierarchically selected from Truven Health's MarketScan Research Databases. Propensity score matching was used to account for selection bias. Adherence to and persistence with the index GLP‐1RA, and switching and augmentation patterns were assessed during the 6‐month post‐index period.
Results
Mean adherence for the matched cohorts was significantly higher for dulaglutide than for exenatide once weekly (0.72 vs 0.61; P < .0001) and liraglutide (0.71 vs 0.67; P < .0001). The percentage of patients achieving PDC ≥ 0.80 was significantly higher for dulaglutide compared with exenatide once weekly (54.2% vs 37.9%; P < .0001) and liraglutide (53.5% vs 44.3%; P < .0001). The mean (standard deviation) days on treatment for all matched patients was significantly higher for patients in the dulaglutide cohort compared with those in the exenatide once‐weekly (148.4 [55.4] vs 123.6 [61.6]; P < .0001) and liraglutide cohorts (146.0 [56.9] vs 137.4 [60.1]; P < .0001). A significantly lower proportion of patients on dulaglutide discontinued treatment compared with those on exenatide once weekly (26.2% vs 48.4%; P < .0001) and those on liraglutide (28.0% vs 35.6%; P < .0001).
Conclusions
Dulaglutide initiators had significantly higher adherence, were more persistent, and had lower discontinuation rates compared with initiators of exenatide once weekly or liraglutide during the 6‐month follow‐up period.
Keywords: adherence, augmentation, glucagon‐like peptide‐1 receptor agonists, persistence, real‐world evidence, switching, type 2 diabetes mellitus
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a chronic, progressive metabolic disease characterized by persistent hyperglycaemia resulting from β‐cell dysfunction and worsening of insulin resistance.1 More than 29.1 million people in the USA have diabetes (21 million diagnosed; 8.1 million undiagnosed), with T2DM accounting for 95% of cases.2 The lifetime risk of developing T2DM has risen significantly, from 20% in the period 1985 to 1989 to 40% in 2000 to 2011 for men and from 27% to 39% for women.3
Optimum glycaemic control in T2DM may be achieved by diet and lifestyle management alone, or along with use of any of several classes of oral antidiabetic drugs (OADs) as monotherapy or combination therapy.4, 5, 6 As the disease progresses, injectable therapies such as insulin and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) can be used, usually as combination treatment.7, 8, 9 Despite the availability of multiple therapeutic options, only 36% of patients with T2DM achieve optimum glycaemic control, and an estimated 14.9% of US adults with uncontrolled diabetes do not take any medications.2, 8
Medication adherence and persistence are critical for the effectiveness of antidiabetic therapies.8 A systematic review of adherence data from retrospective/prospective electronic monitoring studies found a wide range of adherence rates to OADs, ranging from 36% to 93%, and 62% to 64% adherence to insulin therapy.10 Increased adherence to antidiabetic agents has been shown to improve glycaemic control and reduce healthcare utilization, mortality and diabetes‐related complications (glycated haemoglobin [HbA1c] levels, diabetic ketoacidosis and cardiovascular disease).10, 11, 12, 13 Medication adherence has also been associated with lower disease‐related healthcare costs.14, 15 Even adherence to treatment regimens with relatively high pharmacy costs has been found to reduce overall healthcare costs.16 In addition, studies have shown simpler and less frequent dosing regimens are associated with increased adherence.17, 18
The GLP‐1RAs, also known as incretin mimetics, are a relatively new class of injectable antidiabetic drugs19 that reduce hyperglycaemia by stimulating glucose‐dependent insulin secretion, suppressing postprandial glucagon release in a glucose‐dependent manner, and inducing satiety.20, 21 Controlled clinical trials and retrospective studies have shown GLP‐1RAs to be an effective therapeutic option for achieving optimum glycaemic targets,22, 23, 24 and they are included in treatment guidelines. For example, the American Association of Clinical Endocrinologists guidelines recommend the use of GLP‐1RAs as the first therapeutic choice after metformin6 and the American Diabetes Association and the European Association for the Study of Diabetes guidelines recommend GLP‐1RAs as one of the treatment options after metformin5, 7 for patients with inadequately controlled T2DM.
During the study period, 5 GLP‐1RAs had been approved by the US Food and Drug Administration (FDA) for the treatment of T2DM: exenatide twice daily (Byetta), exenatide once weekly (Bydureon), liraglutide once daily (Victoza), albiglutide once weekly (Tanzeum), and dulaglutide once weekly (Trulicity). A sixth GLP‐1RA, lixisenatide (Adlyxin), received approval from the FDA on July 28, 2016, after the study was completed.25 Previous studies have shown significant differences in adherence among patients with T2DM newly initiating exenatide once weekly, exenatide twice daily, or liraglutide.23, 26, 27 Some studies suggested better adherence with exenatide once weekly than liraglutide,23, 27 and 1 study found better adherence with liraglutide than with exenatide twice daily.26 Because of its relatively recent entry into the market, little information is available on adherence associated with dulaglutide vs other GLP‐1RAs. In the present retrospective analysis we sought to address this gap in the literature. The key objectives of the study were to compare adherence, persistence and treatment patterns of dulaglutide vs exenatide once weekly and dulaglutide vs liraglutide. Secondary objectives included comparison of the 5 GLP‐1RAs with respect to those measures. Exenatide once weekly and liraglutide were selected as the main and appropriate real‐world comparators to dulaglutide because exenatide once weekly has once‐weekly dosing like dulaglutide, and liraglutide is the most used GLP‐1RA currently on the market.
2. METHODS
2.1. Data source
This retrospective observational cohort study used administrative medical and pharmacy claims data from the Truven Health MarketScan Commercial Claims and Encounters (Commercial) and the Medicare Supplemental and Coordination of Benefits (Medicare) databases between May 2014 and October 2015. This Early View version maximized available follow‐up data and reflected a pharmacy claims completion rate of >97% with all claims paid and adjudicated prior to their inclusion in the database. The databases included inpatient and outpatient medical and outpatient prescription claims of individuals with employer‐sponsored primary or Medicare supplemental health insurance. The databases have been used in multiple published studies related to medication adherence.23, 28, 29, 30, 31, 32 The databases satisfy the conditions of statistically de‐identified data as set forth in the Health Insurance Portability and Accountability Act of 1996. As this study used only de‐identified patient data, it was exempted from institutional review board approval.
2.2. Patient selection
Patients with at least 1 prescription claim for a GLP‐1RA during the patient selection period of November 5, 2014 to April 30, 2015 were considered for study inclusion. (The beginning of sample selection corresponded to the US launch date of dulaglutide; the end date was based on available data at the time this study was conducted). To maximize the sample size for newer agents, patients were selected hierarchically and placed in the first of the following cohorts for which they qualified: dulaglutide; albiglutide; exenatide once weekly; exenatide twice daily; or liraglutide. The index date was the date of the first claim for the assigned GLP‐1RA. Patients eligible for inclusion were new to the index GLP‐1RA (i.e., no claim for the same medication in the prior 6 mo), with at least 1 medical claim with a diagnosis of T2DM (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD‐9‐CM] 250.x0, 250.x2) during the 6 months pre‐index, who had continuous enrolment with both medical and pharmacy benefits 6 months before and after the index date. Patients aged <18 years on the index date or with a diagnosis of gestational diabetes (ICD‐9‐CM 648.8x) during the 6 months pre‐index were excluded.
2.3. Outcome measures
Adherence, measured by proportion of days covered (PDC), was calculated as the number of days in the 6‐month post‐index period that a patient had the index drug on hand divided by the number of days in the period. Overlapping days covered by 2 consecutive prescriptions of the same medication were not double counted in the PDC calculation. PDC thresholds of ≥0.80 and ≥0.90 were calculated. A patient with PDC ≥ 0.80 was considered adherent.
Persistence (days on treatment) was measured in days from the index date to the end of days’ supply of the last claim before the 60‐day gap in the 6 months post‐index period. Patients were considered as discontinued if there was no claim for the index drug beyond the 60‐day gap. The percentage of patients discontinuing their index drug in the 6 months post‐index period was reported. Sensitivity analysis using a 45‐day gap to define discontinuation was also performed. Treatment modification was the first event of change in the medication (switching and augmentation) in the 6 months post‐index. Switching was the discontinuation of the index GLP‐1RA and the start of another GLP‐1RA or class of antidiabetic medication not present in the 6‐month pre‐index period. Augmentation indicated the addition of another antidiabetic medication class not present in the 6‐month pre‐index period.
2.4. Statistical analysis
Baseline demographics and clinical characteristics were summarized and compared using a t‐test for continuous variables and a chi‐squared test for categorical variables. Because patients were not randomized to treatment, propensity score matching was used to adjust for possible treatment selection bias for each comparison: dulaglutide vs exenatide once weekly and dulaglutide vs liraglutide. The propensity score was defined as the probability of assignment to the dulaglutide cohort given the baseline characteristics. The characteristics listed in Tables 1 and 2 served as covariates in a logistic regression to generate a propensity score for each patient. These variables were selected a priori based on previously identified variables of interest in a comparison of liraglutide and exenatide once weekly.27 Patients on dulaglutide were matched 1:1 to those on exenatide once weekly using a greedy matching algorithm with a caliper of 0.2x standard deviation of the logit of the propensity score.33 In addition, patients were matched exactly on prior GLP‐1RA use to ensure balance with this variable, given the hierarchical nature of the selection. A similar separate process matched patients initiated on dulaglutide with those initiated on liraglutide. Standardized differences of <0.10 were considered to denote balance in baseline characteristics between the cohorts.34, 35 The propensity score matching was finalized before the analysis of the outcomes was conducted.
Table 1.
Patient characteristics of dulaglutide vs exenatide once‐weekly cohorts pre‐ and post‐propensity matching
| Characteristics | All patients | Matched patients | ||||
|---|---|---|---|---|---|---|
| Dulaglutide | Exenatide once weekly | Standardized difference | Dulaglutide | Exenatide once weekly | Standardized difference | |
| n = 2470 | n = 5022 | n = 2415 | n = 2415 | |||
| Mean (s.d.) age, years | 55.3 (10.3) | 54.9 (10.0) | 0.04 | 54.3 (9.9) | 54.4 (9.8) | 0.01 |
| Women, n (%) | 1311 (53.1) | 2688 (53.5) | 0.01 | 1266 (52.4) | 1254 (51.9) | 0.01 |
| Geographic region, n (%) | ||||||
| Northeast | 397 (16.1) | 835 (16.6) | 0.01 | 460 (19.0) | 461 (19.1) | 0.00 |
| North Central | 505 (20.5) | 1006 (20.0) | 0.01 | 398 (16.5) | 379 (15.7) | 0.02 |
| South | 1333 (54.0) | 2664 (53.0) | 0.02 | 1332 (55.2) | 1347 (55.8) | 0.01 |
| West | 233 (9.4) | 512 (10.2) | 0.03 | 222 (9.2) | 226 (9.4) | 0.01 |
| Unknown | 2 (0.1) | 5 (0.1) | 0.01 | 3 (0.1) | 2 (0.1) | 0.01 |
| Primary insurance payer, n (%) | ||||||
| Commercial | 2111 (85.5) | 4364 (86.9) | 0.04 | 2152 (89.1) | 2149 (89.0) | 0.00 |
| Medicare | 359 (14.5) | 658 (13.1) | – | 263 (10.9) | 266 (11.0) | – |
| Comorbid conditions, n (%) | ||||||
| Cardiovascular disease | 281 (11.4) | 550 (11.0) | 0.01 | 267 (11.1) | 273 (11.3) | 0.01 |
| Dyslipidaemia | 1549 (62.7) | 3112 (62.0) | 0.02 | 1612 (66.7) | 1625 (67.3) | 0.01 |
| Hypertension | 1643 (66.5) | 3330 (66.3) | 0.00 | 1595 (66.0) | 1635 (67.7) | 0.04 |
| Nephropathy | 231 (9.4) | 475 (9.5) | 0.00 | 229 (9.5) | 225 (9.3) | 0.01 |
| Neuropathy | 427 (17.3) | 833 (16.6) | 0.02 | 432 (17.9) | 436 (18.1) | 0.00 |
| Retinopathy | 132 (5.3) | 280 (5.6) | 0.01 | 133 (5.5) | 144 (6.0) | 0.02 |
| Mean (s.d.) Deyo–Charlson comorbidity index | 1.9 (1.4) | 1.8 (1.4) | 0.02 | 1.8 (1.4) | 1.9 (1.4) | 0.01 |
| Antidiabetic medications during the pre‐index period, n (%) | ||||||
| Metformin | 1449 (58.7) | 3041 (60.6) | 0.04 | 1484 (61.4) | 1526 (63.2) | 0.04 |
| Sulphonylureas | 708 (28.7) | 1650 (32.8%) | 0.09 | 723 (29.9) | 804 (33.3)* | 0.07 |
| DPP‐4 inhibitor | 526 (21.3) | 1022 (20.4%) | 0.02 | 540 (22.4) | 549 (22.7) | 0.01 |
| SGLT2 | 422 (17.1) | 850 (16.9%) | 0.00 | 661 (27.4) | 643 (26.6) | 0.02 |
| Non‐index GLP‐1RA | 372 (15.1) | 742 (14.8%) | 0.01 | 760 (31.5) | 760 (31.5) | 0.00 |
| Insulin | 923 (37.4) | 1871 (37.2%) | 0.00 | 1025 (42.4) | 1011 (41.9) | 0.01 |
| Othera | 296 (12.0) | 575 (11.5) | 0.02 | 304 (12.6) | 294 (12.2) | 0.00 |
| Endocrinologist visit during pre‐index period, n (%) | 622 (25.2) | 1234 (24.6) | 0.01 | 908 (37.6) | 893 (37.0) | 0.01 |
Abbreviation: DPP‐4, dipeptidyl peptidase‐4; s.d., standard deviation.
*P < .05.
Other antidiabetics include: α‐glucosidase inhibitors, amylin analogues, meglitinides, or thiazolidinediones.
Propensity scores were calculated using the baseline covariates age, gender, geographic location, and health plan type on the index date; Charlson comorbidity index, presence of cardiovascular disease, hyperlipidaemia, obesity, gastrointestinal diagnosis or medication; total copayment and coinsurance across all pharmacy claims observed during the baseline period; total copayment and coinsurance on index claim; number of prescription drug classes, use of non‐index GLP‐1 (exact match), insulin, SGLT2, and DPP‐4 inhibitor during the baseline period; presence of HbA1c test claim pre‐index; presence and number of endocrinologist visits pre‐index; and number of inpatient admissions and office visits pre‐index.
Table 2.
Patient characteristics of dulaglutide vs liraglutide cohorts pre‐ and post‐propensity matching
| Characteristics | All patients | Matched patients | ||||
|---|---|---|---|---|---|---|
| Dulaglutide | Liraglutide | Standardized difference | Dulaglutide | Liraglutide | Standardized difference | |
| n = 2470 | n = 8705 | n = 2037 | n = 2037 | |||
| Mean (s.d.) age, years | 55.3 (10.3) | 54.9 (10.2) | 0.03 | 54.3 (10.1) | 54.1 (10.0) | 0.01 |
| Women, n (%) | 1311 (53.1) | 4656 (53.5) | 0.01 | 1074 (52.7) | 1068 (52.4) | 0.01 |
| Geographic region, n (%) | ||||||
| Northeast | 397 (16.1) | 1421 (16.3) | 0.01 | 392 (19.2) | 370 (18.2) | 0.03 |
| North Central | 505 (20.5) | 1760 (20.2) | 0.01 | 333 (16.3) | 333 (16.3) | 0.00 |
| South | 1333 (54.0) | 4649 (53.4) | 0.01 | 1119 (54.9) | 1122 (55.1) | 0.00 |
| West | 233 (9.4) | 868 (10.0) | 0.02 | 191 (9.4) | 210 (10.3) | 0.03 |
| Unknown | 2 (0.1) | 7 (0.1) | 0.00 | 2 (0.1) | 2 (0.1) | 0.00 |
| Primary insurance payer, n (%) | ||||||
| Commercial | 2111 (85.5) | 7462 (85.7) | 0.01 | 1805 (88.6) | 1791 (87.9) | 0.02 |
| Medicare | 359 (14.5) | 1243 (14.3) | – | 232 (11.4) | 246 (12.1) | – |
| Comorbid conditions, n (%) | ||||||
| Cardiovascular disease | 281 (11.4) | 954 (11.0) | 0.01 | 220 (10.8) | 206 (10.1) | 0.02 |
| Dyslipidaemia | 1549 (62.7) | 5436 (62.4) | 0.01 | 1353 (66.4) | 1353 (66.4) | 0.00 |
| Hypertension | 1643 (66.5) | 5804 (66.7) | 0.00 | 1363 (66.9) | 1373 (67.4) | 0.01 |
| Nephropathy | 231 (9.4) | 892 (10.3) | 0.03 | 198 (9.7) | 216 (10.6) | 0.03 |
| Neuropathy | 427 (17.3) | 1420 (16.3) | 0.03 | 379 (18.6) | 335 (16.4) | 0.06 |
| Retinopathy | 132 (5.3) | 507 (5.8) | 0.02 | 107 (5.3) | 121 (5.9) | 0.03 |
| Mean (s.d.) Deyo–Charlson comorbidity index | 1.9 (1.4) | 1.8 (1.4) | 0.01 | 1.9 (1.4) | 1.9 (1.4) | 0.00 |
| Antidiabetic medications during the pre‐index period, n (%) | ||||||
| Metformin | 1449 (58.7) | 5232 (60.1) | 0.03 | 1238 (60.8) | 1285 (63.1) | 0.05 |
| Sulphonylureas | 708 (28.7) | 2880 (33.1)† | 0.10 | 603 (29.6) | 702 (34.5)† | 0.10 |
| DPP‐4 inhibitor | 526 (21.3) | 1809 (20.8) | 0.01 | 496 (24.3) | 520 (25.5) | 0.03 |
| SGLT2 | 422 (17.1) | 1449 (16.6) | 0.01 | 529 (26.0) | 557 (27.3) | 0.03 |
| Non‐index GLP‐1RA | 372 (15.1) | 1302 (15.0) | 0.00 | 363 (17.8) | 363 (17.8) | 0.00 |
| Insulin | 923 (37.4) | 3212 (36.9) | 0.01 | 850 (41.7) | 865 (42.5) | 0.01 |
| Othera | 296 (12.0) | 873 (10.0)* | 0.06 | 244 (12.0) | 210 (10.3) | 0.05 |
| Endocrinologist visit during pre‐index period, n (%) | 622 (25.2) | 2141 (24.6) | 0.01 | 743 (36.5) | 786 (38.6) | 0.04 |
*P < .05.
†P < .001.
Other antidiabetics include: α‐glucosidase inhibitors, amylin analogues, meglitinides, or thiazolidinediones.
Propensity scores were calculated using the baseline covariates age, gender, geographic location, and health plan type on the index date; Charlson comorbidity index, presence of cardiovascular disease, hyperlipidaemia, obesity, gastrointestinal diagnosis or medication; total copayment and coinsurance across all pharmacy claims observed during the baseline period; total copayment and coinsurance on index claim; number of prescription drug classes, use of non‐index GLP‐1 (exact match), insulin, SGLT2 and DPP‐4 inhibitor during the baseline period; presence of HbA1c test claim pre‐index; presence and number of endocrinologist visits pre‐index; and number of inpatient admissions and office visits pre‐index.
The matched cohorts were compared on adherence measures; specifically, mean PDC and the proportion of patients with PDC ≥ 0.80 and ≥ 0.90. Persistence measures, including days to discontinuation and percentage of patients who discontinued, were also compared for the 2 matched cohorts. Kaplan–Meier estimates and Cox regression were used in the analysis of persistence (days to discontinuation) and logistic regression was used in the analysis of adherence (PDC ≥ 0.80). Comparisons of the 5 cohorts (albiglutide, dulaglutide, exenatide twice daily, exenatide once weekly and liraglutide) were also conducted using inverse probability treatment weighting (IPTW) with propensity score.36, 37, 38, 39 A multinomial logistic regression was used to generate the propensity scores with the baseline characteristics listed in the footnotes of Tables 1 and 2 as covariates. P values <.05 were taken to indicate statistical significance between the treatment cohorts in all comparisons. No adjustments were made for multiple comparisons. Analyses were conducted in SAS version 9.4 (Cary, NC, USA).
3. RESULTS
Between November 5, 2014 and April 30, 2015, 2470 patients met the inclusion criteria for the dulaglutide cohort; 1350 for albiglutide; 5022 for exenatide once weekly; 1369 for exenatide twice daily, and 8705 for liraglutide. After matching, the dulaglutide and exenatide once‐weekly comparison included 2415 patients in each cohort and the dulaglutide and liraglutide comparison included 2037 patients in each cohort (Figure S1). Among matched patients in the exenatide once‐weekly cohort, 666 (27.6%) initiated treatment with the vial formulation and 1749 (72.4%) initiated treatment with the pen formulation.
Tables 1 and 2 show the demographic and clinical characteristics of the dulaglutide vs exenatide once‐weekly and dulaglutide vs liraglutide cohorts before and after propensity score matching, respectively. During the pre‐index period, 42.4% of matched dulaglutide patients were prescribed insulin, 27.4% were prescribed a sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor, and 31.5% were prescribed a non‐index GLP‐1RA compared with 41.9%, 26.6% and 31.5% of matched patients in the exenatide once‐weekly cohort, respectively (Table 1). For the second matched cohort, 41.7% of patients on dulaglutide were prescribed insulin, 26.0% were prescribed an SGLT2 inhibitor, and 17.8% a non‐index GLP‐1RA compared with 42.5%, 27.3%, and 17.8% of matched patients in the liraglutide cohort, respectively. After matching, all key demographics and pre‐index characteristics were balanced.
A list of prescribed antidiabetic medications used in the post‐index period for both matched cohorts is shown in Table S1. Across both matched cohorts, the most frequent medications used during the post‐index period were metformin, sulphonylureas, insulin and SGLT2 inhibitors.
3.1. Adherence
Table 3 shows the adherence and persistence for the 2 matched comparisons. In the dulaglutide vs exenatide once‐weekly matched comparison, the dulaglutide cohort had statistically significantly higher adherence (mean PDC: dulaglutide = 0.72; exenatide once weekly = 0.61), percentage of patients with PDC ≥ 0.80 (54.2% vs 37.9%), and PDC ≥ 0.90 (37.3% vs 26.5%) than the exenatide once‐weekly cohort (all P < .0001). Patients in the dulaglutide cohort also had significantly better adherence (mean PDC: dulaglutide = 0.71; liraglutide = 0.67), percentage of patients with PDC ≥ 0.80 (53.5% vs 44.3%) and PDC ≥ 0.90 (36.4% vs 29.4%) than matched patients in the liraglutide cohort (all P < .0001).
Table 3.
Adherence and persistence of matched patients during the 6‐month post‐index period
| Outcome Variable | Dulaglutide n = 2415 | Exenatide, once weekly n = 2415 | Dulaglutide n = 2037 | Liraglutide n = 2037 |
|---|---|---|---|---|
| PDC | ||||
| Mean (s.d.) | 0.72 (0.27) | 0.61 (0.29) | 0.71 (0.28) | 0.67 (0.28) |
| ≥0.80, n (%) | 1310 (54.2) | 915 (37.9) | 1090 (53.5) | 903 (44.3) |
| ≥0.90, n (%) | 902 (37.3) | 639 (26.5) | 742 (36.4) | 599 (29.4) |
| Mean (s.d.) days on treatment for all patients in the matched cohorts | 148.4 (55.4) | 123.6 (61.6) | 146.0 (56.9) | 137.4 (60.1) |
| Patients who discontinued during the 6‐mo post‐index period, n (%) | 632 (26.2) | 1170 (48.4) | 570 (28.0) | 725 (35.6) |
All P < .0001.
3.2. Persistence
The mean (standard deviation [s.d.]) number of days on treatment for all matched patients in the dulaglutide cohort was higher compared with those in the exenatide once‐weekly cohort (148.4 [55.4] vs 123.6 [61.6]; P < .0001). The mean (s.d.) days on treatment for all matched patients in the dulaglutide cohort was 146.0 (56.9) days compared with 137.4 (60.1 days; P < .0001) for those in the liraglutide cohort.
Over the 6‐month post‐index period, 26.2% of dulaglutide and 48.4% of exenatide once‐weekly patients discontinued treatment (P < .0001). Significantly fewer patients discontinued dulaglutide compared with liraglutide (28.0% vs 35.6%; P < .0001). Sensitivity analysis carried out by shortening the treatment gap for persistence to 45 days resulted in a slight increase in proportion of patients who were considered discontinued in all matched cohort comparisons (dulaglutide vs exenatide once weekly: 30.7% vs 52.7%, P < .0001; dulaglutide vs liraglutide: 32.2% vs 41.6%, P < .0001).
Patients on dulaglutide were less likely to discontinue than patients on exenatide once weekly during the 6 months after initiation (hazard ratio [HR] 0.48; 95% confidence interval [CI] (0.44, 0.53); Figure 1A) and compared with patients on liraglutide (HR 0.79; 95% CI (0.71, 0.88); Figure 1B).
Figure 1.
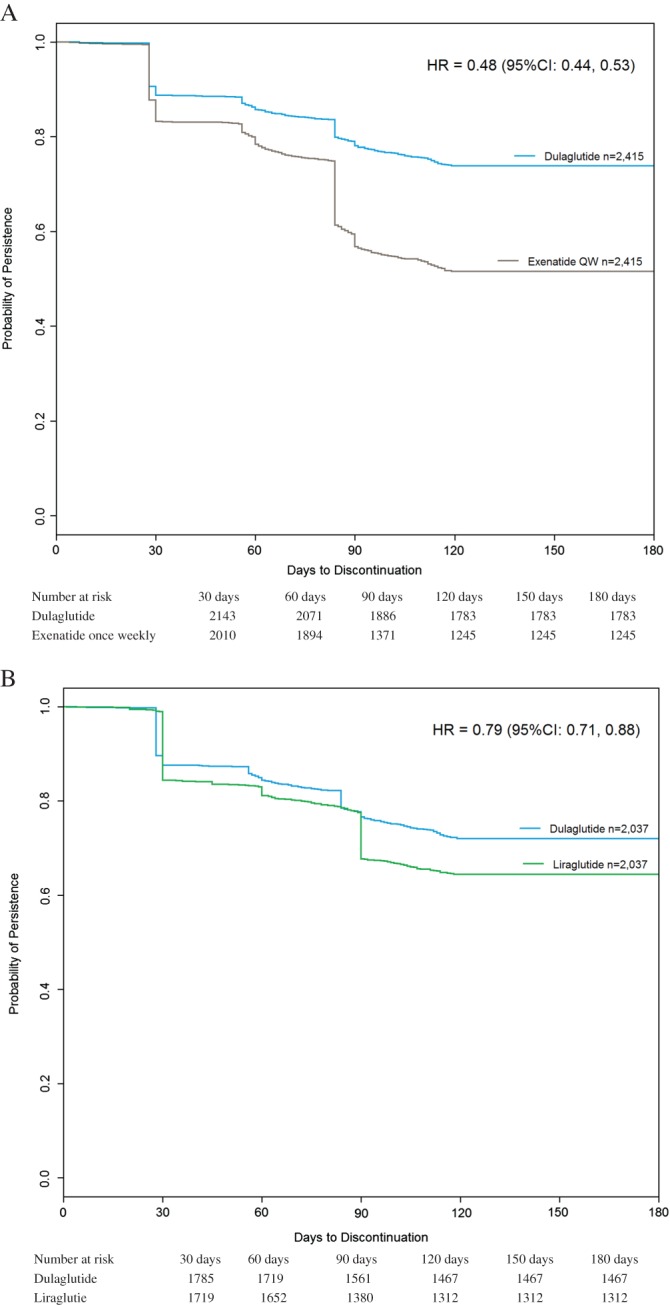
A, Kaplan–Meier persistence curves for the matched dulaglutide and exenatide once weekly patients during the 6‐month post‐index period. B, Kaplan–Meier persistence curves for the matched dulaglutide and liraglutide patients during the 6‐month post‐index period
3.3. Treatment switching
A total of 6.6% of matched patients in the dulaglutide cohort and 11.4% (P < .0001) of those in the exenatide once‐weekly cohort switched antidiabetic agents during the post‐index period. Among matched patients, 2.3% of dulaglutide patients switched to a different GLP‐1RA during the post‐index period, compared with 3.8% of patients on exenatide once weekly (P = .0034). Liraglutide was the most commonly switched to GLP‐1RA in both cohorts (1.1% from dulaglutide and 1.9% from exenatide once weekly; P = .0231; Figure S2A). Patients in the exenatide once‐weekly cohort more frequently switched to another antidiabetic medication class than those in the dulaglutide cohort (8.2% vs 4.8%; P < .0001). SGLT2 inhibitors were the most frequently switched‐to medication class for both cohorts (1.1% dulaglutide, 2.7% exenatide once weekly, P < .0001; Figure S2A).
In the matched dulaglutide and liraglutide comparison, 7.1% of dulaglutide and 7.1% (P = 1.0000) of patients in the liraglutide cohort switched medications during the post‐index period. A total of 2.6% of patients in the dulaglutide cohort switched to a non‐index GLP‐1RA compared with 1.2% of those in the liraglutide cohort (P = .0019). The highest proportion of patients in the dulaglutide cohort switched to liraglutide (1.1%), while dulaglutide was the most commonly switched‐to GLP‐1RA for those in the liraglutide cohort (0.7%, Figure S2B). Switching to another antidiabetic medication class was observed for 5.2% of matched patients in the dulaglutide cohort, compared with 5.9% of matched patients in the liraglutide cohort (P = .3056). Dulaglutide patients most frequently switched to metformin (1.2%) followed by SGLT2 inhibitors (1.1%), while patients in the liraglutide cohort most frequently switched to insulin (1.6%), followed by SGLT2 inhibitors (1.5%; Figure S2B).
3.4. Treatment augmentation
A total of 4.3% of matched patients in the dulaglutide cohort and 9.4% (P < .0001) of patients in the exenatide once‐weekly cohort augmented their index drug with another antidiabetic medication during the post‐index period. Among both cohorts, the highest proportion of patients augmented with SGLT2 inhibitors (1.5% for dulaglutide, 2.9% exenatide once weekly; P = .0006; Figure S3A). In the matched comparison between dulaglutide and liraglutide, 4.6% of patients in the dulaglutide cohort and 6.2% (P = .0265) of those in the liraglutide cohort augmented therapy during the post‐index period. SGLT2 inhibitors were the most frequent augmentation class for patients on dulaglutide (1.6%), while those on liraglutide most frequently augmented with insulin (2.0%; Figure S3B).
3.5. Analysis of all 5 GLP‐1RAs
The findings of improved adherence for dulaglutide in the 2 separate matched comparisons above were consistent with the analysis results that included all 5 GLP‐1RAs. In the IPTW logistic model, the odds of adherence (PDC ≥ 0.80) were lower for albiglutide (odds ratio [OR] 0.63; 95% CI 0.55, 0.73), exenatide twice daily (OR 0.32; 95% CI 0.28, 0.37), exenatide once weekly (OR 0.48; 95% CI 0.43, 0.53), and liraglutide (OR 0.65; 95% CI 0.59, 0.71) compared with dulaglutide (Figure S4). In the IPTW Cox regression model, patients on dulaglutide were significantly less likely to discontinue treatment over the 6‐month post‐index period than patients on albiglutide, exenatide twice daily, exenatide once weekly, and liraglutide (Figure S5).
4. DISCUSSION
To our knowledge, this is the first study to directly compare dulaglutide with exenatide once weekly and liraglutide in terms of medication adherence and persistence using real‐world data. Within this study population, patients with T2DM initiating treatment with dulaglutide showed considerably better adherence and persistence over 6 months post‐index, with lower rates of treatment discontinuation, than either propensity‐score‐matched patients in the exenatide once‐weekly cohort or those in the liraglutide cohort. The results from the analyses including all 5 marketed GLP‐1RAs were consistent with the findings from the 2 propensity‐score‐matched comparisons that included only exenatide once weekly and liraglutide.
Adherence to prescribed therapies is a primary determinant of treatment effectiveness in patients with T2DM.39 Treatment non‐adherence among patients with T2DM has been associated with increased rates of hospitalization and mortality.12, 40 By contrast, an increase in adherence and persistence is likely to result in better glycaemic control11, 22, 41 and economic outcomes.42
The present analysis compared 2415 matched patients for dulaglutide vs exenatide once weekly and 2037 matched patients for dulaglutide vs liraglutide on adherence and persistence outcomes. Patients with T2DM initiating treatment with dulaglutide had significantly higher adherence rates over 6 months post‐index compared with patients initiating exenatide once weekly or liraglutide. Patients treated with dulaglutide had lower discontinuation rates compared with those treated with exenatide once weekly and those treated with liraglutide during the 6‐month follow‐up period. The analysis results including all 5 marketed GLP‐1RAs also showed a lower rate of discontinuation and higher odds of adherence for patients on dulaglutide compared with those initiated on other GLP‐1RAs. Analysis of treatment patterns among matched patients in the dulaglutide and exenatide once‐weekly cohorts showed that during 6 months post‐index, patients who initiated dulaglutide were less likely to switch to a different GLP‐1RA or another antidiabetic medication class compared with those who initiated exenatide once weekly. Among the matched dulaglutide and liraglutide cohorts, switching to a different class of antidiabetic medication also was less common among patients on dulaglutide than those on liraglutide. Rates of therapy augmentation with a different GLP‐1RA or another class of antidiabetic medication also were higher for patients in the exenatide once weekly and those in the liraglutide cohorts, as compared with the matched dulaglutide cohort.
A limited number of previous studies used administrative claims databases to compare adherence and persistence rates of various GLP‐1RAs in patients with T2DM.23, 26, 27 One retrospective analysis comparing 12‐month adherence rates of liraglutide 1.8 mg once daily and exenatide 10 µg twice daily among treatment‐naïve patients with T2DM found liraglutide to have superior adherence rates compared with exenatide twice daily.26 Another retrospective study reported significantly higher adjusted odds of adherence among patients with T2DM treated with exenatide once weekly compared with other GLP‐1RAs.23 In a more recent study, patients with T2DM treated with exenatide once weekly had slightly higher adherence and slightly lower persistence profiles compared with liraglutide;27 however, these studies used data obtained prior to the availability of dulaglutide in the USA.
Higher adherence and persistence with dulaglutide over liraglutide may be related to its simplified dosing—once weekly for dulaglutide vs once daily for liraglutide.43 Dulaglutide's ready‐to‐use single‐dose pen that does not require handling of the needle or reconstitution, vs the exenatide once‐weekly pen or the exenatide once‐weekly vial/syringe kit that requires reconstitution and delivery by a 23‐gauge needle, could have been another factor in the greater adherence and persistence rates seen with dulaglutide.
The observations reported from the present study should be interpreted in the context of the limitations associated with retrospective databases, including variability in the completeness of the data, coding imperfections, and potential for unmeasured confounding. This analysis was limited to individuals with commercial health coverage or private Medicare supplemental coverage and, thus, results may not be generalizable to patients with T2DM with other types of insurance or without insurance. The analysis relied on paid prescription claims; therefore, it was not possible to account for medication samples or cash‐pay low‐cost generic drugs, potentially underestimating the use of additional medication. Misclassification of T2DM diagnosis, covariates, or study outcomes was possible as claims data are subject to coding limitations and data entry error. More than 97% of outpatient prescription drug claims are adjudicated within ~1–2 months after the fill date, but the Early View data used for the present analysis may not have included some claims adjudicated later. Finally, because of the recent launch of dulaglutide and data availability at the time of the study, the follow‐up period of 6 months was relatively short. Future analyses with longer follow‐up periods would foster a better understanding of comparative adherence and persistence over time.
Despite the aforementioned limitations, the present study does have important strengths. It included a large sample size of patients with T2DM enrolled in diverse health plans across the USA. Propensity score matching methodologies balanced baseline covariates between comparators. The study assessed adherence and persistence for all 5 currently available GLP‐1RAs among patients with T2DM from real‐world clinical practice.
In summary, the results of the present study suggest that patients receiving dulaglutide for T2DM are significantly more likely to adhere to and persist with medication during the initial 6 months after medication initiation than patients treated with exenatide once weekly or liraglutide. Given the importance of medication adherence in improving glycaemic control and ultimately preventing chronic complications attributable to T2DM, dulaglutide may be an important treatment option to help improve clinical and economic outcomes for patients with diabetes. Future research with longer follow‐up would be useful to determine if the higher adherence and persistence associated with dulaglutide, as compared with other GLP‐1RAs, translates to healthcare resource utilization and cost savings over time.
Supporting information
Figure S1. Patient selection.
Figure S2A. Treatment switching patterns of dulaglutide and exenatide QW during the 6‐month post‐index period.
Figure S2B. Treatment switching patterns of dulaglutide and liraglutide patients during the 6‐month post‐index period.
Figure S3A. Treatment augmentation patterns of dulaglutide and exenatide QW patients who augmented during the 6‐month post‐index period.
Figure S3B. Treatment augmentation patterns of dulaglutide and liraglutide patients who augmented during the 6‐month post‐index period.
Figure S4. Odds ratio of adherence (PDC ≥ 0.80) from IPTW logistic regression during the 6‐month post‐index period, comparing albiglutide, exenatide BID, exenatide QW, and liraglutide to dulaglutide.
Figure S5. Kaplan‐Meier persistence curves of the 5 GLP‐1 RAs during the 6‐month post‐index period.
Table S1. Prescribed antidiabetic medications during the 6‐month post‐index period.
ACKNOWLEDGEMENTS
The authors would like to thank the following Truven Health colleagues for their assistance with this work: Alice Huang (study design, analysis), George Shrady (programming), Santosh Tiwari (manuscript preparation) and Brian Davis (results interpretation, manuscript preparation).
Conflict of interest
C. Alatorre, L. Fernández Landó, M. Yu, K. Brown, R. Mody are employees of Eli Lilly and Company and own stock in the company. R. Swindle was an employee of Eli Lilly and Company at the time of study design and execution. L. Montejano, P. Juneau are employees of Truven Health Analytics, an IBM Company, which received funding from Eli Lilly and Company to conduct this study.
Author contributions
C. Alatorre, L. Fernández Landó, M. Yu, K. Brown, R. Swindle, contributed to study design, participated in interpretation of results, and helped to draft the manuscript. R. M. participated in interpretation of results and drafting of the manuscript. L. M. and P. J. contributed to study design, study conduct, data analysis, result interpretation and writing of the paper. All authors participated in critical reviewing of and approving the final version of the manuscript and take full responsibility for the content.
Alatorre C, Fernández Landó L, Yu M, Brown K, Montejano L, Juneau P, Mody R and Swindle R. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon‐like peptide‐1 receptor agonists: Higher adherence and persistence with dulaglutide compared with once‐weekly exenatide and liraglutide , Diabetes Obes Metab, 2017;19:953–961. https://doi.org/10.1111/dom.12902.
Funding information Funding for this study was provided to Truven Health Analytics by Eli Lilly and Company. The analysis was conducted independently by Truven Health. Lilly and Truven Health collaborated on study design and interpretation of results.
REFERENCES
- 1. Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 3. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867‐874. [DOI] [PubMed] [Google Scholar]
- 4. Lew KN, Wick A. Pharmacotherapy of type 2 diabetes mellitus: navigating current and new therapies. Medsurg Nurs. 2015;24(6):413‐419, 438. [PubMed] [Google Scholar]
- 5. American Diabetes Association . Standards of medical care in diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1‐S112. [DOI] [PubMed] [Google Scholar]
- 6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016;22(1):84‐113. [DOI] [PubMed] [Google Scholar]
- 7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38(1):140‐149. [DOI] [PubMed] [Google Scholar]
- 8. Miller BR, Nguyen H, Hu CJ, Lin C, Nguyen QT. New and emerging drugs and targets for type 2 diabetes: reviewing the evidence. Am Health Drug Benefits. 2014;7(8):452‐463. [PMC free article] [PubMed] [Google Scholar]
- 9. Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218‐1224. [DOI] [PubMed] [Google Scholar]
- 11. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74‐109. [DOI] [PubMed] [Google Scholar]
- 12. Encinosa WE, Bernard D, Dor A. Does prescription drug adherence reduce hospitalizations and costs? The case of diabetes. Adv Health Econ Health Serv Res. 2010;22:151‐173. [DOI] [PubMed] [Google Scholar]
- 13. Hayward RA, Reaven PD, Wiitala WL, et al. Follow‐up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197‐2206. [DOI] [PubMed] [Google Scholar]
- 14. Banerji MA, Dunn JD. Impact of glycemic control on healthcare resource utilization and costs of type 2 diabetes: current and future pharmacologic approaches to improving outcomes. Am Health Drug Benefits. 2013;6(7):382‐392. [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia‐Perez LE, Alvarez M, Dilla T, Gil‐Guillen V, Orozco‐Beltran D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521‐530. [DOI] [PubMed] [Google Scholar]
- 17. Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31(3):213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollack M, Chastek B, Williams S, Moran J. Impact of treatment complexity on adherence and glycemic control: an analysis of oral antidiabetic agents. J Clin Outcomes Manag. 2010;17(6):257‐265. [Google Scholar]
- 19. Ross SA, Ballantine J. Early use of glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) in type 2 diabetes. Curr Med Res Opin. 2013;29(12):1617‐1626. [DOI] [PubMed] [Google Scholar]
- 20. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696‐1705. [DOI] [PubMed] [Google Scholar]
- 21. Koliaki C, Doupis J. Incretin‐based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2(2):101‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeKoven M, Lee WC, Bouchard J, Massoudi M, Langer J. Real‐world cost‐effectiveness: lower cost of treating patients to glycemic goal with liraglutide versus exenatide. Adv Ther. 2014;31(2):202‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon‐like peptide‐1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31(11):1119‐1133. [DOI] [PubMed] [Google Scholar]
- 24. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Food and Drug Administration . FDA approves Adlyxin to treat type 2 diabetes. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm513602.htm. Accessed July 28, 2016.
- 26. Malmenas M, Bouchard JR, Langer J. Retrospective real‐world adherence in patients with type 2 diabetes initiating once‐daily liraglutide 1.8 mg or twice‐daily exenatide 10 mug. Clin Ther. 2013;35(6):795‐807. [DOI] [PubMed] [Google Scholar]
- 27. Yu M, Xie J, Fernandez Lando L, Kabul S, Swindle RW. Liraglutide versus exenatide once weekly: Persistence, adherence, and early discontinuation. Clin Ther. 2016;38(1):149‐160. [DOI] [PubMed] [Google Scholar]
- 28. Chandran A, Bonafede MK, Nigam S, Saltiel‐Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148‐158. [PMC free article] [PubMed] [Google Scholar]
- 29. Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29(10):1275‐1286. [DOI] [PubMed] [Google Scholar]
- 30. Farr AM, Sheehan JJ, Curkendall SM, Smith DM, Johnston SS, Kalsekar I. Retrospective analysis of long‐term adherence to and persistence with DPP‐4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31(12):1287‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carls GS, Roebuck MC, Brennan TA, Slezak JA, Matlin OS, Gibson TB. Impact of medication adherence on absenteeism and short‐term disability for five chronic diseases. J Occup Environ Med. 2012;54(7):792‐805. [DOI] [PubMed] [Google Scholar]
- 32. Ye X, Qian C, Liu J, St Peter WL. Lower risk of major cardiovascular events associated with adherence to colesevelam HCI. Pharmacotherapy. 2013;33(10):1062‐1070. [DOI] [PubMed] [Google Scholar]
- 33. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Imai K, van Dyk DA. Causal inference with general treatment regimes. J Am Stat Assoc. 2004;99(467):854‐866. [Google Scholar]
- 37. Imbens GW. The role of the propensity score in estimating dose‐response functions. Biometrika. 2000;87(3):706‐710. [Google Scholar]
- 38. Imbens GW. Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat. 2004;86(1):4‐29. [Google Scholar]
- 39. Kirkman MS, Rowan‐Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35(6):1279‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnston SS, Nguyen H, Cappell K, Nelson JK, Chu BC, Kalsekar I. Retrospective study comparing healthcare costs and utilization between commercially insured patients with type 2 diabetes mellitus who are newly initiating exenatide once weekly or liraglutide in the United States. J Med Econ. 2015;18(9):666‐677. [DOI] [PubMed] [Google Scholar]
- 42. Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amblee A. Mode of administration of dulaglutide: implications for treatment adherence. Patient Prefer Adherence. 2016;10:975‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient selection.
Figure S2A. Treatment switching patterns of dulaglutide and exenatide QW during the 6‐month post‐index period.
Figure S2B. Treatment switching patterns of dulaglutide and liraglutide patients during the 6‐month post‐index period.
Figure S3A. Treatment augmentation patterns of dulaglutide and exenatide QW patients who augmented during the 6‐month post‐index period.
Figure S3B. Treatment augmentation patterns of dulaglutide and liraglutide patients who augmented during the 6‐month post‐index period.
Figure S4. Odds ratio of adherence (PDC ≥ 0.80) from IPTW logistic regression during the 6‐month post‐index period, comparing albiglutide, exenatide BID, exenatide QW, and liraglutide to dulaglutide.
Figure S5. Kaplan‐Meier persistence curves of the 5 GLP‐1 RAs during the 6‐month post‐index period.
Table S1. Prescribed antidiabetic medications during the 6‐month post‐index period.


