Abstract
Peritoneum is the most common site for ovarian cancer metastasis. Here we investigate how cancer epigenetics regulates reciprocal tumor-stromal interactions in peritoneal metastasis of ovarian cancer. Firstly, we find that omental stromal fibroblasts enhance colony formation of metastatic ovarian cancer cells, and de novo expression of transforming growth factor-alpha (TGF-α) is induced in stromal fibroblasts co-cultured with ovarian cancer cells. We also observed an over-expression of tumor necrosis factor-alpha (TNF-α) in ovarian cancer cells, which is regulated by promoter DNA hypomethylation as well as chromatin remodeling. Interestingly, this ovarian cancer-derived TNF-α induces TGF-α transcription in stromal fibroblasts through nuclear factor-κB (NF-κB). We further show that TGF-α secreted by stromal fibroblasts in turn promotes peritoneal metastasis of ovarian cancer through epidermal growth factor receptor (EGFR) signaling. Finally, we identify a TNFα-TGFα-EGFR interacting loop between tumor and stromal compartments of human omental metastases. Our results therefore demonstrate cancer epigenetics induces a loop of cancer-stroma-cancer interaction in omental microenvironment that promotes peritoneal metastasis of ovarian cancer cells via TNFα-TGFα-EGFR.
Introduction
Ovarian cancer is a serious health problem worldwide. A majority (>75%) of ovarian cancer patients were diagnosed at late stage (stage III and IV) at which cancer cells have already disseminated and metastasized to the peritoneum and/or distant organs.1 Although about 80% of the patients with advanced ovarian cancers initially respond to the first-line treatment (including surgical debulking and platinum-based post-operative chemotherapy), residual diseases will progress into chemo-resistant ovarian cancer and relapse within 16–22 months in most of the patients. This is the reason why the 5-year survival rate of patients with stage III and IV ovarian cancer remains in an unsatisfactory level (17–39% www.cancer.org).2, 3 By understanding the cellular and molecular mechanism of ovarian cancer metastasis in peritoneum, it will provide insights into developing novel treatment to compensate current standard-of-care treatments for ovarian cancer.
Ovarian cancer metastasizes generally through direct dissemination from the primary site into peritoneal cavity, without intravasation and extravasation of blood vessels.4 In fact, most of patients with advanced ovarian cancer present with omental metastasis.5, 6 In omentum, stromal fibroblasts are the second most numerous cell types.7 The role of stromal fibroblasts and cancer-associated fibroblasts (CAFs) in tumor progression has been described. The factors, secreted by stromal fibroblasts or CAFs, transduce signals to cancer cells as well as to themselves establishing reciprocal reinforcement of growth and progression signals in various types of cancer.1, 8 The growth of metastatic cancer cells in distant sites after dissemination termed ‘metastatic colonization’. This process is thought to be critical for the survival of remaining microscopic tumor residuals after surgical debulking and development of chemo-resistance ovarian tumor.9, 10 The molecular mechanism of how stromal fibroblasts promotes metastatic colonization of ovarian cancer in omental tissue microenvironment, however, remains largely unknown.7
Three-dimensional (3D) organoid models emulate a more physiologically relevant microenvironment in cancer than two-dimensional (2D) monolayer cell culture.7, 11, 12 In this study, we applied a 3D organoid co-culture model to investigate if normal stromal fibroblasts promote metastatic colonization of ovarian cancer, and to investigate the reciprocal paracrine signaling between cancer cells and stromal fibroblasts that promotes peritoneal metastasis of ovarian cancer.
Results
Stromal fibroblasts enhance colony formation of metastatic ovarian cancer cells in 3D organoid model
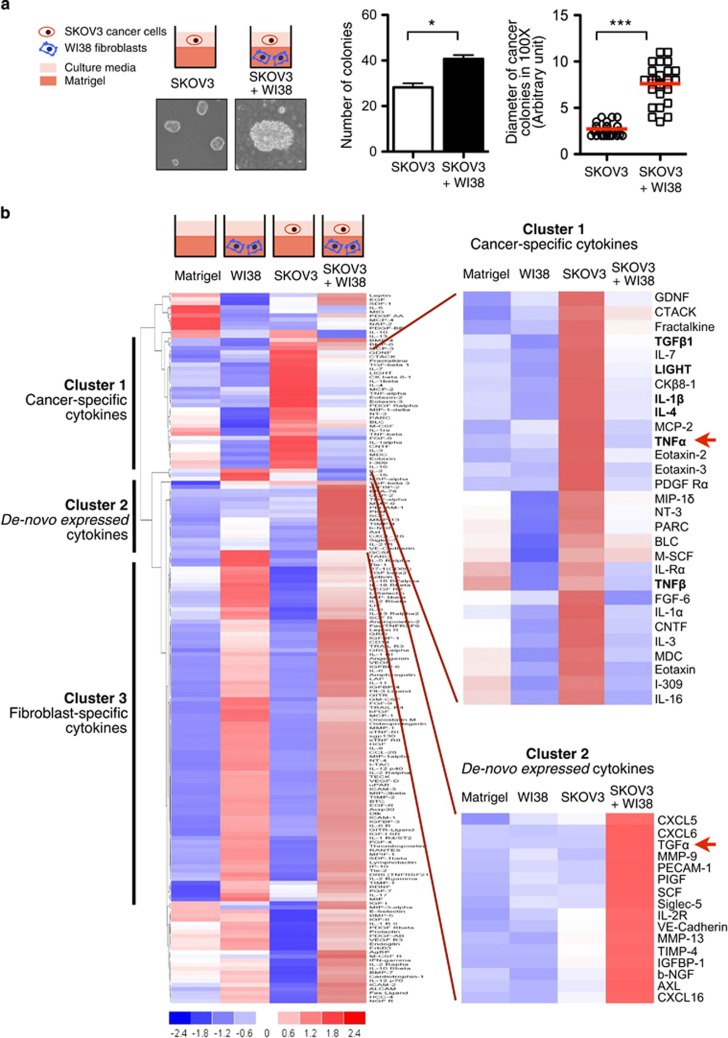
To investigate how the omental microenvironment (that is, stromal fibroblasts) affects metastatic colonization of ovarian cancer, we used a 3D organoid co-culture model based on the ‘seed and soil’ hypothesis.13 We embedded normal human stromal fibroblasts (WI38) with extracellular matrix (ECM; Matrigel) in culture chambers, followed by overlaying a single-cell suspension of metastatic human ovarian cancer cells (SKOV3; a human ovarian cancer adenocarcinoma cell line derived from ascites) on the ECM-fibroblast mixture. Our results showed that SKOV3 cells formed colonies with or without WI38 fibroblasts. The number of SKOV3 cancer colonies formed in ECM with WI38 fibroblasts was significantly higher than that in ECM without WI38 fibroblasts, Moreover, the colonies co-cultured with WI38 fibroblasts were significantly larger in size (Figure 1a). Our results indicated that stromal fibroblasts enhance the colony formation of metastatic ovarian cancer cells in 3D organoid culture.
Figure 1.
Stromal fibroblasts enhance colony formation of metastatic ovarian cancer cells in three-dimensional (3D) organoid model with de-novo expression of 16 cytokines. (a) Schematic representation of 3D organoid co-culture model (left, upper panel). Normal stromal fibroblasts (WI38) were mixed with extracellular matrix (ECM; BD Matrigel™) and placed at the bottom of chamber slides. Single-cell suspension of metastatic ovarian cancer cells (SKOV3) was then overlaid on top of ECM with WI38. Monoculture of SKOV3 with ECM alone was served as controls. The data represents means±s.e.m. from three independent experiments. ***P<0.001, *P<0.05 (Mann-Whitney U test). (b) Conditioned medium (CM) from ECM alone (Matrigel), 3D monoculture of WI38, 3D monoculture of SKOV3, and 3D organoid co-culture model (SKOV3+WI38) were collected on day 4 (left, upper panel) and their relative protein expressions of a total of 174 cytokines were compared using cytokine antibody array (RayBiotech AAH-CYT-G2000-4). The signal was then normalized by the internal positive control and subject to clustering analysis using DChip software (left, lower panel). A panel of cancer-specific cytokines was identified in the CM of 3D organoid monoculture of SKOV3 ovarian cancer cells (right, upper panel). TNF-α was indicated by a red arrow. De-novo expression of 16 cytokines was identified in the CM of 3D organoid co-culture (right, lower panel). TGF-α was indicated by a red arrow. (Blue: low expression level; White: no change; Red: high expression level).
De novo expression of a group of cytokines in 3D organoid co-culture of metastatic ovarian cancer cells and stromal fibroblasts
We then compared the cytokine production of 3D organoid co-culture (SKOV3+WI38) with 3D monocultures (WI38 or SKOV3 alone) by cytokine antibody array. Out of the 174 cytokines being detected, 116 were co-expressed by the conditioned medium (CM) of the stromal fibroblast monoculture (WI38) and co-culture (SKOV3+WI38), suggesting these cytokines are derived from stromal fibroblasts (Figure 1b; Cluster 3: Fibroblast-specific cytokines). A group of 25 cytokines was predominantly expressed by the monoculture of metastatic ovarian cancer cells (SKOV3; Figure 1b; Cluster 1: Cancer-specific cytokines). Notably, a group of 16 cytokines was uniquely expressed by the co-culture (SKOV3+WI38), including CXCL5, CXCL6, TGF-α, MMP-9, PECAM-1, PIGF, Siglec-5, IL-2 R, VE-cadherin, MMP-13, TIMP-4, IGFBP-1, b-NGF, Axl, and CXCL16 (Figure 1b; Cluster 2: De novo expressed cytokines).
De novo expression of TGF-α is induced in stromal fibroblasts co-cultured with metastatic ovarian cancer cells
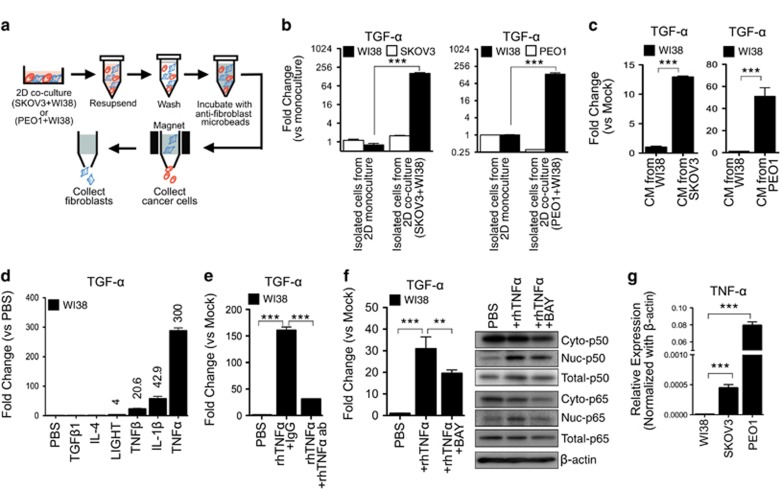
Transforming growth factor alpha (TGF-α) is a key growth factor in ovarian cancer microenvironment.14, 15, 16 Here we investigated the source of de novo expression of TGF-α in our 2D co-culture models, including 1) SKOV3+WI38, and 2) PEO1 (another metastatic human ovarian cancer cell line derived from ascites17)+WI38. By ELISA, we confirmed higher levels of soluble TGF-α in CM from 2D co-cultures (SKOV3+WI38 and PEO1+WI38) when compare to CM of 2D monoculture (Supplementary Figure 1a). We isolated the metastatic ovarian cancer cells from stromal fibroblasts by magnetic microbeads isolation kit (Figure 2a). Purity of the isolation was high. When applying cell surface expression of CD44 as a marker of human stromal fibroblasts,18 we detected 85% of CD44-positive cells in the fibroblast isolations by flow cytometry (Supplementary Figure 2). We then measured the mRNA levels of TGF-α of isolated metastatic ovarian cancer cells and stromal fibroblasts from 2D co-cultures and compared to those from monocultures of SKOV3, PEO1 or WI38 alone (Figure 2a). The mRNA levels of TGF-α of isolated WI38, but not ovarian cancer cells, from the 2D co-cultures (SKOV3+WI38 and PEO1+WI38), were significantly higher than those from 2D monocultures (Figure 2b and Supplementary Figure 1b). Our results indicated that stromal fibroblasts co-cultured with metastatic ovarian cancer cells induce de novo expression of TGF-α.
Figure 2.
De-novo expression of TGF-α in stromal fibroblasts was induced by TNF-α secreted by ovarian cancer cells through NF-κB signaling. (a) Schematic representation depicting the separation between stromal fibroblasts (blue) and ovarian cancer cells (red) from two-dimensional (2D) co-culture by magnetic micro-beads isolation kits (MACS). Ovarian cancer cells were first isolated by fibroblasts depletion and stromal fibroblasts were isolated by positive selection using anti-fibroblast microbeads. (b) The mRNA expression levels of TGF-α of the isolated cells (cancer cells or fibroblasts) from 2D co-cultures (SKOV3+WI38 or PEO1+WI38) were determined by qRT-PCR and compared to those from 2D monocultures (SKOV3, PEO1 or WI38 alone). (c) The mRNA expression levels of TGF-α were examined in WI38 fibroblasts after treatments with CM of SKOV3 or PEO1 cells. Treatments with CM of WI38 fibroblasts were served as controls. (d) The mRNA expression levels of TGF-α were determined in WI38 fibroblasts after treatments of 100 ng/ml recombinant human cytokines (TGF-β1, IL-4, LIGHT, TNF-β, IL-1β and TNF-α) for 24 h. PBS was served as controls. Fold change against PBS treatment was measured. (e) The mRNA expression levels of TGF-α were determined in WI38 fibroblasts after treatments of rhTNFα (100 ng/ml) with or without rhTNFα neutralizing antibodies (rhTNFα ab; 10 ng/ml) for 24 h. Human IgG1 (IgG) was served as controls. (f) The mRNA expression levels of TGF-α (left panel), and protein expression of cytoplasmic (Cyto) and nuclear (Nuc) p50 and p65 (right panel) were detected in WI38 fibroblasts after treatments of rhTNFα (100 ng/ml) for 24 h with or without pre-treatment of BAY 11-7082 (BAY, a NF-κB inhibitor; 1 μM) for 1 h. The mRNA of TGF-α expression was determined by qRT-PCR. Protein expression of p50 and p65 in cytoplasmic and nuclear fraction was detected by Western blotting. β-actin was used for equal loading. (g) The mRNA expression levels of endogenous TNF-α in 2D monoculture of WI38, SKOV3 and PEO1 cells were detected by qRT-PCR. All data represents means±s.e.m. from three independent experiments. Statistical difference was determined by Mann-Whitney U test. **P<0.01; ***P<0.001.
Ovarian cancer cells secrete TNF-α that induces de novo expression of TGF-α in stromal fibroblasts through NF-κB signaling
Next, we examined if the cancer-specific cytokine(s), which were predominantly expressed by the 3D monoculture of SKOV3 cells (Figure 1b; Cluster 1: Cancer-specific cytokines), are involved in inducing the TGF-α transcription in stromal fibroblasts. We treated WI38 fibroblasts with the conditioned medium of metastatic ovarian cancer cells (SKOV3 or PEO1), and found that TGF-α levels in WI38 fibroblasts were significantly up-regulated (Figure 2c). Treatment with recombinant human (rh) cancer-specific cytokines, including TNF-α (tumor necrosis factor alpha; 300 fold), IL-1β (interleukin-1 beta; 42.9 fold), and TNF-β (lymphotoxin; 20.6 fold), also showed significant up-regulation of TGF-α in WI38 fibroblasts (Figure 2d). We then showed the treatment with TNF-α neutralization antibodies abolished the rhTNFα-induced TGF-α expression in WI38 fibroblasts (Figure 2e), confirming that TNF-α is the key cytokine that induces TGF-α induction in stromal fibroblasts.
Besides, in our cytokine antibody array data, TNF-α protein expression level in the conditioned medium decreased substantially in co-culture when compared to that of SKOV3 ovarian cancer cell monoculture. However, our qRT-PCR data showed that there was no change in the mRNA level of TNF-α in either mono- or co-culture cells (Supplementary Figure 3). Since we showed that TNF-α is the key cytokine that induced TGF-α in stromal fibroblasts, our results suggested that the fibroblasts actively uptake the TNF-α in conditioned medium.
The classical TNFα-regulated gene expression involves activation of NF-κB signaling pathway,19 and three critical NF-κB binding sites were identified in the human TGF-α promoter region.20 Here we inhibited the NF-κB signal pathway in WI38 fibroblasts by Bay 11-7082 (BAY) prior to rhTNF-α stimulation, and found that the treatment of BAY significantly reduced the TNFα-induced TGF-α expression (Figure 2f). For p65/p50 heterodimer is the most common form of NF-κB in fibroblasts,21 we had studied the protein expression of cytoplasmic and nuclear p50 and p65 in stromal fibroblasts. Nuclear translocation of p50 and p65 was detected in TNFα-stimulated WI38 fibroblasts, while pre-treatment with BAY reduced such nuclear translocation (Figure 2f). Additionally, the mRNA level of TNF-α was significantly higher in metastatic ovarian cancer cells (SKOV3 and PEO1) when compared to those in stromal fibroblasts (WI38; Figure 2g). Taken together, our results suggested that TNF-α secreted by metastatic ovarian cancer cells induces de novo expression of TGF-α in stromal fibroblasts through NF-κB signaling.
Promoter DNA hypomethylation and chromatin remodeling regulate TNF-α over-expression in human ovarian cancer cells
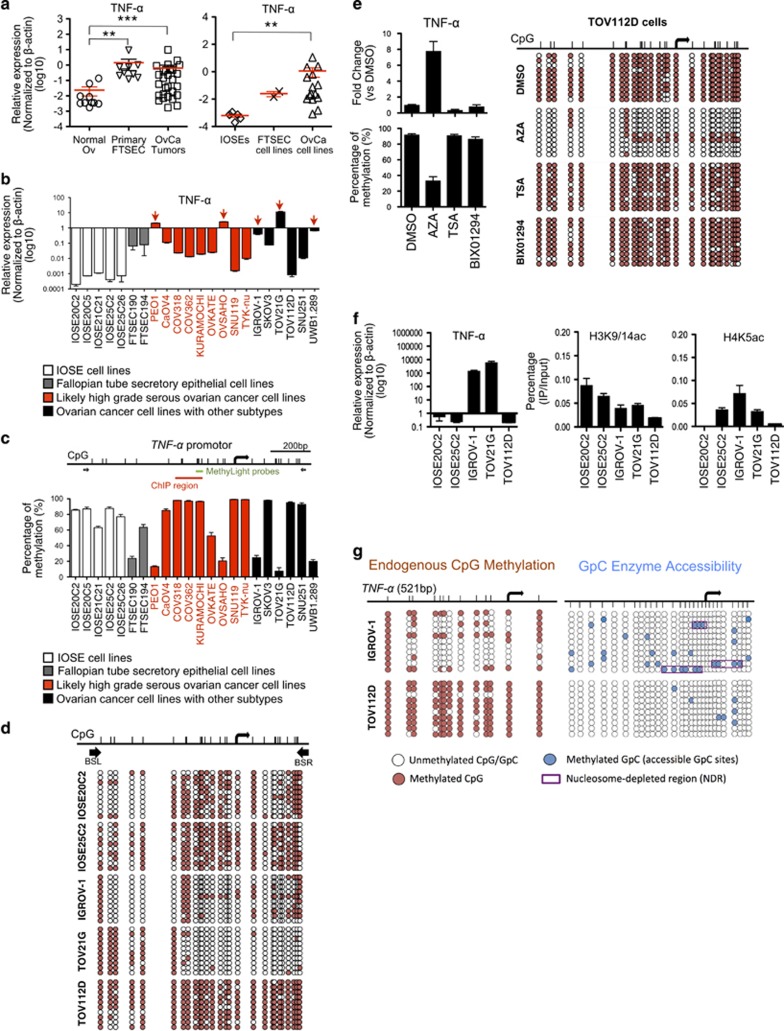
Although TNF-α is over-expressed in ovarian cancer,22 its underlying mechanism is not known. Here, we confirmed that TNF-α is significantly over-expressed in human ovarian cancer by comparing the mRNA levels of normal ovarian surface epithelial tissue (Normal Ov; n=10) to tumor tissue (OvCa Tumors; n=26) from primary ovarian cancer (including serous subtypes; Figure 3a). A recent integrative proteomic profiling study of ovarian cancers indicates human fallopian tube secretory epithelial cells (FTSEC) are one possible origin of high-grade serous ovarian cancer (HGSOC).23 Therefore, we included primary FTSEC (n=9) in the TNF-α mRNA expression study, and found high expression levels of TNF-α in primary FTSEC (Figure 3a). TNF-α is also over-expressed in human ovarian cancer cell lines (n=15) when compared with non-malignant hTERT-immortalized human ovarian surface epithelial (IOSE) cell lines (n=5) and immortalized FTSEC cell lines (n=2; Figure 3a). Individual analysis showed that TNF-α is over-expressed in 5 human ovarian cancer cell lines, including IGROV-1, PEO1 (likely high-grade serous cell line), TOV21G, OVSAHO (likely high-grade serous cell line), and UWB1.289 cells (Figure 3b), whereas low levels of TNF-α were detected in all IOSE cell lines and FTSEC cell lines being examined.
Figure 3.
Promoter DNA hypomethylation and chromatin remodeling regulate TNF-α over-expression in human ovarian cancer cells. (a) The mRNA expression levels of TNF-α was compared between normal ovarian tissue (Normal Ov), micro-dissected fallopian tube secretory epithelial cells from primary normal fallopian tube (Primary FTSEC) and micro-dissected tumor tissue from primary ovarian cancers (OvCa tumors) (left), and between non-malignant hTERT-immortalized human ovarian surface epithelial (IOSE) cell lines, immortalized fallopian tube secretory epithelial cells cell lines (FTSEC cell lines) and human ovarian cancer cell lines (OvCa cell lines)(right), by qRT-PCR. **P<0.01 and ***P<0.001 (Mann-Whitney U test). The data represents means±s.e.m. from three independent experiments. (b) The mRNA expression levels of TNF-α in individual IOSE, FTSEC cell lines and human ovarian cancer cell lines were determined by qRT-PCR. (c) DNA methylation levels of TNF-α promoter in IOSE, FTSEC cell lines and human ovarian cell lines were determined by MethyLight assay using sequence-specific probe (indicated by the green bar). (d) DNA methylation status of TNF-α promoter in IOSE (IOSE20C2 and IOSE25C2) and human ovarian cell lines (IGROV-1, TOV21G, and TOV112D) was investigated by bisulfite genomic sequencing. (White circles, unmethylated CpGs; Red circles, methylated CpGs). Bisulfite-specific PCR primers (BSL and BSR) was indicated by black arrows. (e) The mRNA expression of TNF-α gene and DNA methylation of TNF-α promoter were determined in TOV112D ovarian cancer cells after treatment of 5-aza-2’-deoxycytidine (AZA), trichostatin A (TSA), or methyltransferase inhibitor (BIX01294) for 72 h. DMSO was served as controls. The mRNA expression levels of TNF-α were determined by qRT-PCR (left, upper panel). DNA methylation status was detected by MethyLight assay (left, lower panel) and bisulfite genomic sequencing (right). (f) Chromatin immunoprecipitation (ChIP) was performed in the cell lines with low (IOSE20C2, IOSE25C2, and TOV112D) and high TNF-α expression (IGROV-1 and TOV21G), using antibodies for acetylated H3K9/14 (H3K9/14ac) (middle) and acetylated H4K5 (H4K5ac) (right). Immunoprecipitated DNA was analyzed by qRT-PCR using primers flanking TNF-α promoter region (as indicated by the red bar in (c). Expression of TNF-α transcript in IOSE20C2, IOSE25C2, IGROV-1, TOV21G and TOV112D was shown for reference (left; excerpt from b). (g) Nucleosome-depleted region (NDR) of TNF-α promoter was detected in cell line with high TNF-α expression (IGROV-1), but not in cell line with low TNF-α expression (TOV112D), using high-resolution nucleosome positioning assay (NOMe-Seq). (White circles, unmethylated CpGs/GPCs; red circles, methylated CpCs; blue circles, methylated GpGs; purple boxes, NDR).
Human TNF-α is located at 6p21.3, whereas DNA hypomethylation at 6p21.3 is commonly found in HGSOC.24 Using MethyLight assay, we showed that high levels of DNA methylation of TNF-α promoter were detected in cell lines with low TNF-α levels, while a significant reduction of DNA methylation corresponded with the ovarian cancer cell lines with high levels of TNF-α (that is, IGROV-1, PEO1, TOV21G, OVSAHO, and UWB1.289 cells; Figure 3c). This observation was confirmed by bisulfite genomic sequencing (Figure 3d), indicating that DNA hypomethylation in TNF-α promoter correlates with TNF-α over-expression in human ovarian cancer cells. We then treated TOV112D, an ovarian cancer cell line that expresses low level of TNF-α, with a DNA methyltranferase inhibitor, 5-aza-2’-deoxycytidine (AZA). AZA significantly up-regulated the TNF-α mRNA level, while reducing the DNA methylation level of TNF-α promoter in the ovarian cancer cells (Figure 3e and Supplementary Figure 4). Moreover, there is a negative correlation between TNF-α mRNA expression and TNF-α promoter methylation in ovarian serous cystadenocarcinoma from TCGA study (Supplementary Figure 5). These results indicate DNA hypomethylation regulates active endogenous gene expression of TNF-α promoter in human ovarian cancer.
Specific histone tail modifications have been shown to be associated with active and inactive CpG promoters.25 For instance, acetylation of histone H3 lysine 9 (H3K9) and methylation of histone H3 lysine 4 (H3K4) are important for the transcriptional activation of CpG island promoters.26 Here, we treated TOV112D cancer cells with histone deacetylase (HDAC) inhibitor trichostatin A (TSA) or histone methyltransferase (HMTase) inhibitor BIX01294, but showed no significant changes in their mRNA levels of TNF-α after treatment (Figure 3e and Supplementary Figure 4). Our ChiP-qPCR analysis also showed no enrichment of acetylated H3K9/14 and acetylated H4K5ac at the TNF-α promoter of the ovarian cancer cell lines with high levels of TNF-α (IGROV-1 and TOV21G) when compared to those with low levels (IOSE20C2, IOSE25C2, and TOV112D; Figure 3f). These results indicate that transcription of TNF-α promoter in human ovarian cancer cells is not regulated by histone acetylation or histone methylation.
Chromatin remodeling that involves nucleosome positioning has been implicated in the regulation of gene expression. In particular, active CpG island promoters contain a nucleosome-depleted region (NDR) upstream to their transcription start sites (TSSs) that allows the access transcriptional machinery to the promoters, whereas inactive CpG island promoters lack this NDR.27 Here we used NOMe-Seq (Nucleosome Occupancy and Methylome sequencing)28 to detect the accessibility of the GpC methyltransferase to methylate GpC sites that are not bound by the nucleosomes in TNF-α promoters of the human ovarian cancer cell lines (IGROV-1 and TOV112D). Our results showed in IGROV-1 cells, the upstream of TSS of TNF-α promoter is accessible to GpC methyltransferase, which indicates NDR is present that might allow the binding of appropriate transcription factors (Figure 3g, purple boxes). The presence of NDRs is correlated with high levels of TNF-α expression and un-methylated status of TNF-α promoter in IGROV-1 cells (refer to Figures 3b–d). In contrast, TOV112D cells, which exhibit extensive methylation at TNF-α promoter and low TNF-α levels, showed almost no accessibility to GpC methyltransferase at regions immediately upstream to the TSS (Figure 3g), indicating NDR is absent.
Taken together, these results showed that promoter DNA hypomethylation and chromatin remodeling regulate TNF-α over-expression in human ovarian cancer cells.
Fibroblast-derived TGF-α promotes colony formation of metastatic ovarian cancer cells in 3D organoid co-culture through EGFR activation
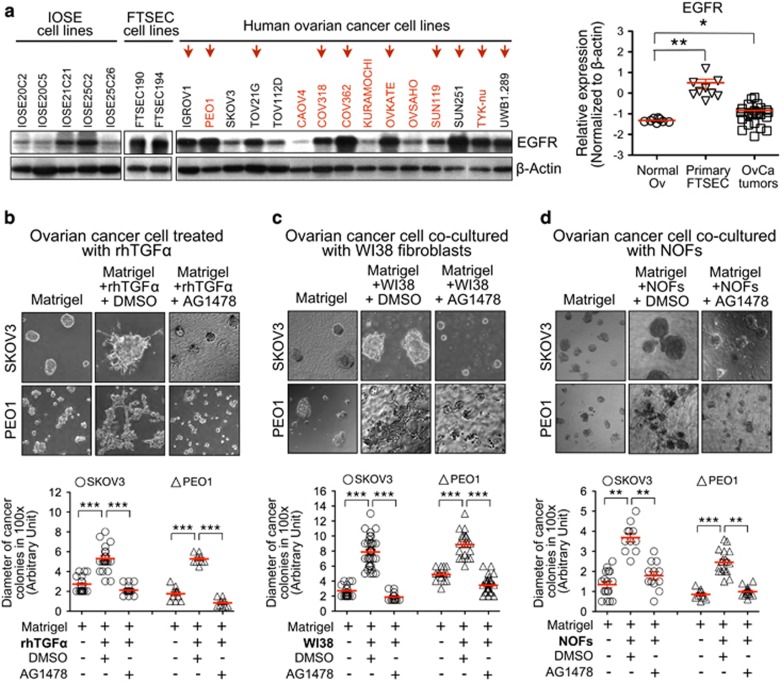
We had shown metastatic ovarian cancer cells induce de novo expression of TGF-α in stromal fibroblasts in co-culture (Figure 1b). Here, we showed that the treatment with rhTGF-α significantly enlarged the colony sizes of metastatic ovarian cancer cells (SKOV3 or PEO1) in 3D monocultures (Figure 4b). We then examined the EGFR (epidermal growth factor receptor) expression in human ovarian cancer cell lines and non-malignant IOSE cells, for EGFR is a receptor for TGF-α29 and is over-expressed by up to 62% of epithelial ovarian cancer and correlated with poor outcome of ovarian cancer.30, 31 We found that EGFR was over-expressed in 10 out of 15 ovarian cancer cell lines and in 2 FTSEC cell lines (Figure 4a). The mRNA levels of EGFR in primary ovarian cancer tissues (OvCa tumors) and primary fallopian tube secretory epithelial cells (FTSECs) were also significantly higher than those in normal ovarian epithelia (Normal Ov; Figure 4a). The pre-treatment of an EGFR tyrosine kinase inhibitor (TKI), Tyrphostin AG1478, significantly abolished the rhTGFα-induced cancer colony formation of the 3D monocultures of metastatic ovarian cancer cells (Figure 4b), as well as the fibroblast-induced colony formation of metastatic ovarian cancer cells in our 3D organoid co-cultures (SKOV3+WI38 and PEO1+WI38; Figure 4c). Given that AG1478 did not affect cellular proliferation of monoculture of WI38 fibroblasts or ovarian cancer cells (SKOV3 and PEO1; Supplementary Figure 6), our results suggested that EGFR is involved in the TGF-α-mediated colony formation of metastatic ovarian cancer.
Figure 4.
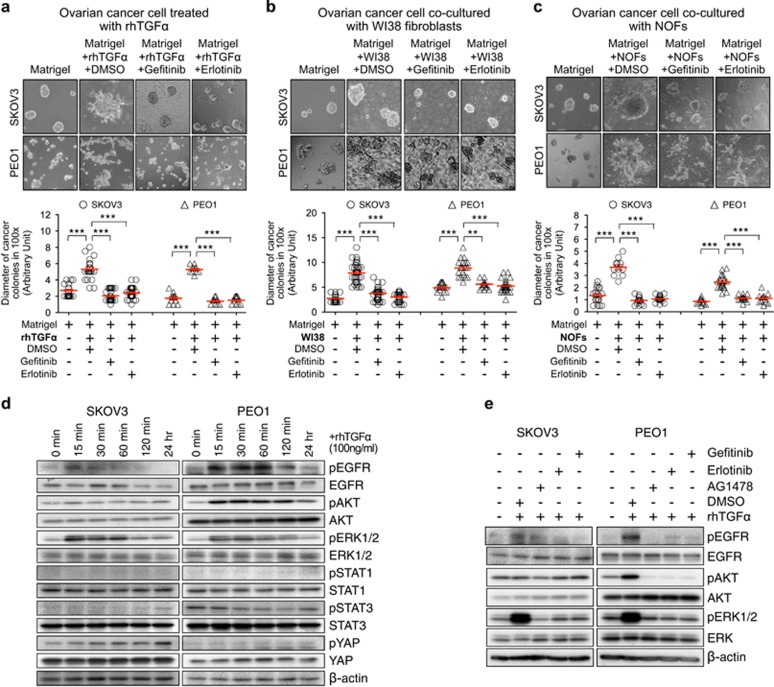
TGF-α and fibroblasts promote colony formation of metastatic ovarian cancer cells in 3D organoid co-culture through activation of EGFR. (a) Protein expression of EGFR in IOSE, FTSEC, and human ovarian cancer cell lines was detected by Western blotting. β-actin was used for equal loading (left panel). mRNA expression of EGFR was compared between normal ovarian tissue (Normal Ov), micro-dissected fallopian tube secretory epithelial cells from primary normal fallopian tube (Primary FTSEC) and micro-dissected tumor tissue from primary ovarian cancers (OvCa tumors) by qRT-PCR (right panel). *P<0.05 and **P<0.01 (Mann-Whitney U test). The data represents means±s.e.m. from three independent experiments. (b–d) Phase-contrast images (100x magnification) of ovarian cancer colonies (SKOV3 or PEO1) were captured on day 8 in 3D organoid cultures, including 3D monoculture of ovarian cancer cells treated with rhTGF-α (b), 3D organoid co-culture with WI38 fibroblasts (c) and 3D organoid co-culture with primary normal omental fibroblasts (NOFs) (d) (upper panels). Treatments of AG1478 were also performed. DMSO was served as vehicle controls. Diameter of cancer colonies in 10 fields was measured (Arbitrary unit) in phase-contrast images, and their differences in treatment groups were compared with the DMSO control groups (lower panels). **P<0.01 and ***P<0.001 (Mann-Whitney U test). The data represents means±s.e.m. from two independent experiments, respectively.
Additionally, the co-culture of primary cultures of normal stromal fibroblasts (NOFs), which were isolated from human omentum, with ovarian cancer cells (PEO1), also showed de novo expression of TGF-α. The treatment with rhTNF-α also induced de novo TGF-α expression in NOFs (Supplementary Figure 7a and b). Here we showed NOFs also promote colony formation of metastatic ovarian cancer cells in the 3D co-cultures (SKOV3+NOFs and PEO1+NOFs), whereas treatment with AG1478 significantly abolished such colony formation (Figure 4d). These results suggested normal omental stromal fibroblasts promote colony formation of metastatic ovarian cancer cells through TGFα-EGFR signaling.
We further repeated our 3D organoid experiments with two clinically used EGFR-specific TKIs (Gefitinib and Erlotinib) (Figure 5). Gefitinib and Erlotinib did not affect cellular proliferation of monoculture of WI38 fibroblasts and ovarian cancer cells (SKOV3 and PEO1; Supplementary Figures 6a and b). Pretreatment with Gefitinib or Erlotinib significantly suppressed the rhTGFα-induced colony formation in 3D organoid monocultures of metastatic ovarian cancer cells (Figure 5a: SKOV3 or PEO1), as well as the fibroblasts-induced colony formation of metastatic ovarian cancer cells in 3D organoid co-cultures (Figure 5b: SKOV3+WI38 and PEO1+WI38; Figure 5c: SKOV3+NOFs and PEO1+NOFs). Our results therefore confirmed that fibroblast-derived TGF-α promotes colony formation of metastatic ovarian cancer cells in 3D organoid co-culture through EGFR activation.
Figure 5.
Fibroblasts-derived TGF-α promotes colony formation of metastatic ovarian cancer cells in 3D organoid co-culture through activation of EGFR, and TGF-α activates AKT and MAPK signaling in metastatic ovarian cancer cells through EGFR. (a-c) Phase-contrast images (100x magnification) of ovarian cancer colonies (SKOV3 or PEO1) were captured on day 8 in 3D organoid cultures, including 3D monoculture of ovarian cancer cells treated with rhTGF-α (100 ng/ml) (a); 3D organoid co-culture with WI38 fibroblasts (b); and 3D organoid co-culture with NOFs (c) (upper panel). Treatment of Gefitinib (1 μM) or Erlotinib (1 μM) was performed. DMSO was served as vehicle controls. Diameter of cancer colonies in 10 fields was measured (Arbitrary unit) in phase-contrast images. The sizes of cancer colonies in the treatment groups were compared with the DMSO control groups (lower panel). **P<0.01 and ***P<0.001 (Mann-Whitney U test). The data represents means±s.e.m. from two independent experiments. (d) Phosphorylation of EGFR, AKT, ERK1/2, STAT1, STAT3 and YAP were examined in ovarian cancer cells (SKOV3 and PEO1) after treatment of rhTGF-α in a time-dependent manner by Western blotting. β-actin was served as loading controls. (e) Phosphorylation of EGFR, AKT, ERK1/2 were examined in ovarian cancer cells (SKOV3 and PEO1) after treatments with 100 ng/ml rhTGF-α±1 μM TKIs (AG1478, Gefitinib or Erlotinib) by Western blotting. DMSO was served as controls. SKOV3 and PEO1 were pre-treated with TKIs for 2 h before stimulating with rhTGF-α for 15 min.
TGF-α activates AKT and MAPK signaling in metastatic ovarian cancer cells through EGFR
The EGFR ligand-dependent activation triggers cell proliferation, invasion and colonization in cancer cells by stimulating various downstream signaling modules, which include phosphatidylinositol 3-kinase (PI3K)/Akt (PKB) pathway, Ras/Raf/MEK/ERK1/2 pathway, STAT pathway, and Hippo/YAP pathway.32, 33, 34, 35 Here, we examined the phosphorylation status of EGFR, AKT, ERK1/2, STAT1, STAT3 and YAP in metastatic ovarian cancer cells after rhTGF-α treatment in a time-dependent manner (Figure 5d). Phosphorylation of EGFR and ERK1/2 were detected in SKOV3 cells after rhTGF-α stimulation, whereas phosphorylation of EGFR, AKT and ERK1/2 were detected in PEO1 cells. No phosphorylation of STAT1, STAT3 and YAP was detected in either PEO1 or SKOV3 cells after rhTGF-α treatment. We further found that treatments of AG1478, Gefitinib, or Erlotinib abolished the TGFα-induced phosphorylation of EGFR and ERK1/2 in SKOV3 cells and phosphorylation of EGFR, AKT and ERK1/2 in PEO1 cells (Figure 5e). These results indicate TGF-α activates AKT and ERK1/2 signaling in metastatic ovarian cancer cells through EGFR.
Stromal fibroblasts promote peritoneal metastasis of ovarian cancer in vivo through activation of EGFR
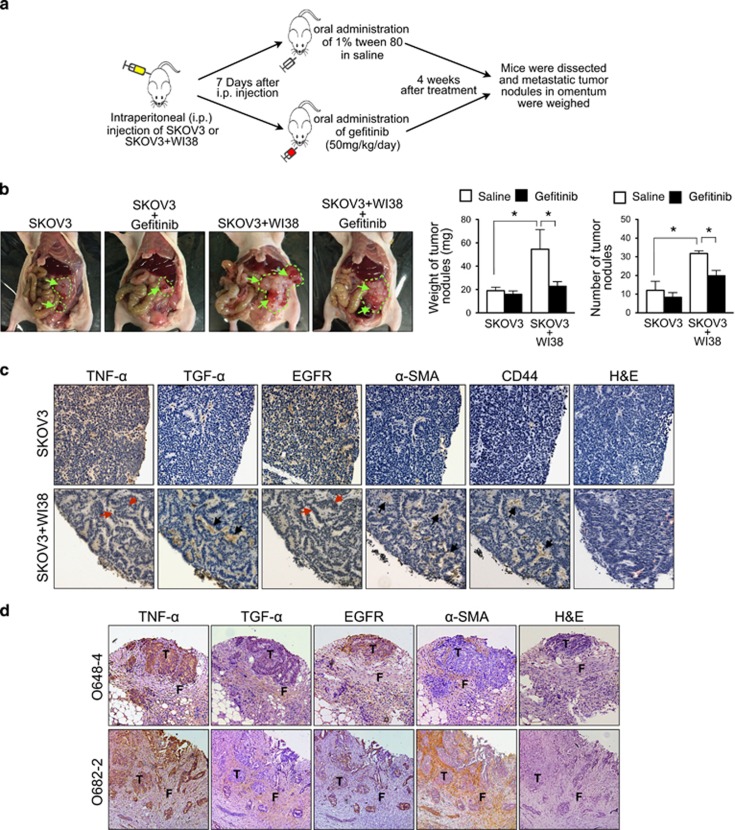
To investigate if stromal fibroblasts promote peritoneal metastasis of ovarian cancer in vivo, we set up the following orthotopic ovarian cancer xenograft experiment. We injected a mixture of metastatic human ovarian cancer SKOV3 cells (1 × 107) and WI38 stromal fibroblasts (1 × 106) intraperitoneally into athymic nude mice. Mice injected with SKOV3 cells alone (1 × 107) were served as controls. Seven days after intraperitoneal injection, the tumor bearing mice (both the groups injected with SKOV3+WI38 and with SKOV3 alone) were treated with either oral administration of Gefitinib (50 mg/kg/day) or 1% tween 80 in saline for 4 weeks (Figure 6a). Metastatic ovarian tumor nodules were found in peritoneal cavity of mice, and they were located mainly in intestine, mesentery, liver, and spleen (Supplementary Figure 8). Our result showed that in the group injected with SKOV3 alone, the tumor weight and number of tumor nodules with Gefitinib treatment (n=5) showed no significant difference from those with saline treatment (n=5). On the other hand, the tumor weight and number of tumor nodule in the saline-treated mice injected with SKOV3+WI38 (n=5) was significantly higher (~2.25 fold and ~2.72 fold, respectively) when compared to the saline-treated mice injected with SKOV3 alone (n=5; Figure 6b), indicating stromal fibroblasts in the peritoneal microenvironment promote metastatic colonization of ovarian cancer in vivo. Furthermore, the tumor weight and number of tumor nodules were significantly lower in the Gefitinb-treated mice injected with SKOV3+WI38 (n=5) when compared to the saline-treated mice injected with SKOV3+WI38 (n=5; Figure 6b), indicating EGFR activation is involved in promoting peritoneal metastasis of ovarian cancer by stromal fibroblasts in vivo.
Figure 6.
Stromal fibroblasts promote peritoneal metastasis of ovarian cancer in vivo through activation of EGFR, and a TNFα-TGFα-EGFR interacting loop presents in the microenvironment of omental metastases of ovarian cancer. (a) Schematic diagram showing the use of orthotopic ovarian cancer xenograft model to evaluate the effect of stromal fibroblasts on peritoneal metastasis of ovarian cancer in vivo, and the effect of EGFR activation on fibroblast-induced peritoneal metastasis of ovarian cancer in vivo. (b) Macroscopical morphology of metastatic tumor nodules in orthotopic xenografts was shown (green arrow and green stroke). The weight and number of dissected metastatic tumor nodules were quantified (right). The weight and number of metastatic tumor nodules were compared between the saline-treated mice injected SKOV3+WI38 (n=5) and the saline-treated mice injected with SKOV3 alone (n=5), and also between the Gefitinib-treated mice injected with SKOV3+WI38 (n=5) and the saline-treated mice injected with SKOV3+WI38 (n=5). *P<0.05 (Student's t test). (c) Immunohistochemical staining for TNF-α, TGF-α, EGFR, CD44 and α-SMA in serial sections of metastatic tumor nodules collected from the saline-treated mice injected with SKOV3 alone or with SKOV3+WI38. Fibroblasts were identified by positive signal of α-SMA and CD44 staining. Image was captured at 200x magnification. (Red arrow: cancer cells and black arrow: fibroblasts) (d) Immunohistochemical staining for TNF-α, TGF-α, EGFR, and α-SMA in serial sections of omental metastases from patients with advanced ovarian cancer. Image was captured at 200x magnification. (T: ovarian tumor cells and F: omental stromal fibroblasts).
A TNFα-TGFα-EGFR interacting loop is present in the microenvironment of omental metastases of ovarian cancer
Using the fibroblast-specific marker (α-SMA) and human fibroblast-specific marker (CD44), we found stromal fibroblasts in the tumor metastases from the mice injected with SKOV3+WI38, but not in those injected with SKOV3 alone. The study of protein expression profiles in omental tumor metastases from the mice injected with SKOV3+WI38 showed that both TNF-α and EGFR were expressed in SKOV3 cancer cells, whereas TGF-α was expressed in α-SMA/CD44-positive stromal fibroblasts (WI38; Figure 6c).
We also examined the protein expression profiles of TNF-α, TGF-α, and EGFR in serial sections of omental metastases from patients with advanced ovarian cancer (16 cases). We scored the percentage of stromal fibroblasts that showed positive TGF-α reactivity, and the intensity of positive TGF-α signal in stromal fibroblasts. We also scored the percentage of ovarian cancer cells that showed positive TNF-α and EGFR reactivity, and the intensity of positive TNF-α and EGFR signal in ovarian cancer cells. Among these 16 samples, two of them (O-648 and O-682) showed both strong TGF-α intensity in high percentage of stromal fibroblasts, and strong TNF-α and EGFR intensities in high percentage of ovarian cancer cells (Supplementary Table 3). Most importantly, in these two omental metastases (O-648 and O-682), both TNF-α and EGFR were expressed in metastatic ovarian cancer cells, whereas TGF-α was expressed in adjacent αSMA-positive omental stromal fibroblasts (Figure 6d). The distinctive localization of TNF-α, TGF-α, and EGFR in tumor metastases suggested a loop of cancer-stroma-cancer interaction, i.e. TNF-α-TGFα-EGFR, is present in the microenvironment of omental metastases of ovarian cancer.
Discussion
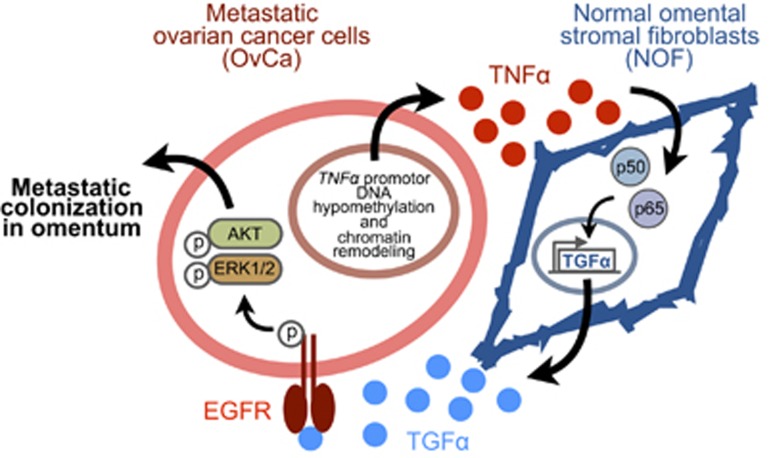
In this study, we uncover a loop of cancer-stroma-cancer interaction that promotes peritoneal metastasis of ovarian cancer via TNFα-TGFα-EGFR. We demonstrate that 1) TNF-α is over-expressed in metastatic ovarian cancer cells by promoter DNA hypomethylaton and chromatin remodeling; 2) TNF-α secreted by ovarian cancer cells then induces de novo expression of TGF-α in omental stromal fibroblasts through NF-κB signaling; 3) finally the fibroblast-derived TGF-α promotes metastasis colonization of ovarian cancer cells through activation of EGFR, AKT and ERK1/2 signaling (Figure 7).
Figure 7.
Proposed mechanism of a loop of cancer-stromal-cancer interaction that promotes peritoneal metastasis of ovarian cancer via TNFα-TGFα-EGFR. 1) The overexpression of TNF-α in metastatic ovarian cancer cells is regulated by DNA hypomethylation and chromatin remodeling of TNF-α promoter; 2) TNF-α secreted from metastatic ovarian cancer cells induces de novo expression of TGF-α in normal omental stromal fibroblasts through NF-κB signaling; and 3) TGF-α secreted by omental stromal fibroblasts promotes metastatic colonization of ovarian cancer cells through the activation of EGFR, AKT and ERK1/2 signaling.
TNF-α is frequently over-expressed in ovarian cancer,36, 37 and its over-expression is associated with the increase of tumor grade,38 but the exact molecular mechanism of TNF-α over-expression is not completely known. One of the mechanisms is that high levels of TNF-α mRNA in ovarian cancer cells is stabilized by autocrine regulation of TNF-α.22 In this study, we showed that over-expression of TNF-α mRNA correlates with DNA hypomethylation and chromatin remodeling in TNF-α promoter region in ovarian cancer cell lines. Interestingly, we have also detected DNA methylation at the promoter region of LTA and exon 4 (CpG 75) of LTB, which are the neighboring genes of TNF-α located at the LTA/TNF/LTB locus at 6p21.3 (Supplementary Figure 9a). DNA methylation status at promoter region of LTA and exon 4 of LTB, however, does not correlate with the over-expression of LTA and LTB in ovarian cancer cell lines, respectively (Supplementary Figures 9 and 10). These results therefore confirmed that DNA hypomethylation of TNF-α promoter at 6p21.3 is specific for TNF-α over-expression in ovarian cancer cells.
A recent report showed hypomethylation of 60 CpGs (1 Mb away from LTA/TNF/LTB locus) located in 6p21.3 is associated with cis up-regulation of genes enriched in immune response processes (TAP1, PSMB8, PSMB9, HLA-DOB1, HLA-DOB2, HLA-DMA, and HLA-DOA), increased CD8-T cell tumor infiltration, trans-regulation of genes in immune-related pathway, and improved prognosis in high-grade serous epithelial ovarian cancer.24 Although expression of TNF-α (due to promoter DNA hypomethylation) is associated with poor outcome, this paradox could be explained by hypomethylation of TNF-α promoter and hypomethylation of those 60 CpGs are independent events in ovarian cancer.
TGF-α is one of the ligands for EGFR, which regulates growth and invasion in ovarian cancer cells through autocrine production of TGF-α and activation of EGFR downstream signaling.16, 39, 40 We have shown that SKOV3 cells express very low levels of TGF-α, whereas PEO1 cells express substantial amount of TGF-α. Moreover, TGF-α expression showed no significant difference after rhTNF-α treatment in both SKOV3 and PEO1 cells (Supplementary Figures 7c and 7d). This result suggests that cancer cell-autonomous TGF-α is not a key event for promoting metastatic colonization of ovarian cancer cells.
EGFR is a potential therapeutic target in ovarian cancer because it is over-expressed in about 30–60% of ovarian cancers and its expression is associated with poor prognosis and decreased therapeutic responsiveness.31, 41, 42 Consistent with our data, several preclinical studies have demonstrated that Gefitinib suppresses ovarian cancer intra-abdominal dissemination or peritoneal metastasis in xenograft models,43, 44, 45 indicating that cell-autonomous EGFR in ovarian cancer cells is critical for their metastatic colonization in peritoneum. Most of the clinical trials, however, showed limited or no clinical response of Gefitinib or Erlotinib in ovarian cancer patients.46, 47, 48 Those results indicated that the biological mechanism of EGFR in ovarian cancer may different from those EGFR-TKIs sensitive disease, such as NSCLC. The limited response can be accounted by the lack of EGFR activating mutation,30, 49 or the presence of alterative activated pathways such as MAPK/ERK and PI3K/AKT/mTOR pathway due to the mutation of KRAS, PIK3CA or PTEN in ovarian cancer.50, 51, 52 These factors may hinder the inhibition of a solitary signal transduction pathway in ovarian cancer by Gefitinib or Erlotinib. Report showed that combination of Gefitinib or Erlotinib and PI3K inhibitor showed synergistic activity against ovarian cancer.53 Furthermore, a recent study demonstrated that combination of IL6 antibodies and Gefitinib showed more effective in reducing ovarian tumor growth when compared with Gefitinib treatment alone.44 These studies further indicated that EGFR participated in the development of ovarian cancer is distinct from the diseases sensitive to TKIs. Therefore, more studies are needed to fully dissect the role of EGFR in ovarian cancer, as a result of developing an ovarian cancer-specific EGFR targeting therapy such as combination therapies or the use of next generation EGFR-TKIs.
Materials and methods
Patients and specimens
A total of 26 specimens of primary ovarian cancer (Supplementary Table 1) and 16 specimens of omental metastases were recruited from patients with epithelial ovarian cancer (Supplementary Table 2). Nine specimens of normal fallopian tube and 10 specimens of normal ovary were recruited from women who underwent oophorectomy for non-malignant conditions. Study subjects were recruited at our unit. Informed consent was obtained from each patient prior surgery. Histological typing of tumor was classified according to the World Health Organization criteria, whereas clinical staging follows the International Federation of Gynecology and Obstetrics standards. The research protocol was approved by the Institutional Clinical Research Ethics Committee.
2D co-culture model
Routine cell culture methods have been reported.54 For 2D co-culture, 2 × 105 of WI38 fibroblasts were first seeded in assigned wells in 6-well plates. One × 105 of ovarian cancer cells (SKOV3 or PEO1) were seeded into the wells. Cancer cells were isolated by fibroblasts depletion and fibroblasts were isolated by positive selection from 2D co-culture (SKOV3+WI38 and PEO1+WI38) using anti-fibroblast microbeads (Miltenyi Biotec, Singapore) after 48 hr co-culture. Purity of isolated fibroblasts was determined by human specific CD44 antibodies (Abcam, Cambridge, UK) using flow cytometry FC500 (Beckmen Coulter, Indianapolis, IN, USA). FCS Express software was used for data analysis.
Isolation and culture of primary omental fibroblasts
Primary normal omental fibroblasts were isolated from non-malignant omental tissues of female patients. Omental tissue was dissociated using gentleMACS dissociators (Miltenyi Biotec). Omental fibroblasts were isolated by positive selection using anti-fibroblast microbeads (Miltenyi Biotec).
3D organoid culture
Fibroblasts at 1 × 105 was mixed with 500 μl extracellular matrix (ECM; Matrigel, BD Biosciences, San Jose, CA, USA) and loaded into each well of chamber slide (Nunc Lab-Tek, Rochester, NY, USA). After the gel solidified, ovarian cancer cells were seeded on top of the gel with a density of 1 × 104 cells in 500 μl complete medium per well and incubated for 8 days. To investigate the effect of rhTGF-α, the 3D organoid monocultures were treated with 100 ng/ml rhTGF-α (R&D System, Minneapolis, MN, USA). To investigate the effect of tyrosine kinase inhibitors, the 3D organoid monocultures or 3D organoid co-cultures were treated with 1 μM of AG1478 (Sigma-Aldrich, Saint Louis, MO, USA), Gefitinib (Toronto Research Chemicals, Canada), or Erlotinib (Toronto Research Chemicals).
Cytokine antibody arrays
Cytokines in conditioned medium from the 3D organoid culture were determined by cytokine antibody array (AAH-CYT-G2000-4, RayBioTech, Norcross, GA, USA). The array data was subjected to the DNA-Chip Analyzer Software (DChip; http://www.dchip.org) for comparative analysis.
Quantitative real-time RT-PCR
Methods of quantitative real-time quantitative reverse transcription–PCR have been reported.54 TaqMan primers and probes for TNF-α, TGF-α, EGFR, and β-actin (Applied Biosystems) were used.
MethyLight
Bisulfite conversion was performed on 2 μg genomic DNA using EZ DNA Methylation Kit (ZYMO Research, Irvine, CA, USA). EpiTect MethyLight PCR Kit (Qiagen, Valencia, CA, USA) was used to analyze promoter DNA methylation of TNF-α. Unmethylated forward primer (GTGTGTTTTTAATTTTTTAAATTTT), unmethylated reverse primer (CAACTACCTTTATATATCCCTAAAAC), and unmethylated probe labeled with VIC (TTTTTGTGATGGAGAAGAAATTGA) were used to detect unmethylated sequence of TNF-α promoter. Methylated forward primer (GTGTGTTTTTAATTTTTTAAATTTT), methylated reverse primer (CAACTACCTTTATATATCCCTAAAAC), and methylated probe labeled with FAM (CGCGATGGAGAAGAAATCGAG) were used to detect methylated sequence of TNF-α promoter.
Bisulfite Genomic Sequencing
The bisulfite converted DNA was amplified by nested PCR that targeting the TNF-α promoter region. Primer sequences for PCR round 1, F: 5′-GTTAAGATTGAAATTAGTATTATGAGT-3′ R: 5′-AAAAATTAAAAACACACAAACATCAAA-3′. Primer sequences for PCR round 2, F: 5′-GTATTTTTGATGTTTGTGTGTTTTTAA-3′ R: 5′-TCACTCAAAATACAACAAACAAAAA-3′. Target PCR products were purified and cloned. Ten clones from each cell line were isolated and sequenced.
Nucleosome occupancy and methylome sequencing (NOMe-Seq)
NOMe-Seq assay (Active Motif, CA, USA) was performed. Bisulfite-specific PCR primers (F: 5′-TTAGAAGGAAATAGATTATAGATTTGG-3′ R: 5′-AAATCAATATATAAAAAAAAAAAAACC-3′) were used to amplify the TNF-α promoter region. Target PCR products were purified and cloned. Ten clones from each cell line were isolated and sequenced. GpC and CpG regions were plotted for analyzing the methylation profiles and nucleosome occupancy.
Western blotting and immunohistochemistry
Methods of Western blotting and immunohistochemistry have been reported.54 Antibodies for p50, p65, AKT, phospho-AKT, ERK, phospho-ERK, STAT1, phospho-STAT1, STAT3, phospho-STAT3, YAP, phospho-YAP, EGFR were purchased from Cell Signaling Technology (Danvers, MA, USA); Antibodies for phospho-EGFR, TGF-α and TNF-α were purchased from Santa Cruz, (Santa Cruz, CA, USA); β-actin antibody was purchased from Sigma-Aldrich; α-SMA and human-CD44 antibody were purchased from Abcam (Cambridge, UK).
Xenograft mouse model
All animal experiments were approved by The Chinese University of Hong Kong Animal Experimentation Ethics Committee. Female nude mice, aged 3 to 4 weeks, were used as xenografts model. Twenty mice were divided into two groups. One group of mice (n=10) were injected intraperitoneally with SKOV3 cells (1 × 107 cell/body). Another group of mice (n=10) were injected intraperitoneally with the mixture of SKOV3 cells (1 × 107 cell/body) and WI38 fibroblasts (1 × 106 cell/body). Seven days after inoculation, mice in each group (SKOV3 and SKOV3+WI38) were randomized into treatment group (n=5) and control group (n=5). For treatment group, mice were oral administered with gefitinib (50 mg/kg/d). For control group, mice were oral administered with equal volume of saline with 1% tween 80. Mice were sacrificed on Day 40 after cells injection. Macroscopic lesions were recorded and metastatic tumor nodules were dissected, weighed and paraffin-embedded for immunohistochemical staining.
Statistical analysis
All data were presented as means±S.E.M. and analyzed using GraphPad Prism 5.0 software (GraphPad Inc., Sandiego, CA, USA) from at least three independent experiments. Statistical difference between groups was determined by Student’s t test or Mann-Whitney rank-sum test. P<0.05 was considered as statistically significant (*), P<0.01 as highly significant (**), and P<0.001 as extremely significant (***).
Acknowledgments
This research was supported by CUHK Research Committee Funding (Direct Grants for Research), Hong Kong Obstetrical and Gynaceological Trust Fund, and The Charlie Lee Charitable Foundation. The authors give special thanks to Alex Shu-Wing NG (Brigham and Women's Hospital, Harvard Medical School) and Ronny Drapkin (The Penn Ovarian Cancer Center, University of Pennsylvanian) for the FTSEC190 and FTSEC194 cell lines. The authors give thanks to Dr Gene Chi-Wai MAN, Mr Jianzhang WANG, Mr Long CUI, and Mr John Chun-Kit LI for technical supports; to Ms. Flora Pui-Ling TAM for processing clinical samples; and to Dr Amy Kit-Ying CHUNG for revising the manuscript.
Author contributions
TSL and JK conceived experiments. TSL, LKYC, CWCH, KS, and JK carried out experiments. TSL, ECHW, and LKYC analyzed data. THC, SFY, JHSL, CSY, Y, and TKHC collected clinical samples. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
The authors declare no conflict of interest.
Supplementary Material
References
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010; 177: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer 2013; 13: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan S, Coward JI, Bast Jr RC, Berchuck A, Berek JS, Brenton JD et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 2011; 11: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Jones LM, Catterall JB, Turner GA. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett 1995; 91: 229–234. [DOI] [PubMed] [Google Scholar]
- Buy JN, Moss AA, Ghossain MA, Sciot C, Malbec L, Vadrot D et al. Peritoneal implants from ovarian tumors: CT findings. Radiology 1988; 169: 691–694. [DOI] [PubMed] [Google Scholar]
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer 2005; 5: 355–366. [DOI] [PubMed] [Google Scholar]
- Cai J, Tang H, Xu L, Wang X, Yang C, Ruan S et al. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis 2012; 33: 20–29. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–572. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest 2014; 124: 4614–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi I, Celli JP, Evans CL, Abu-Yousif AO, Muzikansky A, Pogue BW et al. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res 2010; 70: 9319–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjabi AR, Sawada K, Jagadeeswaran S, Eichbichler A, Kenny HA, Montag A et al. Thrombin induces tumor invasion through the induction and association of matrix metalloproteinase-9 and beta1-integrin on the cell surface. J Biol Chem 2008; 283: 2822–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 2011; 128: 2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauknecht T, Kommoss F, Birmelin G, von Kleist S, Kohler M, Pfleiderer A. Expression analysis of EGF-R and TGFa in human ovarian carcinomas. Anticancer Res 1991; 11: 1523–1528. [PubMed] [Google Scholar]
- Kohler M, Bauknecht T, Grimm M, Birmelin G, Kommoss F, Wagner E. Epidermal growth factor receptor and transforming growth factor alpha expression in human ovarian carcinomas. European Journal of Cancer 1992; 28 A: 1432–1437. [DOI] [PubMed] [Google Scholar]
- D'Antonio A, Losito S, Pignata S, Grassi M, Perrone F, De Luca A et al. Transforming growth factor alpha, amphiregulin and cripto-1 are frequently expressed in advanced human ovarian carcinomas. International Journal of Oncology 2002; 21: 941–948. [PubMed] [Google Scholar]
- Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res 1988; 48: 6166–6172. [PubMed] [Google Scholar]
- Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 2011; 103: 197–208. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annual Review of Immunology 2000; 18: 621–663. [DOI] [PubMed] [Google Scholar]
- Karki P, Johnson Jr J, Son DS, Aschner M, Lee E. Transcriptional Regulation of Human Transforming Growth Factor-alpha in Astrocytes. Molecular neurobiology 2016, e-pub ahead of print 21 January 2016 doi:10.1007/s12035-016-9705-9. [DOI] [PMC free article] [PubMed]
- Qwarnstrom EE, Ostberg CO, Turk GL, Richardson CA, Bomsztyk K. Fibronectin attachment activates the NF-kappa B p50/p65 heterodimer in fibroblasts and smooth muscle cells. J Biol Chem 1994; 269: 30765–30768. [PubMed] [Google Scholar]
- Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F et al. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Molecular Cancer Therapeutics 2006; 5: 382–390. [DOI] [PubMed] [Google Scholar]
- Coscia F, Watters KM, Curtis M, Eckert MA, Chiang CY, Tyanova S et al. Integrative proteomic profiling of ovarian cancer cell lines reveals precursor cell associated proteins and functional status. Nat Commun 2016; 7: 12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cicek MS, Charbonneau B, Kalli KR, Armasu SM, Larson MC et al. Tumor hypomethylation at 6p21.3 associates with longer time to recurrence of high-grade serous epithelial ovarian cancer. Cancer Res 2014; 74: 3084–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H, Bergman Y, Linking DNA. methylation and histone modification: patterns and paradigms. Nat Rev Genet 2009; 10: 295–304. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z et al. High-resolution profiling of histone methylations in the human genome. Cell 2007; 129: 823–837. [DOI] [PubMed] [Google Scholar]
- Lin JC, Jeong S, Liang G, Takai D, Fatemi M, Tsai YC et al. Role of nucleosomal occupancy in the epigenetic silencing of the MLH1 CpG island. Cancer cell 2007; 12: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TK, Liu Y, Lay FD, Liang G, Berman BP, Jones PA. Genome-wide mapping of nucleosome positioning and DNA methylation within individual DNA molecules. Genome Res 2012; 22: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006; 366: 2–16. [DOI] [PubMed] [Google Scholar]
- Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. Journal of Molecular Medicine 2006; 84: 671–681. [DOI] [PubMed] [Google Scholar]
- Brustmann H. Epidermal growth factor receptor expression in serous ovarian carcinoma: an immunohistochemical study with galectin-3 and cyclin D1 and outcome. Int J Gynecol Pathol 2008; 27: 380–389. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12: 553–563. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacological Research 2014; 79: 34–74. [DOI] [PubMed] [Google Scholar]
- Andersen P, Pedersen MW, Woetmann A, Villingshoj M, Stockhausen MT, Odum N et al. EGFR induces expression of IRF-1 via STAT1 and STAT3 activation leading to growth arrest of human cancer cells. Int J Cancer 2008; 122: 342–349. [DOI] [PubMed] [Google Scholar]
- He C, Lv X, Hua G, Lele SM, Remmenga S, Dong J et al. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene 2015; 34: 6040–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 2002; 13: 135–141. [DOI] [PubMed] [Google Scholar]
- Kwong J, Chan FL, Wong KK, Birrer MJ, Archibald KM, Balkwill FR et al. Inflammatory cytokine tumor necrosis factor alpha confers precancerous phenotype in an organoid model of normal human ovarian surface epithelial cells. Neoplasia 2009; 11: 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MS, Stamp GW, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest 1993; 91: 2194–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura H, Sasano H, Sato S, Yajima A. Expression of epidermal growth factor-related proteins and epidermal growth factor receptor in common epithelial ovarian tumors. Int J Gynecol Pathol 1997; 16: 60–68. [DOI] [PubMed] [Google Scholar]
- Qiu X, Cheng JC, Klausen C, Fan Q, Chang HM, So WK et al. Transforming growth factor-alpha induces human ovarian cancer cell invasion by down-regulating E-cadherin in a Snail-independent manner. Biochemical and Biophysical Research Communications 2015; 461: 128–135. [DOI] [PubMed] [Google Scholar]
- Stadlmann S, Gueth U, Reiser U, Diener PA, Zeimet AG, Wight E et al. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol 2006; 19: 607–610. [DOI] [PubMed] [Google Scholar]
- Psyrri A, Kassar M, Yu Z, Bamias A, Weinberger PM, Markakis S et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res 2005; 11: 8637–8643. [DOI] [PubMed] [Google Scholar]
- Ohta T, Ohmichi M, Shibuya T, Takahashi T, Tsutsumi S, Takahashi K et al. Gefitinib (ZD1839) increases the efficacy of cisplatin in ovarian cancer cells. Cancer biology and Therapy 2012; 13: 408–416. [DOI] [PubMed] [Google Scholar]
- Milagre CS, Gopinathan G, Everitt G, Thompson RG, Kulbe H, Zhong H et al. Adaptive Upregulation of EGFR Limits Attenuation of Tumor Growth by Neutralizing IL6 Antibodies, with Implications for Combined Therapy in Ovarian Cancer. Cancer Res 2015; 75: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Wu J, Liu L, Tian Y, Buettner R, Hsieh MY et al. Synergistic anti-tumor effect of combined inhibition of EGFR and JAK/STAT3 pathways in human ovarian cancer. Molecular Cancer 2015; 14: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui T, Shen K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiology 2012; 36: 490–496. [DOI] [PubMed] [Google Scholar]
- Vergote IB, Jimeno A, Joly F, Katsaros D, Coens C, Despierre E et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol 2014; 32: 320–326. [DOI] [PubMed] [Google Scholar]
- Blank SV, Christos P, Curtin JP, Goldman N, Runowicz CD, Sparano JA et al. Erlotinib added to carboplatin and paclitaxel as first-line treatment of ovarian cancer: a phase II study based on surgical reassessment. Gynecol Oncol 2010; 119: 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen KD, Waldstrom M, Olsen DA, Corydon T, Lorentzen KA, Knudsen HJ et al. Mutant epidermal growth factor receptor in benign, borderline, and malignant ovarian tumors. Clin Cancer Res 2008; 14: 3278–3282. [DOI] [PubMed] [Google Scholar]
- Teplinsky E, Muggia F. EGFR and HER2: is there a role in ovarian cancer? Transl Cancer Res 2015; 4: 107–117. [Google Scholar]
- Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer 2014; 120: 3446–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Archives of Gynecology and Obstetrics 2014; 290: 1067–1078. [DOI] [PubMed] [Google Scholar]
- Glaysher S, Bolton LM, Johnson P, Atkey N, Dyson M, Torrance C et al. Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer 2013; 109: 1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau TS, Chung TK, Cheung TH, Chan LK, Cheung LW, Yim SF et al. Cancer cell-derived lymphotoxin mediates reciprocal tumour-stromal interactions in human ovarian cancer by inducing CXCL11 in fibroblasts. J Pathol 2014; 232: 43–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.