Abstract
Objectives
Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) can generate any given cell type in the human body. One challenge for cell-replacement therapy is the efficient differentiation and expansion of large quantities of progenitor cells from pluripotent stem cells produced under good manufacturing practice (GMP). FOXA2 and SOX17 double positive definitive endoderm (DE) progenitor cells can give rise to all endoderm-derived cell types in the thymus, thyroid, lung, pancreas, liver, and gastrointestinal tract. FOXA2 is a pioneer transcription factor in DE differentiation that is also expressed and functionally required during pancreas development and islet cell homeostasis. Current differentiation protocols can successfully generate endoderm; however, generation of mature glucose-sensitive and insulin-secreting β-cells is still a challenge. As a result, it is of utmost importance to screen for small molecules that can improve DE and islet cell differentiation for cell-replacement therapy for diabetic patients.
Methods
The aim of this study was to identify and validate small molecules that can induce DE differentiation and further enhance pancreatic progenitor differentiation. Therefore, we developed a large scale, high-content screen for testing a chemical library of 23,406 small molecules to identify compounds that induce FoxA2 in mouse embryonic stem cells (mESCs).
Results
Based on our high-content screen algorithm, we selected 84 compounds that directed differentiation of mESCs towards the FoxA2 lineage. Strikingly, we identified ROCK inhibition (ROCKi) as a novel mechanism of endoderm induction in mESCs and hESCs. DE induced by the ROCK inhibitor Fasudil efficiently gives rise to PDX1+ pancreatic progenitors from hESCs.
Conclusion
Taken together, DE induction by ROCKi can simplify and improve current endoderm and pancreatic differentiation protocols towards a GMP-grade cell product for β-cell replacement.
Keywords: Rock inhibition, Fasudil, Pancreatic progenitors, Anterior definitive endoderm, Differentiations
Highlights
-
•
High content screen of 23,406 small molecules identifies novel definitive endoderm inducers Fasudil and RKI-1447 in mESCs.
-
•
Fasudil and RKI-1447 induce anterior definitive endoderm differentiation in mESCs and hESCs through ROCK inhibition.
-
•
Fasudil and RKI-1447 further differentiates the ADE cells into PDX1+ pancreatic progenitors.
1. Introduction
The self-renewal capacity and the wide differentiation potential of pluripotent ESC lines have great therapeutic potential to treat chronic diseases such as diabetes mellitus, blindness, or neurodegeneration. Islet transplantation can cure brittle type 1 diabetic patients; however, donor islets are rare and preclude therapy for many patients. ESCs can represent an attractive source to produce functional β-cells from stem cells in the culture dish. Current problems include the expansion of large quantities of progenitor cells using GMP facilities and the generation of functional mature β-cells for transplantation [1]. The existing differentiation protocols are directly translated from developmental studies in vivo [2], [3] and act by mimicking the developmental programs in vitro to facilitate generation and upscaling of pancreatic β-cells [4], [5]. A major drawback of these protocols is the use of recombinant proteins and ligands that show variable activity and stability and are often exposed to animal products that might be contaminated with yet unidentified pathogens [4], [5]. One strategy to overcome this problem and implement cheap and efficient GMP-grade ESC differentiation protocols is to replace biologics by small molecule compounds with stable and reproducible activity.
During embryogenesis, different developmental pathways regulate definitive endoderm (DE) formation and patterning, including the Wnt, fibroblast growth factor (FGF), transforming growth factor β (TGF- β)/Nodal/ActivinA (AA), bone morphogenic protein (BMP), and AKT/PI3K [6], [7], [8], [9]. Modulating the signaling transduction events and genes involved in these pathways can help recapitulate the developmental processes in vitro. In current protocols, efficient DE induction is achieved by direct exposure of ESCs and iPSCs to recombinant proteins, including Wnt3a and AA [8]. However, these proteins can exhibit highly variable activity in culture, due to incomplete post-translational modifications, lack of stability of biological activity as well as batch-to-batch variations. As it is well known that Wnt and Nodal/TGF-β signaling gradients induce and pattern the mesoderm and endoderm germ layer [10], this can result in variable efficiencies of DE patterning in vitro from one week to another. Induction of heterogeneous DE populations can lead to a great inconsistency in establishing long-term differentiation protocols over 20–40 days towards one particular cell fate [4], [5].
Small molecules can serve as tools to replace current proteins and induce the differentiation of ESCs. These molecules can effectively act on target proteins thereby modulating different signaling pathways [11]. The major advantage of using small molecules is that they can be synthesized in high amounts and with higher purity and stored in a way that the substances have reproducible activity. High-throughput screens to monitor directed endodermal differentiation have been reported previously [11], [12]. These screens introduce small molecules that modulate the TGF-β pathway, replacing the use of AA in differentiation cocktails to induce endoderm; however, there is still a great need to identify novel potent endoderm inducers that can effectively augment terminal pancreatic differentiation protocols [4], [5], [11], [13], [14].
Towards this aim, we set-up a high-content screen in mESCs and tested 23,406 small molecules. We identified the Rho associated coiled like protein kinase (ROCK) inhibitor Fasudil as a small molecule that efficiently induces DE in both mESCs and hESCs. Moreover, when compared with the traditional Wnt3a and AA endoderm induction cocktail, ROCKi treated cells showed similar differentiation towards DE. We show that another analogue of Fasudil, RKI-1441, showed similar differentiation efficiencies of mESCs and hESCs towards DE indicating that ROCKi is sufficient to induce DE in culture. Furthermore, the ROCKi differentiates the PSCs towards anterior definitive endoderm (ADE), which gives rise to thymus, thyroid, lung, liver, and pancreas. We found that ROCKi does not induce extraembryonic visceral endoderm or mesoderm in the cell culture system. Additionally, ROCKi-induced DE from hESCs differentiated efficiently into pancreatic progenitors (PP), suggesting a supportive role of ROCKi in pancreatic differentiation. Altogether, we introduce a family of small molecule ROCKis and a novel mechanism that can robustly induce DE/ADE differentiation of PSCs in culture thereby replacing biologics in the differentiation medium.
2. Methods and materials
2.1. Culture, maintenance, and differentiation of mouse and human embryonic stem cells
In-house made (IDG) mESCs (FoxA2-Venus/Oct3/4-RFP) were thawed on mitomycin treated feeders and maintained undifferentiated in ES medium based on DMEM (41966-052; Gibco) containing 15% FCS (PAA, A15-108), mLIF (self-made), 12 ml HEPES (2503024, Gibco), 5 ml Penicillin/Streptomycin (15140122; Gibco), and 1 ml 2-mercaptoethanol (Gibco, 31350-010). In vitro differentiation of the mESCs towards endoderm was carried out in monolayer on 0.1% gelatine coated dishes. The cells were mouse embryo fibroblast feeder cells (MEF) depleted and cultured for few consecutive passages on gelatine and ES medium. On the day of differentiation, mESCs were seeded on gelatine coated dishes directly in serum free medium (SFM). SFM was based on advanced DMEM/F-12 (12634028, Gibco) and advanced RPMI (12633020, Gibco) in ratio 1:1.500 ml of SFM was supplemented with 10 ml GlutaMax™ (35050061, Gibco), 12 ml HEPES (2503024, Gibco), 5 ml Penicillin/Streptomycin (15140122, Gibco), Cytidine 20 ng/ml final concentration (C122106, Sigma Aldrich). For control endoderm differentiation, SFM was supplemented with 1 ng/ml of murine Wnt3a (1324 WN-CF, R and D systems) and 12.5 ng/ml of Activin A (338-AC, R and D systems). Freshly prepared SFM supplemented with Wnt3a and Activin A was added every day until day 4 to achieve optimum differentiation of mESCs towards definitive endoderm. Compound induced differentiation was achieved by adding 3 μM of Fasudil or other ROCK inhibitors to the SFM instead of Wnt3a and Activin A. The differentiation efficiency was assessed based on endogenous expression of FoxA2 and Sox17.
For human ESCs (H9), the cells were thawed on 1:30 geltrex coated dishes along with 10 μM of Y compound for 1 day. The cells were maintained in iPS-Brew medium (130-104-368; Miltenyi Biotech). For differentiation, single cells were seeded on geltrex coated 6-well plates (2 million cells/well). On day 1, the cells were treated with 20 ng/ml WNT3A and 100 ng/ml AA or 10 μM of the ROCK inhibitors for 4 days for DE induction.
2.2. Screening setup for unknown novel compounds that can induced endoderm differentiation in mESCs
2.2.1. Assay seeding and medium change with re-addition of fresh compound solution
For screening, 384 well Cell Carrier plates (Perkin Elmer) were coated with 0.1% gelatine solution. After discarding coating solution, compounds were spotted by echo liquid handling system (Labcyte Echo 550). For primary screening, 250 nl of 20 mM compound solution in 100% DMSO was spotted to the compound area. Coated assay plates with fresh compound solution were stored at 16 °C over night. 1–2 h before seeding the ESCs onto assay plates, ESC medium was changed. Directly before seeding, the cells were washed with PBS (without Ca2+ and Mg2+) and trypsinized. Cells were resuspended in SFM, and cell density was adjusted to 220.000–280.000 cells/ml (optimized for each batch). For the compound area, 50 μl of the cell suspension was added using Multidrop (Thermo Labsystems Type 832). The following controls were used for the assay: a) Differentiation control: Activin A and Wnt3a (final concentrations of 12.5 ng/ml (Activin A) and 1 ng/ml (Wnt3a) plus 0.5% DMSO, b) pluripotency control: GSK3β Inhibitor (CHIR 99021) at 3 μM final concentration in 0.5% DMSO, c) negative controls: 0.5% DMSO).
In the primary screen and hit confirmation single dose, compounds were tested at a final concentration of 10 μM. On day 2 post seeding, the medium was changed (fresh SFM + compound or control substances). For the medium change procedure a pre dilution plate (with compounds) was prepared in advance: Here, compounds (300 nl/well) were spotted into sterile round bottom PP 384 well plates (Greiner, 784201). Then, 60 μl SFM was added using Multidrop to give final compound concentration of 10 μM at 0.5% DMSO. For control wells SFM was supplemented with growth factors, GSK3β Inhibitor or DMSO, 60 μl of control medium cocktail was added to the appropriate wells of the pre-dilution plate (according to the plate layout): a) differentiation control: 12.5 ng/ml Activin A + 1 ng/ml Wnt3a, b) pluripotency control: 3 μM GSK3β Inhibitor (CHIR 99021) and, c) 0.5% DMSO solvent control (Supplementary Figures 3 and 4). For complete medium change, the medium of the assay plate was discharged and fresh medium from the pre-dilution plate was transferred to the assay plate using Perkin Elmer Janus Mini AJ SM001/384 Tip MDT with sterile Tips (slowest dispensing speed, without touching the cells). After medium change assay, plates were immediately placed into an incubator (37 °C and 5% CO2) until day 5 after seeding.
2.3. Assay plate processing and immunostaining
On day 5 post seeding, assay plates were washed 1× with PBS and fixed for 5 min at RT with 4% PFA, rinsed 1× in PBS and 2× in PBST (PBS + 0.1% Tween20) and permeabilized for 10 min in PBS containing 0.1 M Glycin/0.1% Triton X-100. After permeabilization, cells were rehydrated for 10 min in PBST containing 0.1 M Glycin without Triton X-100 and blocked for 30 min with PBST+ 10% FCS, 0.1% BSA, 3% donkey serum. Primary antibodies were diluted in blocking solution (anti-FoxA2 goat polyclonal AB (Santa Cruz, sc6554) and anti-Oct-3/4 mouse monoclonal AB (Santa Cruz, sc5279), (both 1:800 diluted). Plates were incubated with 20 μl of the antibody dilution for 1 h at 37 °C, rinsed with 1× with PBST and washed 2× for 7 min in PBST. Secondary antibodies (Alexa555 anti-mouse IgG and Alexa488 donkey anti-goat, Invitrogen) were diluted in blocking solution (1:800). Secondary antibody solution was added to each well and incubated for 1 h. Secondary antibody dilution was discarded, replaced by PBST+ containing DAPI, and incubated for 15 min. Plates were rinsed once with PBST and washed twice for 10 min in PBS. All washing steps were done with 80 μl/well. Washing solution was added manually or using Perkin Elmer Janus (384 well Head, slowest dispensing speed).
2.4. High content image acquisition
Stained plates were imaged on the Opera confocal imaging system (PerkinElmer) using 10× objective (UPlanSApo_NA = 0.4). A set of 12 image fields were collected from each well covering the complete well. Filter and Camera selection were optimized by using the Opera-Filter-Selection-Visualization Tool (PerkinElmer). For each measurement, laser power and exposure times were adjusted to the linear detection range. Acquired images were captured and analyzed using Columbus 2.0 software (PerkinElmer) (Supplementary Figure 1).
2.5. Image analysis method developed on Columbus (PerkinElmer, Platform)
As pluripotent cells grow in colonies and differentiated cells grow in monolayer, an image analysis strategy was developed and realized using predefined building blocks and machine learning algorithms from Columbus image analysis options (PerkinElmer). The strategy involved the identification of different cell populations and colonies using the “Find texture regions,” which allows the separation of different textures using an interactive training mode (PhenoLOGIC™). Training sets were performed on representative images in which some examples of a texture class had been selected. Aggregated cells region consisted of one class and single cells region of another. The method ‘Find a class’ was chosen based on the DAPI staining channel. After the training phase, each image was automatically divided into two regions each being like the particular examples of the training sets. The two resulting output populations, namely “colony-like region and “isolated” cells, were further analyzed to obtain the related morphology and intensity properties. In subsequent building blocks, the populations of interest were selected in order to calculate the intensity and morphology properties. Both enabled the introduction of filtering steps to refine the analysis and identify accurately each object of the populations of interest. The most relevant phenotypic descriptors were then selected on the basis of subsequent quantification (Supplementary Figure 2).
2.6. Immunocytochemistry analysis
2.6.1. Detailed mechanistic studies following the primary screening and hit confirmation stage
mESCs were differentiated for 4 days towards definitive endoderm. The cells were washed and fixed at room temperature for 10 min in 4% Formalin solution (HT-501128, Sigma Aldrich), washed 3X with PBS and permeabilized with 0.01% triton in PBS for 1 h at RT. Immunostaining was carried out with goat antibodies against Sox17 (1:800 dilution; GT 15094; Neuromics), rabbit antibodies against FoxA2 (1:1000; ab4874, Abcam), goat antibodies against brachyury (T) (1:100; sc-17743, Santa Cruz), and mouse antibodies against Oct3/4 (1:1000; sc-5279, Santa Cruz). Fluorescence labeled secondary antibodies were Alexa Fluor 488 (green), Alexa Fluor 555 (red) and Alexa Fluor 647 (magenta) (Invitrogen, all diluted 1:1000). Nuclei were stained with DAPI (1:10,000). Images were captured using an epifluorescence microscope (Epifluorescence, Zeiss, Germany) and analyzed with Axiovision software (Zeiss).
2.7. Immunoblotting and protein analysis
Protein samples were isolated from differentiated cells with different drugs using RIPA buffer and transferred from the SDS-PAGE to nitrocellulose membrane (Millipore) for electroblotting. Proteins were detected with affinity-purified antibody against FoxA2 (1:1000; ab4874, Abcam), Sox17 (1:1000; GT 15094, Neuromics). Membranes were incubated in the primary antibodies overnight at 4 °C. HRP-conjugated secondary antibodies included HRP-anti rabbit IgG (1:10,000; 12-348, Millipore) and HRP-anti goat IgG (1:10,000; AP106P, Millipore). Gapdh or β-actin were used as loading controls.
2.8. Quantitative real-time RT-PCR (qPCR)
Total RNA was isolated from differentiated cells using RNeasy Mini Kit (74104, Qiagen) according to manufacturer's protocol. A commercial array card (Invitrogen) containing almost all the known endoderm genes, mesoderm genes, neuroectoderm genes, and pluripotency genes was used to run qPCR. The list of the primer sequences is mentioned in supplementary methods. 1 μg of total RNA was reverse transcribed using Go-script transcription Kit (Promega) according to manufacturer's protocol. Tenfold dilutions of the transcribed cDNA were mixed with Taqman Advanced Master Mix (4444556, Invitrogen) for amplifications. Internal standards (housekeeping genes) and samples were amplified simultaneously. The expression of each gene transcript was calculated by normalizing to the respective housekeeping gene. Undifferentiated H9 mRNA was used for normalizing the gene expression. Relative gene expression was expressed as a ratio of target gene concentration to house-keeping gene GAPDH concentration.
2.9. Differentiation of hESCs towards pancreatic progenitors (PDX1+)
Differentiation of H9 cells towards pancreatic progenitors was carried out using an already published protocol [5]. H9 cells were seeded on geltrex coated 6 wells plates with a seeding density of 2 × 106/well. The cells were treated with Fasudil and RKI-1447 for 4 days to induce DE/ADE differentiation. 20 ng/ml WNT3A, and 100 ng/ml AA was used as a differentiation control. The basal medium used contained RPMI supplemented with 0.5% BSA, 1× Glutamax. After 4 days, the medium was changed to RPMI supplemented with 0.5% BSA, 10 nM Glucose, 1× Glutamax, 50 ng/ml FGF7, and 0.25 mM ascorbic acid for 2 days to induce primitive gut tube. For the next 2 days, to induce posterior foregut, the cells were treated with RPMI supplemented with 1× Glutamax, 10 mM final glucose concentration, 2% BSA, 0.25 mM ascorbic acid, 50 ng/ml FGF7, 0.25 μM SANT-1, 1 μM retinoic acid, 100 nM LDN193189, 1:200 ITS, and 200 nM TPB. Pancreatic progenitor was induced in the culture by treating the cells additionally for 3 days with RPMI supplemented with 1× Glutamax, 10 mM final glucose concentration, 2% BSA, 0.25 mM ascorbic acid, 2 ng/ml of FGF7, 0.25 μM SANT-1, 0.1 μM retinoic acid, 200 nM LDN193189, 1:200 ITS-X, and 100 nM TPB.
2.10. Statistical analysis
If not otherwise stated, for comparisons of two groups, levels of significance were calculated using the unpaired Student's t test. *, P < 0.01; ***, P < 0.01; ***, P < 0.0001 were used as levels of significance.
3. Results
3.1. High content screening identifies novel compounds that induce definitive endoderm
To facilitate high content screening (HCS) of novel compounds that induce DE, we analyzed exit of pluripotency and induction of endoderm in mESC using immunofluorescence staining against Oct3/4 and FoxA2, respectively. Previous data indicate that FoxA2 is highly expressed in mesendoderm and DE at gastrulation-stage [15]. The in vitro endoderm differentiation protocol used in the screen was based on previously published protocols [8] [6], [12]. To directly test the effect of the small molecules on DE induction and exclude the influence of non-defined material, we used serum-free medium for the screen. Chemically defined medium supported normal growth of mESCs, cultured as a monolayer in 384-well plates (data not shown). The TGF-β pathway ligand Activin A and the Wnt pathway inducer Wnt3a efficiently induce mesendoderm and DE around day 4 in in vitro differentiated mESCs as monitored by FoxA2 and Oct3/4 antibody stainings (Figure 1A,B). FoxA2+ cells also showed induction of the DE transcription factor Sox17, indicating efficient DE induction in the HCS assay format using Wnt3a and AA (data not shown).
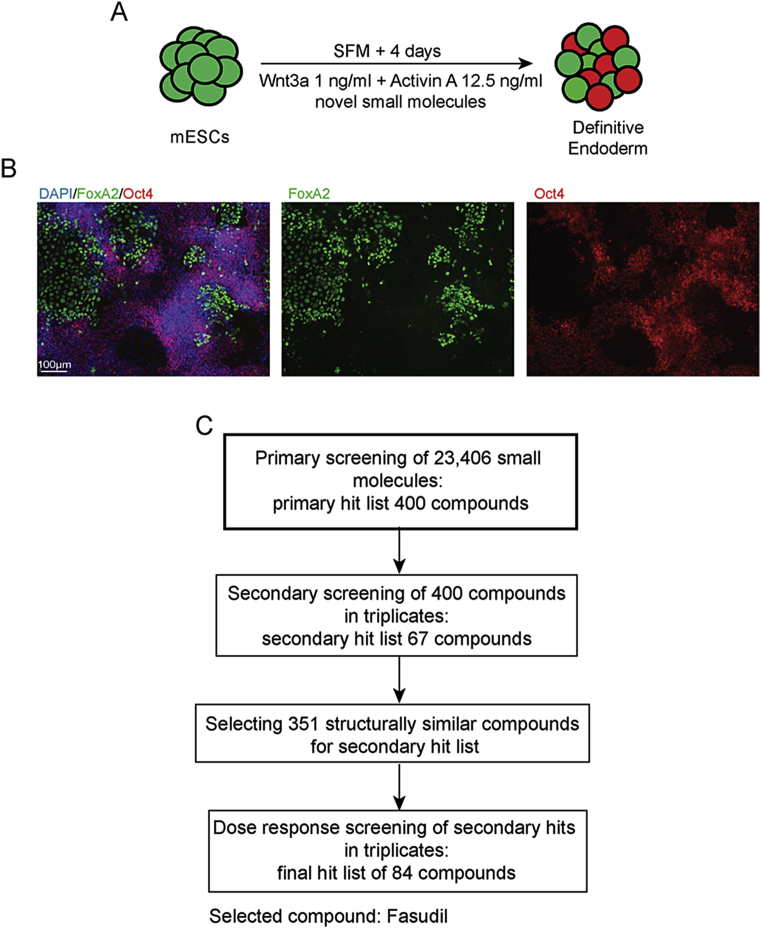
Figure 1.
Screening setup for identification of candidate small molecules that aid in differentiation of mESCs towards definitive endoderm (DE). (A) Schematic representation of ESCs differentiation towards DE. (B) Representative images mESCs treated with drugs indicate the differentiation and pluripotency states of the cells. The differentiation is indicated by the expression of endogenous FoxA2-Venus reporter (green). The pluripotency is marked by endogenous Oct-4-RFP reporter (red). DAPI positive cells are depicted in blue. (C) A schematic approach for systematic identification of compounds that can drive endoderm differentiation. Identification of the hit compounds was based on the above images through high content analysis. The final selected compound from the screen is Fasudil.
The initial screen was performed with 23,406 small molecules selected on the basis of chemical diversity and excluding structural features associated with well described interference effects [16]. From the primary screening results covering 23,406 compounds, 400 putative hits were selected for re-test in Hit confirmation study in triplicates (single dose) to confirm their capacity to induce FoxA2 expression in mESCs (Figure 1C). The compounds which gave at least 20% more FoxA2 marker mean signal intensity as obtained in the DMSO treated mESCs were selected. This selection was refined to compounds where three replicate values showed less than 30% standard deviation or when two of the replicates gave values above the threshold close to each other. In total, 67 compounds were identified to follow-up in dose–response studies.
A further tranche of 351 analogues of the primary hits were also sourced from commercial providers profiled. Initial hits and analogues were tested in triplicates in seven-point dose response studies (final concentrations: 10, 5, 2.5, 1.3, 0.6, 0.3, 0.2 μM). In parallel, the compounds were tested in a cell viability assessment (CellTiter-Glo Luminescent Assay (Promega). Based on these results, we selected 84 compounds, all inducers of FoxA2 and DE differentiation and without any adverse effects on cell viability or proliferation (Supplementary Table). One of the most striking effects on DE differentiation was observed with Fasudil, the only compound with a known molecular target based on the searches performed using public databases such as ChEMBL and PubChem. All other compounds currently have no described direct molecular targets. Fasudil (HA-1077) is a potent ROCK inhibitor [17]. The effect of ROCK inhibitors on DE differentiation of ESCs was not previously described and we selected it for follow-up experiments.
3.2. Fasudil induces definitive endoderm in mouse embryonic stem cells
Fasudil increases the survival of single cells during passaging of human ESCs (hESCs) by blocking apoptosis and thereby increasing plating efficiency [18]. The effects of ROCK inhibition on ESC differentiation are not well studied; thus, we analyzed the effect of long-term exposure of Fasudil and its analogues (1,4-diazepine derivatives) on mESCs. Remarkably, Fasudil showed a similar degree of DE induction when compared to the combination of Wnt3a and AA. mESCs responded in a dose-dependent manner to Fasudil treatment with an EC50 of 2.328 μM (Figure 2A,B). Wnt3a/AA and DMSO were used as positive and negative controls, respectively. Treatment of mESCs with Fasudil resulted in approx. 27% of FoxA2+ cells, which was comparable to the induction of FoxA2-induced DE by Wnt3a/AA (23%) on day 4 of mESC differentiation (Figure 2C).
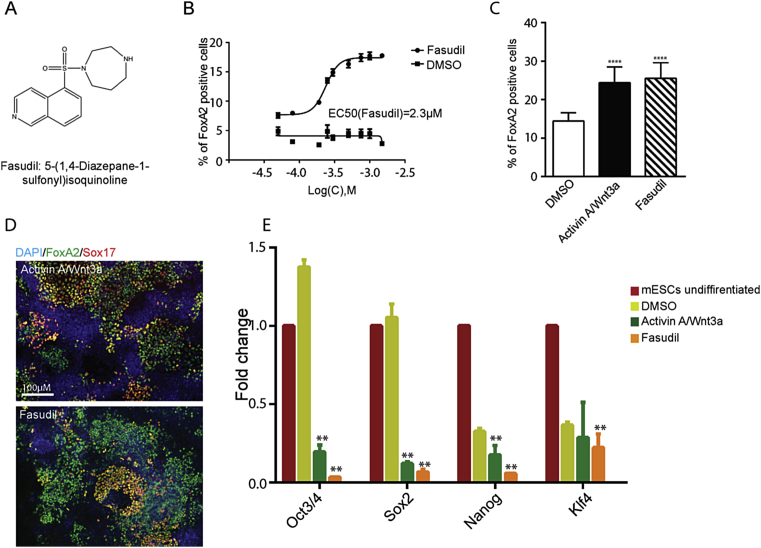
Figure 2.
Fasudil and its analogs induce endoderm differentiation. (A) Chemical structure of Fasudil. (B) Dose response curve representing effect of Fasudil on mESCs endoderm differentiation. In total, 9 concentrations of Fasudil were tested (circles). In parallel, 9 corresponding concentrations of DMSO were tested (squares). Increasing concentration of Fasudil increased the FoxA2 induction in mESCs indicating differentiation towards endoderm. An increase in DMSO concentration did not affect mESCs differentiation towards endoderm. (C) Comparison of FoxA2 induction in mESCs when treated with Wnt3a/AA combination, Fasudil, and DMSO, mean ± SDV, ****P < 0.0005. The cells were treated with DMSO, Activin A 12.5 ng/ml, and Wnt3a 1 ng/ml, 3 μM Fasudil. (D) Representative images of the cells differentiated with Wnt3a/AA and Fasudil. The cells were stained against DAPI (blue), FoxA2 (green), and Sox17 (red). (E) qPCR quantification showing treatment of Fasudil reduces the expression of pluripotency target genes such as Oct3/4, Sox2, Nanog and Klf4 compared to DMSO and Wnt3a/AA treatment, mean ± SDV, ****P < 0.0005.
For the HCS, we used FoxA2 as marker for DE differentiation. However, apart from DE, FoxA2 is also expressed in the mesendoderm. To confirm induction of DE, we combined FoxA2 and Sox17 staining [19]. Moreover, Sox17 is expressed in later stages of endoderm-derived organ development [20], [21]. Fasudil induced endoderm cells were double positive for FoxA2 and Sox17 (Figure 2D). Treatment with Fasudil and Wnt3a/AA reduced the expression levels of pluripotency markers Oct3/4, Sox2, Nanog, and Klf4 significantly, suggesting efficient endoderm differentiation and exit from pluripotency (Figure 2E).
3.3. ROCK inhibition leads to the formation of anterior definitive endoderm (ADE)
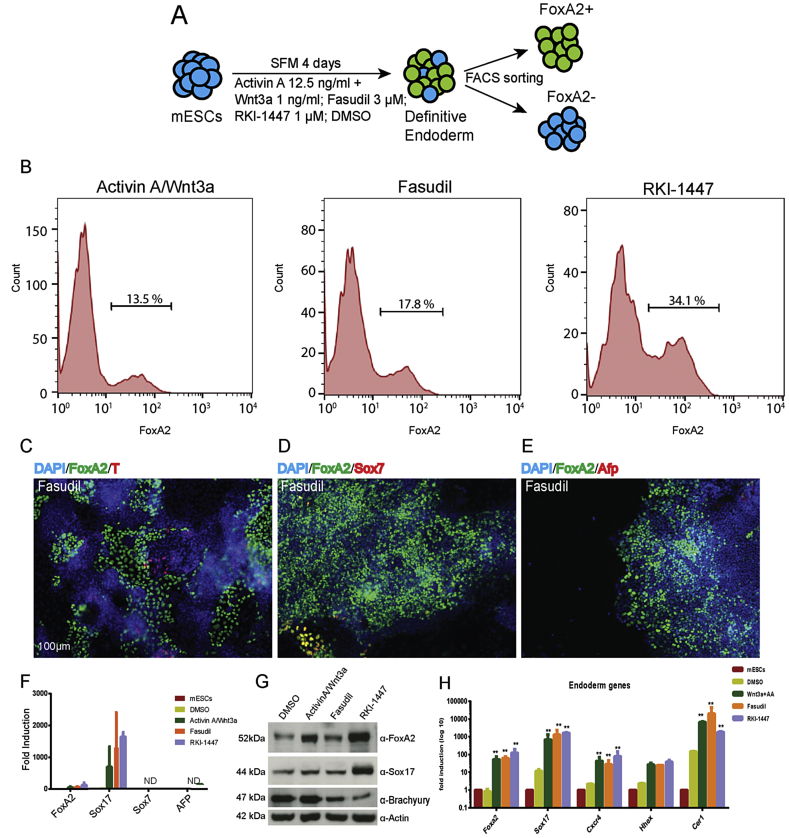
Previously, it has been shown that mesendoderm and endoderm are induced by high levels of Wnt3a and AA [22]. Furthermore, Wnt3a and Nodal/TGF-β signaling are known to pattern the endoderm in a dose-dependent fashion [10]. To test the effect of ROCKi on endoderm induction and patterning, we assessed mESCs using the FoxA2-Venus fusion (FVF) reporter mESC line [15]. During endoderm development and differentiation, expression of FVF marks the mesendoderm (FVFlow) and DE (FVFhigh) in a dose-dependent fashion. We treated the FVF mESC line using selected ROCK inhibitors to induce endoderm lineage (Figure 3A). A common Wnt3a/AA DE induction paradigm (1 ng/ml Wnt3a + 12.5 ng/ml AA) was used for comparison to benchmark DE induction efficiency. It is well known that lower levels of AA induce mesoderm and higher levels of AA induce DE [6]. We also used another ROCKi, RKI-1447, to assure that ROCK inhibition is the mechanistic principal that underlies mesendoderm and DE induction. Both Fasudil and RKI-1447 induced expression of FoxA2 and Sox17 (Figure 3B,F,G). However, the expression of FoxA2 and Sox17 was relatively higher in RKI-1447 treated cells (Figure 3F,G). To assess the induction of mesoderm or mesendoderm cells, we looked for the induction of mesoderm marker Brachyury (T). T+ cells give rise to mesoderm, while FoxA2+ cells give rise to endoderm and FoxA2+/T+ double positive cells mark mesendoderm [23]. Exposure of the cells to Fasudil and RKI-1447 resulted in a low amount of T induction in comparison to the classical Wnt3a/AA induction, indicating fewer mesendoderm cells (Figure 3C,G). To eliminate the possibility that compound exposure led to the generation of visceral endoderm, we also tested two visceral endoderm markers, Sox7 and alpha-fetoprotein (Afp). The cells treated with Fasudil and RKI-1447 expressed almost no Sox7 and Afp mRNA, clearly indicating that pluripotent ESCs differentiated into the DE lineage (Figure 3D–F). Most cells that expressed FoxA2 also expressed significantly higher levels of other endoderm marker genes, such as Sox17 and Cxcr4 (Figure 3H). qPCR quantification of ADE genes, such as Hhex and Cer1, showed similar expression in the chemically induced cells than in Wnt3a/AA treated cells, indicating the efficient generation of ADE by the treatment of mESCs with ROCKi (Figure 3H).
Figure 3.
Fasudil efficiently differentiates mESCs towards anterior definitive endoderm (ADE) (A) Schematic representation of experimental approach. The heading on top and bottom of the arrow represents the culture conditions during differentiation. (B) FACS plots showing the percentage of endoderm induction after differentiation of mESCs using the above mentioned culture conditions. Addition of ROCK inhibitors (Fasudil: 17.8% and RKI-1447: 34.1%) to the medium differentiated the mESCs more towards endoderm than Wnt3a/AA (13.5%). The differentiation is adjudged by the expression of endogenous Foxa2-Venus (Alexa-488) on the x axis. (C–E) Immunofluorescence stainings of Day 4 cells expressing low T and Sox7 but almost no Afp. (F) qPCR analysis indicating suppression of visceral endoderm markers like Sox7 and Afp. (G) Western blot analysis after treatment with Fasudil and RKI-1447 showing similar expression of FoxA2 and Sox17 but lower expression of T compared to Wnt3a/AA treatment. (H) mRNA analysis shows almost 100 fold upregulation of mRNA transcripts of the classical definitive endoderm (Foxa2, Sox17, Cxcr4) and ADE (Hhex, Cer1) genes.
3.4. Inhibition of ROCK pathway can prime mESCs towards DE
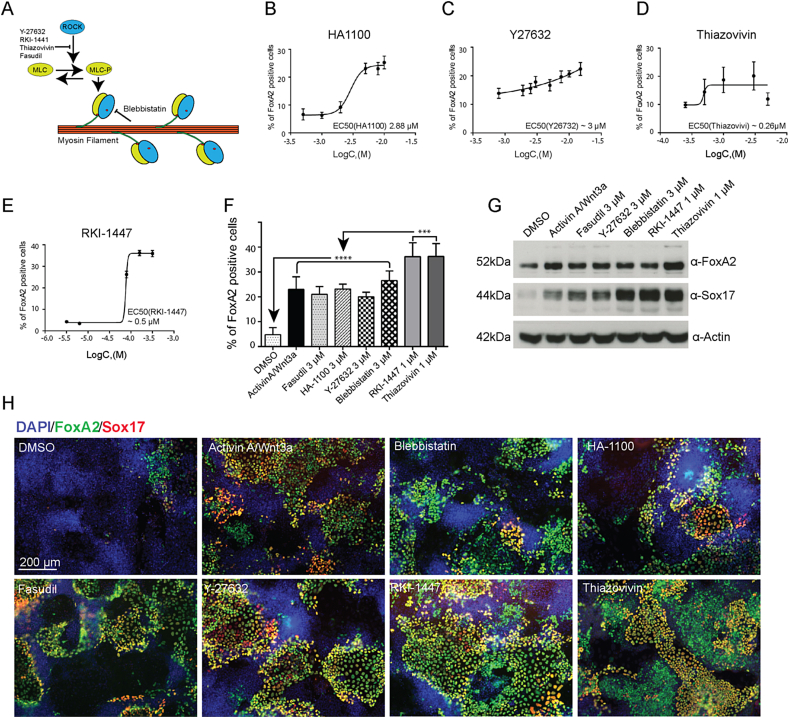
The small GTPases Rho, Rac, and Cdc42 are important regulators of cell polarity, adhesion, and migration via cytoskeletal remodeling, which can also influence gene transcription and differentiation [24]. ROCK is a direct downstream target of the small GTPase RhoA that controls stress fibre formation and cellular contractility. Along with its downstream effectors LIM kinase and Cofilin/ADF, ROCK inhibits actin depolymerization leading to actin filament stabilization and stress fiber formation [25]. ROCK also stimulates myosin II activity, directly by phosphorylation of myosin light chain (MLC) and indirectly by inactivation of MLC phosphatase target subunit I (MYPT1), which dephosphorylates and thus negatively regulates MLC (Figure 4A) [26]. Our screen detected ROCK inhibitor Fasudil as an inducer of DE differentiation. To test whether the effect of Fasudil on DE differentiation is due to ROCK inhibition, we treated both mESCs with Fasudil and several other ROCK inhibitors: HA-1100, Y-27632, RKI-1447, and Thiazovivin. All selected inhibitors were tested in dose response DE differentiation cultures. The EC50 values of HA1100, Y27632 were approximately 3 μM while that of Thiazovivin was around 0.3 μM and RKI-1447 was 0.5 μM (Figure 4B–E). Strikingly, all ROCK inhibitors initiated DE differentiation and led to increased number of FoxA2+ cells on day 4 of mESC differentiation in a dose-dependent manner (Figure 4F).
Figure 4.
Differentiation of mESCs towards definitive endoderm by Fasudil is directed by Rock inhibition. (A) Schematic overview of mechanism of ROCK inhibitors (Y-compound, Fasudil, and RKI-1441), which block phosphorylation of MLC (myosin light chain) and blebbistatin, which inhibits non-muscle myosin II, leading to decreased actin stress fiber formation. (B–E) Dose response curves representing effects of HA1100 (B), Y27632 (C), Thiazovivin (D), and RKI1447 (E) on mESCs differentiation towards endoderm indicated here by expression of endogenous FoxA2, mean ± SDV. (F) Quantification of FoxA2+ cells analyzed by FACS show increase in endoderm differentiation of mESCs by different ROCK inhibitors and Blebbistatin; mean ± SDV, ****P < 0.0005, ***P < 0.005. (G) Endogenous protein levels of FoxA2 and Sox17 were confirmed based on western blotting. The mESCs were treated with Wnt3a/AA, ROCK inhibitors or Blebbistatin respectively. (H) Representative images of cells differentiated in presence of Activin A/Wnt3a, Fasudil, Y27632, blebbistatin, HA1100, RKI1447, Thiazovivin, and DMSO control towards DE. The cells were stained for DAPI (blue), FoxA2 (green) and Sox17 (red).
To test if downstream mediators of ROCK also induced DE we tested the effect of blebbistatin, a direct inhibitor of myosin II activity on mESCs. Interestingly, blebbistatin could also induce DE differentiation in a similar way compared to the Wnt3a/AA positive control (Figure 4F–H). The most prominent effect on endoderm differentiation was observed with the two potent ROCK inhibitors Thiazovivin and RKI-1447, which inhibit ROCK activity at 0.5 nM and 14.5 μM, respectively. Treatment with these small compounds significantly increased the amount of FoxA2+ cells in comparison to the Wnt3a/AA (Figure 4F). However, FoxA2 protein levels in cell lysate of differentiated cells were similar between all compounds and Wnt3a/AA (Figure 4G). Interestingly, we observed a slight induction in Sox17 levels in the differentiated cells using the compounds compared to Wnt3a/AA, suggesting better induction of DE after the treatment of mESCs with these compounds (Figure 4G). As blebbistatin, a modulator of actin cytoskeleton could induce DE equally; we speculated that the ROCKi used in this experiments can induce DE by modulation of the actin cytoskeleton triggered by ROCK or myosin II inhibition.
3.5. Fasudil directs the differentiation of human embryonic stem cells towards ADE
As the differentiation between mESCs and hESCs differ due to the state of pluripotency (naïve vs primed), we also tested the effect of Fasudil and RKI-1447 on hESC differentiation. To test the variability of WNT3A/AA induced DE differentiation, we analyzed all the differentiations together based on the FOXA2 expression. The variability in differentiation efficiencies is comparatively less in Fasudil and RKI-1447 cells compared to WNT3A/AA treated cells (Figure 5A). This can be ascribed to the variation of biological activities between different batches of recombinant proteins compared to small molecules. Based on the EC50 values of the drugs on the mESCs, we treated the hESCs with 3 μM of Fasudil and RKI-1447 for 4 days to induce DE. However, we could not see any induction of DE in hESCs (data not shown). Based on already published data, we decided to use ROCKis in the range of 10 μM for the induction of DE. FACS analysis of H9 hESC line treated with 10 μM of Fasudil and 10 μM RKI-1447 indicated significant increase in the percentage of DE cells when compared to undifferentiated controls (Figure 5B). Moreover, the percentage of FOXA2+ and SOX17+ cells induced by Fasudil and RKI-1447 was similar to WNT3A/AA (Figure 5C). Finally, mRNA quantification of hESCs treated with Fasudil and RKI-1447 revealed significantly higher induction of FOXA2 and SOX17 compared to WNT3A/AA controls (Figure 5D–E), indicating that prolonged exposure to ROCKi can effectively induce DE in human pluripotent stem cells.
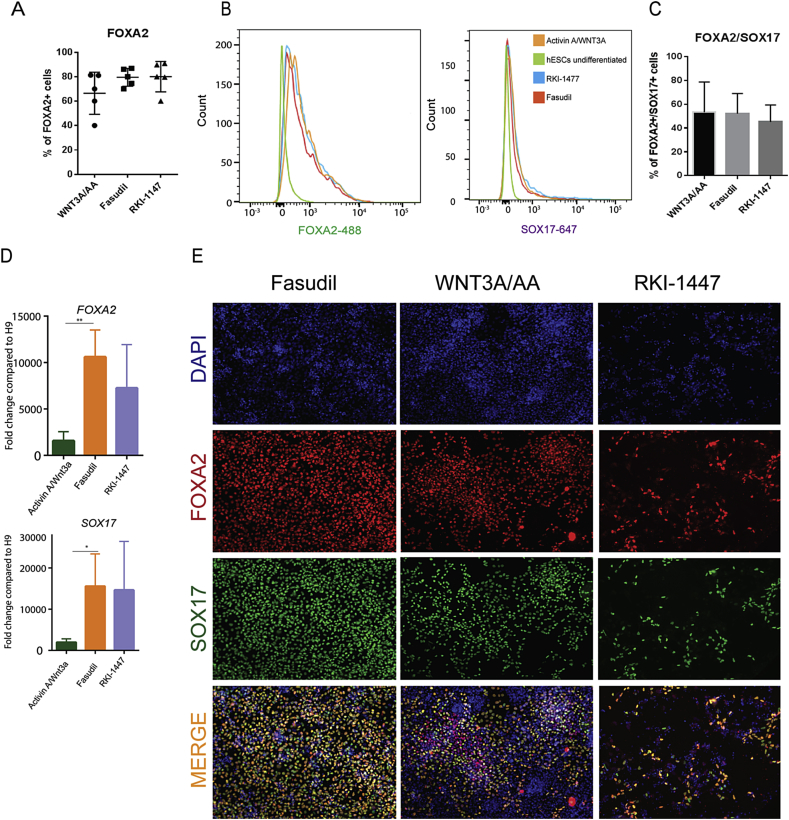
Figure 5.
Rock inhibitors Fasudil and RKI-1447 induce endoderm differentiation of hESCs. (A) Graphical quantification of FACS plots showing the variability in WNT3A/AA differentiation induced in hESCs compared to the differentiations induced by the treatment of Fasudil and RKI-1447. (B) FACS plots showing the differentiation of hESCs towards FOXA2 and SOX17. The differentiation towards DE is assessed by the analysis of FOXA2-488 and SOX17-647 conjugated antibodies. (C) Graphical representation of FACS data showing the percentage of FOXA2+/SOX17 + cells. (D) qPCR quantification of ROCKi treated cells showing similar expression of FOXA2 and SOX17 compared to WNT3A/AA. (E) Representative stainings of H9 treated with Fasudil, RKI-1447, and WNT3A/AA showing the expression of FOXA2 (red) and SOX17 (green).
3.6. ROCK inhibition promotes the differentiation of hESCs towards pancreatic progenitor stage
The main goal of the screen was to identify small molecules that can not only induce DE but can also efficiently give rise to pancreatic progenitors. To assess the quality of the DE induced by ROCKi, we decided to test its differentiation potential towards pancreatic progenitors. After initial differentiation towards the DE using ROCK inhibitors Fasudil and RKI-1447, the protocol was switched to the recently published pancreatic progenitor differentiation protocol [5]. After 8 days of differentiation, the cells treated with 10 μM Fasudil and RKI-1447 induce comparable levels of the pancreatic progenitor marker PDX1 when paralleled to control WNT3A/AA treatment in qPCR analysis (Figure 6B). This was confirmed by FACS analysis, which revealed that the PDX1 levels were slightly lower in Fasudil (80%) and RKI-1447 (85%), when compared to WNT3A/AA treatment (Figure 6E–H). Notably, both ROCKi-treated cultures showed a PDX1low population, suggesting that pancreatic differentiation was efficiently induced but slightly delayed (Figure 6I). We also analyzed the expression of FOXA2 that is expressed throughout endoderm induction and endoderm-derived organ formation in ROCKi-treated cells [15]. The expression of FOXA2 was significantly elevated in Fasudil and RKI-1447 treated cells compared to WNT3A/AA treated cells (Figure 6C). Interestingly, we observed significantly lower expression of the liver markers AFP and TTR in Fasudil and RKI-1447 treated cells in comparison to WNT3A/AA treated cells (Figure 6D). Taken together, these results suggest that ROCKi can direct the differentiation of DE towards pancreatic progenitors.
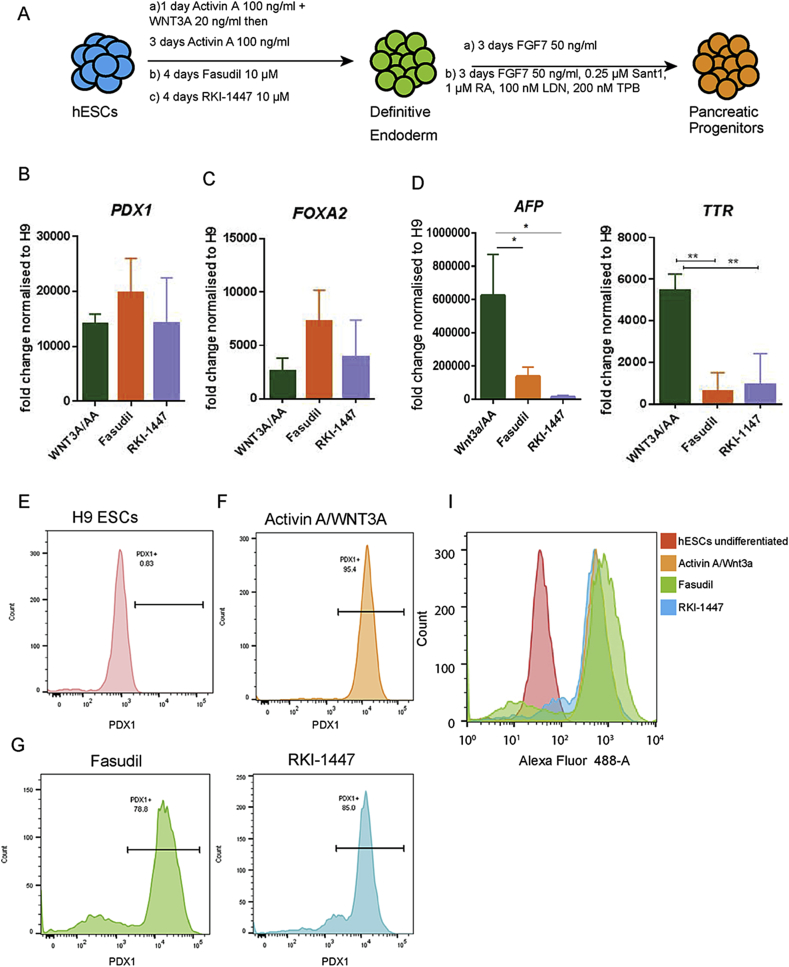
Figure 6.
Rock inhibitors Fasudil and RKI-1447 induce pancreatic progenitor differentiation. (A) Schematic representation of the experimental procedure for pancreatic differentiation. (B–C) Fasudil and RKI-1447 induced DE successfully differentiates into PDX1 and FOXA2 pancreatic progenitors. (D) Liver progenitor markers, AFP, TTR expression is significantly reduced in Fasudil treated cells compared to WNT3A/AA treatment. (E–H) Individual FACS profiles of the cells treated with WNT3A/AA, Fasudil and RKI-1447. (I) FACS plots comparing the differentiation efficiencies towards the PDX1; Fasudil (green) RKI-1447 (blue) and WNT3A/AA (orange) mean ± SDV, ***P < 0.0005.
4. Discussion
The efficient differentiation of ESCs into definitive endoderm is a crucial step in generating organ progenitors for the thymus, thyroid, lung, liver, pancreas, and gastrointestinal tract. Directed differentiation and expansion of these DE progenitors to generate terminally differentiated organ cell types is essential to treat diseases such as chronic liver disease or diabetes mellitus. Most current protocols use recombinant proteins such as WNT3A and AA to induce DE cells from hPSCs. However, to introduce xeno-free conditions in human differentiation culture, recent publications have introduced a plethora of small molecules targeting WNT3A and TGF-β pathways [4], [5], [14]. In mouse, the gradients of Tgf-β/Wnt3a and Bmp4 are known to regulate DE formation and patterning [27]. A recently published protocol on generation of pancreatic β cells from hESCs used GDF8; an ALK inhibitor instead of AA and MCX-928; a GSK3β inhibitor instead of recombinant WNT3A [5]. Although these compounds activate the same signaling pathways, the different concentrations and durations used in published protocols may result in variable endoderm patterning and induction of organ progenitors generated in vitro. Thus, we introduce a novel family of ROCK inhibitors that can effectively induce DE in mESCs and hESCs by modulating the cytoskeleton of the PSCs. One advantage of using ROCKi over recombinant proteins can be stable induction of FOXA2- and SOX17-mediated endoderm patterning. The increased use of stable and defined small molecules in the process of PSC differentiation can help in simplifying and devising robust differentiation protocols.
We report two potent ROCK inhibitors, Fasudil and RKI-1447 that can direct mESCs differentiation in vitro towards ADE. Both the compounds were also equally effective in directing the differentiation of hESCs into DE. In mESCs, at a concentration of 3 μM Fasudil and RKI-1447 induced DE in absence of LIF. In hESCs, slightly higher concentrations (10 μM) of these compounds were found to induce differentiation. Another ROCK inhibitor, Y-27632, at 10 μM is used to prevent dissociation induced apoptosis during plating of hESCs in particular [28]. It is reported that treatment of hESCs with Y-27632 inhibits ROCK1/2, thereby suppressing an epithelial-to-mesenchymal transition through inhibition of key ligands of TGFβ1, GDF6, and WNT signaling [29]. Therefore, we treated hESCs with prolonged exposure of 10 μM of ROCKis Fasudil and RKI-1447. The extended treatment using 10 μM ROCK inhibitors for a period of 4 days leads to formation of DE in hESCs. The efficiency of differentiation in both mESCs and hESC systems was equally comparable to the classical Wnt3A/AA induction. This differentiation towards DE/ADE can be attributed to ROCK inhibition.
It has been already reported that ROCKi's are known to alter the actin cytoskeleton of the cells. This affects remodeling of the actin cytoskeleton, thereby provoking differentiation in these cells [26]. The changes induce the differentiation process in the pluripotent cells by activating the PI3Kinase/AKT activity [30]. Also, it has been reported that ROCK inhibitor Y-27632 promotes activation of AKT [31]. Apart from the changes in cytoskeleton, there are several mechanisms by which the ROCK inhibitors RKI-1447 and Fasudil could facilitate differentiation in ESCs of mouse and human origin. The two widely described mechanisms are the effects of ROCK inhibition on β-catenin translocation and TGF-β pathway activity. A discovery in zebrafish embryos revealed that ROCK2 inhibits mesoderm formation through the control of the TGFβ pathway [32]. Inhibition of ROCK in embryonal carcinoma stem cells has shown to induce mesoderm and neuro-ectodermal lineages by blocking endoderm lineage formation [26]. However, in both the studies the concentration of the ROCK inhibitors used is more than 10 μM. At higher concentrations of ROCK inhibitors we have also seen the formation of mesoderm (data not shown). This can be due to the lack of subcellular translocation of β-catenin from the cell junctions to the nucleus, thereby blocking endoderm formation [26]. In regards to the results of this study, it would be interesting to know if high levels of ROCK inhibition have similar influences on the TGFβ pathway in hESCs and whether the used ROCK inhibitors, especially RKI-1447 would influence this regulation.
Furthermore, we tested the differentiation potential of the chemically-induced endoderm towards pancreatic progenitor cells [5]. Fasudil and RKI-1447-induced ADE efficiently generated PDX1+ pancreatic progenitors. When compared to the standard WNT3A/AA conditions, the efficiency of Fasudil and RKI-1447-induced PDX1+ cells was similar. Previous data have suggested that Rho-ROCK signaling regulates Hippo signaling pathway that, in turn, plays pivotal roles in cell lineage specification of the mouse blastocyst [33]. The major components of Hippo signaling, YAP-TEAD proteins, are also known to regulate pancreatic lineages during development [34]. A study on the enhancer network of human embryonic pancreatic progenitors recently has shown the presence of YAP in the nucleus of the hESCs. Upon differentiation of exocrine and endocrine pancreatic lineages, the nuclear localization of YAP is abolished, suggesting the activation of Hippo pathway in these cells [34]. Therefore, it is tempting to assume that the alteration of the actin cytoskeleton due to the administration of ROCK inhibitors might induce the Hippo pathway driving the differentiation of ADE cells towards the pancreatic lineage.
Thus, differentiation using ROCK inhibitors can play an important role in early lineage formation and specification of stem cells towards the DE and pancreatic lineage. The addition of single small molecules to current differentiation protocols can further simplify the existing ones and make them more robust for future GMP-production of cell products.
Author contribution
AK and PUM designed and executed experiments, analyzed the data, and wrote the manuscript. HL designed the experiments and wrote the manuscript. OK designed, executed, and analyzed high content experiments. PG and OK wrote the high throughput part of the manuscript.
Acknowledgements
A.K and P.M are supported by a grant from BMBF: European Screening Port (FKZ:0315023) and HumEN consortitum (602889) under European Stem Cell Network respectively. The high content image analysis script development and the high throughput screen was performed at the Department of Fraunhofer-Institut für Molekularbiologie und Angewandte Ökologie IME, ScreeningPort, Hamburg Germany. The assay development was done at the Department of Assay Development and Screening Platform, Helmholtz Zentrum München, Germany. The study was supported by grants from: HumEN consortitum of the European Union (602889) and a BMBF: European drug Screening port (ESP: FKZ: 0315023) grant to support academic drug screening projects. We would like to acknowledge the excellent assistance provided by Francoise Halley and Roderick Porter for the screening and medicinal chemistry analyses, respectively. The authors will like to thank Dr. Mostafa Bakhti, Donna Marie Thompson and Noel Moya Betancourt for through reading of the manuscript.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.04.009.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnology. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 2.Pan F.C., Wright C. Pancreas organogenesis: from bud to plexus to gland. Developmental Dynamics. 2011;240(3):530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 3.Pagliuca F.W., Melton D.A. How to make a functional β-cell. Development. 2013;140(12):2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagliuca Felicia W., Millman Jeffrey R., Gürtler M., Segel M., Van Dervort A., Ryu Jennifer H. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 6.Kubo A., Shinozaki K., Shannon J.M., Kouskoff V., Kennedy M., Woo S. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131(7):1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 7.McLean A.B., D'Amour K.A., Jones K.L., Krishnamoorthy M., Kulik M.J., Reynolds D.M. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25(1):29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 8.D'Amour K.A., Agulnick A.D., Eliazer S., Kelly O.G., Kroon E., Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnology. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 9.Yasunaga M., Tada S., Torikai-Nishikawa S., Nakano Y., Okada M., Jakt L.M. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nature Biotechnology. 2005;23(12):1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 10.Arnold S.J., Robertson E.J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nature Reviews Molecular Cell Biology. 2009;10(2):91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 11.Borowiak M., Maehr R., Chen S., Chen A.E., Tang W., Fox J.L. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4(4):348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S., Borowiak M., Fox J.L., Maehr R., Osafune K., Davidow L. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chemical Biology. 2009;5(4):258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 13.Russ H.A., Parent A.V., Ringler J.J., Hennings T.G., Nair G.G., Shveygert M. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. The EMBO Journal. 2015;34(13):1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nostro M.C., Sarangi F., Yang C., Holland A., Elefanty Andrew G., Stanley Edouard G. Efficient generation of NKX6-1(+) pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports. 2015;4(4):591–604. doi: 10.1016/j.stemcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burtscher I., Lickert H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development. 2009;136(6):1029–1038. doi: 10.1242/dev.028415. [DOI] [PubMed] [Google Scholar]
- 16.Baell J.B., Holloway G.A. New substructure filters for removal of Pan Assay Interference Compounds (PAINS) from screening libraries and for their exclusion in bioassays. Journal of Medicinal Chemistry. 2010;53(7):2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita K., Kotani Y., Nakajima Y., Shimazawa M., Yoshimura S.-i., Nakashima S. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Research. 2007;1154:215–224. doi: 10.1016/j.brainres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Claassen D.A., Desler M.M., Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Molecular Reproduction and Development. 2009;76(8):722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niakan K.K., Ji H., Maehr R., Vokes S.A., Rodolfa K.T., Sherwood R.I. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes & Development. 2010;24(3):312–326. doi: 10.1101/gad.1833510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spence J.R., Lange A.W., Lin S.-C.J., Kaestner K.H., Lowy A.M., Kim I. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Developmental Cell. 2009;17(1):62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Asakura M., Inoue H., Nakamura T., Sano M., Niu Z. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(10):3859–3864. doi: 10.1073/pnas.0609100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D.H., Chung H.M. Differentiation into endoderm lineage: pancreatic differentiation from embryonic stem cells. International Journal of Stem Cells. 2011;4(1):35–42. doi: 10.15283/ijsc.2011.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inman K.E., Downs K.M. Localization of Brachyury (T) in embryonic and extraembryonic tissues during mouse gastrulation. Gene Expression Patterns. 2006;6(8):783–793. doi: 10.1016/j.modgep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 25.Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285(5429):895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 26.Krawetz R.J., Taiani J., Greene A., Kelly G.M., Rancourt D.E. Inhibition of Rho kinase regulates specification of early differentiation events in P19 embryonal carcinoma stem cells. PLoS One. 2011;6(11):e26484. doi: 10.1371/journal.pone.0026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorn A.M., Wells J.M. Vertebrate endoderm development and organ formation. Annual Review of Cell and Developmental Biology. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 29.Croze R.H., Buchholz D.E., Radeke M.J., Thi W.J., Hu Q., Coffey P.J. ROCK inhibition extends passage of pluripotent stem cell-derived retinal pigmented epithelium. Stem Cells Translational Medicine. 2014;3(9):1066–1078. doi: 10.5966/sctm.2014-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo H.J., Choi D.-K., Lim J.S., Park J.-S., Lee S.-H., Song S. ROCK suppression promotes differentiation and expansion of endothelial cells from embryonic stem cell–derived Flk1+ mesodermal precursor cells. Blood. 2012;120(13):2733–2744. doi: 10.1182/blood-2012-04-421610. [DOI] [PubMed] [Google Scholar]
- 31.Aly H., Rohatgi N., Marshall C.A., Grossenheider T.C., Miyoshi H., Stappenbeck T.S. A novel strategy to increase the proliferative potential of adult human beta-cells while maintaining their differentiated phenotype. PLoS One. 2013;8(6):e66131. doi: 10.1371/journal.pone.0066131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Li X., Qi J., Wang J., Liu X., Zhang H. Rock2 controls TGFβ signaling and inhibits mesoderm induction in zebrafish embryos. Journal of Cell Science. 2009;122(13):2197–2207. doi: 10.1242/jcs.040659. [DOI] [PubMed] [Google Scholar]
- 33.Kono K., Tamashiro D.A.A., Alarcon V.B. Inhibition of RHO–ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Developmental Biology. 2014;394(1):142–155. doi: 10.1016/j.ydbio.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cebola I., Rodriguez-Segui S.A., Cho C.H.H., Bessa J., Rovira M., Luengo M. TEAD and YAP regulate the enhancer network of human embryonic pancreatic progenitors. Nature Cell Biology. 2015;17(5):615–626. doi: 10.1038/ncb3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.