Abstract
Objective
Upregulation of uncoupling protein 2 (UCP2) is associated with impaired glucose-stimulated insulin secretion (GSIS), which is thought to be an important contributor to pathological β cell failure in obesity and type 2 diabetes (T2D); however, the physiological function of UCP2 in the β cell remains undefined. It has been suggested, but not yet tested, that UCP2 plays a physiological role in β cells by coordinating insulin secretion capacity with anticipated fluctuating nutrient supply, such that upregulation of UCP2 in the inactive/fasted state inhibits GSIS as a mechanism to prevent hypoglycemia. Therefore, we hypothesized that daily cycles of GSIS capacity are dependent on rhythmic and predictable patterns of Ucp2 gene expression such that low Ucp2 in the active/fed phase promotes maximal GSIS capacity, whereas elevated Ucp2 expression in the inactive/fasted phase supresses GSIS capacity. We further hypothesized that rhythmic Ucp2 expression is required for the maintenance of glucose tolerance over the 24 h cycle.
Methods
We used synchronized MIN6 clonal β cells and isolated mouse islets from wild type (C57BL6) and mice with β cell knockout of Ucp2 (Ucp2-βKO; and respective Ins2-cre controls) to determine the endogenous expression pattern of Ucp2 over 24 h and its impact on GSIS capacity and glucose tolerance over 24 h.
Results
A dynamic pattern of Ucp2 mRNA expression was observed in synchronized MIN6 cells, which showed a reciprocal relationship with GSIS capacity in a time-of-day-specific manner. GSIS capacity was suppressed in islets isolated from wild type and control mice during the light/inactive phase of the daily cycle; a suppression that was dependent on Ucp2 in the β cell and was lost in islets isolated from Ucp2-βKO mice or wild type islets treated with a UCP2 inhibitor. Finally, suppression of GSIS capacity by UCP2 in the light phase was required for the maintenance of normal patterns of glucose tolerance.
Conclusions
Our study suggests that Ucp2/UCP2 in the β cell is part of an important, endogenous, metabolic regulator that controls the temporal capacity of GSIS over the course of the day/night cycle, which, in turn, regulates time-of-day glucose tolerance. Targeting Ucp2/UCP2 as a therapeutic in type 2 diabetes or any other metabolic condition must take into account the rhythmic nature of its expression and its impact on glucose tolerance over 24 h, specifically during the inactive/fasted phase.
Keywords: Uncoupling protein 2, Glucose-stimulated insulin secretion, Glucose tolerance, Pancreatic islets, β cells
Abbreviations: UCP2, Uncoupling protein 2; GSIS, Glucose-stimulated insulin secretion; LG, Low glucose; HG, High glucose; ZT, Zeitgeber time; MIN6, Mouse insulinoma 6; Ucp2-βKO, β cell-specific Ucp2 knockout; Ins2-cre, Ins2 promoter-driven cre recombinase; i.p.GTT, intraperitoneal glucose tolerance test; T2D, Type 2 diabetes; WT, wild type
Highlights
-
•
Ucp2 mRNA expression in MIN6 β cells and isolated islets is dynamic and rhythmic over 24 h.
-
•
Daily cycles of glucose-stimulated insulin secretion capacity are dependent on rhythmic Ucp2 expression and UCP2 activity.
-
•
Loss of rhythmic Ucp2 mRNA expression triggers glucose intolerance only in the light/inactive phase of the daily cycle.
-
•
UCP2 is part of an endogenous diurnal metabolic regulator that coordinates islet function with the daily cycle of fasting and feeding.
1. Introduction
Uncoupling protein 2 (UCP2) is a member of the mitochondrial anion carrier superfamily that, when activated, can partially uncouple aerobic respiration from ATP synthesis [1], [2], [3]. Ucp2 is expressed in many tissues, including the pancreatic β cell [4], which relies heavily on glucose metabolism coupled to ATP production for efficient and appropriate insulin secretion. Not surprisingly, numerous gain-of-function [5], [6], [7], [8], [9], [10] and loss-of-function [11], [12], [13], [14], [15], [16] studies have been used to interrogate the function of UCP2 in the β cell and, for the most part, have established a negative relationship between UCP2 and glucose-stimulated insulin secretion (GSIS). For example, whole body (Ucp2-/-) [11], [12] and β cell Ucp2 knockout (Ucp2-βKO) mice [14] show augmented GSIS, whereas overexpression of Ucp2 in clonal β cell cultures and rat islets impairs GSIS [5], [9]. Furthermore, Ucp2 mRNA and protein are markedly upregulated in isolated islets from animal models of type 2 diabetes (T2D) and obesity [11], [12], [17], [18], and treatment of isolated islets in vitro with high glucose and elevated lipid concentrations significantly increases Ucp2 expression [13], [19], [20]. There is also sufficient evidence that suppression of insulin secretion in humans at risk for T2D is associated with higher expression levels of Ucp2 [21], [22], [23].
The negative relationship between Ucp2 expression and GSIS has been dubbed a “surprising whim of nature” [24], [25] and has provoked us and other research groups to ask an important evolutionary question: Why do β cells express Ucp2 if its activity is detrimental to insulin secretion and metabolic health? It is thought that Ucp2 must have an important physiological role in the β cell or it would have been selected against during evolution. Notably, obesity and over-nutrition are relatively ‘modern’ phenomena; humans evolved in an environment that was prone to prolonged periods of fasting and, in some cases, starvation [26]. The metabolic response to fasting is tightly regulated by insulin such that suppression of insulin during fasting is essential to allow for efficient endogenous glucose production and lipolysis to release metabolic fuels when dietary glucose is not sufficiently available. As such, it has been suggested that the true physiological function of Ucp2/UCP2 is to coordinate insulin secretion capacity with fluctuating nutrient supply, such that upregulation of Ucp2/UCP2 during fasting inhibits insulin secretion to promote fuel mobilization and prevent hypoglycemia [25], [27], [28]. Interestingly, Ucp2 mRNA is upregulated in islets and whole pancreas of starved mice [29], and rats [30] and fasted Ucp2-/- mice display higher serum insulin levels and blunted lipid metabolic responses [25]. Only a few studies have examined the role of UCP2 in the β cell during fasting, and all have employed either long-term (i.e. >24 h) or supra-physiological fasts (i.e. artificially fasting the animals overnight during the normal wake/fed period); however, our diurnal nature dictates that we cycle between acute periods of fasting and feeding on a daily basis, and therefore, UCP2 may play an important physiological role in coordinating insulin secretion with the acute phases of fasting and feeding experienced on a daily basis. The temporal expression of Ucp2 and its contribution to GSIS capacity over the course of a daily cycle in a healthy β cell has not yet been assessed.
In this study, we hypothesized that Ucp2 mRNA is dynamically expressed in healthy β cells over the course of a 24 h daily cycle such that Ucp2 expression is low in the fed/active phase to promote maximal GSIS capacity and upregulation of Ucp2 in the fasted/inactive phase suppresses GSIS capacity. To test this, we used synchronized MIN6 clonal β cells and islets isolated from wild type (WT) and Ucp2-βKO mouse models to define the daily expression patterns of Ucp2 mRNA, temporal GSIS capacity and glucose tolerance over 24 h. We have further explored the mechanistic contribution of UCP2 to the control of GSIS by examining changes in glucose-induced ATP production over the course of the 24 h cycle. Here, we demonstrate that Ucp2/UCP2 plays an important role in the regulation of temporal GSIS capacity, which is vital for the maintenance of glucose tolerance in the inactive/fasted phase of the daily cycle.
2. Materials & methods
2.1. Animals
Ucp2-βKO mice were generated and characterized previously [14]. Ins2-cre and Ucp2-βKO mice were maintained in closed colonies and were age-matched (12–24 weeks old) for experimentation. Note that based on similar results obtained between these mice (Ins2-cre and Ucp2-βKO) and other models of experimentation (MIN6 cells and C57BL6 mice), we have no reason to suspect that genetic background or genomic variation in these colonies is a confounding issue in this study. C57BL6 mice were obtained from breeding colonies housed at the University of Manitoba Central Animal Care Facility. Only male mice were used for experimentation. All animal experiments were approved by the University of Manitoba Animal Care Committee, and animals were handled according to the guidelines of the Canadian Council of Animal Care.
2.2. MIN6 cells and synchronization
MIN6 cells were cultured in DMEM (4500 mg/l glucose and l-glutamine) (Gibco, Thermo Fisher, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher, Waltham, MA, USA) and 1% penicillin/streptomycin (VWR, Radnor, PA, USA). MIN6 cells were plated in 6-well tissue culture dishes for approximately 2 days, until they were 60–70% confluent. MIN6 cells were synchronized prior to experimentation using a previously described serum-starve/serum-shock protocol [31]. For genipin-treated cells, 50 μM genipin (Wako Chemicals, Richmond, VA, USA) (dissolved in 70% ethanol) was applied 1 h prior to the start of the experiment and maintained throughout. Control cells in these experiments were treated with the same volume of 70% ethanol only.
2.3. Islet isolations
Islets were isolated by collagenase IV (Sigma, Oakville, ON, CA) digestion of the pancreas, according to our previously established protocol [14]. Once digested, islets were hand-picked 3–4 times into fresh RPMI 1640 media (11.1 mM glucose; Gibco, Thermo Fisher, Waltham, MA, USA), supplemented with 10% FBS (Thermo Fisher, Waltham, MA, USA), 1% PenStrep (VWR, Radnor, PA, USA) and 1% l-Gluatmine (Hyclone. GE Healthcare, Logan, Utah, USA). Picked islets were allowed to rest for 1 h at 37 °C/5% CO2 and then immediately used for experimentation. For genipin treatment of isolated islets, 50 μM genipin (Wako Chemicals, Richmond, VA, USA) (dissolved in 70% ethanol) was applied 1 h prior to the start of the experiment and maintained throughout.
2.4. Quantitative real-time PCR (qPCR)
RNA was isolated from MIN6 cells and islets (∼200 islets) using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA) or the RNeasy Micro Plus Kit (Qiagen, Germantown, MD, USA), respectively. RNA was reverse transcribed into cDNA using the Quantitect Reverse Transcription kit (Qiagen, Germantown, MD, USA) according to the manufacturer's instructions. 50–100 ng of cDNA was used as the template for qPCR reaction using the Quantitect SYBR green PCR kit (Qiagen, Germantown, MD, USA) in a MicroAmp Optical 96-well reaction plate with barcode (Applied Biosciences). qPCR reactions were run on an ABI 7500 real time system (Applied Biosystems, Thermo Fisher, Waltham, MA, USA) using the following program: 95 °C for 15 min, followed by 40 cycles of: 95 °C for 15 s, 57 °C for 30 s, and 72 °C for 30 s. Data was normalized to Eif2α mRNA and expressed relative to the expression level at time zero of the experiment, or otherwise indicated, to determine the 2−ΔΔCT. Ucp2 primers: (F) GAGATGTAGGGAAACTCCAGAAC and (R) GCCTTGTCTTCTGTAGGTCTATG; Eif2α primers: (F) CCTGAAGTGTGATCCTGTGTTT and (R) CCAAATCCAGCCAGCACTAATA (Invitrogen, Thermo Fisher, Waltham, MA, USA).
2.5. Glucose tolerance tests (GTT) and blood glucose measurements
Prior to beginning the GTT, mice were fasted for 3 h to ensure baseline blood glucose levels immediately prior to the time of day that the GTT was performed. i.p. glucose was administered at 1 g/kg and blood glucose was measured using a glucometer at the tail vein at 10, 20, 30, 60 and 120 min after i.p. injection.
2.6. Glucose-stimulated insulin secretion assay
GSIS assays were performed as described previously [14]. Insulin concentration was measured using a mouse insulin ELISA kit (ALPCO, Salem, NH, USA).
2.7. ATP content measurements
MIN6 cells or 10–15 islets were incubated in either 2.8 mM glucose or stimulated with 16.7 mM glucose (as described in [14]). The media was removed and the cells/islets were washed two times with sterile PBS. Cells were lysed with 300 μl or 100 μl, respectively, of 0.1 M NaOH/0.5 mM EDTA buffer for 20 min at 60 °C and stored at −80 °C until assayed. The ATP Bioluminescent Assay Kit (Sigma, Oakville, ON, CA) was adapted to a 96-well plate format and the assay performed according to the manufacturer's instructions. Luminescence was detected and quantified using a Fluostar Optima microplate reader (BMG Labtech, Ortenberg, Germany). ATP concentrations were normalized to total μg of DNA.
2.8. Statistics
Statistical significance was assessed by two-tailed Student t-test or one- or two-way ANOVA for repeated measures, followed by a Bonferroni post-test comparisons using Prism 6 software (GraphPad, San Diego, CA, USA). A value of P < 0.05 was considered significant. All data are expressed as the mean +/− SEM.
3. Results
3.1. Ucp2 mRNA expression is dynamic over 24 h and regulates inverse patterns of GSIS capacity in both MIN6 cells and isolated islets
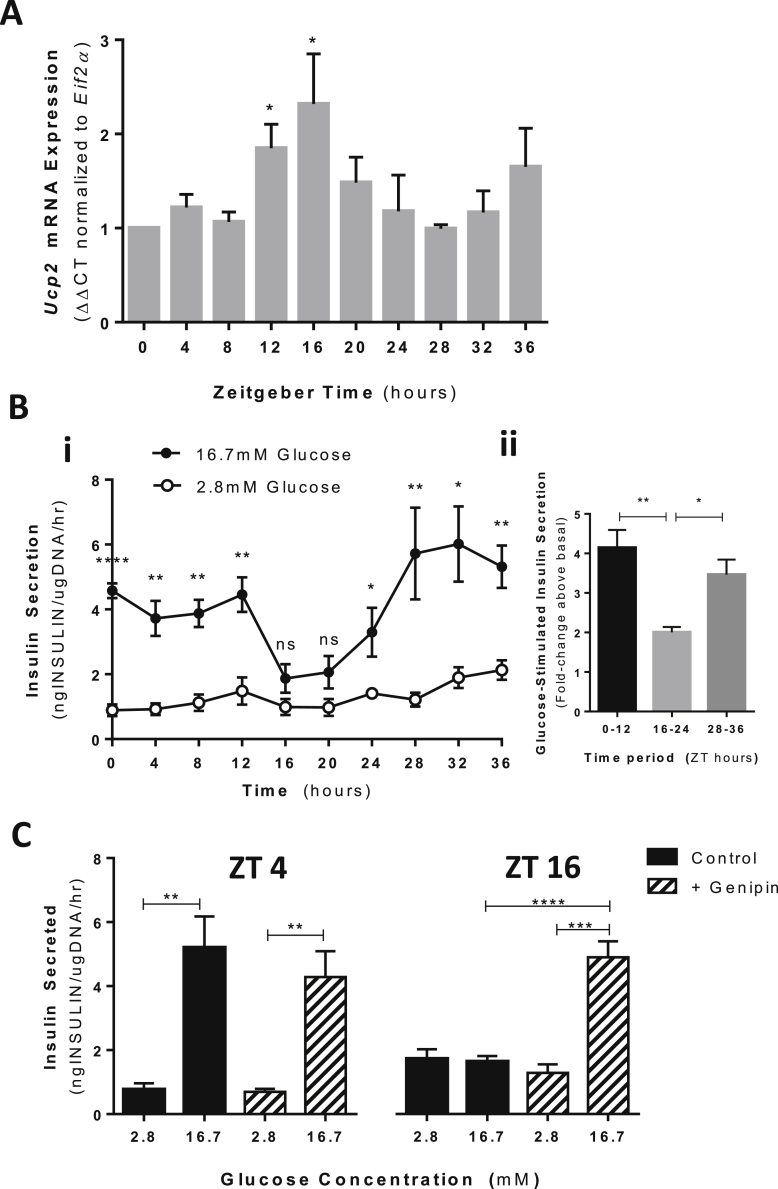
We first set out to determine the dynamics of Ucp2 mRNA expression over a 24 h period using MIN6 cells. To accomplish this, MIN6 cells were synchronized using an established serum-starve/serum-shock protocol [31] and lysates collected every 4 h over 36 h for gene expression analyses by qPCR. Ucp2 mRNA transcript levels displayed a dynamic and rhythmic pattern showing approximate 12 h repeating and alternating patterns of low and elevated Ucp2 mRNA expression (Figure 1A). At Zeitgeber time (ZT) 0, 4, and 8 (note: Zeitgeber time refers to time post-entrainment; in this case, after serum shock), Ucp2 mRNA expression remained low and unchanged. At ZT 12 and 16, Ucp2 mRNA levels increased 1.85 (+/− 0.254; p = 0.0102) and 2.318 (+/− 0.533; p = 0.0259) –fold, respectively, compared to time 0. Between ZT 24 and ZT 28, Ucp2 mRNA expression levels returned to baseline and gradually increased over the next 12 h (up to 36 h). Notably, beyond ZT 24, a trend towards a subsequent increase in Ucp2 mRNA was observed up to ZT 36, although not statistically significant by 36 h, likely due to waning synchronicity of the MIN6 cultures.
Figure 1.
Dynamic expression pattern of Ucp2 regulates the temporal capacity of GSIS in MIN6 cells. (A)Ucp2 mRNA expression levels at 4 h intervals over 36 h in synchronized MIN6 cells. Ucp2 mRNA shown is expressed relative to Eif2α levels and normalized to mRNA levels at time 0 (immediately after synchronization). N = 4–6 independent experiments. (B) i. GSIS capacity of synchronized MIN6 cells every 4 h over 36 h as measured by static incubation assay. Open circles represent insulin secreted when MIN6 cells are exposed to low (2.8 mM) glucose. Closed circles represent insulin secretion when MIN6 cells are exposed to high glucose (16.7 mM). N = 7–8 independent experiments. ii. Average GSIS capacity over segmented 12 h periods. GSIS is presented as the fold-change in insulin secretion above basal (2.8 mM glucose) when stimulated with high (16.7 mM) glucose. (C) GSIS capacity at 4 and 16 h post-synchronization in MIN6 cells treated with (hatched bars) and without genipin (solid black bars), a UCP2 activity inhibitor. Genipin was applied at a final concentration of 50 μM, 1 h before start of GSIS assay and remained present throughout the assay. N = 5. *p < 0.01; **p < 0.01; ****p < 0.0001.
Next, the temporal capacity of GSIS over 24 h in synchronized MIN6 cells was established by performing static incubation assays in low (2.8 mM) and high (16.7 mM) glucose concentrations every 4 h over a 36-hour time course. GSIS capacity varied in a repeatable pattern over 36 h (Figure 1B): From ZT 0 to ZT 12, stimulation with high glucose triggered an average 4.12-fold increase in insulin secretion; whereas during the next 12 h period (from ZT 16 to ZT 24), GSIS was significantly suppressed, showing only a 2-fold increase when stimulated with high glucose. Beyond 24 h (ZT 28 to ZT 36), GSIS capacity was again significantly increased, showing an average 3.47-fold increase when stimulated with high glucose. For the most part, the observed rhythmic pattern of GSIS capacity (Figure 1B) was opposite to the expression level of Ucp2 mRNA (Figure 1A), with the exception of the 12 h time point. At this time, Ucp2 mRNA increased almost 2-fold, but suppression of GSIS was not yet observed (suppression observed at 16 h). This lag in suppression of GSIS likely represents a lag in the translation of Ucp2 mRNA into functional protein. Unfortunately, protein levels were not measured due the lack of an available antibody that has sufficient sensitivity and specificity for UCP2. To demonstrate that rhythmic GSIS was dependent on rhythmic UCP2 activity, we performed GSIS assays at ZT 4 and ZT 16 (time points that correspond to robust and suppressed GSIS, respectively) in the presence of genipin, a well-established pharmacological UCP2 inhibitor [32], [33], [34]. Application of genipin prevented the suppression of GSIS in control MIN6 cells at ZT 16 but had no effect on GSIS capacity at ZT 4 when Ucp2 mRNA expression was low (Figure 1C), suggesting that UCP2 activity at ZT 16 contributes to the suppression of GSIS at this time point. Together, these data demonstrate that rhythmic GSIS capacity is dependent on inverse rhythms of Ucp2 expression/UCP2 activity in MIN6 cells.
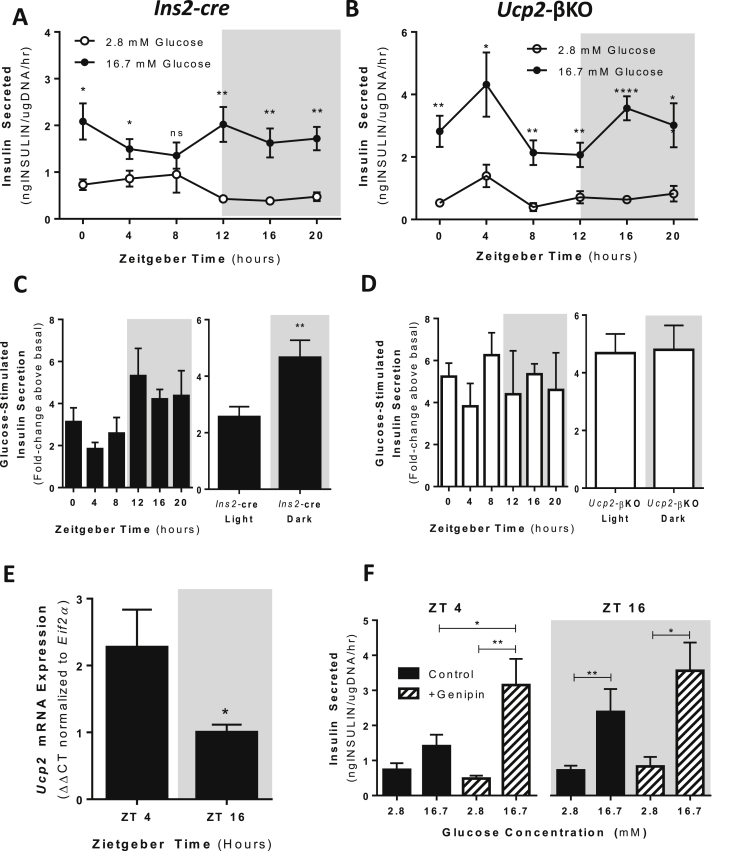
To determine if rhythmic GSIS capacity is dependent on rhythmic Ucp2 expression in primary β cells, we exploited our previously generated and characterized β cell Ucp2 knockout mice (Ins2Cre:Ucp2fl/fl; commonly referred to as Ucp2-βKO) [14]. Similar to MIN6 cells, control (Ins2Cre:Ucp2+/+; commonly referred to as Ins2-cre) islets isolated at 4 h intervals over 24 h (lights on at 6am = ZT 0; lights off at 6pm = ZT12) displayed a rhythmic capacity for GSIS that was suppressed in the light/inactive phase of the daily cycle (ZT 0, 4, 8), but became more robust in the dark/active phase (ZT 12, 16, 20) (Figure 2A,C). Suppression of GSIS in isolated Ins2-cre islets at ZT 4 was associated with elevated islet Ucp2 mRNA expression, whereas the more robust GSIS observed at ZT 16 was associated with reduced islet Ucp2 mRNA (Figure 2E). Importantly, daily rhythms of GSIS were lost in islets isolated from Ucp2-βKO mice, which displayed robust GSIS across the full 24 h time course (Figure 2B,D). No differences in fasted plasma insulin levels or fasted blood glucose levels were observed over 24 h between the Ins2-cre control and the Ucp2-βKO mice (data not shown).
Figure 2.
Daily rhythms of GSIS capacity are dependent on Ucp2/UCP2 in isolated islets. (A) GSIS capacity assessed every 4 h over 24 h in Ins2-cre (control) isolated islets. Data for insulin secretion capacities at 2.8 mM glucose (open circles) and 16.7 mM glucose (black circles) are shown for each time point. N = 4–5 mice per time point. (B) GSIS capacity assessed at 4 h intervals over 24 h in islets isolated from Ucp2-βKO mice. Data for insulin secretion capacities at 2.8 mM glucose (open circles) and 16.7 mM glucose (black circles) are shown for each time point. N = 4–13 mice per time point. (C) Left panel: GSIS capacity presented as the fold-change in insulin secretion above basal (2.8 mM glucose) when stimulated with high (16.7 mM) glucose at each time point in islets isolated from Ins2-cre mice. Right panel: Average GSIS capacity of isolated islets from Ins2-cre mice in the light vs. dark periods. (D) Left panel: GSIS capacity presented as the fold-change in insulin secretion above basal (2.8 mM glucose) when stimulated with high (16.7 mM) glucose at each time point in islets isolated from Ucp2-βKO mice. Right panel: Average GSIS capacity of isolated islets from Ucp2-βKO mice in the light vs. dark periods. (E) Islet Ucp2 mRNA levels are significantly elevated at ZT 4 (light/inactive phase) compared to ZT 16 (dark/active phase) in Ins2-cre control mice. Ucp2 mRNA shown is expressed relative to Eif2α levels and normalized to Ucp2 mRNA levels ZT 16. Islets were isolated from 3 to 5 mice at each time point. *p < 0.05. (F) Insulin secretion capacity measured in islets isolated from WT (C57BL6) mice at ZT4 (10 am) and ZT16 (10 pm). Application of genipin (50 μM, 1 h before assay) shows that inhibition of UCP2 at ZT4 prevents suppression of GSIS at this time point. N = 5–9 mice per time point. *p < 0.05; **p < 0.01; ****p < 0.0001.
To reduce genotype and strain effects, we also isolated islets from wild type (WT) C57BL6 mice and treated them with and without genipin to manipulate UCP2 activity prior to performing insulin secretion assays. Similar to Ins2-cre islets, C57BL6 islets displayed suppressed GSIS at ZT 4 in the light/inactive phase and robust GSIS capacity at ZT 16 in the dark/active phase (Figure 2F, black bars). Pre-treatment of isolated C57BL6 islets with genipin reversed the suppression of GSIS observed at the ZT 4 time point (Figure 2F, striped bars). Together, these data suggest that UCP2 plays an important physiological role in controlling the temporal capacity of GSIS such that increased Ucp2 expression/UCP2 activity are required for the normal suppression of GSIS in the light/inactive phase of the daily cycle.
3.2. Daily rhythms of UCP2 activity contribute to the temporal control of glucose-induced ATP production in both MIN6 cells and isolated islets
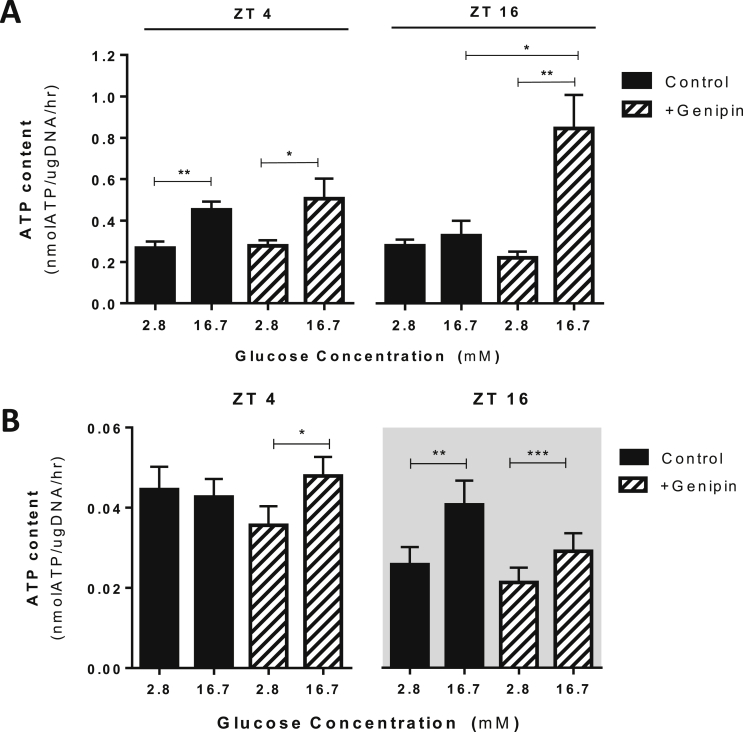
Currently, the contribution of β cell UCP2 to the regulation of mitochondrial ATP production remains debatable, with some groups demonstrating that manipulation of Ucp2 levels impact β cell ATP content [6], [11], [12], [35] while others show no impact on ATP [14], [36]; however, all studies performed to date have only examined ATP at a single time point and dynamic Ucp2 expression may contribute to these debateable findings. To determine if rhythmic UCP2 contributes to the control of cellular ATP levels in a temporal manner, we examined glucose-stimulated ATP content in both synchronized MIN6 cells and islets isolated at key time points (ZT 4 and ZT 16) plus or minus genipin treatment. In synchronized MIN6 cells at ZT 4 (a time point when MIN6 cells have a high GSIS capacity), 16.7 mM glucose significantly increased cellular ATP content; however, at ZT 16 (a time point when GSIS is impaired), cellular ATP content did not increase with high glucose exposure (Figure 3A). Application of genipin had no impact on cellular ATP content at ZT 4, suggesting no available target for genipin at this time point (i.e. endogenously low UCP2 activity), but reversed the impairment observed at ZT 16 (Figure 3A). Similar results were seen in islets isolated from C57BL6 mice at ZT 4 (light/inactive phase) and ZT 16 (dark/active phase) (Figure 3B). Collectively, these findings demonstrate that UCP2 controls the capacity of ATP production in a temporal manner, which is an important regulatory signal for GSIS.
Figure 3.
Daily rhythms of UCP2 activity control the temporal capacity of glucose-induced ATP content in MIN6 cells and isolated islets. (A) Measurement of ATP content in MIN6 cells after exposure to low (2.8 mM) and high (16.7 mM) glucose concentrations. Glucose-induced ATP content was impaired at ZT 16 compared to ZT 4. Application of genipin (50 μM, 1 h before assay) prevented the impairment of glucose-induced ATP content at ZT 16. N = 6–12 independent experiments. (B) Measurement of ATP content in islets isolated from WT C57BL6 mice at ZT 4 (10am) and ZT 16 (10 pm). Application with genipin (50 μM, 1 h before assay) prevented ATP content suppression at ZT 4 but had no impact at ZT 16. n = 12–18 mice per time point. *p < 0.05; **p < 0.01; ***p < 0.001.
3.3. Dynamic Ucp2 expression is required for normal cycles of glucose tolerance over 24 h
It is well-known that glucose tolerance fluctuates over a 24-hour period such that mild glucose intolerance occurs in the fasting/inactive phase of the daily cycle [37], [38], [39], [40] and our results so far suggest that UCP2 plays an important role in controlling the glucose-stimulated insulin secretion response. To determine if dynamic Ucp2 expression contributes to the overall pattern of glucose tolerance over a 24 h cycle, we performed i.p. glucose tolerance tests (GTTs) every 4 h over 24 h in Ucp2-βKO and Ins2-cre control mice. Note that mice were given ad libitum access to food, but were fasted 3 h prior to the GTT to ensure baseline blood glucose levels at the start of the test. Ins2-cre control (Figure 4A – light panels; Figure 4B) and C57BL6 mice (not shown) both displayed normal rhythms of glucose tolerance, which include improved glucose tolerance in the dark/active phase and mild glucose intolerance in the fasted/inactive phase. Interestingly, Ins2-cre control and Ucp2-βKO mice displayed no difference in glucose tolerance in the dark/active phase of the daily cycle (Figure 4A – dark panels; Figure 4B); however, Ucp2-βKO mice displayed greater glucose intolerance in the light/inactive phase (Figure 4A – light panels; Figure 4B). Rhythmic Ucp2 expression, particularly the upregulation of Ucp2 expression in the light/inactive phase, appears to be required for the regulation of normal cycles of glucose tolerance over a 24 h period.
Figure 4.
UCP2 deficiency impairs glucose tolerance only in the light phase of the daily cycle. (A) Assessment of glucose tolerance by i.p. GTT test was performed at 4 h intervals over 24 h at the following times: i) ZT0 (6 am – lights on), ii) ZT4 (10 am), iii) ZT 8 (2 pm), iv) ZT12 (6 pm – lights off), v) ZT16 (10 pm), and vi) ZT20 (2 am). Open squares are Ins2-cre mice; Closed squares are UCP2-βKO mice. (B) AUC calculations of each glucose tolerance curve in A. N = 18–25 mice per group. *p < 0.05; **p < 0.01.
4. Discussion
Glucose tolerance in healthy human subjects is greatest in the morning, just before the onset of the feeding phase, whereas glucose tolerance declines significantly in the evening/night, at the onset of the fasted phase [37], [38], [39], [40]. While daily fluctuations in peripheral insulin sensitivity likely contribute to this phenomenon, the “capacity” of GSIS varies over 24 h and contributes significantly to diurnal variations in glucose tolerance [41], [42], [43], [44]. These daily rhythms of GSIS are highly robust and persist in islets isolated from rodent models [43], [44], suggesting that an endogenous diurnal metabolic regulator in islets controls the temporal capacity of GSIS and ultimately glucose tolerance; however, the intrinsic metabolic regulators that control daily fluctuations in GSIS capacity and glucose tolerance have not yet been identified. Here, we have demonstrated in synchronized MIN6 cells and isolated islets that Ucp2 mRNA displays a rhythmic and predictable expression pattern required for the temporal control of GSIS capacity and glucose tolerance over 24 h, suggesting that Ucp2 is an important component of the intrinsic metabolic regulator that controls daily fluctuations of GSIS. We also show that fluctuations in GSIS capacity are associated with UCP2-mediated fluctuations in ATP production, which helps to shed some light on current debatable findings in the literature regarding the role of UCP2 in regulating ATP in the β cell. Some studies have demonstrated that Ucp2 knockout augments ATP production [11], [13], while others demonstrate no impact on ATP [14], [36]. Here, we show that the impact of UCP2 on glucose-induced ATP production is dynamic over a 24 h time course, suggesting that previous discrepancies in the literature may relate to time of day differences when performing actual assessments.
While this study demonstrates that Ucp2 expression is dynamic in β cells, it is not clear what drives this rhythmic expression. Recent studies have demonstrated that pancreatic islets have remarkably robust endogenous circadian oscillators [35], [45], [46], [47]. Cell-autonomous expression of CLOCK/BMAL1 (core transcriptional activator of the circadian machinery) drives 24 h rhythms of GSIS in isolated wild type islets [48]. Interestingly, it has been established that ∼27% of the β cell transcriptome displays circadian oscillations [48] and that a number of biological pathways involved in the regulation of insulin secretion and oxidative phosphorylation display enriched expression profiles in the dark/active phase of the daily cycle [49]. Additionally, the Ucp2 promoter contains tandem double E-box elements [50], which are known binding sites for the CLOCK/BMAL1 complex [48]; however, chromatin immuno-precipitation (ChIP) of INS1 832/13 cells with anti-BMAL1 antibody and qPCR with primers flanking the putative BMAL1-binding E-Box elements did not show enrichment of Ucp2 [46], suggesting that Ucp2 may not be a direct transcriptional target of BMAL1. Notably, these studies were performed in an unsynchronized clonal β cell line and temporal analyses in primary β cells may yield different results, preventing the exclusion of this possibility. While circadian clock-controlled expression of Ucp2 is an attractive possibility, it is also possible that temporal Ucp2 expression is dictated by daily fluctuations in fasting/feeding rhythms and nutrient availability. Exposure to elevated free fatty acids has been shown to induce upregulation and activation of UCP2 in β cells [13], [20], [50], [51], and circulating levels of free fatty acids are significantly increased during fasting. Clearly, further studies are needed to determine what drives the daily cycles of Ucp2 expression in the pancreatic β cell, but nutritional and circadian cycles are a logical place to start.
At the outset of this study, we hypothesized that rhythmic expression of Ucp2 would not only regulate daily cycles of insulin secretion but also daily cycles of glucose tolerance. In a 2011 study [14], we assessed glucose tolerance in Ins2-cre and Ucp2-βKO mice at a single time point (which corresponded with ZT 4 in the current study) and showed impaired glucose tolerance in Ucp2-βKO mice despite them having significantly elevated GSIS capacity. In this earlier study, we further demonstrated that excessive glucagon secretion in Ucp2-βKO mice, which resulted from aberrant intra-islet reactive oxygen species signaling originating from Ucp2-deficient β cells, was an important contributing factor to glucose intolerance development in these mice. In the current study, we have demonstrated a similar phenomenon where Ucp2-βKO mice displayed impaired glucose tolerance despite experiencing augmented GSIS capacity, but only in the light phase on the daily cycle. No difference in glucose tolerance was observed in Ucp2-βKO and Ins2-cre in the dark/active phase, a phase when both groups of mice displayed similar GSIS capacities and have low (or no) Ucp2 expression. These findings suggest that β cell Ucp2 deletion does not chronically impair glucose tolerance but rather selectively contributes to the control of glucose tolerance in the light/inactive phase as the animal enters into an acute period of fasting. Based on our previous studies on the Ucp2-βKO mouse, it would appear that β cell Ucp2-mediated regulation of glucagon secretion is an important contributor to the control of glucose tolerance and in the light/inactive phase; however, it is not currently clear how rhythmic expression of Ucp2 in the β cell affects the 24 h function of the α cell. Further experimentation, which is beyond the scope of this current study, is required to delineate this β cell Ucp2-α cell axis in the regulation of 24 h glucose tolerance.
In terms of limitations of this study, we acknowledge that our Ucp2-βKO mouse was created using the Ins2 promoter to drive cre recombinase expression. Unfortunately, it has been demonstrated that Ins2 is also expressed in glucose-sensing regions of the brain [52], and we have previously demonstrated minor Ucp2 deletion occurs in the hypothalamus of the Ucp2-βKO mouse [14]. Although the identity, function, and contribution of the Ins2-expressing neurons to glucose-sensing in the hypothalamus remains unknown, we cannot rule out that Ucp2 deletion in the brain may contribute to the results of this study, particularly the assessment of whole body glucose tolerance. Notably, we were able to demonstrate that daily rhythms of Ucp2 expression contribute to daily cycles of GSIS capacity both in islets ex vivo as well as in clonal cell lines; models that are removed from central nervous system influence. The use of an Ins1 cre model for β cell-specific Ucp2 deletion (as Ins1 is not expressed in the brain [53]) is suggested to validate the glucose tolerance findings in this paper.
5. Conclusions
For almost two decades, islet biologists have been in pursuit of the physiological function of UCP2 in pancreatic β cells and many suggested functions have been hypothesized. Here, we explored the endogenous patterns of Ucp2 expression in both isolated islets and mouse clonal MIN6 cells and demonstrated that Ucp2 mRNA is not only dynamically and rhythmically expressed, but also that this rhythmic expression is required for the temporal control of glucose-stimulated ATP production, normal rhythms of GSIS capacity, and, ultimately, normal rhythms of glucose tolerance. From this, we conclude that UCP2 is part of an important, endogenous, diurnal metabolic regulator that coordinates islet function with anticipated changes and cycling of nutrient availability to regulate temporal glucose tolerance. Given its negative impact on GSIS and its associated upregulation in obesity and diabetes, several groups have suggested that Ucp2/UCP2 would make an attractive target for the creation of novel T2D therapies; however, given this novel physiological function of UCP2, targeting Ucp2/UCP2 as a therapy for T2D or other metabolic diseases must take into account the rhythmic nature of its expression and its impact on glucose tolerance over 24 h, specifically during the fasted phase.
Acknowledgements
This work was supported by research grants awarded to C.A.D. from the National Sciences and Engineering Research Council of Canada (NSERC; Discovery Grant RGPIN-2015-06551), the Manitoba Medical Services Foundation (8-2014-06), the Children's Hospital Research Institute of Manitoba (2013-13) and the Thorlakson Foundation (University of Manitoba). As well, both S.C. and M.E.J. received summer studentship scholarships from the Children's Hospital Research Institute of Manitoba. The authors would like to acknowledge Mark Mutawe and Thomas Mahood for their technical assistance on this work.
Conflict of interest
The authors declare no conflicts of interest associated with the work in this manuscript.
References
- 1.Brand M.D., Esteves T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metabolism. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Echtay K.S., Roussel D., St-Pierre J., Jekabsons M.B., Cadenas S., Stuart J.A. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 3.Echtay K.S., Esteves T.C., Pakay J.L., Jekabsons M.B., Lambert A.J., Portero-Otin M. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. The EMBO Journal. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nature Genetics. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 5.Chan C.B., MacDonald P.E., Saleh M.C., Johns D.C., Marban E., Wheeler M.B. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–1486. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- 6.Chan C.B., De Leo D., Joseph J.W., McQuaid T.S., Ha X.F., Xu F. Increased uncoupling protein-2 levels in β-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- 7.Hong Y., Fink B.D., Dillon J.S., Sivitz W.I. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142:249–256. doi: 10.1210/endo.142.1.7889. [DOI] [PubMed] [Google Scholar]
- 8.Kashemsant N., Chan C.B. Impact of uncoupling protein-2 overexpression on proinsulin processing. Journal of Molecular Endocrinology. 2006;37:517–526. doi: 10.1677/jme.1.02091. [DOI] [PubMed] [Google Scholar]
- 9.McQuaid T.S., Saleh M.C., Joseph J.W., Gyulkhandanyan A., Manning-Fox J.E., MacLellan J.D. cAMP-mediated signaling normalizes glucose-stimulated insulin secretion in uncoupling protein-2 overexpressing β-cells. The Journal of Endocrinology. 2006;190:669–680. doi: 10.1677/joe.1.06723. [DOI] [PubMed] [Google Scholar]
- 10.Wang M.Y., Shimabukuro M., Lee Y., Trinh K.Y., Chen J.L., Newgard C.B. Adenovirus-mediated overexpression of uncoupling protein-2 in pancreatic islets of Zucker diabetic rats increases oxidative activity and improves β-cell function. Diabetes. 1999;48:1020–1025. doi: 10.2337/diabetes.48.5.1020. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C.Y., Baffy G., Perret P., Krauss S., Peroni O., Grujic D. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 12.Joseph J.W., Koshkin V., Zhang C.Y., Wang J., Lowell B.B., Chan C.B. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- 13.Joseph J.W., Koshkin V., Saleh M.C., Sivitz W.I., Zhang C.Y., Lowell B.B. Free fatty acid-induced β-cell defects are dependent on uncoupling protein 2 expression. The Journal of Biological Chemistry. 2004;279:51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- 14.Robson-Doucette C.A., Sultan S., Allister E.M., Wikstrom J.D., Koshkin V., Bhattacharjee A. Β-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60:2710–2719. doi: 10.2337/db11-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.C., Robson-Doucette C.A., Wheeler M.B. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. The Journal of Endocrinology. 2009;203:33–43. doi: 10.1677/JOE-09-0117. [DOI] [PubMed] [Google Scholar]
- 16.Krauss S., Zhang C.Y., Scorrano L., Dalgaard L.T., St-Pierre J., Grey S.T. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic β cell dysfunction. The Journal of Clinical Investigation. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassis N., Bernard C., Pusterla A., Casteilla L., Penicaud L., Richard D. Correlation between pancreatic islet uncoupling protein-2 (UCP2) mRNA concentration and insulin status in rats. International Journal of Experimental Diabetes Research. 2000;1:185–193. doi: 10.1155/EDR.2000.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winzell M.S., Svensson H., Enerback S., Ravnskjaer K., Mandrup S., Esser V. Pancreatic β-cell lipotoxicity induced by overexpression of hormone-sensitive lipase. Diabetes. 2003;52:2057–2065. doi: 10.2337/diabetes.52.8.2057. [DOI] [PubMed] [Google Scholar]
- 19.Laybutt D.R., Sharma A., Sgroi D.C., Gaudet J., Bonner-Weir S., Weir G.C. Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. The Journal of Biological Chemistry. 2002;277:10912–10921. doi: 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- 20.Patane G., Anello M., Piro S., Vigneri R., Purrello F., Rabuazzo A.M. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes. 2002;51:2749–2756. doi: 10.2337/diabetes.51.9.2749. [DOI] [PubMed] [Google Scholar]
- 21.Krempler F., Esterbauer H., Weitgasser R., Ebenbichler C., Patsch J.R., Miller K. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002;51:3331–3335. doi: 10.2337/diabetes.51.11.3331. [DOI] [PubMed] [Google Scholar]
- 22.Sasahara M., Nishi M., Kawashima H., Ueda K., Sakagashira S., Furuta H. Uncoupling protein 2 promoter polymorphism -866G/A affects its expression in β-cells and modulates clinical profiles of Japanese type 2 diabetic patients. Diabetes. 2004;53:482–485. doi: 10.2337/diabetes.53.2.482. [DOI] [PubMed] [Google Scholar]
- 23.Sesti G., Cardellini M., Marini M.A., Frontoni S., D'Adamo M., Del Guerra S. A common polymorphism in the promoter of UCP2 contributes to the variation in insulin secretion in glucose-tolerant subjects. Diabetes. 2003;52:1280–1283. doi: 10.2337/diabetes.52.5.1280. [DOI] [PubMed] [Google Scholar]
- 24.Nedergaard J., Cannon B. The 'novel' 'uncoupling' proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Experimental Physiology. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- 25.Sheets A.R., Fulop P., Derdak Z., Kassai A., Sabo E., Mark N.M. Uncoupling protein-2 modulates the lipid metabolic response to fasting in mice, American journal of physiology. Gastrointestinal and Liver Physiology. 2008;294:G1017–G1024. doi: 10.1152/ajpgi.00016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Rourke R.W. Metabolic thrift and the genetic basis of human obesity. Annals of Surgery. 2014;259:642–648. doi: 10.1097/SLA.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan C.B., Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochemical Society Transactions. 2006;34:802–805. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- 28.Affourtit C., Brand M.D. On the role of uncoupling protein-2 in pancreatic β cells. Biochimica et Biophysica Acta. 2008;1777:973–979. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Bordone L., Motta M.C., Picard F., Robinson A., Jhala U.S., Apfeld J. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biology. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gremlich S., Nolan C., Roduit R., Burcelin R., Peyot M.L., Delghingaro-Augusto V. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146:375–382. doi: 10.1210/en.2004-0667. [DOI] [PubMed] [Google Scholar]
- 31.Greco J.A., Oosterman J.E., Belsham D.D. Differential effects of omega-3 fatty acid docosahexaenoic acid and palmitate on the circadian transcriptional profile of clock genes in immortalized hypothalamic neurons, American journal of physiology. Regulatory, Integrative and Comparative Physiology. 2014;307:R1049–R1060. doi: 10.1152/ajpregu.00100.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C.Y., Parton L.E., Ye C.P., Krauss S., Shen R., Lin C.T. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced β cell dysfunction in isolated pancreatic islets. Cell Metabolism. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Parton L.E., Ye C.P., Coppari R., Enriori P.J., Choi B., Zhang C.Y. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 34.Allister E.M., Robson-Doucette C.A., Prentice K.J., Hardy A.B., Sultan S., Gaisano H.Y. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013;62:1623–1633. doi: 10.2337/db12-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J., Kim M.S., Li R., Liu V.Y., Fu L., Moore D.D. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets. 2011;3:381–388. doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochimica et Biophysica Acta. 2009;1787:377–383. doi: 10.1016/j.bbabio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Saad A., Dalla Man C., Nandy D.K., Levine J.A., Bharucha A.E., Rizza R.A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61:2691–2700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll K.F., Nestel P.J. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22:333–348. doi: 10.2337/diab.22.5.333. [DOI] [PubMed] [Google Scholar]
- 39.Van Cauter E., Desir D., Decoster C., Fery F., Balasse E.O. Nocturnal decrease in glucose tolerance during constant glucose infusion. The Journal of Clinical Endocrinology and Metabolism. 1989;69:604–611. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 40.Van Cauter E., Blackman J.D., Roland D., Spire J.P., Refetoff S., Polonsky K.S. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. The Journal of Clinical Investigation. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polonsky K.S., Given B.D., Hirsch L., Shapiro E.T., Tillil H., Beebe C. Quantitative study of insulin secretion and clearance in normal and obese subjects. The Journal of Clinical Investigation. 1988;81:435–441. doi: 10.1172/JCI113338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polonsky K.S., Given B.D., Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. The Journal of Clinical Investigation. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picinato M.C., Haber E.P., Carpinelli A.R., Cipolla-Neto J. Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. Journal of Pineal Research. 2002;33:172–177. doi: 10.1034/j.1600-079x.2002.02925.x. [DOI] [PubMed] [Google Scholar]
- 44.Delattre E., Cipolla-Neto J., Boschero A.C. Diurnal variations in insulin secretion and K+ permeability in isolated rat islets. Clinical and Experimental Pharmacology & Physiology. 1999;26:505–510. doi: 10.1046/j.1440-1681.1999.03073.x. [DOI] [PubMed] [Google Scholar]
- 45.Marcheva B., Ramsey K.M., Buhr E.D., Kobayashi Y., Su H., Ko C.H. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J., Moulik M., Fang Z., Saha P., Zou F., Xu Y. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Molecular and Cellular Biology. 2013;33:2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadacca L.A., Lamia K.A., deLemos A.S., Blum B., Weitz C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perelis M., Marcheva B., Ramsey K.M., Schipsma M.J., Hutchison A.L., Taguchi A. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rakshit K., Qian J., Ernst J., Matveyenko A.V. Circadian variation of the pancreatic islet transcriptome. Physiological Genomics. 2016;48:677–687. doi: 10.1152/physiolgenomics.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medvedev A.V., Robidoux J., Bai X., Cao W., Floering L.M., Daniel K.W. Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. The Journal of Biological Chemistry. 2002;277:42639–42644. doi: 10.1074/jbc.M208645200. [DOI] [PubMed] [Google Scholar]
- 51.Lameloise N., Muzzin P., Prentki M., Assimacopoulos-Jeannet F. Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes. 2001;50:803–809. doi: 10.2337/diabetes.50.4.803. [DOI] [PubMed] [Google Scholar]
- 52.Wicksteed B., Brissova M., Yan W., Opland D.M., Plank J.L., Reinert R.B. Conditional gene targeting in mouse pancreatic ss-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson J.D. A practical guide to genetic engineering of pancreatic β-cells in vivo: getting a grip on RIP and MIPE. Islets. 2014;6:e944439. doi: 10.4161/19382014.2014.944439. [DOI] [PMC free article] [PubMed] [Google Scholar]