Abstract
Objective
We developed a specific cognitive–existential intervention to improve existential distress in nonmetastatic cancer patients. The present study reports the feasibility of implementing and evaluating this intervention, which involved 12 weekly sessions in both individual and group formats, and explores the efficacy of the intervention on existential and global quality of life (QoL) measures.
Method
Some 33 nonmetastatic cancer patients were randomized between the group intervention, the individual intervention, and the usual condition of care. Evaluation of the intervention on the existential and global QoL of patients was performed using the existential well-being subscale and the global scale of the McGill Quality of Life (MQoL) Questionnaire.
Results
All participants agreed that their participation in the program helped them deal with their illness and their personal life. Some 88.9% of participants agreed that this program should be proposed for all cancer patients, and 94.5% agreed that this intervention helped them to reflect on the meaning of their life. At post-intervention, both existential and psychological QoL improved in the group intervention versus usual care (p = 0.086 and 0.077, respectively). At the three-month follow-up, global and psychological QoL improved in the individual intervention versus usual care (p = 0.056 and 0.047, respectively).
Significance of results
This pilot study confirms the relevance of the intervention and the feasibility of the recruitment and randomization processes. The data strongly suggest a potential efficacy of the intervention for existential and global quality of life, which will have to be confirmed in a larger study.
Keywords: Cognitive–existential intervention, Existential, Quality of life, Cancer
INTRODUCTION
Quality of life is defined as a complex combination of physical, psychological, social, and existential well-being (Wilson & Cleary, 1995). A cancer diagnosis may disrupt these various dimensions in many different ways (e.g., pain, psychological distress, financial burden) (Yalom, 1980; Trijsburg et al., 1992; Fawzy et al., 1995; Lepore, 2001; Wong, 1997; Fillion et al., 2006; Andersen, 1992; Fawzy, 1995; Fawzy & Fawzy, 1998; Goodwin, 2004). The existential dimension is an integral part of human experience, including but not limited to spiritual and religious aspects, which attempts to address questions about human existence and all that is connected to one’s reason for being (Yalom, 1980; LeMay & Wilson, 2008; Goodwin et al., 2001; Hellman et al., 1990). The concept of existential distress remains more universal and neutral than the concepts of spiritual distress or religious crisis. People diagnosed with a life-threatening disease such as cancer may have to learn to cope with loss of meaning and empowerment, which can compromise quality of life (Aaronson et al., 1993; Parle & Maguire, 1995; Somerfield, 1997; Manne et al., 1994). Questions regarding “Why me?” along with universal existential concerns about death, loneliness, freedom and autonomy, finitude, dignity, losses and changes, the search for meaning, mystery, quality of relationships, and a sense of control over one’s life, often constitute the principal source of overall suffering (Sauer & Seitz, 1988; Cole & Pargament, 1999; Park & Folkman, 1997; Moadel et al., 1999; Spiegel & Classen, 2000; Yalom, 1980; Mok et al., 2010; Frankl, 1997; Kissane, 2012). Since there is no single and identifiable cause of cancer, those existential questions are commonly observed among patients who demand specific interventions to properly address this central issue (Réseau canadien du cancer du sein et l’Initiative Ontarienne de Recherche Communautaire sur le cancer du sein, 2003). In fact, the search for meaning is a significant need among more than 40% of those with cancer (Moadel et al., 1999; Fawzy et al., 1995).
The cognitive–behavioral approach has revealed itself to be useful for improving emotional quality of life (QoL) and for development of active coping strategies (Chochinov, 2000; Cunningham, 2002). Psychological interventions have been shown to effectively treat emotional distress but usually fail to address the existential dimension (Trijsburg et al., 1992; Lindemalm et al., 2012; Fawzy et al., 1995; Jacobsen & Hann, 1998; Levin & Kissane, 2006; Andersen, 1992; Fawzy & Fawzy, 1998; Fekete et al., 2007; Le-May & Wilson, 2008; Sheard & Maguire, 1999). Several studies have emphasized that the existential dimension should be addressed in psychotherapy within the scope of a life-threatening illness (Spiegel & Classen, 2000; Yalom, 1980; Classen et al., 2001). The existential approach addresses a central issue of survivorship in cancer and can be employed to help patients find meaning in the middle of a crisis, which appears promising for improving existential and global quality of life (Lee et al., 2006). While existential interventions are sometimes proposed for advanced cancer patients, nonmetastatic cancer patients also have existential and spiritual needs that often remain unexplored, unfulfilled, and understudied (LeMay & Wilson, 2008; Moadel et al., 1999). Interventions including existential dimensions (e.g., support–expressive groups) suffer from several limitations (Spiegel & Classen, 2000; Classen et al., 2008; Breitbart, 2002; Breitbart et al., 2004; Spiegel et al., 1989; Xiao et al., 2013), usually including a lack of demonstrated efficacy in reducing existential distress. In addition, as a limitation, many studies focus on breast cancer, and consequently solely on women (Kissane et al., 1997; 2003; 2004a,b; 2007; Cunningham et al., 1998; Spiegel et al., 1999; Classen et al., 2001; 2008). Other studies have indicated different points to improve in existential studies, such as contrasting individual versus group format (Breitbart et al., 2010), a recommendation that we followed in the present study. There is also empirical support for interventions combining both cognitive–behavioral and existential approaches aimed at decreasing emotional distress in cancer patients (Jacobsen & Hann, 1998; LeMay & Wilson, 2008; Fekete et al., 2007; Cunningham, 2005; Chochinov et al., 2005; Kissane et al., 2003). Very few studies have tested the efficacy of interventions that include both an emotional and an existential dimension (Cunningham, 2005; Gagnon et al., 2008; Kissane, 2000; Kissane et al., 2003). The literature points to the relevance of combining cognitive–behavioral and existential approaches (Breitbart, 2002; Cunningham, 2005).
As the model of Cunningham (2005) suggests and as we noticed in our population, patients first need to get some sense of control and understanding of their situation, hence the components of education and cognitive behavioral therapy at the beginning of an intervention. This can naturally and smoothly pave the way for a more existential component that follows, though it remains present throughout the intervention.
This paper reports the results of a randomized controlled trial pilot study of a cognitive–existential intervention aimed at improving existential and global quality of life in cancer patients. Our primary objectives were: (1) to evaluate the feasibility of the recruitment and randomization processes, the acceptability of the intervention format and content, the dropout rate, and the overall satisfaction of participants; and (2) to explore statistical trends in existential and global QoL measures according to the assigned condition with nonmetastatic cancer patients.
For the first objective, the research questions were: Is the recruitment and randomization process feasible? Is the intervention format and content acceptable? Is the dropout rate acceptable? Are the participants satisfied with the intervention? For the second objective, the hypothesis was that participants in the experimental groups (group and individual cognitive–existential intervention) would record higher scores on the existential and global QoL measures, as compared to those in the usual-care group.
METHODS
Study Design
A longitudinal design was chosen for the first and second objectives. The repeated measurement format included six test timepoints: baseline (T0), mid-intervention (6 weeks; T1), post-intervention (12 weeks; T2), 3-month follow-up (T3), 6-month follow-up (T4), and 12-month follow-up (T5). For the first objective, a descriptive approach utilizing quantitative indicators was employed to evaluate the feasibility and acceptability of a cognitive–existential intervention (CEI). For the second objective, a randomized controlled trial was designed to pretest the efficacy of the CEI on existential and global QoL.
For efficacy pretesting, patients were randomized into one of three experimental conditions: (1) the group cognitive–existential intervention, consisting of 12 weekly 2-hour sessions, (2) the individual CEI, including 12 weekly 1-hour sessions, or (3) the usual-care condition without CEI. The necessary time to fill out questionnaires was 30 to 45 minutes at T0 and 20–30 minutes for the other timepoints (T1–T5). Participants and research assistants were blinded at baseline. Participants received an explanation of the three experimental conditions. Randomization to study conditions was performed after completion at T0. To increase participant retention in the usual-care condition, participants received the CEI manual as an incentive at T3. A shortened version of the intervention (four unstructured group meetings with a therapist of the program) was offered at T3 as well. There was a high risk that patients would not adhere to the follow-up questionnaires if no meetings were proposed. Accordingly, the last two data collection points and analyses (T4 and T5) were only exploratory and descriptive.
Recruitment
People were recruited from all over the Québec City area using different strategies (healthcare professionals, advertisements posted at participating centers and information through local media). The inclusion criteria were: (1) to be at least 18 years of age, (2) to speak French, and (3) to have received a diagnosis of nonmetastatic cancer. The only exclusion criterion was a depressive mood (score above 10 on the Hospital Anxiety and Depression Scale’s depressive subscale; Zigmond & Snaith, 1983), which could interfere with the intervention (Scogin et al., 2007; Savard et al., 1998; Yang et al., 2009). As a matter of fact, depression can lead to a state that is not conducive to properly address existential issues; a patient with more severe depressive symptoms must first regain control of their emotions to actively embark on existential therapy, especially in a group format, which at times might bring up more anxiogenic issues.
The Intervention
The intervention was developed during a two-year period by a multidisciplinary committee of experts that included social workers, psychologists, spiritual care providers, psychiatrists, nurses, a nurse navigator, and co-investigators. We designed the intervention as a 12-week cognitive–existential intervention comprised of 12 modules (Gagnon et al., 2008). Deciding on the length of an intervention is always a difficult issue, raising both conceptual and pragmatic questions. This 12-session format was chosen over a longer one so as to reflect clinical reality and to provide a more cost-effective intervention. A metaanalysis suggested that short-term interventions with qualified therapists are more effective than long-term ones with less well-trained therapists (Sheard & Maguire, 1999). Some studies have proposed interventions that are clearly too long (Jacobsen & Hann, 1998; Cunningham, 2002; Cunningham et al., 2000; 1998; Edmonds et al., 1999; Edwards, 2004; Goodwin et al., 2001; Kissane et al., 1997; 2003; Classen et al., 2001), usually a year or more, to be integrated into the current healthcare system. We had already designed a four-session clinical intervention based on cognitive–behavioral therapy, which we employed along with Breitbart’s eight-session existential model, which resulted in the final 12-session model. The final model carries an intrinsic pragmatic value since it can be easily integrated within a normal “session” of the calendar, fall or winter/spring, thus avoiding overlapping summer and the holiday season, when several absentees were expected. Figuratively speaking, one participant commented, “We spent a day together” (12 sessions × 2 hours = 24 hours).
The first module is an introduction of the 12-week intervention that presents the 12 modules and explains the functioning of the group in accordance with the randomization. The first three modules involve cognitive–behavioral techniques promoting the use of behavioral (e.g., relaxation, activation) and emotional (e.g., cognitive restructuring) coping strategies. The next three modules, inspired by empirically validated interventions (Cunningham, 2002), further explore emotional strategies. The final six modules specifically address the existential dimension. They were modified from logotherapy techniques initially developed by Frankl (1997), adapted for our study intervention, and empirically tested by Breitbart and colleagues (Breitbart, 2002; Breitbart et al., 2004). The existential component was further adapted for a French-Canadian population by our team (Fillion et al., 2006). All 12 modules were standardized in two manuals. Following Breitbart’s structure, the participant manual included didactic material, exercises, and home assignments, while the manual for therapists included instructions for facilitating the sessions and a section dedicated to training. The training sessions were led by two healthcare professionals (psychiatrist and psychologist). Regardless of the modality (group or individual), the content remained identical. Individual and group sessions were video- or audiotaped to ascertain intervention integrity. Supervisory meetings were held on a regular basis with the therapists and the principal investigator to provide a rigorous application of the intervention.
Questionnaires
Global Satisfaction Questionnaire
The Global Satisfaction Questionnaire is a 20-item questionnaire with a 5-point Likert-type scale and 4 short questions that measures the global satisfaction of the patients who received the intervention.
McGill Quality of Life Questionnaire (MQoL)
The MQoL is a patient-reported instrument that employs 16 items plus a single-item global scale specifically developed to measure the quality of life of patients at all stages of a life-threatening illness, from diagnosis to cure or the palliative stage (Cohen et al., 1997; 1995). Four subscales corresponding to four QoL domains (physical well-being, psychological well-being, existential well-being, and social support) are included. An overall index score can be calculated from the means of the four subscales. Pearson correlation coefficients between MQoL data and a self-reported change scale ranged from 0.56 (existential well-being) to 0.66 (total MQoL score). The strongest evidence of validity comes from a comparison with the single-item QoL measure. The correlation with this single-item instrument (measured after completing the full questionnaire) was highest with total MQoL (0.66), followed by physical well-being (0.59), existential well-being (0.53), psychological well-being (0.44), and social support (0.41). The internal consistency of the 16-item MQoL instrument and the subscales was examined and found to be good (Cronbach’s α of 0.86 for the existential well-being subscale and 0.83 for the global subscale) (Cohen et al., 1997). This tool was validated in both French and English with similar psychometric properties.
Statistical Analysis
For the first objective, data were tabulated and summarized using descriptive statistics. For the second objective, to pretest the efficacy of the CEI, data were analyzed with intention to threat. Mixed-model repeated measures analysis of covariance was employed to compare the post-intervention and follow-up QoL means as measured by the psychological well-being and existential well-being subscales of the MQoL, while controlling for baseline values. The global QoL was analyzed in similar fashion. This model controls for heterogeneous variances and correlations among the measures assessed at different time intervals. The finding of significant differences between groups was followed by point and interval estimations of the mean differences using the Bonferroni correction for multiple comparisons. Means and standard deviations were provided for continuous variables. Counts and percentages were listed for categorical variables. Most statistical analyses were performed using SAS software (v. 9.1). Effect sizes were calculated using GPower (v. 3.1). All tests were performed with an overall significance level of 5%, i.e., a p value less than 0.05 was considered statistically significant.
RESULTS
Participants (see Fig. 1 and Table 1)
Fig. 1.
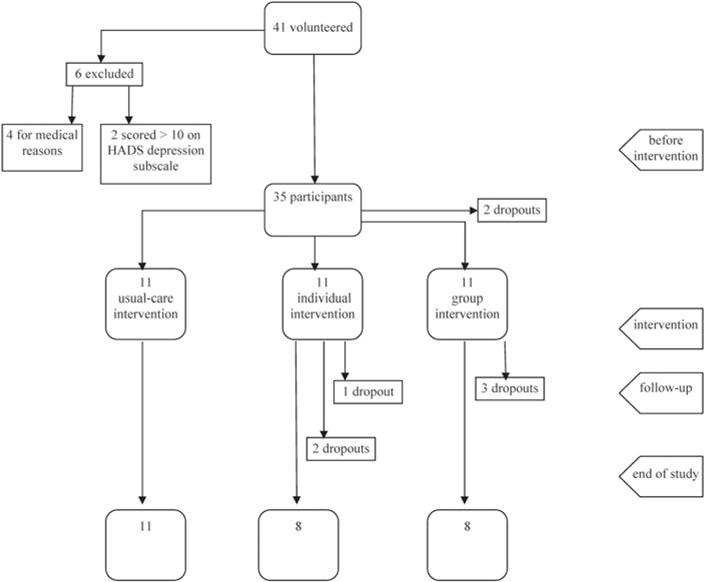
Flowchart of participants.
Table 1.
Sociodemographic characteristics of the participants (n = 33)
| n (%) | |
|---|---|
| Age | 33 |
| Mean ± SD | 58.4 ± 9.3 |
| Sex | 33 |
| Female | 21 (63.6) |
| Male | 12 (36.4) |
| Education (highest level) | 33 |
| Primary/secondary | 6 (18.2) |
| Post-secondary/college | 7 (21.2) |
| University | 20 (60.6) |
| Employment | 33 |
| Retired | 13 (39.4) |
| Sick leave | 8 (24.2) |
| Working | 6 (18.2) |
| Others | 5 (15.2) |
| Not specified | 1 (3.0) |
| Tumor type | 38 |
| Breast | 12 (31.6) |
| Prostate | 7 (18.4) |
| Head and neck | 3 (7.9) |
| Renal | 3 (7.9) |
| Gastrointestinal | 3 (7.9) |
| Others | 10 (26.3) |
| Actively in treatment? | 33 |
| Yes | 10 (30.3) |
| No | 23 (69.7) |
During a two-week period, 41 potentially eligible participants volunteered to participate in our study. Six were excluded from the study: four for medical reasons (metastatic cancer) and two for presenting a depressive mood state. Some 35 nonmetastatic cancer patients were randomized to each experimental condition, using an equal allocation ratio (1:1:1). Two participants dropped out before the first session, so that 33 participants actually began the intervention. The mean age of participants was 58 years (from 37 to 77 years; ±9.3 years), and 64% were women. Some 18% had a high school degree or less, 21% had a professional or college degree, 39% had a bachelor’s degree, and 21% had a master’s or doctorate degree. Some 41% of participants were retired, 25% were on sick leave, 19% were currently working, and 16% were unemployed. Some 32% of participants had breast cancer, 18% had prostate cancer, 8% had head and neck cancer, 8% had gastrointestinal cancer, 8% had renal cancer, and 26% had some other type of cancer. Fully 70% of participants were not undergoing active medical treatment during the study.
Feasibility and Acceptability Indicators
Recruitment and Adherence
The adherence rate for the protocol was 95.5%. Participants attended sessions 94% of the time, and the completion rate for the questionnaires was 96.7%. The dropout rate during the intervention was 12.1% (3 in the group and 1 in the individual intervention) and 6.1% at follow-up (2 in the individual intervention), for a total of 18.2%. Patients were aware of the three conditions before randomization. The first module is an introduction to the 12-week intervention. The 12 modules are present and the functioning of the group is explained; hence, the preparation, consisting of instructions given during the first meeting, could only be realized after the randomization. Reasons for dropping out included external factors (car accident, death, timetable conflict, new treatments) and differences in expectations about the content of the intervention. Since it was a pilot study, a focus group with therapists was conducted and the fidelity in application of the intervention was judged to be satisfactory on a qualitative evaluation by a consensus conference. We did not proceed with quantitative data to evaluate this aspect.
Satisfaction
All participants agreed that their participation in the program helped them deal with both their illness and their personal life. They also stated that the topics were interesting, the activities pleasant, and that the themes addressed were relevant. In addition, 88.9% of participants agreed that the intervention should be proposed to all cancer patients, and 94.5% agreed that the intervention helped them to reflect on the meaning of their lives. Furthermore, all participants agreed that the therapists mastered the procedures and made the content interesting and lively. Participants allowed themselves to be video- and/or audiotaped, which allowed us to document that the intervention was implemented as described in the treatment manual. No negative or unexpected psychological reactions were observed or reported.
Efficacy Outcomes
Improving Quality of Life
Our results showed a significant trend on the McGill existential well-being subscale (Table 2). Some differences between the intervention groups and the usual-care group were observed at post-intervention (T2) and at the 3-month follow-up (T3). At post-intervention (T2), existential and psychological QoL improved in the group intervention versus usual care (existential well-being: t(19) = 0.91, p = 0.086; psychological well-being: t(19) = 0.25, p = 0.077, respectively). At the 3-month follow-up (T3), when comparing individual CEI to the usual-care condition, global QoL improved (t(18) = 1.93, p = 0.056) and psychological QoL improved significantly (t(18) = 2.01, p = 0.047). Furthermore, when effect sizes were calculated, 67 and 83% of the relationships showed large and combined medium and large effect sizes, respectively (see Table 2).
Table 2.
Simple summary statistics and comparison of the pilot study post-intervention means (T2, 12 weeks) and 3 months later (T3, 24 weeks) for the McGill Quality of Life Questionnaire (MQoL)
| Variable (Condition) | T0, Baseline
|
T2, 12 Weeks
|
T3, 24 Weeks
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | Contrast | t (df) | p | d | n | Mean ± SD | Contrast | t (df) | p | d | |
| Psychological well-being | |||||||||||||
| Usual care (U) | 6.3 ± 2.8 | 11 | 6.0 ± 2.9 | U vs. I | 0.25 (19) | 0.800 | 0.14 | 11 | 4.7 ± 2.6 | U vs. I | 2.01 (18) | 0.047 | 0.91 |
| Individual (I) | 6.7 ± 1.6 | 10 | 6.4 ± 3.0 | I vs. G | 1.51 (16) | 0.133 | 9 | 7.1 ± 2.7 | I vs. G | 0.34 (15) | 0.731 | ||
| Group (G) | 6.8 ± 2.3 | 8 | 8.1 ± 1.8 | G vs. U | 1.78 (17) | 0.077 | 0.87 | 8 | 6.5 ± 2.8 | G vs. U | 1.51 (17) | 0.117 | 0.67 |
| Existential well-being | |||||||||||||
| Usual care (U) | 7.2 ± 1.4 | 11 | 6.6 ± 1.6 | U vs. I | 0.91 (19) | 0.365 | 0.39 | 11 | 6.3 ± 2.4 | U vs. I | 1.27 (18) | 0.207 | 0.54 |
| Individual (I) | 6.9 ± 1.4 | 10 | 7.3 ± 2.0 | I vs. G | 0.86 (16) | 0.394 | 9 | 7.3 ± 1.1 | I vs. G | 0.20 (15) | 0.842 | ||
| Group (G) | 6.6 ± 1.8 | 8 | 8.1 ± 1.0 | G vs. U | 1.73 (17) | 0.086 | 1.1 | 8 | 7.4 ± 1.7 | G vs. U | 1.43 (17) | 0.155 | 0.53 |
| Global | |||||||||||||
| Usual care (U) | 6.6 ± 1.4 | 11 | 6.2 ± 2.2 | U vs. I | 0.06 (19) | 0.955 | 0.05 | 11 | 5.5 ± 1.6 | U vs. I | 1.93 (18) | 0.056 | 1.06 |
| Individual (I) | 6.5 ± 1.2 | 10 | 6.3 ± 2.1 | I vs. G | 0.75 (16) | 0.458 | 9 | 7.1 ± 1.4 | I vs. G | 0.65 (15) | 0.516 | ||
| Group (G) | 6.2 ± 1.7 | 8 | 7.0 ± 0.8 | G vs. U | 0.81 (17) | 0.420 | 0.48 | 8 | 6.5 ± 1.6 | G vs. U | 1.19 (17) | 0.238 | 0.63 |
DISCUSSION
The goal of our pilot study was to evaluate the feasibility and acceptability of the cognitive–existential intervention and to pretest its efficacy to improve existential and global QoL.
Our results confirmed the feasibility of the study in terms of: recruitment; randomization to group intervention versus individual intervention versus usual care; training of therapists; delivery, standardization, and quality control of the intervention, including supervision by senior therapists; video- and audiotaping; adverse reactions, acceptability, and interest pertaining to the intervention, including participant satisfaction, duration of the program (12 weeks), length and content of sessions, level of therapist activity, and relevance of the manual distributed; acceptability and time needed to administer the instruments; and adherence to follow-up measures. Data were also provided to show the potential impact on existential and global quality of life and allowed calculation of the study sample size required for a full-scale efficacy study.
We found that existential concerns addressed in the CEI are relevant to cancer patients and that there is a desire to discuss these different topics with health professionals. The intervention was well received, and participant comments indicate that they considered their experience in the research project to be life changing.
The results obtained on selected outcomes also suggest the efficacy of the CEI to potentially impact existential and global QoL. The statistical analyses revealed some improvement trends in existential and global quality of life. On the post-intervention measure (T2), participants randomized in the group condition had higher existential well-being and fewer psychological symptoms than participants in the usual-care group. At the three-month post-intervention follow-up, participants assigned to the individual CEI group had better global and psychological quality of life than patients assigned to the usual-care condition. Compared to participants in the usual-care condition, those receiving group CEI also had higher existential, psychological, and global QoL.
Even if statistically significant differences were not found on the existential well-being subscale of the MQoL, the medium and large effect sizes indicated that a larger sample would be able to demonstrate statistically significant differences between the usual-care and intervention groups. Patients in group and individual interventions tended to have a better existential QoL than usual-care patients at post-intervention (T2) and at 3-month follow-up (T3). The cognitive–existential intervention may be associated with therapeutic gain in existential, psychological, and global quality of life. However, a larger sample size is required to further investigate these findings.
A larger effect size for the group intervention may reflect the therapeutic mechanisms usually described in group formats, such as sharing of experiences and strategies, including sources of meaning, support between peers, universalization, and emotional expression. It may also depend partially on such non-specific factors as more intense involvement and therapeutic work, as well as the longer time spent in group (24 hours) versus individual formats (12 hours). There is also an increase in the control arm, which is not surprising considering that the simple fact of going through the recruitment and follow-up processes may provide some nonspecific support and prompt patients to seek support outside the protocol.
LIMITATIONS
Our sample was clearly biased toward more educated, better-functioning patients, though we strove to include patients with varied educational backgrounds. The content of the intervention and the commitment required seem to fit better with better-educated patients, at least for the recruitment process. Hence, efforts have to be undertaken to improve the therapeutic goals of the therapy, which could benefit all patients struggling with universal existential issues.
It is well known that women are more likely to seek psychological help than men. This bias may explain the larger number of women recruited for our study.
We chose to exclude patients with a higher level of depression in order to increase the homogeneity of the group and avoid the presence of highly distressed patients who could destabilize such a small group. This is a difficult decision since we have to balance, on one hand, the specific goals of the therapy with, on the other hand, a research and public-health perspective to show results for more distressed patients. Some authors argue to include more distressed patients. Kissane and colleagues showed that supportive–expressive group therapy not only improved depressive symptoms but actually prevented the development of new cases of depression (Kissane et al., 2007). In addition, psychotherapeutic researchers and the National Institutes of Health have been insisting that distressed patients be targeted for intervention studies so as to optimize effect sizes and eliminate the flooring effect introduced by inclusion of resilient and well-functioning patients. This has to be carefully evaluated with each trial.
The act of providing the manual and the possibility of attending four informational sessions at T3 reduces the comparison power of the control arm. This remains a difficult editorial choice. The advantages involve increasing the inclusion rate and enhancing adherence to the protocol. It also allows exploration of the relative efficacy of a very short, cheap, and informational intervention mainly based on bibliotherapy. Once again, the researcher has to prioritize the different requirements of psychotherapeutic research through a randomized trial with the different goals of showing efficacy and effectiveness, and testing many modalities and feasibility. It is especially timely to undertake these different goals during a pilot trial.
CONCLUSION
The cognitive–existential intervention appears feasible, acceptable, and enabling of quality of life. This intervention possesses many advantages. It integrates the existential dimension into medical care. It considers the person in his entirety (physical, psychological, social, existential, and spiritual spheres), and thus contributes to the humanization of care. It offers a new outlook for ill persons and their families to cope with the difficulties faced over the course of the illness and in its aftermath. It provides tools for mental health professionals to intervene on a dimension usually not addressed by standard psychotherapeutic interventions. It constructs links and dialogue among the medical, psychosocial, and spiritual disciplines. This intervention is in concordance with the values of modern oncology: empowerment of people through the development of skills and active coping strategies; comprehensive treatment of patients with cancer in all their dimensions; and flexible, transposable interventions with an impact at all points along the care continuum.
From a clinical point of view, this intervention helps patients adopt a sensible, reflective, dignified, and courageous attitude in the face of inevitable suffering. Instead of passive endurance, they can choose to go deeper in search of a meaning in their relationships, in the here and now, and in their engagements. It affords them the opportunity to appreciate the beauty that surrounds them; to revisit and review their values; to be aware of their own finitude and “detoxify” death; to explore transcendence and spirituality; and even, for some patients, to “develop a user’s guide to better living.”
The next step in the validation process of this intervention should be to evaluate the efficacy of the CEI with a larger sample of cancer patients in order to detect statistically significant changes in existential and global quality of life.
Acknowledgments
The project was supported by the Canadian Institutes of Health Research through NET End-of-Life Care. We are indebted to the patients, therapists, and nursing and medical staff for their invaluable support and assistance in carrying out this study.
References
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ–C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Andersen BL. Psychological interventions for cancer patients to enhance the quality of life. Journal of Consulting and Clinical Psychology. 1992;60(4):552–568. doi: 10.1037//0022-006x.60.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W. Spirituality and meaning in supportive care: Spirituality- and meaning-centered group psychotherapy interventions in advanced cancer. Supportive Care in Cancer. 2002;10(4):272–280. doi: 10.1007/s005200100289. [DOI] [PubMed] [Google Scholar]
- Breitbart W, Gibson C, Poppito SR, et al. Psychotherapeutic interventions at the end of life: A focus on meaning and spirituality. Canadian Journal of Psychiatry. 2004;49(6):366–372. doi: 10.1177/070674370404900605. [DOI] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Gibson C, et al. Meaning-centered group psychotherapy for patients with advanced cancer: A pilot randomized controlled trial. Psycho-Oncology. 2010;19(1):21–28. doi: 10.1002/pon.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chochinov HM. Psychiatry and terminal illness. Canadian Journal of Psychiatry. 2000;45(2):143–150. doi: 10.1177/070674370004500204. [DOI] [PubMed] [Google Scholar]
- Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: A novel psychotherapeutic intervention for patients near the end of life. Journal of Clinical Oncology. 2005;23(24):5520–5525. doi: 10.1200/JCO.2005.08.391. [DOI] [PubMed] [Google Scholar]
- Classen C, Butler LD, Koopman C, et al. Supportive–expressive group therapy and distress in patients with metastatic breast cancer: A randomized clinical intervention trial. Archives of General Psychiatry. 2001;58(5):494–501. doi: 10.1001/archpsyc.58.5.494. [DOI] [PubMed] [Google Scholar]
- Classen CC, Kraemer HC, Blasey C, et al. Supportive–expressive group therapy for primary breast cancer patients: A randomized prospective multicenter trial. Psycho-Oncology. 2008;17(5):438–447. doi: 10.1002/pon.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SR, Mount BM, Strobel MG, et al. The McGill Quality of Life Questionnaire: A measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliative Medicine. 1995;9(3):207–219. doi: 10.1177/026921639500900306. [DOI] [PubMed] [Google Scholar]
- Cohen SR, Mount BM, Bruera E, et al. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: A multi-centre Canadian study demonstrating the importance of the existential domain. Palliative Medicine. 1997;11(1):3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- Cole B, Pargament K. Recreating your life: A spiritual/psychotherapeutic intervention for people diagnosed with cancer. Psycho-Oncology. 1999;8(5):395–407. doi: 10.1002/(sici)1099-1611(199909/10)8:5<395::aid-pon408>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ. Bringing spirituality into your healing journey. Toronto, Ontario: Key Porter Books; 2002. [Google Scholar]
- Cunningham AJ. Integrating spirituality into a group psychological therapy program for cancer patients. Integrative Cancer Therapies. 2005;4(2):178–186. doi: 10.1177/1534735405275648. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Edmonds CV, Jenkins GP, et al. A randomized controlled trial of the effects of group psychological therapy on survival in women with metastatic breast cancer. Psycho-Oncology. 1998;7(6):508–517. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<508::AID-PON376>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Edmonds CV, Phillips C, et al. A prospective, longitudinal study of the relationship of psychological work to duration of survival in patients with metastatic cancer. Psycho-Oncology. 2000;9(4):323–339. doi: 10.1002/1099-1611(200007/08)9:4<323::aid-pon465>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Edmonds CV, Lockwood GA, Cunningham AJ. Psychological response to long-term group therapy: A randomized trial with metastatic breast cancer patients. Psycho-Oncology. 1999;8(1):74–91. doi: 10.1002/(SICI)1099-1611(199901/02)8:1<74::AID-PON339>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Edwards AG, Hailey S, Maxwell M. Psychological interventions for women with metastatic breast cancer. Cochrane Database of Systematic Reviews. 2004;(3) doi: 10.02/14651858.CD004253.pub3. [DOI] [PubMed] [Google Scholar]
- Fawzy NW. A psychoeducational nursing intervention to enhance coping and affective state in newly diagnosed malignant melanoma patients. Cancer Nursing. 1995;18(6):427–438. [PubMed] [Google Scholar]
- Fawzy FI, Fawzy NW. Group therapy in the cancer setting. Journal of Psychosomatic Research. 1998;45(3):191–200. doi: 10.1016/s0022-3999(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Fawzy FI, Fawzy NW, Arndt LA, et al. Critical review of psychosocial interventions in cancer care. Archives of General Psychiatry. 1995;52(2):100–113. doi: 10.1001/archpsyc.1995.03950140018003. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Antoni MH, Schneiderman N. Psychosocial and behavioral interventions for chronic medical conditions. Current Opinions in Psychiatry. 2007;20(2):152–157. doi: 10.1097/YCO.0b013e3280147724. [DOI] [PubMed] [Google Scholar]
- Fillion L, Dupuis R, Tremblay I, et al. Enhancing meaning in palliative care practice: A meaning-centered intervention to promote job satisfaction. Palliative & Supportive Care. 2006;4(4):333–344. doi: 10.1017/s1478951506060445. [DOI] [PubMed] [Google Scholar]
- Frankl VE. Man’s search for meaning. New York: Washington Square Press; 1997. [Google Scholar]
- Gagnon P, Girard M, Fillion L, et al. La recherche de sens à la suite d’un diagnostic de cancer: Une intervention pour améliorer la qualité de vie existentielle et globale. Les Cahiers Francophones de Soins Palliatifs. 2008;9(1):57–69. [Google Scholar]
- Goodwin PJ. Support groups in breast cancer: When a negative result is positive. Journal of Clinical Oncology. 2004;22(21):4244–4246. doi: 10.1200/JCO.2004.06.926. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in meta-static breast cancer. The New England Journal of Medicine. 2001;345(24):1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- Hellman CJ, Budd M, Borysenko J, et al. A study of the effectiveness of two group behavioral medicine interventions for patients with psychosomatic complaints. Behavioral Medicine. 1990;16(4):165–173. doi: 10.1080/08964289.1990.9934605. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Hann DM. Cognitive–behavioral interventions. In: Holland J, et al., editors. Psycho-Oncology. New York: Oxford University Press; 1998. pp. 719–729. [Google Scholar]
- Kissane DW. Psychospiritual and existential distress. The challenge for palliative care. Australian Family Physician. 2000;29(11):1022–1025. [PubMed] [Google Scholar]
- Kissane DW. The relief of existential suffering. Archives of Internal Medicine. 2012;172(19):1501–1505. doi: 10.1001/archinternmed.2012.3633. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Bloch S, Miach P, et al. Cognitive–existential group therapy for patients with primary breast cancer: Techniques and themes. Psycho-Oncology. 1997;6(1):25–33. doi: 10.1002/(SICI)1099-1611(199703)6:1<25::AID-PON240>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Bloch S, Smith GC, et al. Cognitive–existential group psychotherapy for women with primary breast cancer: A randomised controlled trial. Psycho-Oncology. 2003;12(6):532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Grabsch B, Clarke DM, et al. Supportive–expressive group therapy: The transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psycho-Oncology. 2004a;13(11):755–768. doi: 10.1002/pon.798. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Love A, Hatton A, et al. Effect of cognitive–existential group therapy on survival in early-stage breast cancer. Journal of Clinical Oncology. 2004b;22(21):4255–4260. doi: 10.1200/JCO.2004.12.129. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Grabsch B, Clarke DM, et al. Supportive–expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcome from a randomized controlled trial. Psycho-Oncology. 2007;16(4):277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- Lee V, Cohen SR, Edgar L, et al. Meaning making and psychological adjustment to cancer: Development of an intervention and pilot results. Oncology Nursing Forum. 2006;33(2):291–302. doi: 10.1188/06.ONF.291-302. [DOI] [PubMed] [Google Scholar]
- LeMay K, Wilson KG. Treatment of existential distress in life threatening illness: A review of manualized interventions. Clinical Psychological Review. 2008;28(3):472–493. doi: 10.1016/j.cpr.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Lepore SJ. A social–cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial interventions for cancer. Washington, DC: American Psychological Association; 2001. pp. 99–116. [Google Scholar]
- Levin T, Kissane DW. Psychooncology: The state of its development in 2006. The European Journal of Psychiatry. 2006;20:183–197. [Google Scholar]
- Lindemalm C, Granstam-Bjorneklett H, Bergkvist L, et al. Existential aspects are neglected in the evaluation of support-intervention in breast cancer patients. Acta Oncologica. 2012;51(6):807–809. doi: 10.3109/0284186X.2012.681699. [DOI] [PubMed] [Google Scholar]
- Manne SL, Sabbioni M, Bovbjerg DH, et al. Coping with chemotherapy for breast cancer. Journal of Behavioral Medicine. 1994;17(1):41–55. doi: 10.1007/BF01856881. [DOI] [PubMed] [Google Scholar]
- Moadel A, Morgan C, Fatone A, et al. Seeking meaning and hope: Self-reported spiritual and existential needs among an ethnically diverse cancer patient population. Psycho-Oncology. 1999;8(5):378–385. doi: 10.1002/(sici)1099-1611(199909/10)8:5<378::aid-pon406>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mok E, Lau KP, Lam WM, et al. Healthcare professionals’ perceptions of existential distress in patients with advanced cancer. Journal of Advanced Nursing. 2010;66(7):1510–1522. doi: 10.1111/j.1365-2648.2010.05330.x. [DOI] [PubMed] [Google Scholar]
- Park CL, Folkman S. Meaning in the context of stress and coping. Review of General Psychology. 1997;1(2):115–144. [Google Scholar]
- Parle M, Maguire P. Exploring relationships between cancer, coping, and mental health. Journal of Psychosocial Oncology. 1995;13(1–2):27–50. [Google Scholar]
- Réseau canadien du cancer du sein et l’Initiative Ontarienne de Recherche Communautaire sur le cancer du sein. “Rien ne me convenait”: Les besoins d’information et de soutien des jeunes canadiennes atteintes du cancer du sein: Consultation nationale menée auprès de jeunes femmes atteintes du cancer du sein. 2003 Available at http://www.cbcn.ca/index.php?pageaction=content.page&id=5722&lang=fr.
- Sauer R, Seitz M. Psychological and social support of cancer patients: Report on a program of the radiotherapy department, Erlangen. Recent Results in Cancer Research. 1988;108:311–315. doi: 10.1007/978-3-642-82932-1_38. [DOI] [PubMed] [Google Scholar]
- Savard J, Laberge B, Gauthier JG, et al. Evaluating anxiety and depression in HIV-infected patients. Journal of Personality Assessment. 1998;71(3):349–367. doi: 10.1207/s15327752jpa7103_5. [DOI] [PubMed] [Google Scholar]
- Scogin F, Morthland M, Kaufman A, et al. Improving quality of life in diverse rural older adults: A randomized trial of a psychological treatment. Psychology and Aging. 2007;22(4):657–665. doi: 10.1037/0882-7974.22.4.657. [DOI] [PubMed] [Google Scholar]
- Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: Results of two meta-analyses. British Journal of Cancer. 1999;80(11):1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerfield MR. The utility of systems models of stress and coping for applied research: The case of cancer adaptation. Journal of Health Psychology. 1997;2(2):133–151. doi: 10.1177/135910539700200202. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Classen C. Group therapy for cancer patients: A research-based handbook of psychosocial care. New York: Basic Books; 2000. [Google Scholar]
- Spiegel D, Bloom JR, Kraemer HC, et al. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2(8668):888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Morrow GR, Classen C, et al. Group psychotherapy for recently diagnosed breast cancer patients: A multicenter feasibility study. Psycho-Oncology. 1999;8(6):482–493. doi: 10.1002/(sici)1099-1611(199911/12)8:6<482::aid-pon402>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Trijsburg RW, van Knippenberg FC, Rijpma SE. Effects of psychological treatment on cancer patients: A critical review. Psychosomatic Medicine. 1992;54(4):489–517. doi: 10.1097/00006842-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. The Journal of the American Medical Association. 1995;273(1):59–65. [PubMed] [Google Scholar]
- Wong PTP. International forum for logotherapy. Vol. 20. Abilene, TX: Viktor Frankl Institute of Logotherapy; 1997. Meaning-centered counseling: A cognitive–behavioral approach to logotherapy; pp. 85–94. [Google Scholar]
- Xiao H, Kwong E, Pang S, et al. Effect of a life review program for Chinese patients with advanced cancer: A randomized controlled trial. Cancer Nursing. 2013;36(4):274–283. doi: 10.1097/NCC.0b013e318268f7ba. [DOI] [PubMed] [Google Scholar]
- Yalom ID. Existential psychotherapy. New York: Basic Books; 1980. [Google Scholar]
- Yang JA, Garis J, Jackson C, et al. Providing psychotherapy to older adults in home: Benefits, challenges, and decision-making guidelines. Clinical Gerontologist. 2009;32(4):333–346. [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


