Total joint arthroplasty (TJA) is one of the most cost-effective and successful interventions in medicine. There are more than 1 million TJAs performed in the United States annually [1], [2]. This number is expected to increase to nearly 4 million by 2030 [3]. Prospective monitoring of these procedures is critical and can serve as an early warning system for faulty implant designs or procedures with higher than expected failure rates. Results presented based on a nationwide perspective, rather than from a single institution or multicenter trials allow for a larger sample size and national representation of procedures being performed [4]. The ideal way to conduct this national assessment is via an arthroplasty registry.
The 2 main purposes of an arthroplasty registry, “are to define the epidemiology of TJR and to monitor its clinical outcomes” [5]. Generally, data from registries are provided back to hospitals, clinics and clinicians which then facilitate comparisons with local, regional, or even national benchmarks. Registries also enable follow-up of large volumes of patients and are a critical element in the area of implant safety surveillance.
Development of American Joint Replacement Registry (AJRR)
The importance of national joint registries has been recognized for decades. Starting with arthroplasty registries in Scandinavia, Australia, and the United Kingdom, it has been clear that registries are a reliable source of descriptive information on the outcomes of joint replacement. Indeed, in 2009, the Council of Presidents, representing 7 international orthopaedic associations, endorsed the establishment and maintenance of national arthroplasty registries [6].
It was clear that a national registry in the United States was needed, called for by William J. Maloney, MD, in 2001, in a Journal of Bone and Joint Surgery manuscript entitled “National Joint Replacement Registries: Has the Time Come?” [7]. Although earlier arthroplasty registry efforts were undertaken in the United States, these efforts were unsuccessful mainly in part because of technical constraints and reliance on a single stakeholder group, surgeons. A multistakeholder initiative would be required to truly advance a national registry. As such, in 2010, the American Academy of Orthopaedic Surgeons (AAOS) undertook an effort to establish a national joint registry in conjunction with stakeholders from all areas of orthopaedics. Key stakeholders, including the American Association of Hip and Knee Surgeons (AAHKS), The Hip Society, The Knee Society, and orthopaedic device manufacturers, supported the development of a national arthroplasty registry. Under the direction of AAOS, a level I pilot project was conducted by 2 staff members during the first 6 months of 2011. Level I data included patient, surgeon, and hospital identifiers, procedure and implant data (Table 1) [5], [8]. With this information, registries are able to monitor revision rates after TJA [5]. This was a critical step forward in light of the 2010 recall of the ASR (DePuy Synthes, Inc., Warsaw, IN) metal-on-metal hip implant.
Table 1.
| Levels of registry data | |
|---|---|
| Level I | Patient, surgeon, and hospital identifiers, procedural and demographic information |
| Level II | Patient comorbidities, American Society of Anesthesiologists Score, surgical factors and perioperative care data, complications and/or adverse events |
| Level III | Patient-reported outcome measures |
This pilot study was conducted at 8 health systems representing 11 hospitals. A registry database software was donated to AJRR by the Harris Orthopaedic Laboratory at Massachusetts General Hospital. The goals of the pilot program included feedback and refinement of the AJRR recruitment process, identification of data submission methods, review and feedback on participation and business agreements, and development of the various hospital functions involved with the recruitment process and their specific roles. In the end, hospitals were able to complete legal agreements and identify means to streamline the enrollment process. At the time, much of the data were manually entered in to the pilot registry platform. However, data were collected on N = 8308 patients from 150 surgeons. The procedural data and demographics were consistent with other large scale studies of TJA in the United States (data not published). Ten years after his first piece, Dr. Maloney, then Vice-Chair of AJRR, reiterated the need for “high-quality, real-time data to identify poorly performing technologies and techniques,” and addressed the need to support AJRR in its early stages [9].
Concurrent with the pilot study, AJRR began a search for a commercial registry product. A workgroup was formed to analyze the requirements for a production registry software package and evaluated both focused orthopaedic charting systems and generic registry applications. AJRR selected a product and launched the application in early 2012. Data from the pilot study were migrated to the new platform. An Executive Director and additional staff were hired in 2011 and 2012 to fully launch a national registry effort. By the end of 2012, the new technology system was underway and over 100 hospitals had enrolled.
Expansion of AJRR
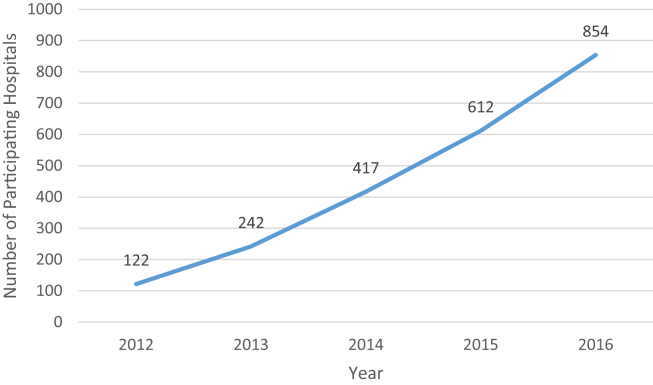
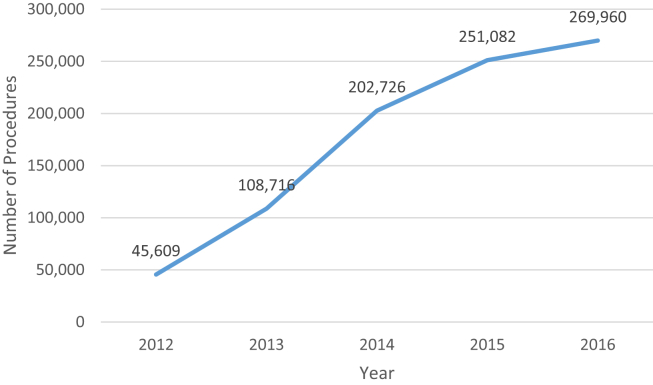
The years 2013-2014 saw greater expansion of AJRR initiatives, hospital participation, and staff. AJRR undertook an effort to test the capture of level II (comorbidities, surgical data, perioperative care, and complications) and level III (patient-reported outcome measures); participated in an external audit of registry data; collected data on over 150,000 procedures and became the national registry, with representation in all 50 states (Figs. 1 and 2). The staff grew to 12 full-time employees. AJRR also sought to expand offerings in 2014 by receiving designation as a Qualified Clinical Data Registry by the Centers for Medicare & Medicaid Services (CMS). Through a collaboration with Premier, Inc. (Charlotte, NC), the Orthopaedic Quality Resource Center was developed to facilitate the submission of Physician Quality Reporting System data to CMS. Finally, in 2014, AJRR released the First AJRR Annual Report on Hip and Knee Arthroplasty Data. This document includes over 43,000 procedures, showing the national scope of AJRR along with its data reporting capabilities. Subsequent annual reports have been released each November, in conjunction with the AAHKS Annual Meeting (http://www.ajrr.net/publications-data).
Figure 1.
AJRR hospital participation as of December 31, 2016.
Figure 2.
AJRR annual procedure count as of March 1, 2017.
On January 1, 2015, AJRR became a self-sustaining organization, no longer a formal entity of AAOS. This transition created new and exciting opportunities for AJRR, in addition to allowing AJRR to have independent budgeting processes, internal payroll, and employee benefits. Primarily, the transition facilitated greater autonomy for AJRR to develop policies and programs to develop the organization's future, its planning, and goals. By the end of 2015, AJRR had enrolled over 600 hospitals; had over 400,000 procedures; concluded requirements to implement International Classification of Disease, Tenth Revision procedure and diagnosis codes along with Current Procedural Terminology codes on AJRR's data system; and completed the transfer of the California Joint Replacement Registry to AJRR. With the inclusion of California Joint Replacement Registry in to AJRR operations, what was 2 separate entities could subsequently operate more efficiently and be more cost-effective than previously. The transfer also facilitated significant growth in California with new hospitals aligning with AJRR.
During 2016, AJRR revamped the original technology platform and approach from 2012. AJRR added a Chief Technology Officer who conducted a full assessment of the existing information technology architecture and developed a multiyear strategic roadmap. A comprehensive overhaul was deemed necessary and became the primary focus of 2016 efforts (see Future of AJRR for further details).
To date, AJRR has presented updates and findings at the International Congress of Arthroplasty Registries. AJRR has begun cross-registry comparisons, publishing an article, and presenting results assessing “revision burden” across 5 international joint registries [10]. Findings indicated hip revision burden appears to be decreasing, whereas knee revision burden has remained relatively constant. Current analysis is focusing on comparing infection burden across the same international joint registries. Abstracts have been submitted for this year's Congress pertaining to surgical treatment and stem designs used for femoral neck fractures and the use of constrained liners and dual mobility articulations in hip revision cases. In the future, AJRR anticipates partnering with other investigators on a variety of studies.
Related initiatives
AJRR's Board of Directors has directed the organization to become a quality improvement effort for orthopaedics. That is, the Board of Directors views AJRR as more than a device registry. Instead, AJRR should evolve to meet the varying needs of the numerous stakeholders involved in the initiative. Some of AJRR's evolving projects are described here.
As mentioned previously, AJRR has been a Qualified Clinical Data Registry since the program's inception in 2014. This effort continues to evolve in the context of the Medicare Access and CHIP Reauthorization Act of 2015 and the Merit-Based Incentive Payment System. With CMS's focus on alternative payment models, AJRR has addressed the needs of hospitals participating in CMS's Comprehensive Care for Joint Replacement bundled payment program. AJRR provides Comprehensive Care for Joint Replacement hospitals with a platform for the capture of risk variables and patient-reported outcome measures along with a reporting template to assist with data submission to CMS. AJRR maintains timely knowledge of emerging quality initiatives and policies and evolves its programs accordingly.
Through a partnership with the International Society of Arthroplasty Registers (ISAR), AJRR is currently developing the International Prosthesis Library. The International Prosthesis Library platform is a collaborative effort between the medical device industry and international registries as a way to maintain global standards and updated device product data through the use of a centralized information management solution. AJRR will be providing the technology, infrastructure, and support resources while ISAR maintains governance oversight and direction.
AJRR was awarded a subcontract for partnership with Weill Cornell Medical College on a grant from the US Food and Drug Administration (U01 FD005478), Creating National Surveillance Infrastructure for Priority Medical Devices. This effort seeks to develop a national device surveillance network in orthopaedics, across a number of local and regional arthroplasty registry initiatives. It also intends to implement a system to link registry and claims data. Ideally, the project will demonstrate the value of linked data for conducting postmarket research. Preliminary results of cross-registry findings from this project will be presented at the 2017 ISAR Congress meeting.
AJRR evolved its relationships and technology vendors to create the Authorized Vendor Program. The registry has agreements with over 20 technology partners to assist participants with data submission. These agreements entail extracting certain levels of client data or sending data on behalf of clients.
As TJAs are being increasingly performed in Ambulatory Surgery Centers, AJRR has an effort with the Ambulatory Surgery Center Association to promote AJRR as an important quality improvement effort across member Ambulatory Surgery Centers.
AJRR and AAHKS have also further strengthened their relationship over the past few years. In 2015, AJRR became the official registry of AAHKS. Furthermore, as this publication can attest, AJRR named Arthroplasty Today as the official journal of the registry. Staff of the 2 organizations are in frequent communication with regular meetings and a strong collective spirit.
Future of AJRR
In summary, AJRR has made significant advances over the past 5 years in building a national arthroplasty registry. AJRR has collected substantial volumes of procedural data and is advancing the quality of arthroplasty care through a variety of ventures. AJRR has become the only entity providing national benchmarks on arthroplasty care in the United States. The 2016 Annual Report covered over 427,000 procedures from 416 hospitals and 3170 surgeons. In the first quarter of 2017, AJRR data systems included over 1 million procedures.
The AJRR technology initiative is a digital transformation effort that introduces a completely new and innovative solution architecture focused on the ability to provide a secure, scalable, and adaptable environment to meet the evolving needs of the orthopaedic community and health care industry. At the core of the technology plan is a data management strategy that aims to build internal capacity in a big data environment coupled with a robust analytics and outcome measurement reporting platform.
The new systems have the capacity to facilitate collaborative research efforts. Those interested in analysis of AJRR data for scholarly efforts will be able to submit applications on a twice-yearly basis for review by an AJRR subcommittee.
AJRR is looking to create more state-based and regional registries. It is a goal to work with states and regions to create additional reporting systems within AJRR's structure. With local orthopaedic leaders championing these regional efforts, AJRR has the ability to provide data at varying geographic and systemic levels to encourage participation and increase data acquisition.
In 2015, Malchau et al [11] proposed that monitoring of new implants by registries be a universal requirement. They suggest that expanding the use of registries is critical in such models and that the role of registries should be expanded. AJRR sees the need to be a strong partner with our orthopaedic colleagues in these types of efforts and in the future of orthopaedic registries.
For more information on AJRR, please visit www.ajrr.net.
Acknowledgements
The authors thank Brian J. McGrory, MD, MS for his guidance and continued support of AJRR.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2017.02.002.
Appendix A. Supplementary data
References
- 1.Williams S.N., Wolford M.L., Bercovitz A. 2000. Hospitalization for total knee replacement among inpatients aged 45 and over: United States, 2000–2010 key findings. https://www.cdc.gov/nchs/data/databriefs/db210.pdf; [accessed 30.01.17] [PubMed] [Google Scholar]
- 2.Wolford M.L., Palso K., Bercovitz A. 2000. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000–2010 key findings. https://www.cdc.gov/nchs/data/databriefs/db186.pdf; [accessed 30.01.17] [PubMed] [Google Scholar]
- 3.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Robertsson O., Lewold S., Knutson K., Lidgren L. The Swedish Knee Arthroplasty Project. Acta Orthop Scand. 2000;71(1):7. doi: 10.1080/00016470052943829. [DOI] [PubMed] [Google Scholar]
- 5.Hansen V.J., Greene M.E., Bragdon M.A. Registries collecting level-I through IV data: institutional and multicenter use. J Bone Joint Surg Am. 2014;96A(e160):1. doi: 10.2106/JBJS.M.01458. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini V.D.J., Marx C., Rankin E.A. Position statement in support of National Joint Registries. J Bone Joint Surg Am. 2009;91(12):2983. doi: 10.2106/JBJS.I.01469. [DOI] [PubMed] [Google Scholar]
- 7.Maloney W.J. National Joint Registries: has the time come? J Bone Joint Surg Am. 2001;83A(10):1582. doi: 10.2106/00004623-200110000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Pugely A.J., Martin C.T., Harwood J. Database and registry research in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(21):1799. doi: 10.2106/JBJS.O.00134. [DOI] [PubMed] [Google Scholar]
- 9.Maloney W.J. The role of orthopaedic device registries in improving patient outcomes. J Bone Joint Surg Am. 2011;93(24):2241. doi: 10.2106/JBJS.9323edit. [DOI] [PubMed] [Google Scholar]
- 10.McGrory B.J., Etkin C.D., Lewallen D.G. Comparing contemporary revision burden among hip and knee joint replacement registries. Arthroplasty Today. 2016;2(2):83. doi: 10.1016/j.artd.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malchau H., Graves S.E., Porter M., Harris W.H., Troelsen A. The next critical role of orthopedic registries. Acta Orthop. 2015;86(1):3. doi: 10.3109/17453674.2014.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.