Abstract
Objective
The prevalence of obesity and related co-morbidities is reaching pandemic proportions. Today, the most effective obesity treatments are glucagon-like peptide 1 (GLP-1) analogs and bariatric surgery. Interestingly, both intervention paradigms have been associated with adaptive growth responses in the gut; however, intestinotrophic mechanisms associated with or secondary to medical or surgical obesity therapies are poorly understood. Therefore, the objective of this study was to assess the local basal endogenous and pharmacological intestinotrophic effects of glucagon-like peptides and bariatric surgery in mice.
Methods
We used in situ hybridization to provide a detailed and comparative anatomical map of the local distribution of GLP-1 receptor (Glp1r), GLP-2 receptor (Glp2r), and preproglucagon (Gcg) mRNA expression throughout the mouse gastrointestinal tract. Gut development in GLP-1R-, GLP-2R-, or GCG-deficient mice was compared to their corresponding wild-type controls, and intestinotrophic effects of GLP-1 and GLP-2 analogs were assessed in wild-type mice. Lastly, gut volume was determined in a mouse model of vertical sleeve gastrectomy (VSG).
Results
Comparison of Glp1r, Glp2r, and Gcg mRNA expression indicated a widespread, but distinct, distribution of these three transcripts throughout all compartments of the mouse gastrointestinal tract. While mice null for Glp1r or Gcg showed normal intestinal morphology, Glp2r−/− mice exhibited a slight reduction in small intestinal mucosa volume. Pharmacological treatment with GLP-1 and GLP-2 analogs significantly increased gut volume. In contrast, VSG surgery had no effect on intestinal morphology.
Conclusion
The present study indicates that the endogenous preproglucagon system, exemplified by the entire GCG gene and the receptors for GLP-1 and GLP-2, does not play a major role in normal gut development in the mouse. Furthermore, elevation in local intestinal and circulating levels of GLP-1 and GLP-2 achieved after VSG has limited impact on intestinal morphometry. Hence, although exogenous treatment with GLP-1 and GLP-2 analogs enhances gut growth, the contributions of endogenously-secreted GLP-1 and GLP-2 to gut growth may be more modest and highly context-dependent.
Keywords: GLP-1R, GLP-2R, GCG, Preproglucagon, Intestinal volume, Obesity, Diabetes, Bariatric surgery
Highlights
-
•
The preproglucagon system does not play an essential role in normal gut development in mice.
-
•
Pharmacological treatment with GLP-1 and GLP-2 analogs show potent intestinotrophic effects.
-
•
VSG-operation, in contrast to RYGB, displays normal gut mucosal and submucosal tissue volume.
1. Introduction
Obesity and type-2 diabetes (T2D) represent increasing health and socio-economic problems worldwide [1]. The currently most effective pharmacological treatments for obesity include peptides stimulating GLP-1 receptor (GLP-1R) function [2], [3], while dual agonists for the GLP-1R and other mechanisms targeting anorexigenic receptors are in development [4]. Furthermore, bariatric surgery has become increasingly attractive providing a significant, rapid, and sustainable weight loss with several positive effects on related morbidities, including resolution of T2D. Although Roux-en-Y gastric bypass (RYGB) has historically been the standard bariatric surgery method, less invasive procedures, e.g. vertical sleeve gastrectomy (VSG), have comparable beneficial metabolic outcomes with reduced perioperative complications, which explains why VSG is now the fastest-growing weight loss surgery option for the treatment of obesity [5]. The underlying molecular mechanisms leading to these marked metabolic effects are not fully elucidated, but several lines of evidence support an important role for nutrient-stimulated gut hormones, such as GLP-1 and GLP-2 [6], [7], [8].
High circulating levels of GLP-1 and GLP-2 have been linked to development of gut hypertrophy following RYGB [9], [10], [11], [12]. The rise in GLP-1 and GLP-2 could provide a positive feedforward mechanism rendering the enlarged intestine more predisposed towards glucose disposal [13], [14] and the release of a plethora of gut hormones with additional metabolic implications. GLP-1 and GLP-2 are co-secreted from enteroendocrine L cells in the gut and released into the circulation following enzymatic cleavage of the common prohormone proglucagon [15] see reviews [16], [17]. While GLP-1 is mainly known for its metabolic effects; i.e. the increase in pancreatic glucose-dependent insulin secretion (the incretin effect), regulation of glucose flux, inhibition of gastric emptying, and reduction of appetite [18], [19], GLP-2 is primarily known for its direct actions on the gut. Accordingly, peripheral administration of GLP-2 exerts potent intestinotrophic effects by increasing mesenteric blood flow and stimulating epithelial proliferation and may constitute the molecular link between nutritional status and commensurate adaptation of mucosal absorptive surface area [9], [20], [21], [22], [23]. Furthermore, GLP-2 reduces gastric emptying and gastric secretion and exhibits anti-inflammatory properties in the intestinal mucosa [24], [25], [26], [27], [28]. A growth-regulating role of GLP-1 has also been reported [29], demonstrating that activity of GLP-1R controls mucosal expansion in both the small and large intestine. However, even though the actions of these peptides are well described, the anatomical distribution and functional implications of the endogenous proglucagon system for normal gut growth and development remains poorly understood.
Traditionally, the ileum and colon have been identified as the primary sites containing the majority of the proglucagon expressing L-cell populations in rat, pig, dog, primate, and man [30], [31], [32], [33], [34]. This general assumption focused greater attention on studies of proglucagon-related biology in the distal gut, with limited analysis of the full gastrointestinal tract. The GLP-1R is known to be expressed in several tissues, including brain, gastrointestinal tract, pancreatic islets, kidney, heart, and lung [35], [36], [37], [38]. However, cellular localization of GLP-1R expression is confounded by the lack of validated, specific antibodies [39], [40], [41]. Similarly, GLP-2 receptor (GLP-2R) expression has been reported previously in gastrointestinal tract, mesenteric lymph nodes, fat, spleen, bladder, and hepatocytes, as well as in the central nervous system [24], [42], [43], [44], [45]. However, the exact local distribution of intestinal GLP-2Rs is disputed and thus remains unresolved [44], [45], [46], [47], [48].
Given the current limitations in our understanding of the localization of GLP-1R and GLP-2R expression in the gut, we aimed to provide a detailed map of Glp1r, Glp2r, and Gcg mRNA expression throughout the complete rostral–caudal axis of the mouse gastrointestinal tract. To gain further insight into the functional relevance of the endogenous GLP-1 and GLP-2 system on intestinal growth, we characterized intestinal volumes in Glp1r−/−, Glp2r−/−, and Gcg−/− mice in comparison to corresponding wild-type littermate controls. Furthermore, since bariatric surgery represents a valuable tool for studying the role of these peptide hormones in intestinal adaptation, we performed a detailed study of intestinal volume in a mouse model of VSG surgery.
2. Materials and methods
2.1. Animals
All animal experiments were approved by the Danish Committee for Animal Research under the personal license of Jacob Jelsing (2015-15-0201-00518) using internationally accepted principles for the use of laboratory animals. All animals were housed in a light-, temperature-, and humidity-controlled room (12-hour light:12-hour dark cycle, lights on/off at 4AM/4PM hour; 22 ± 1 °C; 50 ± 10% relative humidity) and offered domestic quality tap water. Mice bred in Toronto were cared for in accordance with animal protocols approved by the Animal Care Committee, Toronto Centre for Phenogenomics, Mt. Sinai Hospital.
2.2. Compounds
The GLP-1 analog liraglutide was acquired commercially (Hørsholm Pharmacy). Native GLP-1, native GLP-2, and the GLP-2 analog teduglutide were prepared by automated solid-phase peptide synthesis (SPPS) using the Fmoc/tBu strategy on pre-loaded PHB TentaGel resin (Rapp polymere GmbH, Tuebingen, Germany). The couplings were performed using Fmoc-Nα-protected amino acids, N,N-diisopropylcarbodiimide and ethyl cyanoglyoxylate-2-oxime (oxyma) in N,N-dimethylformamide (Iris Biotech GmbH, Marktredwitz, Germany) for 2 × 2 h. The N-deprotections were performed using 40% piperidine in N-methyl-2-pyrrolidione (Iris Biotech GmbH, Marktredwitz, Germany) for 3 min followed by 20% piperidine in N-methyl-2-pyrrolidione for 17 min. Finally, the peptide was simultaneously side-chain deprotected and released from the solid support by a TFA cocktail containing trifluoro acetic acid (TFA) (Iris Biotech GmbH, Marktredwitz, Germany), triethylsilane (Sigma–Aldrich, Brøndby, Denmark), and H2O (95/2.5/2.5) as scavengers for 2 h. The peptide was precipitated by the addition of diethylether (Sigma–Aldrich, Brøndby, Denmark). The peptide was purified by RP-HPLC and identified by LC-MS. The final products were obtained with >95% purity.
2.3. Sub-chronic treatment in C57BL/6J mice
C57BL/6J mice (Janvier Labs, Saint Berthevin, Cedex, France), 8 weeks of age, were fed a regular chow diet (Altromin 1324, Brogaarden A/S, Denmark). Mice were randomized according to body weight into four individual study groups (n = 10 per group): Group 1: Vehicle (SC, BID), Group 2: liraglutide (0.2 mg/kg, SC, BID), Group 3: teduglutide (1 mg/kg, SC, BID), Group 4: liraglutide (0.2 mg/kg, SC, BID) + teduglutide (1 mg/kg, SC, BID). Compounds were dissolved in PBS buffer containing 3% mannitol and 0.6% L-His (pH 9.0), and dosing volume was 5 ml/kg. On day 8, animals were fasted for 4 h before being sacrificed during the light phase. The intestines were collected, and the length of the small and large intestine was measured. Intestines were cleaned by flushing with saline and finally the weight was measured. Intestines were placed in 10% natural buffered formalin until further processing. For description of mice treated with native GLP-1 and GLP-2, see supplementary information.
2.4. Histology and stereology
The gut was dissected into small and large intestine, and the lengths were measured. The intestine was sampled using systematic uniform random sampling (SURS) principles, providing a minimum of 8 systematically placed biopsies from both small and large intestine. All biopsies were embedded in blocks of paraffin enabling later identification of individual biopsies. Paraffin blocks were sectioned into 5 μm thick sections and stained with hematoxylin-eosin for subsequent stereology-based volume estimations. Stereological volume estimations were performed by point-counting on digitally scanned slides using the newCAST system (Visiopharm, Denmark) [49], [50], [51]. For studies involving double KO (Glp1r−/−:Glp2r−/−) mice or mice treated with native GLP-1 and GLP-2 peptides, weights of saline-flushed intestines were used (see supplementary information).
2.5. In situ hybridization (ISH)
Single-cell ISH was performed on paraffin-embedded intestinal tissue biopsies from two C57BL/6J mice using the RNAscope 2.5 HD – RED Assay (Advanced Cell Diagnostics) to visualize cellular mRNA using specific probes directed against selected genes. Slides with tissue biopsies were treated according to RNAscope 2.5 HD – RED Assay user manual. In brief, tissue sections were pretreated, including target retrieval, hydrogen peroxide treatment, and protease treatment. Then, the specific probe was hybridized to the mRNA target, and the signal was amplified and visualized using Fast Red substrate. A probe against bacterial dapB mRNA was used as negative control, whereas a mouse probe against Ppib was used as positive control. Custom-made specific probes against Glp1r (REF418851), Glp2r (REF447061), and Gcg (REF400601) mRNA were employed on sections covering the entire gastro-intestinal tract (glandular and non-glandular stomach, duodenum with Brunner's glands, caudal duodenum without Brunner's glands, jejunum, ileum, caecum, and colon, for overview see supplementary information). Following ISH, the slides were counterstained in Gill's hematoxylin and coverslipped. Finally, slides were scanned under a 20× objective in a ScanScope AT slide scanner (Aperio).
2.6. VSG surgery
C57BL/6J mice were made obese by provision of a high-fat diet at least 6 weeks before surgery. In the peri-surgery period (day −3 to 4), the mice in all groups were offered a liquid diet (Osmolite). Mice were randomized based on body weight on day −1 into the following experimental groups: VSG or sham. The VSG procedure was performed as previously described [52]. In brief, mice had ∼80% of the stomach resected along the major curvature and the incision was closed with staples. In sham-operated mice the stomach was taken out of the abdominal cavity and then repositioned without being cut. Pain relief was provided by subcutaneous injections of Metacam (0.25 mg/100 g body weight) from day 0 until day 4 post-surgery. On day 10 post-surgery animals were terminated, and intestines were placed in formalin. For a description of RYGB procedures, see supplementary information.
2.7. Knockout (KO) mice
Glp1r−/− and Glp2r−/− mice and age-matched wild-type (WT) littermates were from the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital (Toronto, Ontario, Canada). Gcg−/− mice and age-matched WT littermates were from the University of Michigan (Ann Arbor, Michigan, US). Single KO mice were generated as described previously [53], [54], [55]. In addition, double KO (Glp1r−/−:Glp2r−/−) mice were generated in Toronto as described in supplementary information.
2.8. Statistics
All data were analyzed using Graph Pad Prism 5.0 software, applying either student's t-test (gut volumes in KO mice vs. WT littermates, and VSG vs. sham controls, respectively) or one-way analysis of variance (ANOVA) with Tukey's post-hoc test (treatment with GLP-1/GLP-2 analogs). Results are presented as mean ± SEM (standard error of the mean). A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Glp1r mRNA expression in the mouse GI tract
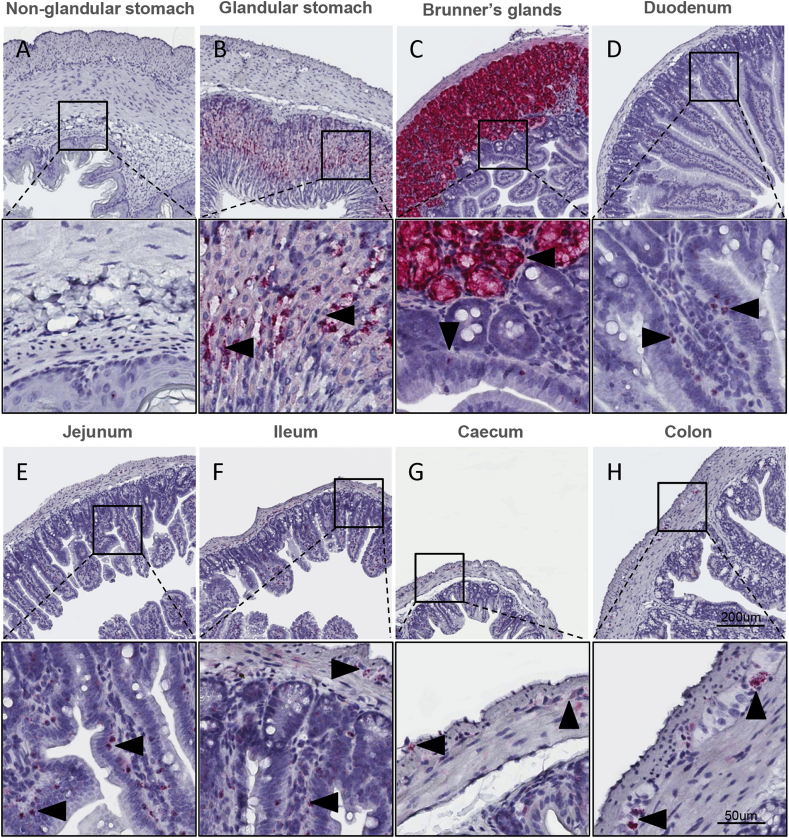
Glp1r expression was virtually absent in the non-glandular stomach (Figure 1A) but highly expressed on gastric parietal cells in the stomach glands (Figure 1B). In the proximal duodenum, Glp1r mRNA transcripts were abundant in the Brunner's glands (Figure 1C) in addition to a low level of expression detected in few scattered cells of the mucosa. In the caudal duodenum, Glp1r expression became more apparent in the mucosa (Figure 1D) with Glp1r-positive cells increasing in density throughout the jejunum (Figure 1E) and in the ileum (Figure 1F). Glp1r expression was also observed in the nerve plexus throughout the glandular stomach, small intestine, and colon (Supplementary Figs. 1A–D). In the caecum, Glp1r expression (Figure 1G) was restricted to the muscular nerve plexus in addition to sporadic expression in the mucosa, whereas in the colon (Figure 1H), Glp1r expression was found predominantly in the nerve plexus with only some expression in the mucosa.
Figure 1.
Expression of Glp1r mRNA in the mouse gastrointestinal tract. Localization of Glp1r mRNA in mouse non-glandular (A), glandular stomach (B), Brunner's glands (C), duodenum (D), jejunum (E), ileum (F), caecum (G) and colon (H) using RNA scope 2.5 in situ hybridization. Inserts magnified below overview.
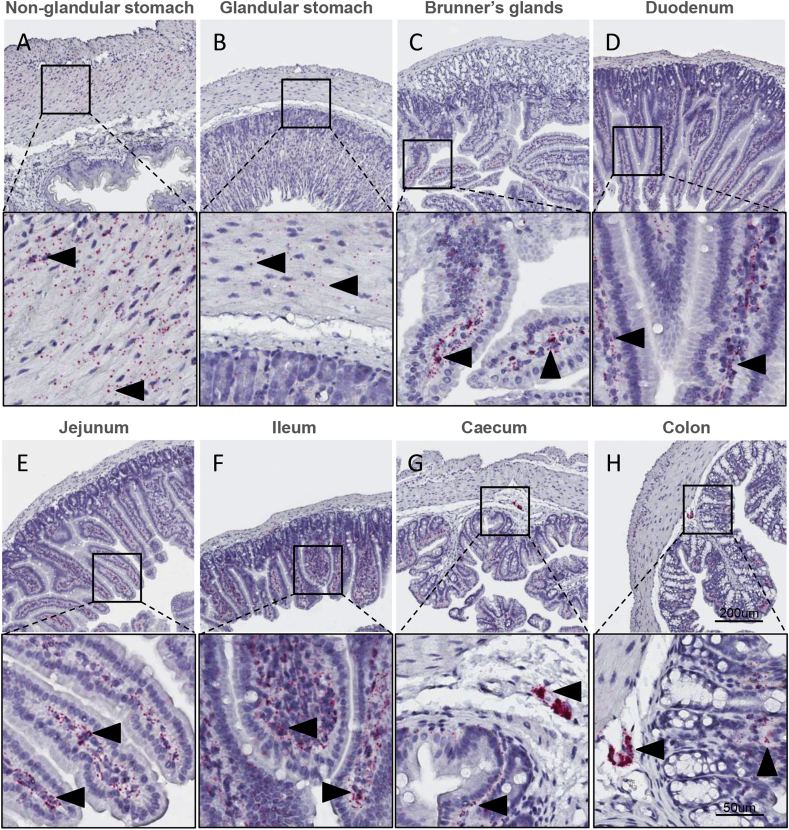
3.2. Glp2r mRNA expression in the mouse GI tract
Glp2r mRNA transcripts were abundant in both the circular and longitudinal muscle layer of the non-glandular (Figure 2A) and less abundantly but still detectable in the glandular part of the stomach (Figure 2B). In contrast to Glp1r, Glp2r was not expressed in the nerve plexuses of the glandular stomach (Supplementary Fig. 1E), nor in the Brunner's glands of duodenum (Figure 2C). Within duodenum, Glp2r was abundantly expressed in the lamina propria of the mucosa layer (Figure 2C), as well as in the circular and longitudinal muscle layers (Supplementary Fig. 1F). A similar cellular expression pattern was observed in the caudal duodenum (Figure 2D) where Glp2r mRNA transcripts were localized to the nerve plexuses (Supplementary Fig. 1G). In the jejunum (Figure 2E) and ileum (Figure 2F), Glp2r was found to be highly expressed in scattered cells within the mucosa and the nerve plexus, with sporadic expression in the muscle cells of the muscularis layer. In the caecum (Figure 2G) and colon (Figure 2H), Glp2r mRNA was expressed in both the mucosa and nerve plexus. In addition to the expression observed in the myenteric plexuses, Glp2r was also detected in submucosal plexuses of the colon (Supplementary Fig. 1H).
Figure 2.
Expression of Glp2r mRNA in the mouse gastrointestinal tract. Localization of Glp2r mRNA in mouse non-glandular (A), glandular stomach (B), Brunner's glands (C), duodenum (D), jejunum (E), ileum (F), caecum (G) and colon (H) using RNA scope 2.5 in situ hybridization. Inserts magnified below overview.
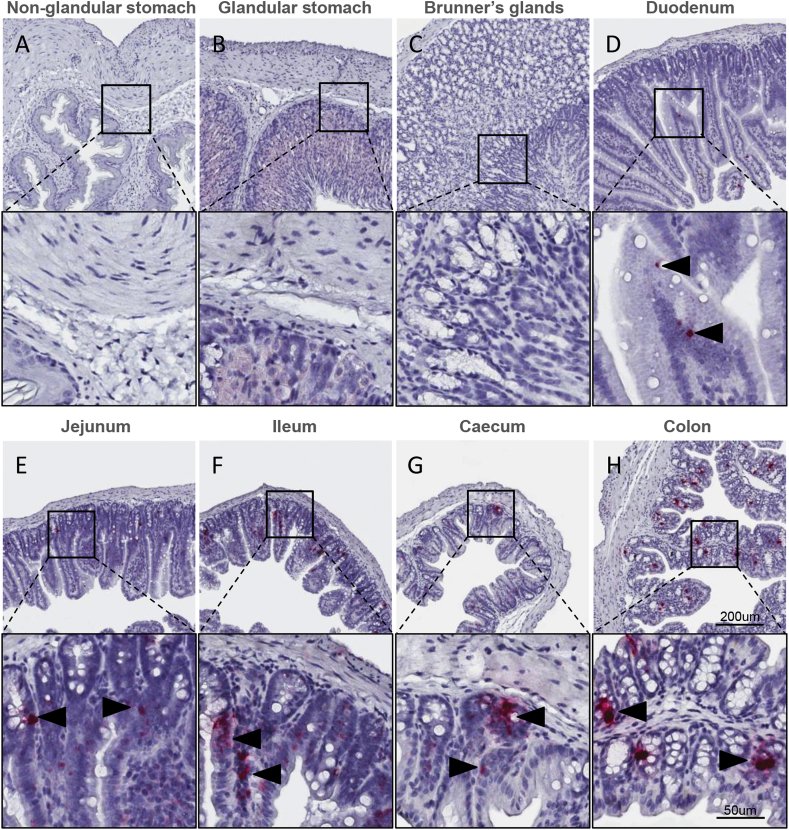
3.3. Gcg mRNA expression in the mouse GI tract
Gcg expression was virtually absent in both the non-glandular stomach (Figure 3A) and the glandular stomach (Figure 3B) as well as in the most proximal part of the small intestine (Figure 3C). A few scattered Gcg-positive cells were detected in the mucosal epithelium in the caudal duodenum (Figure 3D). In the jejunum (Figure 3E), Gcg expression was confined to single cells of the epithelium with an increased density caudally towards the ileum (Figure 3F) and throughout the colon (Figure 3H). Likewise, Gcg expression was found in the cecal mucosa (Figure 3G).
Figure 3.
Expression of Gcg mRNA in the mouse gastrointestinal tract. Localization of Gcg mRNA in mouse non-glandular (A), glandular stomach (B), Brunner's glands (C), duodenum (D), jejunum (E), ileum (F), caecum (G) and colon (H) using RNA scope 2.5 in situ hybridization. Inserts magnified below overview.
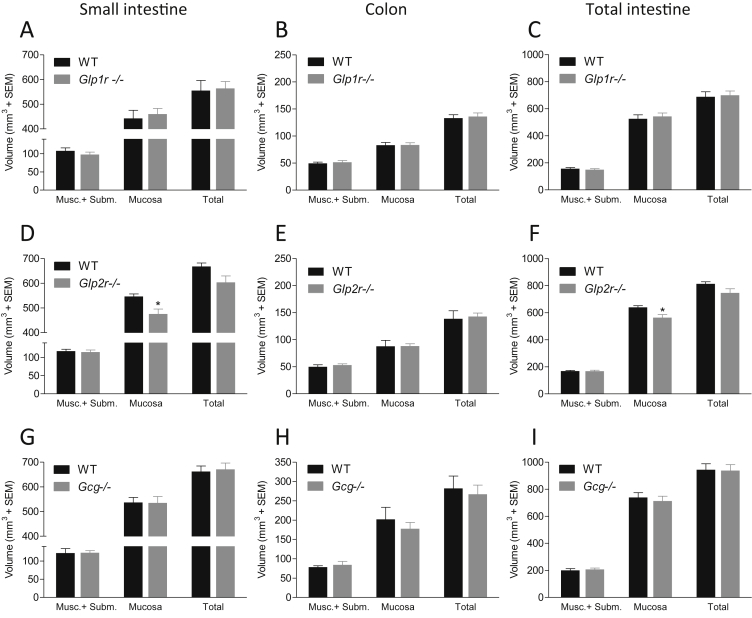
3.4. Analysis of intestinal volume in Glp1r−/−, Glp2r−/− and Gcg−/− mice
Stereological estimates of compartmental gut volumes in Glp1r−/−, Glp2r−/−, and Gcg−/− mice, as compared to WT littermate control mice, are indicated Figure 4. Glp1r−/− mice had similar small intestine, colon, and total intestine volumes, compared to wild-type controls (small intestine, 555 ± 41 mm3 vs. 564 ± 28 mm3, p = 0.86; colon, 133 ± 7 mm3 vs 136 ± 7 mm3, p = 0.95; total intestine, 688 ± 37 mm3 vs 700 ± 31 mm3, p = 0.81), see Figure 4A–C.
Figure 4.
Glp1r−/−and Gcg−/−mice show normal gut development while Glp2r−/−mice displays a slightly reduced mucosal volume. Intestinal volume in Glp1r−/− (A, B and C), Glp2r−/− (D, E and F) and Gcg−/− (G, H and I) mice as estimated by stereology in WT C57BL/6J or KO mice. Small intestine volume in panel A, D and G, colon volume in panel B, E and H and total intestine volume in panel C, F and I. Volume of muscularis + submucosa (Musc. + Subm.) and mucosa layers was measured separately.
In contrast, Glp2r−/− mice exhibited a slight, statistically significant, reduced volume of the mucosal layer of the small intestine (13 ± 3.7%, 547 ± 10 mm3 vs 475 ± 20 mm3, p = 0.01) and total intestine (12 ± 3.6%, 640 ± 12 mm3 vs 564 ± 22 mm3, p = 0.01), (Figure 4D). Total volumes of the small intestine (668 ± 14 mm3 vs 603 ± 26 mm3, p = 0.06) and whole intestine (814 ± 15 mm3 vs. 746 ± 30 mm3, p = 0.09) in Glp2r−/− mice trended lower, while the colon volume was unaltered (139 ± 15 mm3 vs. 143 ± 6 mm3, p = 0.79, see Figure 4E). Glp2r−/− mice exhibited a non-significant increase in volume of immune cells of the small intestine (4.3 ± 0.8 mm3 vs. 9.2 ± 2.1 mm3, p = 0.06), see Supplementary Figs. 2A–C).
Similar to Glp1r−/− mice, Gcg-deficient mice also showed normal gut development, as compared to wild-type controls (Figure 4G–I) (small intestine, 662 ± 23 mm3 vs. 671 ± 26 mm3, p = 0.79; colon, 282 ± 32 mm3 vs. 267 ± 24 mm3, p = 0.71; total intestine, 944 ± 43 mm3 vs. 938 ± 44 mm3, p = 0.92).
As single disruption of either the Glp1r or Glp2r alone might result in compensatory gut growth arising from enhanced activation of the remaining functional receptor, we generated double knock out (DKO) Glp1r−/−:Glp2r−/− mice. A very modest reduction of small intestine weight (14 ± 2.1%, 1215 ± 44 mg vs. 1047 ± 25 mg, p = 0.002), thus also being reflected in total intestine weight (1466 ± 44 mg vs. 1301 ± 28 mg, p = 0.003; see Supplementary Fig. 3A), was observed in Glp1r−/−:Glp2r−/− mice. Colon weight (251 ± 10 mg vs 254 ± 9 mg, p = 0.80) and body weight (41 ± 1.5 g vs 38 ± 1.2 g, p = 0.18) were not different in Glp1r−/−:Glp2r−/− mice (Supplementary Figs. 3A–B).
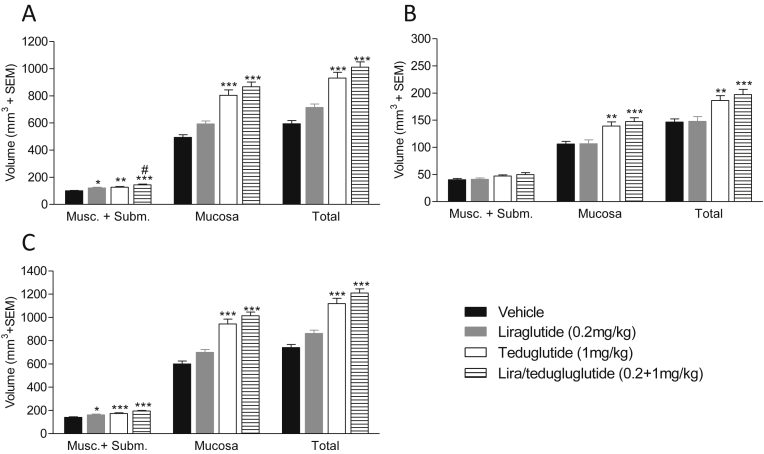
3.5. GLP-1 and GLP-2 analogs increase gut volumes in C57BL/6J mice
The lack of major changes in gut volumes in mice with disruption of the endogenous proglucagon system prompted us to reassess the pharmacological consequences of GLP-1R and GLP-2R agonism in the intestine. The long-acting GLP-1 analog (liraglutide) and GLP-2 analog (teduglutide) were administered individually or in combination to male C57BL/6J mice for 7 days (Figure 5A–C). Liraglutide monotherapy induced a non-significant 20 ± 4.3% increase in total small intestinal volume (p > 0.05, one-way ANOVA). When analyzed by individual t-test, however, small intestine volume was increased significantly by liraglutide compared to vehicle treatment (p = 0.003, students t-test). In contrast, teduglutide treatment led to a significant and robust 57 ± 7.1% increase in small intestine volume (p = 0.001, one-way ANOVA) while combined treatment with liraglutide and teduglutide led to a further increase (70 ± 6.5%, p = 0.001, one-way ANOVA), indicating a nearly additive effect of the drug combination treatment. Similar changes in intestinal volumes were detected in the colon (Figure 5B) but were most apparent in the small intestine, specifically in the mucosa layer (Figure 5A). The increase in small intestine, colon, and total intestine volume was also observed following 7-days treatment with the native GLP-1 and GLP-2 peptides (Supplementary Fig. 4); however, the effect was less pronounced compared to treatment with stable analogs.
Figure 5.
GLP-1 and GLP-2 analogs significantly increase gut volumes in C57BL/6J mice. The effect of liraglutide, teduglutide and co-agonism on small intestine (A), colon (B) and total intestinal volume (C) as estimated by stereology. Volume of muscularis + submucosa (Musc. + Subm.) and mucosa layers was measured separately.
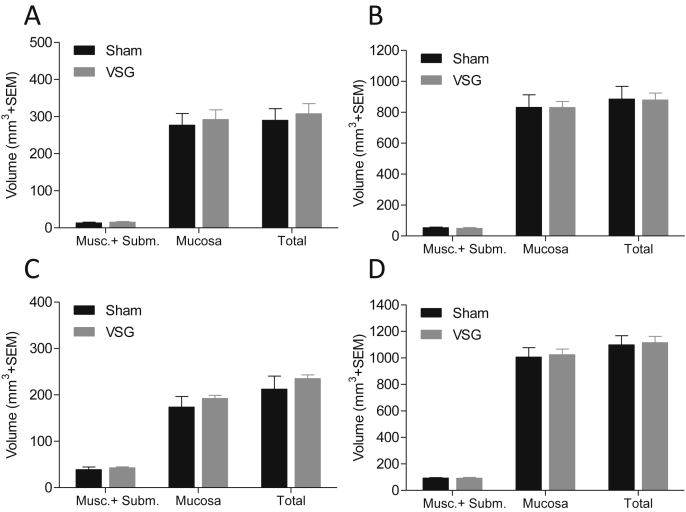
3.6. Intestinal growth after vertical sleeve gastrectomy
VSG led to a significant reduction (14 ± 2.8%, p = 0.002) in body weight as compared to sham-operated control mice (Supplementary Fig. 5A). Similarly, a significant weight loss (7.3 ± 0.9%, p = 0.002) was observed after RYGB surgery in rats (Supplementary Fig. 5B). At the time of termination (10 days post-surgery), stereological assessment of the duodenum (first 5 cm of the small intestine), the total small intestine, colon, and total intestine did not reveal any volume alterations in any segments or layers investigated (duodenum: 290 ± 31 mm3 vs. 308 ± 27 mm3, p = 0.67, total small intestine: 885 ± 82 mm3 vs. 879 ± 44 mm3, p = 0.95, colon: 213 ± 28 mm3 vs. 235 ± 8 mm3, p = 0.46; Figure 6A–D). In contrast, RYGB surgery led to a marked increase in alimentary limb area in the rat (Supplementary Figs. 6A–D).
Figure 6.
Mice subjected to VSG surgery do not display increased gut volume. The effect of VSG or sham surgery on duodenum (A), total small intestine (B), colon (C) and total intestine (D) volume as estimated by stereology. Volume of muscularis + submucosa (Musc. + Subm.) and mucosa layers was measured separately.
4. Discussion
By use of highly sensitive in situ hybridization techniques, we here report a widespread and distinct expression pattern of Glp1r, Glp2r, and Gcg mRNAs throughout the mouse gastrointestinal tract. As previously demonstrated in rodents, monkey, and man [39], [56], we confirm that Glp1r mRNA is expressed in gastric parietal cells in mice, with a weak staining in smooth muscle cells and stomach mucosal structures. This distinct receptor expression corresponds well with GLP-1's inhibitory effects on gastric acid production and gastric emptying [18], [19]. In addition, we confirm Glp1r to be highly expressed in Brunner's glands of the proximal duodenum [36], [57], consistent with a role for GLP-1 in the regulation of intestinal mucin production [38]. Glp1r was also localized to nerve plexuses of the submucosa and muscularis along the full rostro-caudal extension of the intestinal tract [17], [39], [58]. As a novel finding, we demonstrate with a high cellular resolution Glp1r expression in scattered cells throughout the small intestinal mucosa. The specific phenotype of these cells is currently unknown but consistent with intraepithelial lymphocytes [59]; however, further studies are needed to confirm this assertion. Similar to Glp1r mRNA, Glp2r expression was observed in smooth muscle cells of the gastric muscular layer, supporting a role for the GLP-2R in inhibition of gastric emptying [24], [60]. In contrast, Glp2r mRNA expression was not detected in Brunner's glands within the duodenum. Glp2r mRNA was, however, markedly expressed in the mucosal lamina propria throughout the intestinal tract, consistent with the distribution of subepithelial myofibroblasts [45], [48]. No Glp2r expression was detected in the intestinal epithelium or in proliferating crypt cells. This is in line with several other reports [45], [61] but contrasts with reports of GLP-2R immunoreactivity in the epithelium from rodents, pigs, and humans [44], [46]. Similar to the distribution of Glp1r, Glp2r mRNA expression was abundantly expressed in nerve plexuses of the submucosa and muscularis, suggesting a potential role of these receptors in the enteric nervous system. In agreement with numerous reports [31], [62], [63], we also demonstrated conspicuous Gcg expression in the epithelium from the caudal duodenum and throughout the gut, with an increasing caudal density gradient reaching highest density in the colon [31], [62]. The Gcg mRNA-positive cells were organized in a clear enteroendocrine pattern with minimal luminal contact intercalated between the epithelial cells of both villi and crypts. In contrast, no Gcg expression was observed in the muscularis or enteric nervous system.
To investigate the relevance of basal GLP-1R and GLP-2R signaling in intestinal growth, we used unbiased stereological techniques to estimate intestinal volumes in Glp1r−/−, Glp2r−/−, and Gcg−/− mice. Mice with GLP-1R or GCG deletion had similar intestinal volumes as compared to corresponding wild-type controls. This is in line with previous findings of normal weight and length of the small intestine in Glp1r−/− mice [29]. It should be noted, however, that loss of GLP-1R expression is associated with reduced intestinal polyp growth in genetic models of small bowel hyperplasia [29], possibly suggesting that intrinsic trophic effects of GLP-1R receptors may be more marked under conditions of pathological gut growth. In contrast, GLP-2R deletion resulted in a slight reduction in small intestine mucosa volume, in agreement with previous reports of gut weights in Glp2r−/− mice [54]. The DKO Glp1r−/−:Glp2r−/− mice also displayed only a slight reduction in small intestine weight, indicating no functionally overlapping compensation between the two receptors.
In comparison to the very limited effects of GLP-2R deletion on gut growth in mice, we demonstrate that exogenous administration of a long-acting GLP-2 analog (teduglutide) alone or in combination with a long-acting GLP-1 analog (liraglutide) leads to markedly increased gut mucosal volumes in mice. Although liraglutide monotherapy did not lead to a significant increase in gut volume in the present experiment (when analyzed by one-way ANOVA), others have reported GLP-1R dependent intestinotrophic effects of the GLP-1R agonist exendin-4 [29]. Similarly, the authors demonstrated that exogenous native GLP-1 and GLP-2 promoted additive hypertrophic effects in the gastrointestinal tract of WT mice. In line with this report, we also observed a significant increase in colon weight following 7 days of treatment with high doses of native GLP-1 (3 mg/kg) (Supplementary Fig. 4). The discrepancies in reported efficacies between experiments may be related to specific compound doses and quantification methods. Hence, while Koehler and coworkers [29] reported changes in gut mass as a function of body weight, we used stereology-based histological methods for quantification of total volumes.
In contrast to the less well described intestinal growth-promoting effects of GLP-1, the intestinotrophic effects of GLP-2 are well-established and thought to be mediated via different signaling mechanisms, which involve induction of growth factor signaling pathways, including those associated with IGF-1, FGF7, and ErbB [48], [64], [65], mediating crypt proliferation and decreased apoptosis [20], [66]. Collectively, our data support a model for GLP-2 action via paracrine, or endocrine mechanisms to stimulate mucosal expansion, as we found no Glp2r expression on epithelial cells or crypt stem cells, putative targets for GLP-2 action. In contrast, strong Glp2r expression was detected in other cells types within mucosa and the enteric nervous system. This localization is consistent with the hypothesis that local release of growth factors together with enteric neuronal stimulation, is a more probable explanation for the intestinotrophic action of GLP-2 [67]. As our data indicate a very limited effect of Gcg, Glp1r, or Glp2r deletion in normal gut development, but pharmacological doses of GLP-1/GLP-2 induced intestinal mucosal expansion, these findings suggest that supraphysiological stimulation of GLP-1R/GLP-2R activity is required for evoking intestinal growth.
It has been demonstrated previously that RYGB surgery causes massive hypertrophy of the alimentary limb in both mice, rats, and pigs [68], [69], and also leads to markedly increased circulating levels of GLP-1 and GLP-2 [9], [10], [11], [12], [70]. Similarly, VSG also increases plasma levels of proglucagon-derived peptides, although to levels not as high as those observed after RYGB [71], [72], [73]. Here, we demonstrate that RYGB induces intestinal hypertrophy already at 10-days post-surgery, i.e. before the animals have returned to consumption of a normal diet (Supplementary Figs. 5 and 6). Whether gut hypertrophy, associated with concomitant improved glucose homeostasis and increased gut hormone secretion, contributes to one or more metabolic effects of RYGB surgery remains unclear [10], [14], [70]. VSG also increases postprandial proglucagon-derived peptide levels [10], prompting us to examine the sub-acute effects of VSG surgery in mice with the use of stereological methods. Our histological data indicate similar gut tissue volumes in VSG and sham-operated mice, which is in agreement with similar observations in a rat model of VSG [74]. Although RYGB and VSG have common beneficial metabolic effects, the absence of gut hypertrophy in VSG-treated mice suggests that gut growth and adaptation is not a prerequisite for improved metabolism after bariatric surgery. In addition, our data in single receptor knockout and Gcg−/− mice suggest that basal signaling through the GLP-1R/GLP-2R receptors is dispensable for normal gut growth.
Although we predominantly studied VSG-treated mice, post-prandial plasma GLP-1 (and by inference GLP-2) levels are higher after RYGB as compared to VSG [75]. This effect may potentially be explained by gut hypertrophy-dependent increases in GCG-expressing cells after RYGB [68], which could contribute to increased GLP-1 secretion. There is evidence from human studies that GLP-1 secretion is strongly associated with the rate of glucose appearance in the intestine [76], [77]. Although it remains to be established, VSG-mediated increased gastric emptying or RYGB-induced redirection of intestinal nutrient flow could therefore possibly lead to enhanced L-cell responsiveness to glucose, as well as amino acids and bile acids. Indeed, VSG surgery procedures promote increased gastric emptying rates in rodents [78], [79] as well as in humans [80], [81], [82]. Nevertheless, the VSG procedure maintains delivery of macronutrients within the proximal duodenum which exhibits very few, if any, GCG-expressing cells. In contrast, RYGB surgery will promote delivery of dietary nutrients to more distal parts of the gut which have a relatively higher density of GCG expressing cells. Thus, it cannot be ruled out that the delivery of macronutrients to the more distal parts of the gut following RYGB may induce higher local release of GLP-1/GLP-2, which could further enhance GLP-1R/GLP-2R trophic signaling to promote hypertrophy of the alimentary and common limb. This notion is also supported by the finding of marked gut hypertrophy following ileal interposition surgery, i.e. in a condition where the ileal segment is transposed to more proximal parts of the gut [83].
5. Conclusion
Collectively, our data suggest that endogenous GLP-1R signaling does not play an essential role in intestinal growth homeostasis, whereas loss of the GLP-2R produces modest reductions in gut mucosal volume. In addition, RYGB, but not VSG, surgery induced marked gut hypertrophy, suggesting that surgically-induced modification of macronutrient entry to more distal intestinal regions exhibiting relatively high L-cell density may underlie enhanced release and intestinotrophic effects of GLP-1/GLP-2. Our findings clearly dissociate pharmacological from physiological actions of GLP-1R/GLP-2R signaling on gut tissue expansion, with potential implications for different therapeutic strategies augmenting L cell activity for the treatment of metabolic disorders.
Funding
P.W. receives grant funding from Innovation Fund Denmark. D.A.S. receives grant funding from NIH-NIDDK (DK107282), Novo Nordisk, Sanofi, and Ethicon Endo-surgery. D.J.D is supported by the Canada Research Chairs program, a Banting and Best Diabetes Centre-Novo Nordisk Chair in Incretin Biology, and Canadian Institutes of Health Research grant 123391. The funding sources were not involved in the study design, the collection, analysis and interpretation of data, in the writing of the report or the decision to submit the article for publication.
Author contribution
P.W., P.B., T.S., L.L.B., and J.A.K. performed the experiments and evaluated the data. P.W., H.B.B., D.J.D., D.A.S, P.B.J, N.V., and J.J. made substantial contributions to the study design, evaluation of data and drafted the manuscript.
Acknowledgements
We wish to thank Søren Ljungberg Pedersen for chemical synthesis of native GLP-1, GLP-2 and teduglutide.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.04.007.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Meier J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nature Reviews Endocrinology. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz M., Evers A., Wagner M. Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorganic & Medicinal Chemistry Letters. 2013;23(14):4011–4018. doi: 10.1016/j.bmcl.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Tschöp M.H., Finan B., Clemmensen C., Gelfanov V., Perez-Tilve D., Müller T.D. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metabolism. 2016;24(1):51–62. doi: 10.1016/j.cmet.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Franco J.V.A., Ruiz P.A., Palermo M., Gagner M. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, roux-en-y gastric bypass and adjustable gastric banding. Obesity Surgery. 2011;21(9):1458–1468. doi: 10.1007/s11695-011-0390-5. [DOI] [PubMed] [Google Scholar]
- 6.le Roux C.W., Aylwin S.J.B., Batterham R.L., Borg C.M., Coyle F., Prasad V. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Annals of Surgery. 2006;243(1):108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of Surgery. 2007;246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 8.Laferrère B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2008;93(7):2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.le Roux C.W., Borg C., Wallis K., Vincent R.P., Bueter M., Goodlad R. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Annals of Surgery. 2010;252(1):50–56. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- 10.Chambers A.P., Stefater M.A., Wilson-Perez H.E., Jessen L., Sisley S., Ryan K.K. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiology and Behavior. 2011;105(1):120–123. doi: 10.1016/j.physbeh.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman L.M., Beckman T.R., Sibley S.D., Thomas W., Ikramuddin S., Kellogg T.A. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN. Journal of Parenteral and Enteral Nutrition. 2011;35(2):169–180. doi: 10.1177/0148607110381403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pournaras D.J., Osborne A., Hawkins S.C., Mahon D., Ghatei M.A., Bloom S.R. The gut hormone response following roux-en-Y gastric bypass: cross-sectional and prospective study. Obesity Surgery. 2010;20(1):56–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]
- 13.Van der Schoor S.R.D., Reeds P.J., Stoll B., Henry J.F., Rosenberger J.R., Burrin D.G. The high metabolic cost of a functional gut. Gastroenterology. 2002;123(6):1931–1940. doi: 10.1053/gast.2002.37062. [DOI] [PubMed] [Google Scholar]
- 14.Saeidi N., Meoli L., Nestoridi E., Gupta N.K., Kvas S., Kucharczyk J. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund P.K., Goodman R.H., Dee P.C., Habener J.F., Biological P., Jan S. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. PNAS. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker D.J., Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annual Review of Physiology. 2014;76:561–583. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 17.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 18.Meier J.J., Gallwitz B., Salmen S., Goetze O., Holst J.J., Schmidt W.E. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2003;88(6):2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 19.Willms B., Werner J., Holst J.J., Ørskov C., Creutzfeldt W., Nauck M.A. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. Clinical Endocrinology and Metabolism. 1996;81(1):327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 20.Drucker D.J., Erlich P., Asa S.L., Brubaker P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(15):7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker P.L., Izzo A., Hill M., Drucker D.J. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. American Journal of Physiology, Endocrinology and Metabolism. 1997;272(6 Pt 1):E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- 22.Jeppesen P.B. Modern treatment of short bowel syndrome. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(5):582–587. doi: 10.1097/MCO.0b013e328363bce4. [DOI] [PubMed] [Google Scholar]
- 23.Bremholm L., Hornum M., Henriksen B.M., Larsen S., Holst J.J. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scandinavian Journal of Gastroenterology. 2009;44(3):314–319. doi: 10.1080/00365520802538195. [DOI] [PubMed] [Google Scholar]
- 24.Guan X., Shi X., Li X., Chang B., Wang Y., Li D. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(7):E853–E864. doi: 10.1152/ajpendo.00245.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagell C.F., Wettergren A., Pedersen J.F., Mortensen D., Holst J.J. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scandinavian Journal of Gastroenterology. 2004;39(4):353–358. doi: 10.1080/00365520410004424. [DOI] [PubMed] [Google Scholar]
- 26.Jeppesen P.B., Hartmann B., Thulesen J., Graff J., Lohmann J., Hansen B.S. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120(4):806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 27.Meier J.J., Nauck M.A., Pott A., Heinze K., Goetze O., Bulut K. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130(1):44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koehler J.A., Baggio L.L., Yusta B., Longuet C., Rowland K.J., Cao X. GLP-1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metabolism. 2015;21(3):379–391. doi: 10.1016/j.cmet.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Bryant M.G., Bloom S.R. Distribution of the gut hormones in the primate intestinal tract. Gut. 1979;20(8):653–659. doi: 10.1136/gut.20.8.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eissele R., Göke R., Willemer S., Harthus H., Vermeer H., Arnold R. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. European Journal of Clinical Investigation. 1992;22(4):283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 32.Polak J.M., Bloom S., Coulling I., Pearse A.G. Immunofluorescent localization of enteroglucagon cells in the gastrointestinal tract of the dog. Gut. 1971;12:311–318. doi: 10.1136/gut.12.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varndell I.M., Bishop A.E., Sikri K.L., Uttenthal L.O., Bloom S.R., Polak M. Localization of glucagon-like peptide (GLP) immunoreactants in human gut and pancreas using light and electron microscopic immunocytochemistry. The Journal of Histochemistry and Cytochemistry. 1985;33(10):1080–1086. doi: 10.1177/33.10.3900195. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien R.M., Granner D.K. Regulation of gene expression by insulin. Physiological Reviews. 1996;76(4):1109–1161. doi: 10.1152/physrev.1996.76.4.1109. [DOI] [PubMed] [Google Scholar]
- 35.Bullock P., Scott R. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):31–33. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 36.Campos R., Lee Y., Druckers D. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134(5):2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 37.Körner M., Stöckli M., Waser B., Reubi J.C. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. Journal of Nuclear Medicine. 2007;48(5):736–743. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- 38.Bang-Berthelsen C.H., Holm T.L., Pyke C., Simonsen L., Søkilde R., Pociot F. GLP-1 induces barrier protective expression in Brunner's glands and regulates colonic inflammation. Inflammatory Bowel Diseases. 2016;22(9):2078–2097. doi: 10.1097/MIB.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 39.Pyke C., Heller R.S., Kirk R.K., Orskov C., Reedtz-Runge S., Kaastrup P. GLP-1 receptor localization in monkey and human tissue; Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 40.Pyke C., Knudsen L.B. The glucagon-like peptide-1 receptor—or not? Endocrinology. 2013;154(1):4–8. doi: 10.1210/en.2012-2124. [DOI] [PubMed] [Google Scholar]
- 41.Panjwani N., Mulvihill E.E., Longuet C., Yusta B., Campbell J.E., Brown T.J. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology. 2013;154(1):127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 42.Lovshin J., Estall J., Yusta B., Brown T.J., Drucker D.J. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. The Journal of Biological Chemistry. 2001;276(24):21489–21499. doi: 10.1074/jbc.M009382200. [DOI] [PubMed] [Google Scholar]
- 43.Lovshin J.A., Huang Q., Seaberg R., Brubaker P.L., Drucker D.J. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology. 2004;145(7):3495–3506. doi: 10.1210/en.2004-0100. [DOI] [PubMed] [Google Scholar]
- 44.Guan X., Karpen H.E., Stephens J., Bukowski J.T., Niu S., Zhang G. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130(1):150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 45.El-Jamal N., Erdual E., Neunlist M., Koriche D., Dubuquoy C., Maggiotto F. Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2014;307(3):G274–G285. doi: 10.1152/ajpgi.00389.2012. [DOI] [PubMed] [Google Scholar]
- 46.Yusta B., Boushey R.P., Drucker D.J. The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. Journal of Biological Chemistry. 2000;275(45):35345–35352. doi: 10.1074/jbc.M005510200. [DOI] [PubMed] [Google Scholar]
- 47.Bjerknes M., Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12497–12502. doi: 10.1073/pnas.211278098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ørskov C., Hartmann B., Poulsen S.S., Thulesen J., Hare K.J., Holst J.J. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regulatory Peptides. 2004;124(1–3):105–112. doi: 10.1016/j.regpep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Gundersen H.J., Jensen E.B.V., Kieu K., Nielsen J. The efficiency of systematic sampling in stereology – reconsidered. Journal of Microscopy. 1999;193(February 1998):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 50.Gundersen H.J., Jensen E.B.V. The efficiency of systematic sampling in stereology and its prediction. Journal of Microscopy. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 51.Hansen C.F., Vrang N., Sangild P.T., Jelsing J. Novel insight into the distribution of L-cells in the rat intestinal tract. American Journal of Translational Research. 2013;5(3):347–358. [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scrocchi L., Brown T., MacLusky N., Brubaker P., Auerbach A., Joyner A. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide receptor gene. Nature Medicine. 1996;2(11):1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.J., Lee J., Li K.K., Holland D., Maughan H., Guttman D.S. Disruption of the murine Glp2r impairs paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology. 2012;153(3):1141–1151. doi: 10.1210/en.2011-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chambers A.P., Sorrell J.E., Haller A., Roelofs K., Hutch C.R., Kim K.-S. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metabolism. 2017 doi: 10.1016/j.cmet.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schepp W., Schmidtler J., Riedel T., Dehne K., Schusdziarra V., Holst J.J. Exendin-4 and exendin-(9-39)NH2: agonist and antagonist, respectively, at the rat parietal cell receptor for glucagon-like peptide-1-(7-36)NH2. European Journal of Pharmacology. 1994;269(2):183–191. doi: 10.1016/0922-4106(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 57.Körner M., Rehmann R., Reubi J.C. GLP-2 receptors in human disease: high expression in gastrointestinal stromal tumors and Crohn's disease. Molecular and Cellular Endocrinology. 2012;364(1–2):46–53. doi: 10.1016/j.mce.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Richards P., Parker H.E., Adriaenssens A.E., Hodgson J.M., Cork S.C., Trapp S. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yusta B., Baggio L.L., Koehler J., Holland D., Cao X., Pinnell L.J. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537–2549. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 60.Amato A., Baldassano S., Serio R., Mule F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2009;296(3):678–684. doi: 10.1152/ajpgi.90587.2008. [DOI] [PubMed] [Google Scholar]
- 61.Pedersen N.B., Pedersen J., Brix S.W., Hartmann B., Petersen B.L., Ørskov C. The glucagon-like peptide 2 receptor is expressed in enteric neurons and not in the epithelium of the intestine. Peptides. 2015;67:20–28. doi: 10.1016/j.peptides.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Van Ginneken C., Verlinden K., Van Meir F., Sys S., Weyns A. A stereologic evaluation of glucagon-like peptide-1 (GLP-1) mucosal cells in the small intestine of the developing pig. Anatomy and Embryology. 2002;205(2):153–157. doi: 10.1007/s00429-002-0235-z. [DOI] [PubMed] [Google Scholar]
- 63.Evans G., Potten C. The distribition of endocrine cells along the mouse intestine: a quantitative immunocytochemical study. Virchows Archive B Cell Pathology Including Molecular Pathology. 1988;56(3):191–199. doi: 10.1007/BF02890017. [DOI] [PubMed] [Google Scholar]
- 64.Dubé P.E., Forse C.L., Bahrami J., Brubaker P.L. The essential role of insulin-like growth Factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131:589–605. doi: 10.1053/j.gastro.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 65.Yusta B., Holland D., Koehler J.A., Maziarz M., Estall J.L., Higgins R. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology. 2009;137(3):986–996. doi: 10.1053/j.gastro.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 66.Tsai C., Hill M., Asa S., Brubacker P., Drucker D. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. American Journal of Physiology. 1997;273(1):E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- 67.Rowland K.J., Brubaker P.L. The “ cryptic ” mechanism of action of glucagon-like peptide-2. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2011;301:1–8. doi: 10.1152/ajpgi.00039.2011. [DOI] [PubMed] [Google Scholar]
- 68.Hansen C.F., Bueter M., Theis N., Lutz T., Paulsen S., Dalbøge L.S. Hypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated rats. PloS One. 2013;8(6):e65696. doi: 10.1371/journal.pone.0065696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barkholt P., Vegge A., Clausen Ryberg T., Birck Muusfeldt M., Fels Josef J., Støckel M. Post-surgical effects of roux- En-Y gastric bypass on glucose homeostasis, intestinal morphology and L-cells in obese Göttingen minipigs. Journal of Obesity and Bariatrics. 2015;1(1):1–8. [Google Scholar]
- 70.Cummings B.P., Bettaieb A., Graham J.L., Stanhope K.L., Kowala M., Haj F.G. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153(8):3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson-Pérez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D.A., Stoffers D. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garibay D., Mcgavigan A.K., Lee S.A., Ficorilli J.V., Cox A.L., Michael M.D. Beta-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology. 2016;157(9):3405–3409. doi: 10.1210/en.2016-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGavigan A.K., Garibay D., Henseler Z.M., Chen J., Bettaieb A., Haj F.G. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2015 doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mumphrey M.B., Hao Z., Townsend R.L., Patterson L.M., Berthoud H.-R. Sleeve gastrectomy does not cause hypertrophy and reprogramming of intestinal glucose metabolism in rats. Obesity Surgery. 2015;25(8):1468–1473. doi: 10.1007/s11695-014-1547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peterli R., Steinert R.E., Woelnerhanssen B., Peters T., Christoffel-courtin C., Gass M. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy : a randomized, prospective trial. Obesity Surgery. 2012;22(5):740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schirra J., Katschinski M., Weidmann C., Schäfer T., Wank U., Arnold R. Gastric emptying and release of incretin hormones after glucose ingestion in humans. Journal of Clinical Investigation. 1996;97(1):92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seimon R.V., Brennan I.M., Russo A., Little T.J., Jones K.L., Standfield S. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. American Journal of Physiology. Endocrinology and Metabolism. 2013;304(3):E294–E300. doi: 10.1152/ajpendo.00533.2012. [DOI] [PubMed] [Google Scholar]
- 78.Chambers A.P., Smith E.P., Begg D.P., Grayson B.E., Sisley S., Greer T. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(4):E424–E432. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kulkarni B.V., LaSance K., Sorrell J.E., Lemen L., Woods S.C., Seeley R.J. The role of proximal versus distal stomach resection in the weight loss seen after vertical sleeve gastrectomy. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2016;311(5):R979–R987. doi: 10.1152/ajpregu.00125.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braghetto I., Davanzo C., Korn O., Csendes A., Valladares H., Herrera E. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obesity Surgery. 2009;19:1515–1521. doi: 10.1007/s11695-009-9954-z. [DOI] [PubMed] [Google Scholar]
- 81.Shah S., Shah P., Todkar J., Gagner M., Sonar S., Solav S. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surgery for Obesity and Related Diseases. 2010;6(2):152–157. doi: 10.1016/j.soard.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Michalsky D., Dvorak P., Belacek J., Kasalicky M. Radical resection of the pyloric antrum and its effect on gastric emptying after sleeve gastrectomy. Obesity Surgery. 2013;23(4):567–573. doi: 10.1007/s11695-012-0850-6. [DOI] [PubMed] [Google Scholar]
- 83.Hansen C.F., Vassiliadis E., Vrang N., Sangild P.T., Cummings B.P., Havel P. The effect of ileal interposition surgery on enteroendocrine cell numbers in UC Davis type 2 diabetes melitus rat. Regulatory Peptides. 2014;189:31–39. doi: 10.1016/j.regpep.2014.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.