Abstract
Circadian systems include slave oscillators and central pacemakers, and the cores of eukaryotic circadian clocks described to date are composed of transcription and translation feedback loops (TTFLs). In the model system Neurospora, normal circadian rhythmicity requires a TTFL in which a White Collar complex (WCC) activates expression of the frequency (frq) gene, and the FRQ protein feeds back to attenuate that activation. To further test the centrality of this TTFL to the circadian mechanism in Neurospora, we used low-amplitude temperature cycles to compare WT and frq-null strains under conditions in which a banding rhythm was elicited. WT cultures were entrained to these temperature cycles. Unlike those normal strains, however, frq-null mutants did not truly entrain to the same cycles. Their peaks and troughs always occurred in the cold and warm periods, respectively, strongly suggesting that the rhythm in Neurospora lacking frq function simply is driven by the temperature cycles. Previous reports suggested that a FRQ-less oscillator (FLO) could be entrained to temperature cycles, rather than being driven, and speculated that the FLO was the underlying circadian-rhythm generator. These inferences appear to derive from the use of a phase reference point affected by both the changing waveform and the phase of the oscillation. Examination of several other phase markers as well as results of additional experimental tests indicate that the FLO is, at best, a slave oscillator to the TTFL, which underlies circadian rhythm generation in Neurospora.
Keywords: FRQ-less oscillator, frq, FRQ
Circadian programs in eukaryotes are widely perceived to be the output of multiple oscillatory systems based on cell intrinsic transcription and translation feedback loops (TTFLs) (1–3). In many animals and fungi, heterodimeric PAS domaincontaining transcription factors drive expression of genes encoding proteins that block the activity of their heterodimeric activators; such negative feedback loops generally are believed to make up the cores of these circadian clocks. In addition to these autonomous biological clocks, slave oscillators also exist within the panoply of circadian systems. Early studies on entrainment in Drosophila gave rise to models in which a pacemaker drove a slave oscillator that directly regulated an overt rhythmic event (4), and noncircadian slaves have since been experimentally described (e.g., ref. 5). However, because there are few molecular descriptions of slave oscillators, their existence and properties have so far chiefly been inferred from the behavior of the circadian system when exposed to Zeitgeber period lengths outside its innate frequency.
At the core of the TTFL in the circadian model system Neurospora crassa are the products of the frequency (frq), white collar-1 (wc-1), and wc-2 genes. Similar to animal systems, Neurospora possesses a feedback loop in which a heterodimeric activator, the White Collar complex (WCC) of the PAS proteins WC-1 and WC-2, activates expression of frq and thus FRQ, which in turn depresses transcriptional activation by WCC (6). In this organism the clock controls several processes including the daily production of asexual spores (conidia). Rhythmic conidiation is visualized in cultures growing along the surface of media as a regularly occurring pattern of aerial hyphae and orange spores (a “band”). In addition to this circadian regulation, the developmental processes leading to conidiation are independently affected by light, temperature, humidity, and media composition, as well as by oscillators that lack full circadian credentials (reviewed in ref. 7). The first such oscillation (in the absence of a fully functioning TTFL) was observed in frq-null strains two decades ago (8–10). Observed oscillations in Neurospora that lack circadian characteristics and can operate absent the core TTFL have been inferred to be underpinned by operation of FRQ-less oscillators (FLOs) (11, 12), several of which are now believed to exist (reviewed in ref. 7).
Despite these conceptual models, molecular mechanisms of FRQ-less oscillations remain cryptic; the molecular relationship between the driver and the slave has never been clear; and the only sporadic appearance of the original FLO under freerunning conditions further rendered its study problematic. To circumvent these obstacles, some studies (e.g., ref. 13) have exploited the observation that, whereas driven rhythms simply respond to environmental cycles, oscillators can entrain to subharmonics of their innate frequencies; that is, a 24-h circadian clock can entrain to a recurring 12-h cycle in the phenomenon known as frequency demultiplication (14). Additionally, temperature cycles have been used to reveal the FLO and to probe the relationship between it and the circadian oscillator (15). This work suggested that the phase of the temperatureinduced FLO varied systematically with the period of the temperature cycle and did so in a manner that paralleled the intact circadian system (15). Such results would indicate that this FLO truly can be entrained by temperature cycles. Normal circadian entrainment in frq-null strains, i.e., strains lacking the TTFL, would have profound implications. In contemplating these implications, including self-sufficiency for the FLO and that the TTFL might not be necessary for circadian rhythmicity, the authors proposed a novel, ingenious, and speculative model in which the FLO was the underlying circadian oscillator and the TTFL part of the entraining mechanism to light (13, 16). Because this model would require major revisions in circadian theory and in the interpretation of a great many previous results, we reexamined the premise and results on which it was based.
Here, we report the results of experiments performed in several laboratories in the United States and United Kingdom exploring the relationship between temperature cycles and conidiation rhythms in several strains of Neurospora, including frq-null strains lacking a functional TTFL. There was no evidence for entrainment by frequency demultiplication using any phase marker. Results using three of four phase markers clearly showed that the frq-null strains did not truly entrain to temperature cycles. Rather, they exhibited a simple readout of the temperature cycle. Previous results suggesting entrainment of the FLO by temperature cycles were, however, replicated by using a fourth phase marker, “onset,” which occurred when conidial production was rising toward its peak. However, special, and unfortunately misleading, properties of this nonstandard phase marker in Neurospora accounted for the unique results obtained with its use. We conclude that, although the Neurospora FLO that is visualized by temperature cycles may play a role in output rhythms, it cannot be the basis of truly circadian oscillations.
Materials and Methods
Strains and Media. All frq alleles isolated or manufactured in Neurospora, including the null alleles frq9 and frq10 involving, respectively, an intra-ORF stop codon and complete gene deletion by molecular replacement (10), are described in refs. 17 and 18. frq9 cultures used in ref. 15 were independently obtained from M. Merrow (Ludwig-Maximilians University, Munich). Medium containing water, 1× Vogel's salts, 1.5% agar, 50 ng/ml biotin, and either 0.3% glucose, 0.5% l-arginine, or half these concentrations of glucose and arginine, were used with identical results.
Temperature Cycles. Temperature gradients, such as those used in ref. 15 or steps between 22°C and 27°C requiring 25 min to reach 90% of the end point, yielded indistinguishable conclusions. To replicate the temperature cycles used previously, incubators (Percival, Perry, IA) were programmed to increase temperature from 22°C in 0.5° steps every 5 min for 10 steps and to decrease from 27°C in 0.5° steps every 10 min for 10 steps. Temperature was recorded inside the incubator every 2 min by using HOBO temperature recorders (Onset Computer Corp., Bourne, MA). Temperature recorded at the agar surface showed an ≈10-min delay from changes seen in the incubator's air temperature.
Phase Analysis. Race tubes were scanned, and the continuous record of the optical density of the culture was used as the output of the clock. This output was averaged across all replicates and is reported in Figs. 1, 2, 3, 4 as the mean (solid line) ± SD (dark gray shading). Phase in Fig. 2 for data from all laboratories was calculated manually by measuring the distance between peaks and the temperature transition. Temperature transitions were either marked individually or estimated after growing the different strains at 22°C and 27°C, calculating a growth ratio, and comparing predicted and calculated growth. Both methods yielded indistinguishable conclusions. For Fig. 3, chrono (19) was used to replot the data with respect to Zeitgeber time (ZT), where ZT0 and ZT12 correspond to the beginning and end of the 22°C part of the cycle, so that corresponding phases in all cultures could be aligned regardless of cycle length. As phase reference points, conidiation onset, “offset,” peak, and trough phases were calculated by using the chrono program's settings. The continuous variable of culture optical density often showed gradual changes over a week of growth. To calculate onset and offset, a running average of optical density first was calculated over a period equaling the cycle length. Onset is defined as the point where the line describing mean optical density moves up through the running average line, and offset is the corresponding downward transition through the nonrhythmic trend (see horizontal lines in Fig. 3 A and C; ref. 15).
Fig. 1.
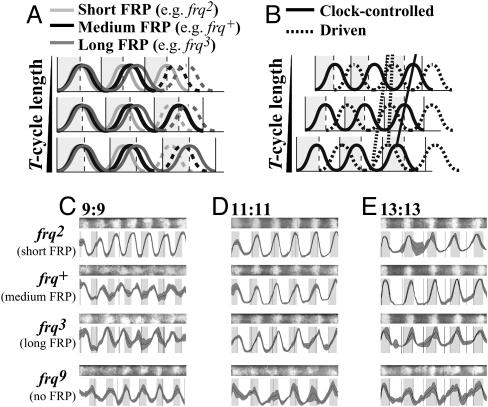
Phase of oscillation shown by systems with short, medium, or long FRPs compared with phases of clock-controlled and driven oscillations. (A) Model representing the effect of different-length temperature cycles on a rhythm regulated by a circadian clock. Phase (the position of the curve relative to the temperature transitions) will vary, reflecting the difference between the oscillator's inherent FRP and the period of the entraining cycle (T). When FRP and T are different, the oscillator adapts to the entraining cycle, and the rhythm assumes a stable phase relationship (seen here as the dashed curves on the 3rd day). Oscillators with different FRPs will establish different phase relationships with entraining cycles of a given length. (B) A rhythm generated by a circadian pacemaker (e.g., clock-controlled conidiation) will have a phase relationship with an entraining cycle such that, when entrained to different T cycles, the peak can occur before, at, or after the temperature transition (solid diagonal line). In a rhythm driven by temperature cycles (e.g., temperaturedriven conidiation), peaks and troughs fall at the same relative points, but the rate of rise and fall is influenced by the temperature. In this example, conidiation is initiated each time the temperature rises. The constant position of the peak with respect to the temperature rise is apparent (vertical dotted line) when the curves are lined up so that the third temperature rises are synchronous. However, if the temperature cycles influence the relative shape of the curve, then phase as determined by a point along the rise of the curve appears to change with T (diagonal dotted line). (C–E) Behavior of WT and frq mutant strains subjected to temperature cycles with different periods. Race tubes (at the top) and densitometric scans (at the bottom) representing Neurospora frq2, frq+, frq3, and frq9 strains grown under temperature cycles of 22°C/27°C of18h(9h/9h)(C), 22 h (11 h/11 h) (D), and 26 h (13 h/13 h) (E). Densitometric scans represent average pixels (darker central line) ± 1 SD (gray shading above and below the average curve) of at least five independent replicates (n ≥ 5 race tubes per genotype for each T cycle). Gray and white shading represents periods of growth at 22°C and 27°C, respectively.
Fig. 2.
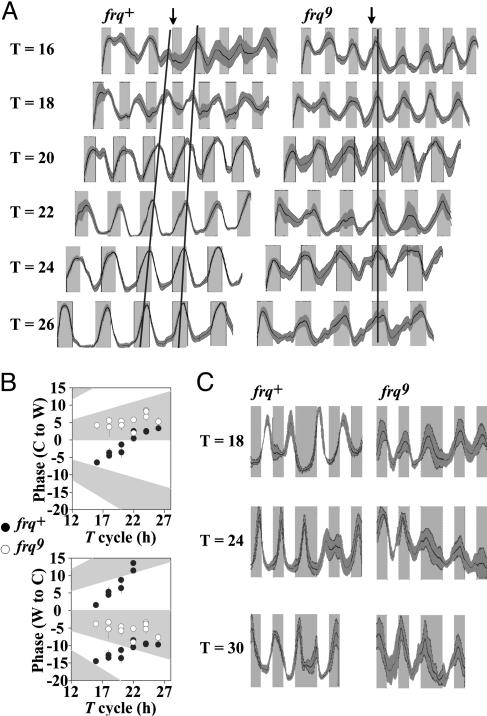
frq+ strains are entrained, and frq9 strains are driven, by temperature cycles. (A) Densitometric tracings of the conidial banding rhythm entrained to 22°C/27°C temperature cycles of varying period length (indicated at left). Shading is as in Fig. 1; middle lines report average (n ≥ 6 race tubes) pixel density, and shading above and below this line marks ± 1 SD. The widths of the cool and warm periods were drawn to scale because the growth rate is higher at the higher temperature. The end of the third warm period in each tube has been aligned as indicated by the vertical arrow, and a line was drawn through the third (and in frq+ fourth) peaks to highlight the trends in phase. This line is sloped in WT (Left) showing the systematic change in phase as a function of T consistent with entrainment, but the line is vertical in the frq-null strain (Right). Similar results were obtained in all three laboratories; plotted data are from one laboratory. (B) The phase of the rhythm peak under different period length (T) cycles was measured as the average number of hours the peaks occurred after (-) or before (+) the cool (22°C) to warm (27°C) (Upper) or warm to cool (Lower) transitions for frq9 (○) and frq+ (•). When plotting the phase relative to the warm to cool transition, the frq+ peak cannot always be unambiguously plotted as occurring before or after the transition. Thus, we plotted the results as delays (negative values) as well as advances (positive values). This plot represents all data collected independently in three different laboratories. Each data point is an average phase value from at least three cycles per race tube from n ≥ 6 race tubes ± 1 SD. To test how well entrainment period length could predict phase, we performed a linear regression analysis and found a highly significant linear relationship (P < 0.001) between phase and T in the frq+ strain. However, in frq9 the relationship was not significant (P > 0.05), indicating that this strain is not entrained. (C) Densitometric scans of frq+ (Left) and frq9 (Right) strains under cycles of 9 h/9 h (Top), 12 h/12 h (Middle), and 15 h/15 h (Bottom) at 22°C and 27°C. After the fourth full temperature cycle (first two cycles not shown), the temperature was held at 22°C for another half-cycle before resuming regular cycling. In frq+, the oscillation continues, whereas in frq9, cycling ceases until the temperature rises again, consistent with the rhythm being driven. Data are from one laboratory, n = 6 race tubes ± 1 SD; see also Fig. 5, which is published as supporting information on the PNAS web site.
Fig. 3.
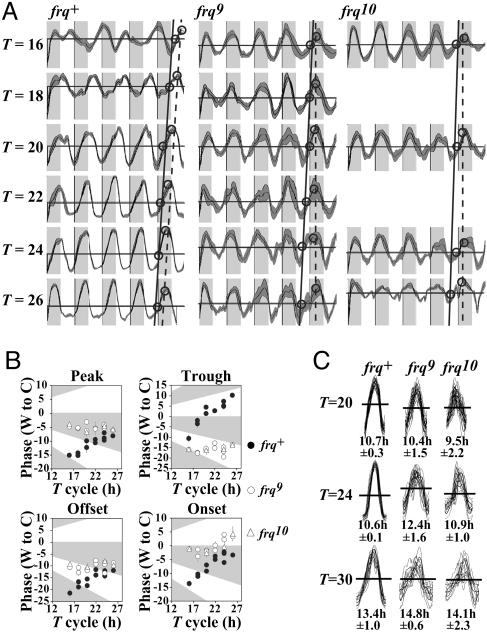
Choice of phase reference point influences the derived phase of the rhythm. (A) Data for frq+ and frq9 from Fig. 2 A, as well as data from the frq-null strain frq10 (10), are drawn with different T cycles scaled to the same size. As phase reference points, “onsets” (solid lines) and peaks (dotted lines, diagonal in frq+ and vertical in frq9 and frq10) were used and are drawn to show the trends. (B) The data from A, as well as corresponding data from all three laboratories, were analyzed with chrono by using phase reference points as shown. Statistics are shown in Table 1, whose data reveal a strong dependence of phase on T for frq+ (i.e., a line whose slope approaches or is >1) consistent with entrainment; in contrast, slopes close to 0, for the frq-null data tabulated, indicate a driven rhythm. Significance reflects the probability that the slope of the line is 0. (C) Conidial density from replicate tubes was averaged, and the profiles from each T were divided into individual days and superimposed. The average width of the bands from each T cycle was measured along the line used by chrono for onset determination and is indicated under each profile as average hours ± 1 SD.
Fig. 4.
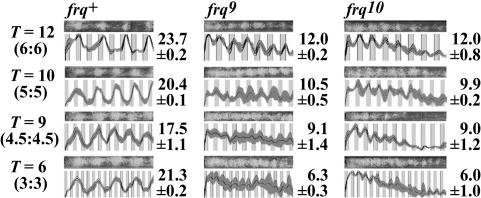
High-frequency temperature cycles cannot elicit demultiplication in the absence of functional frq. frq+ and frq-null strains were subjected to temperature cycles (T) whose durations ranged downward from the circadian range, as indicated on the far left of the figure. Whereas frq+ is able to demultiply to 18-, 20-, and 24-h periodicities, and free-runs in shorter duration cycles, both frq-null strains show conidiation being driven by temperature cycles for all T's applied. On the right of each profile is the period length (in hours) ± 1 SD estimated from at least six race tubes. Similar experiments yielded equivalent results independently in two laboratories; data reported are from one laboratory.
Results
Strains Lacking a Functional frq Gene Fail to Exhibit Circadian Entrainment to Temperature Cycles as Measured by Standard Criteria. In true entrainment of an oscillator to a cycle with discrete transitions, the period of the observed rhythm is the same as that of the entraining cycle, but the phase adopts a characteristic relationship with the entraining cycle: When the oscillator's free running period (FRP) is less than the duration of the entraining cycle (T), the oscillator's endogenous cycle will finish and begin again before the entraining cycle is complete, so that any point on the observed rhythm will phase-lead the corresponding point in entraining cycle, i.e., be phase-advanced by a number of hours equaling T - FRP (Fig. 1 A and B). Conversely, if FRP is longer than T, the endogenous rhythm will not have completed its cycle when the entraining cycle restarts, so observed rhythms will constantly phase-lag behind the entraining cycle by a number of hours equaling FRP - T (20, 21). These changes in phase angle are characteristic of entrainment and do not occur in the absence of an oscillator that can generate a free-running rhythm.
Primary data showing the behavior of clock-mutant and wild-type (WT) strains exposed to low-amplitude temperature cycles are shown in Fig. 1 C–E. In strains bearing functional frq alleles, frq2 (FRP ≈ 18 h), frq+ (FRP ≈ 21 h), and frq3 (FRP ≈ 24 h), peaks scan through the warm and cool periods, phase leading or lagging transitions depending on the relationship between the FRP and T (Fig. 1 C–E). However, this behavior is not seen in the frq-null allele frq9: Peaks and troughs of conidiation always occur during the cool and warm phases, respectively (see also Fig. 2). These data provide information concerning the nature of the oscillators responding to cycling temperature and suggest that there may be no need to invoke the existence of a FLO underlying the biological cycles observed in frq-null strains, even though the banding seen in these strains under temperature cycles shows a frq-less oscillation.
It is perhaps valuable to compare these rhythmic phenomena (see also ref. 15) with what occurs in a more typical case in light/dark cycles. In clock WT Neurospora cycling in darkness, minimal FRQ levels coincide with the peak in conidiation that normally occurs ≈11 h after the light-to-dark transfer. FRQ levels are high in extended light and fall upon transfer to darkness, with new synthesis of FRQ not observed until a half-circadian cycle later (≈10–14 h after the light-to-dark transition). The FRQ/WCC TTFL responds to shifts in temperature, with FRQ levels increasing rapidly as temperature rises and falling rapidly when the temperature decreases (22). Thus, in a WT strain subjected to 22-h temperature cycles, the shift from cool to warm after 11 h coincides with the FRQ minimum and reinforces the natural momentum of FRQ kinetics; the peak of the conidiation band occurs at the upward temperature transition (Fig. 1D). In longer temperature cycles (T > FRP; Fig. 1E), the peak must occur before the transition, because the negative feedback mechanism dictates when FRQ reaches its minimum; conversely, under shorter cycles (T < FRP; Fig. 1C) the peak occurs after the transition. For strains with functional circadian clocks but different period lengths, this explanation still holds. For example, in frq2 (FRP ≈ 18 h) the peak occurs at the cool-to-warm transition when the entraining cycle has 9 h at 22°C and 9 h at 27°C (9:9, Fig. 1C) and occurs before the transition in longer cycles (Fig. 1 D and E). Absent functional FRQ, temperature cycles still elicit cyclic spore production (Fig. 1 C–E and ref. 15), but in this case spore production appears to respond directly to temperature: Peaks of conidiation always occur in the cool sections of the cycle regardless of cycle length, although the duration of spore production increased with increasing cycle length. Generally, therefore, whether temperature is driving or entraining the clock can be assessed by comparing the phase of the rhythm when exposed to cycles of different period lengths (Fig. 1B).
To accurately assign the function of frq in the circadian system, we examined in greater detail the behavior of both the intact (frq+) circadian system and the FLO within a broad range of temperature cycles (Fig. 2). As before (Fig. 1 and ref. 15), 22°C/27°C temperature cycles were used (Fig. 2 A). It is clear that in frq+, rhythm peaks and troughs scan across the warm and cool periods depending on period length of the entraining cycle; this behavior exemplifies typical entrainment. In the FLO, exposed in a frq-null (frq9) strain, the peak of the rhythm occurs in the cool period, ≈5 h after the warm-to-cool transition, and similarly the trough of the rhythm invariably is found in the midst of the warm period. This invariant behavior indicates that the FLO does not show typical entrainment. All results from WT and frq9 strains from three different laboratories were pooled, and the peaks in the conidiation bands were plotted (Fig. 2B). In this manner of plotting the data, a sloped line reflects a systematic dependence of oscillator phase on the period of the temperature cycle, indicative of entrainment; a flat line reflecting no dependence of phase on T indicates a direct response to, but no apparent entrainment by, the temperature cycle. Whereas the clock WT strain shows a distinctly sloped line, phase data from the frq9 strain describe a line with a slope statistically indistinguishable from 0 (Fig. 2B). These data indicate a lack of entrainment in frq9 and are consistent with direct effects of temperature on development or with temperature cycles driving the (by definition) frq-less oscillation.
Rhythms in frq-Null Strains Are Driven by Temperature Changes. Additional experiments suggested that the rhythm in the null mutant was responding acutely to temperature steps and that without such steps it might cease to cycle altogether. This idea was examined in a final test of entrainment: Temperature cycles were applied for 4 days, and then the strains were left at the lower temperature for an additional half-cycle (e.g., for an extra 9, 12, or 15 h) before again raising the temperature and resuming regular temperature cycles (Fig. 2C). We predicted that this additional half-cycle exposure to cool temperatures would act as a brief release into constant conditions. In this case, a normal clock would continue to cycle until it was entrained by the reestablished T cycle; conversely, a strain whose conidiation was driven would cease to cycle until the T cycle was reestablished. These results were seen, respectively, in the frq+ and frq9 strains: The frq+ strain relaxed into its free-running 21-h periodicity and continued to cycle, rising during the extended cool interval; however, the frq9 strain stopped cycling during the extended cool interval, and the rhythm returned only after the resumption of the temperature cycle. See also Fig. 5.
Use of a Nonstandard Phase-Reference Point Can Yield Misleading Results. Previous studies also used temperature cycles to address the relationship between phase and cycle length (e.g., refs. 13 and 15) but reached conclusions different from ours. To understand the bases of these differences, we repeated the analysis of data shown here from the three laboratories using the chrono program (19). Also included were data generated with a strain bearing an alternative frq-null allele, frq10 (10). Each cycle from all period lengths was rescaled to the same width (equivalent ZTs) so that results across different cycle lengths could be more readily aligned and compared (see Fig. 4A). We measured phase relative to the warm-to-cool transition by using as phase reference points the standard measures of phase represented by the peaks and troughs of conidiation, as well as the nonstandard reference points of onsets and offsets (Fig. 3B, Table 1). As implemented in chrono, these are the points along the curves where the average level of conidiation is crossed (for details, see Materials and Methods and ref. 15). Because all reference points are surrogate markers of phase of the underlying oscillator, all should yield similar results to the extent that they faithfully report phase independently of other influences. Analyses using three of the four phase markers agreed in showing no dependence of phase on cycle length in frq-null strains, indicative of a lack of entrainment (Fig. 3B). This result also was confirmed by visual inspection of the primary data (Figs. 1 C–E and 2 A). However, by using onset as a reference point in frq-null strains, we observed a dependence of phase on the period of the driving cycle. This result would be unremarkable in and of itself, given the agreement in lack of entrainment by every other measure of phase. However, the onset of conidiation was the sole reference point used previously (13, 15) whose behavior led to the suggestion of circadian entrainment in frq-null strains. Why does the onset phase marker give results different from those obtained with the other phase markers? After close inspection of the primary data, we believe the resolution of the paradox lies in the realization that the waveform of the rhythm in each cycle is not symmetrical, and the temperature cycle is sculpting the waveform: The rate of the rise to the peak is quite different under different temperature cycles (Fig. 3C). Consequently, the apparent behavior of phase under changing conditions, when onset is used as the reference point (as in ref. 15), is affected by the changing waveform as well as by oscillator phase.
Table 1.
Statistical analysis of data in Fig. 3B
| Slope | SE | Significance* | |
|---|---|---|---|
| frq+ | |||
| Trough | 1.70 | 0.12 | 4.9 × 10-19 |
| Onset | 1.05 | 0.07 | 1.6 × 10-19 |
| Peak | 0.80 | 0.06 | 6.4 × 10-19 |
| Offset | 0.91 | 0.06 | 6.8 × 10-19 |
| frq9 and frq10 combined | |||
| Trough | 0.20 | 0.06 | 0.001 |
| Onset | 0.50 | 0.07 | 1.3 × 10-9 |
| Peak | -0.05 | 0.05 | 0.33 |
| Offset | 0.16 | 0.07 | 0.02 |
Data from all three laboratories (26 separate estimates of phase as a function of genotypes and T) were compiled, 11 for frq+ and 15 collectively for frq9 and frq10. Phase was estimated for each individual race tube from cycling during at least 4 days from a total of 51 race tubes for frq+ and 75 for frq9 and frq10.
To examine the effect of increasing cycle length on the shape of the curves, we plotted and superimposed the densitometric scans of the bands from each day of temperature cycles of 20, 24, and 30 h for both WT and frq-null strains (Fig. 3C). In frq-null strains in particular, there was a marked increase in band width with increasing cycle length. This result also was evidenced in the variability in the shape of the bands in the frq-null vs. WT strains. It clearly becomes problematic to identify phase-reference points that are biologically equivalent, other than peak and trough, among curves (bands) of different shape and size. More specifically, use of points along the rise of bands with different widths and slopes leads to different phase estimates, even though peak positions are constant, thereby confounding interpretation of the relationship between phase and cycle length.
Functional FRQ Is Required for Circadian Entrainment by Frequency Demultiplication. Many circadian oscillators can show entrainment to light or temperature cycles that are harmonics of the FRP, for instance, cycles that are about half the length of a normal circadian cycle (14, 20). This phenomenon, known as frequency demultiplication, affords an independent evaluation of the oscillator in frq-null strains as compared with WT. For instance, when clock WT Neurospora is exposed to cycles of 6-h warm/6-h cool, it will exhibit a 24-h rhythm just as if the entraining cycle was 12-h warm/12-h cool (20). We reasoned that this phenomenon would provide another independent test of whether the FLO can exhibit normal entrainment or would instead simply passively respond to the temperature cycles. The period length of the FLO in frq9 is uncompensated, so it depends on temperature and nutrition (8, 9) and can be as short as 12 h (8, 9, 15); alternatively, the frq-less oscillations have been modeled as arising from a noise-driven damped harmonic oscillator of 21-h period length (23). We thus used a range of cycle lengths, as short as 6 h (3:3), which would allow entrainment of a 12-h rhythm by demultiplication, and as long as 12 h (6:6), which would demultiply to yield entrainment to 24 h. As expected, clock WT (frq+) strains successfully entrained by demultiplication. Warm/cool cycles of 4.5/4.5 yielded a rhythm of ≈18 h, 5/5 cycles led to a rhythm of ≈20 h, and 6/6 cycles yielded a rhythm of ≈24 h (Fig. 4); 3/3 cycles could in principle demultiply to a 12-h rhythm, but 12 h is outside of the WT limits of entrainment, so the observed rhythm simply free runs at its normal FRP of ≈21 h. In contrast, we saw no evidence for frequency demultiplication in the frq-null strains to any cycle lengths in this range (see also ref. 13). Instead, at all frequencies, the rhythms observed in frq-null strains simply assumed the periodicity of the driving temperature cycle, again showing that strains lacking frq are incapable of normal entrainment. A similar loss of demultiplication-driven entrainment has been observed in per-null mutants of Drosophila (24).
Discussion
The developmental processes that culminate in the production of aerial hyphae and conidiation occur both in clock-enabled strains of Neurospora and strains bearing the null-alleles frq9 or frq10 that lack a circadian core TTFL. In the presence of frq-encoded functions, including those specified by any of several (nonnull) missense mutations in this gene (17, 18), conidiation is regulated in a manner bearing all of the hallmarks of a normal circadian rhythm: a robust self-sustained oscillation ≈21 h in length, with precise control of phase, period, and entrainment to environmental cues, as well as compensation of period against differences in ambient temperature or nutrition. Absent frq functions, all of this is lost. Nevertheless, rhythmic output from a frq-less oscillator still can sometimes be observed under permissive conditions (8–10). However, the overt rhythm controlled by such a FLO has lost its robustness, precision of phase, and all aspects of temperature and nutritional compensation; moreover, the period becomes highly variable among consecutive days and shortens with increasing temperature (8–10). However, a rhythm can be dependably visualized by exposing frq-null cultures to low-amplitude temperature cycles (15). This process makes it possible to ask whether a bona fide FLO underlies this aspect of frq-less rhythmicity by following surrogate markers of it, the various phase reference points. Among these, peaks and troughs are reliable and relevant to the biology of the organism. Onset, however, is not a reliable marker, because it is influenced directly by environmental factors as well as being putatively controlled by a FLO. In any case, by using temperature cycles and reference points of peak and trough as well as offset, we have shown that the FLO is not capable of normal circadian entrainment and, by inference, that the TTFL is required for such entrainment. Given these consistent results from standard reference points, the data suggest that the apparent entrainment seen by using onset is an artifact of the altered waveform. Our results also provide an alternative to “normal entrainment” (13, 15) as the interpretation of the behavior of frq-null strains in temperature cycles by showing how use of the onset reference point confounded those phase estimates. Additionally, because we show that the previous results (15) can be duplicated by using the onset reference point, it is unlikely that the discrepancy between our conclusions and those previously proposed lies in any subtle differences in strains, growth conditions, or experimental setup among different laboratories. Finally, we believe that our more extensive data provide more consistent and compelling conclusions.
The data obtained and analyzed in the current study are most consistent with the frq-less oscillation being driven by changes in temperature, because all our experiments have failed to find evidence for circadian entrainment of the FLO that was inferred to regulate these biological cycles (15). Absent these data, a role for this FLO as a “circadian rhythm generator,” as previously suggested (13, 16), seems unlikely. An extension of this conclusion is that the FRQ/WCC TTFL is not simply required for light input to a postulated temperature-entrainable oscillator (13) but has a central role in the circadian oscillator.
These considerations leave open the question of the identity of this temperature-influenced frq-less oscillation. At present, no known molecular components can be assigned to a FLO (9, 25), so one can only guess about its importance, and even its existence, in WT cells. Without temperature cycles, for instance, oscillations appear in only a fraction of frq-null cultures and only after several days of growth (8, 9, 25). An analogous phenomenon is known in the field of chemical oscillators: When conditions are changed in a mixture capable of robust oscillation, a delayed and sporadic appearance of oscillations often signals the organization of an alternative oscillatory state, one not formed when the regular components are present, which is nucleated after the system has passed an “induction period” (26). From this analogy, Christensen et al. (27) have suggested that a FLO may not normally exist and, instead, represents an unnatural oscillatory state formed when normal connections cannot be made. Alternatively, perhaps the FLO is always present but not always overtly manifest. Or it could reflect developmental circuits that normally are organized by the circadian circuitry but, absent a TTFL, can instead be ordered by temperature cycles. It is tempting to liken FLO to an hourglass that can be flipped at an environmental transition; the experiments in Fig. 2C in which FLO ceased to cycle as soon as the temperature cycle disappeared would be consistent with this hypothesis. However, when a FLO does appear during growth in constant conditions, the oscillation generally continues for several days (9, 25). This finding suggests that some oscillator, however weak, must still exist there to restart the cycle again each day.
More generally, it is now known that Neurospora harbors a number of FLOs that can appear in the absence of the TTFL (e.g., refs. 27–29 and reviews in refs. 7 and 18). Although none of these can yet be characterized as wholly circadian, and not any are known to be essential for expression of circadian rhythms, each can display some circadian characteristics. Our data do not preclude a role for other FLOs in the circadian system, but at present a simple and plausible view unifying all of the FLOs is that these are oscillators that can be coupled to a FRQ/WCC- associated circadian core (7). Absent the FRQ/WCC loop, they can run on their own in a noncircadian manner. Whether such FLOs evolved before or after the circadian mechanism, and what their contribution is, if any, to the normal circadian program, are questions that remain unanswered.
Supplementary Material
Acknowledgments
We thank Carl Johnson and Martin Zatz for advocating this collaboration to reexamine “entrainment” in frq-null strains, and the members of the Neurospora and rhythms communities who have provided input or commented on the data. This work was supported by National Institutes of Health Grants MH44651 (to J.C.D. and J.J.L.), R37 GM34985 (to J.C.D.), and NS39546 and GM58529 (both to D.B.P.); National Science Foundation Grant MCB-0084509 (to J.J.L.); Biotechnology and Biological Sciences Research Council Grant 34/G17511, Nuffield Foundation Grant NAL/00493/G, and a grant from the University of Manchester Fund (to C.H.).
Author contributions: A.M.P. and N.P.-L. designed research; A.M.P., N.P.-L., D.B.-P., C.H., and J.C.D. performed research; A.M.P. N.P.-L., D.B.-P., C.H., J.J.L., and J.C.D. analyzed data; and A.M.P., D.B.-P., C.H., J.C.D., and J.J.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FLO, FRQ-less oscillator; FRP, free running period; TTFL, transcription and translation feedback loop; ZT, Zeitgeber time.
References
- 1.Dunlap, J. C. (1998) Science 280, 1548-1549. [DOI] [PubMed] [Google Scholar]
- 2.Young, M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 2, 702-715. [DOI] [PubMed] [Google Scholar]
- 3.Sehgal, A. (2003) Molecular Biology of Circadian Rhythms (Wiley, New York).
- 4.Pittendrigh, C. S. (1960) Cold Spring Harbor Symp. Quant. Biol. 25, 159-184. [DOI] [PubMed] [Google Scholar]
- 5.Heintzen, C., Nater, M., Apel, K. & Staiger, D. (1997) Proc. Natl. Acad. Sci. USA 94, 8515-8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Froehlich, A. C., Loros, J. J. & Dunlap, J. C. (2003) Proc. Natl. Acad. Sci. USA 100, 5914-5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlap, J. C. & Loros, J. J. (2004) J. Biol. Rhythms 19, 414-424. [DOI] [PubMed] [Google Scholar]
- 8.Loros, J. J., Richman, A. & Feldman, J. F. (1986) Genetics 114, 1095-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loros, J. J. & Feldman, J. F. (1986) J. Biol. Rhythms 1, 187-198. [DOI] [PubMed] [Google Scholar]
- 10.Aronson, B. D., Johnson, K. A. & Dunlap, J. C. (1994) Proc. Natl. Acad. Sci. USA 91, 7683-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McWatters, H., Dunlap, J. C. & Millar, A. (1999) Curr. Biol. 9, 633-635. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki, H. & Dunlap, J. C. (2000) Curr. Opin. Microbiol. 3, 189-196. [DOI] [PubMed] [Google Scholar]
- 13.Roenneberg, T. & Merrow, M. (2001) in Zeitgebers, Entrainment and Masking of the Circadian System, eds. Honma, K. & Honma, S. (Hokkaido Univ. Press, Sapporo, Japan), pp. 113-129.
- 14.Bruce, V. G. (1960) Cold Spring Harbor Symp. Quant. Biol. 25, 29-48. [Google Scholar]
- 15.Merrow, M., Bruner, M. & Roenneberg, T. (1999) Nature 399, 584-586. [DOI] [PubMed] [Google Scholar]
- 16.Roenneberg, T. & Merrow, M. (1999) J. Biol. Rhythms 14, 449-459. [DOI] [PubMed] [Google Scholar]
- 17.Loros, J. J. & Dunlap, J. C. (2001) Annu. Rev. Physiol. 63, 757-794. [DOI] [PubMed] [Google Scholar]
- 18.Dunlap, J. C., Loros, J. J., Denault, D., Lee, K., Froehlich, A. F., Colot, H., Shi, M. & Pregueiro, A. (2004) in The Mycota III, eds. Brambl, R. & Marzluf, G. A. (Springer, Berlin), pp. 209-229.
- 19.Roenneberg, T. & Taylor, W. (2000) Methods Enzymol. 305, 104-119. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, C. H., Elliott, J., Foster, R., Honma, K.-I. & Kronauer, R. (2004) in Chronobiology: Biological Timekeeping, eds. Dunlap, J. C., Loros, J. J. & Decoursey, P. (Sinauer, Sunderland, MA), p. 406.
- 21.Aschoff, J., ed. (1981) in Biological Rhythms: Handbook of Behavioral Neurobiology (Plenum, New York), Vol. 4, pp. 81-93. [Google Scholar]
- 22.Liu, Y., Merrow, M., Loros, J. J. & Dunlap, J. C. (1998) Science 281, 825-829. [DOI] [PubMed] [Google Scholar]
- 23.Bain, E. L., Millar, A. J. & Turner, M. S. (2004) J. Theor. Biology 229, 413-420. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler, D. A., Hamblen-Coyle, M., Dushay, M. & Hall, J. C. (1993) J. Biol. Rhythms 8, 67-94. [DOI] [PubMed] [Google Scholar]
- 25.Loros, J. J. (1986) Dissertation (University of California, Santa Cruz, CA).
- 26.Field, R. J. & Burger, M. (1985) Oscillations and Traveling Waves in Chemical Systems (Wiley, New York). [DOI] [PubMed]
- 27.Christensen, M., Falkeid, G., Hauge, I., Loros, J. J., Dunlap, J. C., Lillo, C. & Ruoff, P. (2004) J. Biol. Rhythms 19, 280-286. [DOI] [PubMed] [Google Scholar]
- 28.Correa, A., Lewis, Z. A., Greene, A. V., March, I. J., Gomer, R. H. & Bell-Pedersen, D. (2003) Proc. Natl. Acad. Sci. USA 100, 13597-13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakin-Thomas, P. (1998) J. Biol. Rhythms 13, 268-277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.