Abstract
Anorexia nervosa (AN) is a psychiatric illness with minimal effective treatments and a very high rate of mortality. Understanding the neurobiological underpinnings of the disease is imperative for improving outcomes and can be aided by the study of animal models. The activity-based anorexia rodent model (ABA) is the current best parallel for the study of AN. This review describes the basic neurobiology of feeding and hyperactivity seen in both ABA and AN, and compiles the research on the role that stress-response and reward pathways play in modulating the homeostatic drive to eat and to expend energy, which become dysfunctional in ABA and AN.
Keywords: activity-based anorexia (ABA) animal model, anorexia, feeding, hyperactivity, neurobiology, reward, stress
INTRODUCTION
Anorexia nervosa (AN) is a poorly understood psychiatric disorder that commonly begins in adolescence and that is more prevalent in women. It involves abnormally restrictive eating behavior leading to cachexia, combined with an irrational fear of weight gain and obsession with body shape.1 Women with AN often have other psychiatric comorbidities, such as depression and anxiety, and those with a diagnosis of anorexia also have the highest mortality rate of all psychiatric illnesses.2,3 The prevalence of AN in developed countries is near 1% of the female population, and family studies have shown 50% genetic contribution to heritability, which combines with environmental pressures to produce illness.4–6 Given the high percentage of genetic influence, it is likely that biological treatments could have good effect, yet few specific treatments are available. Pharmacologic studies in AN have been confusing at best, with conflicting results between studies and minimal improvement in disease symptoms or weight restoration. Thus, it is imperative to better understand the pathophysiology involved in order to develop new treatment targets. Doing so requires insight into the biology and neural circuitry that govern reduced feeding and related behaviors.
Much work has been done in animal models to understand the physiology of normal feeding behavior. AN is complicated by its constellation of symptoms that go along with low weight, as well as by select predisposing factors, including excessive exercise and motor restlessness, an anxious or obsessive temperament, extreme self-control and reward insensitivity, and cognitive inflexibility.7–10 Some of the traits seen in AN, such as an obsession with thinness and fear of gaining weight, are impossible to model in animals. Interesting work studying circulating biomarkers of energy balance and of stress in AN patients has not yet yielded viable treatment targets.11–13 Studies of brain activity abnormalities related to fear, reward, and cognition using fMRI point to the utility of more invasive study in animal models.14–16
To better understand how all of these components fit together to affect feeding behavior in AN, it is important to grasp how the drive to eat is developed. Energy homeostasis is the balance between energy intake, or feeding, and energy expenditure, the combination of internal body heat production and external physical activity. When energy intake is less than energy expended, as occurs in AN, one is said to be in negative energy balance, which triggers the sensations that go along with hunger. For mammals under normal conditions, the outcome is to feed.
Researchers have tried to use food-restriction paradigms to model eating disorders, but these efforts are stymied by the animals’ innate preference for homeostasis. Unlike people with AN, who combine food restriction with excess activity to optimize weight loss, rodents given unrestricted access to food and a running wheel in their cages eat more to compensate for increased energy expenditure.17 When food is freely available in the natural habitat, physically healthy animals do not voluntarily restrict food intake. The signaling milieu (hormones and neuropeptides) that develops during a fast leads to optimized energy balance when food is presented, so body-weight change is minimal.17–21 This homeostatic balance is upset in the activity-based anorexia (ABA) model. In the ABA model, food restriction to one hour per day (rats) or two to four hours per day (mice) is combined with unlimited access to a running wheel. In this paradigm, a rodent’s food intake declines strikingly in combination with elevated running-wheel activity, leading to weight loss exceeding 30% of original weight.22 Animals also begin to engage in stereotyped activity—namely, hyperactivity prior to presentation of food, known as food-anticipatory activity.
By recapitulating both the overactivity and the undereating components of AN, the ABA model mimics AN fairly well. Much like AN, age and gender play a role in the susceptibility to ABA development; female adolescent rats are more likely to develop ABA and tolerate the paradigm.23,24 Early-life stressors, such as early weaning or cold temperatures, also increase susceptibility to development of ABA behavior in rats, and this behavior is ameliorated by environmental enrichment.25–28 It takes approximately one week to develop ABA behavior in rodents, which is roughly equivalent to a few months for humans. Some animals progress to self-starvation and death, similar to the near 10% of patients with AN who die from suicide, starvation, or complications of electrolyte imbalance. Importantly, ABA rodents overcome the basic homeostatic mechanism for survival, reducing their food intake in the presence of hunger and body-weight loss, in combination with increased energy expenditure through increased locomotor activity.29 ABA is the only known model where nonhuman mammals choose self-starvation over homeostatic balance. The ABA model also recapitulates a number of endocrinologic findings that are seen in AN patients through the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal axes.30,31
Much like people, not all animals exposed to the ABA paradigm develop ABA. A population is therefore available to study the differences in susceptibility to development of disease, including the role of genetic versus environmental influences (which can be better controlled in animal populations). Equivalent research would be nearly impossible to coordinate in humans, as it would require a massive cohort and the enrollment of children. Given that a major research question in the field of eating disorders is how much of the disease pathology is related to the state or scar of malnutrition, an animal study that can distinguish this factor prospectively would provide a huge boon to the field. Investigating circulating hormones, neuropeptide and receptor expression, and brain circuit connectivity both prior to and after disease onset in rodents—and comparing these findings to those animals that are resistant to the development of ABA— is providing rich information useful for prevention or early treatment of AN. Recent, elegant studies to determine what underlying factors lead animals to be susceptible to the development of ABA have laid the groundwork for the utility of the ABA model in prevention and treatment studies that will be applicable to human disease.32
What makes modeling the pathophysiology of AN uniquely difficult is the influence of socio-environmental and psychological factors, some of which are mediated by fear- and stress-response pathways, such as the obsession with thinness and extreme fear of weight gain, both of which clash with the neurobiological drive to eat.33,34 The motivation and emotions associated with eating—in particular, non-homeostatic feeding—also offset energy demands that drive homeostatic feeding.35–37 With non-homeostatic feeding, the endogenous energy-regulatory signals are thought to become ineffective at transmitting feedback to the central nervous system (CNS), and feeding is potentially regulated through some other CNS circuitry. This additional circuitry is either directly or indirectly connected with hypothalamic circuitry to modulate feeding behavior; the reductionist methods afforded by the ABA model may prove to be the most effective way of unraveling these interactions.
This review will discuss in detail the neurobiology of feeding behavior originating in the hypothalamus, and the way in which non-homeostatic signals coming from stress and reward pathways impinge on the physiologic homeostatic pathways of metabolism. It will focus on the parallels between the ABA rodent model and human AN, with a discussion as to how ABA is the best current model for improving biological understanding and for developing new treatment options for AN.
HOMEOSTATIC FEEDING AND ACTIVITY: MAJOR CIRCULATING HORMONES
Peripherally derived signals that modulate metabolic neuropeptide activity in the hypothalamus include hormones such as leptin, ghrelin, and corticosterone, as well as sex hormones, such as estrogen.
Leptin is produced by adipocytes in fat stores. Circulating leptin concentration is reflective of the total amount of body fat and is highly correlated to energy stores in adipose tissues. Leptin expression levels rise with body fat status, whereas fasting reduces its availability.38–40 High levels of circulating leptin serve to promote satiation and heat production, and animals lacking leptin are hypoactive.41 Adaptive responses to low leptin levels in negative energy balance include decreased energy expenditure, suppressed gonadal- and thyroid-axis function, and increased activation of the adrenal axis.39,42 Furthermore, treatment with leptin in mice with low leptin levels restores normal functioning of the HPA, hypothalamic-pituitary-gonadal, and thyroid axes.39 Because people with AN have low levels of body fat, they have reduced leptin levels in both plasma and cerebrospinal fluid.43–47 ABA rats also have reduced leptin systemically, and exogenously applied leptin reduces hyperactivity, decreases food intake, and increases thermogenesis in the model.21
Ghrelin is produced in the gastrointestinal tract and is negatively correlated with energy balance such that when the stomach is empty, ghrelin is secreted. Ghrelin signals to increase hunger and stimulates locomotor activity and reward pathways.48–51 Similar to leptin, but with opposite function, circulating ghrelin levels reflect changes in body weight; high ghrelin levels are seen after weight loss due to food restriction or deprivation.52,53 However, ghrelin also suppresses brown adipose tissue activity, a source of heat production, quieting energy expenditure while promoting food intake. In AN patients, ghrelin levels are elevated compared to normal-body-weight and obese subjects.54,55 Ghrelin levels are also found to be increased in ABA mice.56 Patients with AN who are treated with ghrelin develop increased appetite and adiposity.57,58 Unfortunately, the fear of weight gain that is pathognomonic for the disease precludes treatment with ghrelin from being a viable option.
Corticosterone (dominant in rodents) or cortisol (dominant in humans) (both referred to as CORT) is important for maintaining glucose availability. CORT is produced by the adrenal gland. Its synthesis and secretion is stimulated by adrenocorticotropin (ACTH), which is secreted from the anterior pituitary gland in response to corticotrophin-releasing hormone (CRH) from the hypothalamus. During a fast or food deprivation, ACTH and CORT levels rise, and in AN, CORT levels are high.59–63 In non-disease states, treatment with CORT increases the size of fat stores.64 Chronic CORT administration stimulates foraging behavior and food intake.65 As stress induces the secretion of corticosteroids, higher levels of CORT increase motivation for comfort-type food in humans and in rats.66 CORT also provides negative feedback to the hypothalamus to decrease production of CRH and to stop production of ACTH, thereby putting the brakes on its own expression. During a fast, CORT is increased in circulation. This effect is seen in both AN and the ABA model; both show similarly increased CORT signaling.67
Estrogen, the female sex hormone, though not specifically a metabolic hormone, does play a role in food intake and body-weight control. Its release from the ovary is regulated by the hypothalamic-pituitary-gonadal, which is affected by body-weight status and the presence of leptin. In women, increased levels of body dissatisfaction have been associated with low levels of estrogen, and the level of body dissatisfaction fluctuates during the menstrual cycle.68 Interestingly, leptin-receptor expression in the hypothalamus also fluctuates with estrogen levels during the menstrual cycle, but no change in associated feeding behavior has been described.68,69 Decreased levels of estrogen after menopause or ovariectomy lead to hyperphagia and weight gain, and deletion of the alpha subtype of the estrogen receptor (the primary form of the receptor found in the hypothalamus) leads to obesity in both male and female mouse models.70,71 Direct application of estrogen to the brain in animals leads to hypophagia.72 In states of negative energy balance, estrogen levels are low, which is thought to be the underlying cause of amenorrhea in patients with AN. No studies have looked at estrogen signaling in the ABA model.
HYPOTHALAMIC NEURONS RESPOND TO CIRCULATING METABOLIC HORMONES TO REGULATE FEEDING AND ACTIVITY
The arcuate nucleus (ARC) of the hypothalamus is a key node in understanding the neural circuit regulating feeding. The ARC lies adjacent to the median eminence, where the blood-brain barrier is relatively permeable for metabolic hormones.73 The endocrine factors that signal energy sufficiency or deficiency act on subsets of neurons in the ARC to effect the electrical and chemical signaling of ARC neurons. These neurons project to, and act on, multiple nuclei within and outside the hypothalamus and can secrete specific neuropeptides that orchestrate feeding behavior and energy expenditure in response to bodily needs (see Figure 1 and Table 1). Two important populations of neurons are found in the ARC and play antagonistic roles in controlling feeding behavior and energy balance.161,162 One type of neuron is orexigenic and co-expresses agouti-related peptide (AgRP), neuropeptide Y (NPY), and GABA; these are commonly referred to as AgRP neurons. The other, referred to as POMC neurons, co-express pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) and are anorexigenic.
Figure 1.
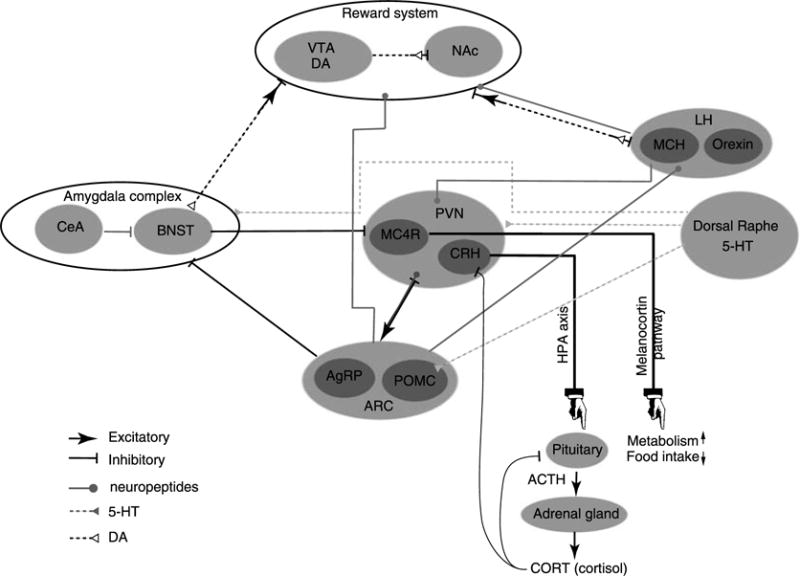
A simplified scheme of the interlinked neuronal circuits implicated in the regulation of feeding, reward, and stress. The scheme selectively highlights the interaction discussed in regard to anorexia nervosa and the activity-based anorexia rodent model. 5-HT, serotonin; ACTH, adrenocorticotropin; AgRP, agouti-related protein; ARC, arcuate nucleus (hypothalamus); BNST, bed nucleus (stria terminalis); CeA, central amygdala; CORT, corticosterone; CRH, corticotrophin-releasing hormone; DA, dopamine; LH, lateral hypothalamus; MC4R, melanocortin 4 receptors; MCH, melanin-concentrating hormone; NAc, nucleus accumbens; POMC, pro-opiomelanocortin; PVN, paraventricular hypothalamus; VTA, ventral tegmental area.
Table 1.
Comparison of Effects of Feeding Neuropeptides in the Hypothalamus When Peptide Is Deleted or Injected, When Neurons Are Stimulated, or in Response to Exogenously Applied Hormones
| Peptide KO | Exogenous application of peptide | Neuron stimulation/activation | Effects of exogenously applied leptin | Effects of ghrelin | Effects of CORT | |
|---|---|---|---|---|---|---|
| General effect of hormone in body | ↓ FI ↑ EE (T) ↓ hyperactivity 21,74 |
↑ FI75,76,77 ↑ LMA48 |
↑ FI ↑ LMA/foraging 65 |
|||
| Neuropeptide | ||||||
| AgRP | = BW78 | ↑ FI ↑ LMA 79,80,81 |
↑ FI ↓ EE 82,83 |
↓84,85,86,87 | ↑88,89,90 | ↑91,92,93 |
| NPY | = BW78 | ↑ FI ↑ LMA 79,80,81 |
Similar to AgRP | ↓ 84,85,86 |
↑88,94 | ↑91,95 |
| POMC | ↑ FI96 ↑ BW ↓ LMA 97 |
↓ FI98,99,100 | ↓ FI ↑ EE 83,87,101,102 |
↑ 87,103,104 |
↓105 | ↓87,106 |
| Beta-endorphins | ↑ FI ↑ BW 107 |
↑ FI108 | Similar to POMC | Similar to POMC | ↓109 | Similar to POMC |
| CART | — | ↓ FI ↓ BW 95,110 ↑ T111 |
↑ T112,113 | ↑39,114,115,116 | ↑117 | ↑93,112,113 |
| Orexin | ↓ FI ↓ BW ↓ LMA ↓ T 118,119,120 |
↑ FI 121,122,123,124 |
↑ FI ↑ EE 125 ↑ LMA 126,127 |
=119,128 | ↑119,128,129,130 | ↑93 |
| MCH | ↓ BW131,132,133 | ↑ FI 134,135,136 |
↑ FI ↓ EE 135 |
↓137 | =137 | =138,139 |
| CRH | = FI =BW 140 |
↓ FI ↑ EE 141,142 |
↓ FI ↑ LMA 143 |
↑144,145 | ↑146 | ↓146 |
| Serotonin | — | ↓ FI 147,148 |
↓ FI 149,150,151,152 |
↑153 | ↓154 | — |
| Dopamine | ↓ FI155 | ↑ FI ↓ BW 156 |
↑FI75,76,77 | ↓157,158,159 | ↑160 | Inconclusive |
AgRP, agouti-related peptide; BW, body weight; CART, cocaine- and amphetamine-regulated transcript; CRH, corticotropin-releasing hormone; EE, energy expenditure; FI, food intake; KO, knockout; LMA, locomotor activity; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; RWA, running-wheel activity; T, thermogenesis. Studies were performed in rats, mice, or humans. Reference numbers for studies not using an animal model (65, 84, 119, and 157) are printed in plain type.
Agouti-Related Peptide and Neuropeptide Y
AgRP neurons are both necessary and sufficient to drive food-seeking activity and consumption.82,83,163,164 Silencing AgRP neurons in fasted mice prevents food intake.82,83 Direct acute stimulation of AgRP neurons drives intense feeding and weight gain within minutes, even in sated mice.82,83 This orexigenic effect is thought to occur by the inverse agonism of the AgRP peptide on melanocortin 3/4 receptor (MC3/4R)–expressing neurons in the paraventricular nucleus of the hypothalamus (PVH).165,166 Before a feeding period, when ghrelin and CORT levels are high and leptin levels are low, the activity of AgRP neurons peaks, causing AgRP and NPY mRNA expression and synthesis to increase.91,101,167–169 Activity of AgRP neurons in brain slices was found to be enhanced following fasting.170,171 In vivo studies show that AgRP neuron activity decreases as quickly as food becomes available.163,164,172
Administration of exogenous AgRP and NPY have a potent stimulatory effect on feeding and locomotor activity.79–81,173,174 However, AgRP- and NPY-deficient mice exhibit normal body weight under ad libitum feeding.78 Notably, ablation of AgRP neurons in adult mice, as opposed to neonates, leads to starvation and death, suggesting that there is compensation for the individual genetic knockout of the peptides but that the neurons are required to drive feeding.175
NPY knockout mice show reduced food intake in response to a fast but no significant decrease in body weight under normal diet conditions, suggesting that NPY may be more important for fasting-induced refeeding than for baseline regulation of food intake.176,177 AgRP and NPY gene expression is increased in states of negative energy balance in rats and in patients with AN compared to healthy controls.62,178 In the ABA model, AgRP and NPY are even more robustly expressed than during a simple fast.178
Injection of leptin suppresses AgRP and NPYexpression, inhibits the spiking activity of AgRP neurons, reduces food intake and meal size, and increases energy expenditure.84–87,121,179 Oppositely, ghrelin increases AgRP activity and food consumption.88–90,180 This effect is most marked when ghrelin is injected in the ARC, indicating a direct role on the AgRP neurons.58 CORT increases AgRP mRNA expression and neuron activity.91,101,167–169 Estrogen suppresses AgRP neuron activation in rodents, and in the murine menstrual cycle, AgRP levels vary inversely with estrogen levels.181
Besides being co-expressed with AgRP in the ARC, NPY is abundantly expressed throughout the brain.167 Ghrelin and CORT enhance NPY orexigenic activity, while leptin decreases it.91,101,167–169,182 Estrogen leads to a decrease in NPY expression in the mouse hypothalamus but an increase in NPY receptor gene expression in rat pituitary cell culture, suggesting altered NPY sensitivity may play a role in estrogen-induced hypophagia.181,183
Pro-opiomelanocortin
Pro-opiomelanocortin (POMC) neuron activation suppresses food intake and stimulates energy expenditure by activation of MC3/4R.83,102 The neuropeptide POMC is cleaved to produce ACTH and beta-lipoprotein. Further processing of ACTH in the ARC produces alpha-melanocyte-stimulating hormone (alpha-MSH), and processing of beta-lipoprotein in the pituitary produces beta-endorphin. Terminals of POMC and AgRP neurons project to similar regions that contain neurons expressing MC3/4R, which are excited by the release of alpha-MSH (and antagonized by AgRP).184 Within the ARC, POMC neuron activity can be modulated by AgRP neurons, but not vice versa.185,186
Deletion of POMC leads to hyperphagia and obesity.96,97 This phenotype can be reversed by exogenous administration of alpha-MSH—which, when injected specifically in the PVH, rapidly and robustly inhibits food intake.98–100 Furthermore, POMC-deficient mice exhibit low levels of circulating CORT, likely due to the role that the POMC-cleavage product, ACTH, plays in CORT secretion.98
The other cleavage products of POMC, beta-lipoprotein and beta-endorphin, are endogenous opioids that interact with opioid receptors to regulate energy intake and utilization through reward-mediated behavior.187,188 Endogenous opioid peptides function as neurotransmitters and are released during intrinsically rewarding activities, such as exercise. Increased levels of endorphins inhibit the experience of pain.189–191 Furthermore, opioid peptides are key mediators of hedonic balance and emotional response in food intake.192 Beta-endorphin terminals are distributed throughout the CNS, including the PVH, where they inhibit the HPA axis.193 Beta-endorphin KO mice are obese and hyperphagic.107 Exogenous administration of beta-endorphin in chicks increases food intake, and pharmacologic activation of the beta-endorphin receptor in mice drives feeding.108,194
Leptin activates POMC neurons by increasing their firing rate and increases POMC mRNA.87,103,104,114,195,196 Estrogen receptors are found on POMC neurons and are thought to play a role in leptin’s effect on POMC expression.197 Estrogen administration centrally also leads to increased excitability of POMC neurons.162 Ghrelin, oppositely, inhibits POMC neuron activity, and application of CORT decreases POMC mRNA and gene expression.105,106,109
Cocaine- and Amphetamine-Regulated Transcript
CART-expressing neurons co-localize with POMC neurons in the ARC. CART-expressing neurons are also found in the paraventricular nucleus of the hypothalamus, the lateral hypothalamus (LH), and the dorsomedial hypothalamus.111,126,198 Injection of CART directly to the PVH increases thermogenesis and decreases feeding and body weight.95,110,111 It has been shown that CART in the PVH interacts with downstream NPY-signaling pathways, and may inhibit feeding through activation of CRH.112,199
CART expression is mediated by leptin and ghrelin. Low leptin levels following fasting suppress CART expression in the ARC, and intracerebroventricular administration of CART increases leptin levels.39,115,116,200 Ghrelin increases CART expression, and refeeding of fasted animals strongly increases CART.200 Distinct CART neurons in different brain regions may respond oppositely to leptin and ghrelin, though it is unclear how this influences energy balance. CORT signaling leads to increased CART expression and neuronal activity, which induces thermogenesis, independent of POMC.112,113,201 Estrogen’s effects on CART expression are known to be site specific and vary by region.202
In states of negative energy balance, POMC and CART expression are decreased in rats and humans.62,178,203 In the ABA model, as well as in patients with AN, POMC and CART are similarly decreased compared to sedentary controls.178 However, in both AN and the ABA model, beta-endorphin levels are high in negative energy balance, which may relate to its role in reward signaling.204–207
Orexin
Outside of the ARC are other hypothalamic populations that fluctuate with the hormones and signals that reflect energy status. Orexin-expressing neurons (also called hypocretin-expressing neurons), localized in the lateral hypothalamus, promote feeding and locomotor activity.118,119 These orexin neurons project to numerous areas in the brain, with direct projections to the ARC.208–210
Deletion of orexin leads to hypophagia and decreased locomotor activity as well as to reduced brown fat thermogenesis.118–120 Injection of orexin directly into the ARC increases food intake by stimulating NPY and inhibiting POMC neural activity.121–124,211,212 Feedback innervation of orexin neurons by NPY inhibits orexin neuronal activity.208,209,213 Orexin activity in the LH is also decreased by intracerebroventricular CART administration, which inhibits both locomotor activity and food intake.125–127
In addition, orexin neurons send projections to the PVH and to several mesolimbic areas where orexin receptors, as well as opioid and dopamine receptors, are densely expressed. These projections provide a neuroanatomical basis for interaction between opioids and non-opioid peptides in both the satiety and the reward centers of the brain.214 In support of this potential interaction, the feeding response induced by central injection of orexin is greatly attenuated by co-administration of an opioid receptor antagonist.215,216 Also, intracerebroventricular administration of orexin increases the motivation for food seeking, particularly for palatable food.217,218
While orexin neurons are insensitive to changes in leptin levels under physiological conditions (i.e., the range of leptin levels induced by the body, as opposed to extreme levels that can be induced by leptin injection), ghrelin stimulates orexin mRNA expression.119,128,219 The orexin neurons are activated by food deprivation through ghrelin and CORT, facilitating locomotor activity and food-seeking behavior under conditions of fasting.92,93,129,130,134,219 Estrogen suppresses orexin expression in ovariectomized rats, which is thought to contribute to the role of estrogen on feeding.202 In states of negative energy balance, there is no change in orexin mRNA expression in male and female rats.178,220 Surprisingly, in patients with AN and in ABA rats, orexin levels are found to be increased compared to sedentary controls.62,178,221,222 This finding points to the LH—and to orexin, in particular—as a potential target for better understanding and possible treatment of AN.
Melanin-Concentrating Hormone
A separate neuronal population in the LH distinct from orexin-expressing neurons expresses melanin-concentrating hormone (MCH). These MCH neurons, like orexin neurons, promote palatable food intake and are stimulated by palatable food.223 They also play an important role in processing hedonic and rewarding behaviors associated with feeding. The projections of MCH and orexin neurons exhibit significant overlap, including projections to the regions of feeding and reward circuitry.134,135,224–226 Like orexin, MCH expression is unchanged by fasting in female rats, but studies in male mice have shown elevated MCH after food restriction. It is unclear if either is generalizable to humans with AN; more gender- and species-specific study is warranted. Orexin and MCH have different, but complementary, effects on behavior, with orexin promoting food seeking and motivation for palatable food and MCH functioning during ongoing food intake, reinforcing the consumption of calorically dense foods.208,219,223
Leptin application decreases MCH and MCH receptor (MCHR1) mRNA levels, but MCH neurons are unaffected by ghrelin administration.137 Intracerebroventricular administration of MCH stimulates feeding, but to a lesser extent than NPY-induced feeding.134–136 MCH-deficient mice are lean, indicating that MCH signaling is important for maintaining energy homeostasis.132,133,227,228 Similarly, chronic administration of an MCHR1 antagonist decreases body weight by reducing food intake.229,230 In rats, adrenalectomy also decreases MCH mRNA levels, but MCH expression is not restored by replacing CORT, suggesting that MCH-driven effects are independent of CORT.138,139 Despite earlier research showing that estrogen inhibits MCH expression, more recent studies show that MCH expression does not change in response to estrogen and that estrogen’s effect on food intake is independent of MCH.131,202,231 Though not studied in AN, in the ABA model, MCH levels, like orexin levels, are increased compared to sedentary, food-restricted female rats.178
In the setting of food restriction and hyperactivity, where energy balance is negative, neither ABA rodents nor AN patients feed to levels that would reestablish energy balance. Neuropeptides in the ARC are expressed at levels that would be expected to increase feeding, but feeding does not increase. Neurons of the LH, which have reciprocal connection with other areas of the hypothalamus and with reward circuitry, do show unexpected elevation of orexigenic neuropeptides. Therefore, it is likely that other signals impinge on these hunger signals downstream and prevent their translation to the act of feeding.
NON-HOMEOSTATIC CONTROL OF FEEDING AND ACTIVITY
Stress: The Hypothalamic-Pituitary-Adrenal Axis and Corticotrophin-Releasing Hormone
Interactions between the hypothalamus, the pituitary, and the adrenal gland control responses to stress and regulate many processes, including energy storage and expenditure. Neurons expressing corticotrophin-releasing hormone are abundant in the PVH, though CRH is also heavily expressed in other brain regions. CRH is released from the hypothalamus with stress and physical activity, which leads to activation of the HPA-axis cascade: CRH stimulates anterior pituitary cells to produce ACTH from POMC, which is released to systemic circulation and stimulates the adrenal cortex to produce CORT, the major stress-response hormone. Circulating CORT acts to decrease the production of CRH, whereas ghrelin increases it.146
Central administration of CRH stimulates the release of CORT acutely and leads to increased energy expenditure and locomotor activity but reduced calorie intake.141–143,232 Chronic continuous CRH administration over two days overrides CORT feedback, leading to further increased levels of circulating CORT.233 Mice that are deficient in CRH exhibit normal body weight and food intake, and CRH expression does not change in a state of negative energy balance.140,178 However, in patients with AN, CRH is increased.62,221 CRH expression remains unchanged at the onset of ABA development but is elevated when ABA rats approach 75% of original body weight.67,178,234,235 These changes in CRH and in other neuropeptides are presented in Table 2.
Table 2.
Comparison of Expression Levels of the Feeding Neuropeptides of the Hypothalamus in Fasted and Disease States
| Peptide/hormone | State of negative energy balance | Anorexia nervosa | Activity-based anorexia rodent model |
|---|---|---|---|
| AgRP | ↑92,93,130,178 | ↑221 | ↑↑178 |
| NPY | ↑178 | ↑221 | ↑↑178 |
| POMC | ↓87,104,178,203 | ↓236 | ↓178 |
| beta-endorphins | ↑205 | ↑204,207 | ↑206 |
| CART | Similar to POMC | Similar to POMC | ↓178 |
| Orexin | =178 | ↑222 | ↑178 |
| MCH | =178 | — | ↑178 |
| CRH | Inconclusive | ↑237,238,239,240 | ↑67,178,234 |
| Serotonin | ↓241 | ↓242 | ↓33 |
| Dopamine | ↓75,77 | ↓ ↑ receptor density 243,244 |
↑33,245 |
| Leptin | ↓246 | ↓43,44,45,46,47 | ↓21,178 |
| Ghrelin | ↑52,53 | ↑54,55 | ↑56 |
| CORT | ↑67 | ↑59,60,61,63 | ↑67 |
| Effects of exogenously applied leptin | ↓ FI ↑ EE 87,101 |
Expected to ameliorate hyperactivity and depression247 | ↓ hyperactivity21,43,248 |
| Effects of ghrelin | ↑ activity AgRP and orexin neurons92,93 | ↑ FI57 | ↑ hyperactivity56 |
| Effects of CORT | ↑ activity AgRP and orexin neurons92,93 | — | — |
AgRP, agouti-related peptide; CART, cocaine- and amphetamine-regulated transcript; CORT, cortisol or corticosterone; CRH, corticotropin-releasing hormone; EE, energy expenditure; FI, food intake; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; POMC, pro-opiomelanocortin. Studies were performed in rats, mice, or humans.
The HPA Axis in Feeding and Activity
ARC neuropeptides have significant effects on HPA-axis activity.249 For example, infusion of AgRP on hypothalamic explants significantly increases CRH release, and central injection of NPY stimulates the HPA axis in rats.250–252 Alpha-MSH and CART increase the circulating levels of ACTH and CORT, and stimulate CRH release from hypothalamic neurons.251,253,254 The implication is that signals produced in both negative energy balance and satiety can induce a stress response. Furthermore, central leptin injection increases CRH mRNA but blunts HPA-axis responses to stress.144,145 Blockade of CRH signaling attenuates leptin-induced and exercise-induced anorexia, implying that CRH interferes with pro-homeostatic signals.144,146,255,256
Different types of stress have different effects on neuropeptides and hormones. Stress-induced modulation of feeding is thought to occur through the HPA axis, due to its proximity to the melanocortin system in the PVH.257–259 Given the variety of stressors that contribute to the development of AN and ABA, the direct results of HPA-axis activation may vary among individuals. The HPA effect on food intake is bidirectional, with both increases and decreases observed, depending on the type of stressor or model studied.259 It is likely that when manifest as AN, the stress-induced chronic activation of the HPA axis does contribute to decreased feeding. What factors influence this susceptibility are not yet known and would be a useful target for study with the ABA model.
The HPA Axis in AN and ABA: The Effect of Stress on Feeding, Activity, Hormones, and Neuropeptides
It has been well documented that the HPA axis is elevated in patients with AN, with increased CRH and CORT levels that then drive the patient’s hyperactivity.237–240 The hypercortisolism seen in AN is associated with increased central CRH and normal circulating levels of ACTH, which indicates a broken feedback loop.60,61 The paradoxical hyperproduction of CRH that causes sustained HPA-axis activity could be due to the continued stress of hyperactivity, food restriction, or emotional stressors.61,260
Many patients with AN have a history of traumatic or other stressful events that may affect stress responsivity in later years.240,261 In animals, early-life stress is recapitulated by early weaning, single housing, or severe food restriction, and the addition of these stressors to the ABA paradigm leads more animals to develop ABA.262
Exercise is itself a physical stressor that can lead to elevated plasma CORT levels.263 In fact, patients with AN who are hyperactive display higher levels of CORT than less active patients with comparable body weights. Treadmill running alone has been found to increase CRH mRNA levels in the PVH.145 Increased levels of CRH and increased activity of the HPA axis result in hyperactive behavior.255 Multiple components of the ABA model are therefore stressful to the animal; together, starvation and hyperactivity have an additive effect on CRH and circulating CORT levels, much like the multiple life stressors that often accompany the development of AN.264
Higher levels of CORT in AN are associated with lower fMRI activity levels in the amygdala, hypothalamus, insula, and prefrontal cortex in response to food imagery.265,266 A palatable meal increases activity of the amygdala in AN patients compared to healthy controls, which may be related to the aversive nature of the palatable food to an AN patient, or to the fear of weight gain.267 Hours after a calorie-controlled meal, CORT remains high for AN compared to healthy controls, with similar hypoactivation of the amygdala and insula on fMRI.266
Chronic stress increases preference for palatable food in young mice.268 Palatable food, such as sucrose or lard, reduces CRH expression in the PVH and resultant anxiety-like behavior.269–274 Yet, under stress there are no differences in plasma CORT levels in young versus aged mice.268 These findings indicate a role for central regulation of other non-homeostatic feeding pathways with the capacity to affect body weight in the setting of elevated stress. More studies may be helpful to determine if the inability to adapt to elevated CRH, along with the signaling cascade it sets off, may directly affect how ABA animals or patients with AN respond to feeding neuropeptides.275
Reward Circuitry: Hyperactivity and the Neuropeptides of Reward
Similar to stress-response pathways, the reward/motivation circuitry has direct connections to the metabolic neurons of the hypothalamus, and affects energy balance. Further, growing evidence suggests that food, exercise, and drugs of abuse have similar rewarding properties and activate overlapping neural systems.276–281 It is thought that AN patients become addicted to physical activity while reviling food reward. Evidence suggests that reward-based associations with activity can also explain the paradox of self-starvation and hyperactivity that leads to physical collapse in the ABA model.282–284
Food anticipatory activity (FAA) in animals is defined as a specific, intrinsically rewarding peak in activity prior to a scheduled feeding.285 This phenomenon may be based in evolution, providing the necessary drive a starved animal would need to continue to search for food to survive.248,286–288 Alternatively, FAA may provide active heat generation that yields purposeful thermogenesis, as opposed to calorie-squandering brown fat activity. Though this increased activity before a meal has no direct correlate in humans, hyperactivity in AN is prominent and is thought to be analogous to FAA, as both represent a choice to engage in activity that is directly at odds with the energy requirements necessary for survival.
The regulation of the rewarding aspects of feeding and activity involve the dopaminergic and serotonergic systems.36,37,289 While dopaminergic signaling is associated with the expression of an appetitive reward system, the serotonergic system signals the prediction of both punishments and rewards.290,291 Importantly, these two systems interact with each other to effect reward.
The reward circuitry includes the following: the ventral tegmental area (VTA), a dopaminergic midbrain area implicated in reward signaling; the nucleus accumbens (NAc), which is implicated in hedonic and motivational aspects of feeding; the amygdala, involved in aversive response learning; and the striatonigral pathway, which is implicated in hedonic evaluation of stimuli and also in transposing stimulus-driven motivation into motor responses.243,244,292–295 The hypothalamus is linked to this “motivational circuitry” both anatomically and functionally by multiple pathways, allowing information regarding energy balance to affect motivation and vice versa. Specifically, the lateral hypothalamus is a crucial area for coordinating motivated feeding behavior since it both receives afferents from the amygdala and projects to the VTA.37,296–299 Moreover, ARC AgRP neurons project directly to the central amygdala, which is implicated in the control of feeding, and to the extended amygdala complex (including the bed nucleus of the stria terminalis), which is implicated in modulating VTA dopaminergic activity.300–303
Dopamine’s Role in Feeding and Activity
The mesolimbic dopamine (DA) system, important in the reward value of food and in addiction behavior, is implicated in feeding and FAA.304–306 DA is correlated to anorexia-associated hyperactivity, and increases during FAA.307 Food restriction results in a decrease in DA.308 However, palatable food selectively enhances release of DA in the NAc.245,308–310 Food-restricted rats given a DA D1-receptor agonist, but not D2-receptor agonist, show increased preference for palatable food.311 Systemic administration of DA receptor antagonist is strongly correlated with a decrease in FAA in food-restricted rats that are presented with palatable food.312 However, the different contributions of individual DA receptor subtypes suggest that it is important to distinguish between the receptor subtype–specific neural pathways to determine DA effects on feeding.
Since the NAc receives both POMC and AgRP projections from the ARC, the convergence of these projections with DA from the VTA may be a mechanism through which hunger states directly modulate the motivation to eat.313,314 Notably, VTA dopaminergic neurons also receive taste information via afferent sensory fibers, which allows for direct integration of food information with motivational behavior.315,316
AN patients show decreased DA metabolites, indicating low DA, as well as increased density of DA receptors, suggesting increased sensitivity to low DA levels.243,244 However, in cognitive and fMRI studies of people with a history of AN who have recovered their weight, there is a decrease in reward sensitivity compared to healthy controls in regard to both food-related and neutral, non-food-related cues.16,265,317 This seeming contradiction of an increase in receptor density but decreased sensitivity to reward may be explained by the alteration in the reward value of food intake, which has been shown to be an aversive stimulus for AN patients.289,318,319 Instead, other stimuli become rewarding, possibly due to the chronic stress that sensitizes DA reward circuitry via the HPA axis.320 Thus, the decreased reward sensitivity seen in humans with AN, tempered by CRH and elevated CORT levels, likely plays a role in dampening the rewarding aspects of feeding.
Alterations in the mesolimbic DA system are reported in the ABA model compared to rats fed ad lib, with increased DA in the NAc during food consumption but not during food anticipation.33,245 Administration of a DA antagonist reduces activity levels and increases body weight and food intake in ABA rats compared to ad lib fed rats, indicating that direct manipulation of reward circuitry can affect metabolic outcomes.156
The mechanisms through which the dopaminergic mesolimbic system reinforces running-wheel behavior during food restriction (and vice versa) may be through its interaction with other homeostatic feeding signals. Ghrelin, which is known to promote feeding, is also linked to FAA: plasma ghrelin levels in ABA rats are highly associated with FAA, and suppression of ghrelin signaling suppresses FAA.56 Ghrelin stimulates food intake primarily via activating the hypothalamic pathway, but it also integrates non-homeostatic feeding through activity in the meso-cortico-limbic pathway, including direct activation of VTA neurons.76 Systemic injection of ghrelin in mice causes an increase in DA neuronal activity and synapse formation, which is blocked by intra-VTA delivery of a selective ghrelin receptor antagonist, indicating co-expression of DA and ghrelin receptors in the mesolimbic system.160 Microinjection of ghrelin in the VTA of rats drives food intake—which is thought to be the basis of reward-driven eating behavior.75,77 In fact, it was shown recently that palatable food feeding does not need to be driven by AgRP neurons but can be induced by ghrelin activity on DA neurons in the VTA.321
Leptin, in addition to suppressing feeding and hyperactivity, is known to attenuate the effects of DA on motivated behaviors in reward-related brain areas.74,157–159 Leptin action in the VTA regulates effort-based responding for food rewards.322 Direct intracerebroventricular administration of leptin to the VTA is sufficient to inhibit feeding behavior and reduce hyperactivity.323 Thus, because of the direct effect of leptin on the midbrain DA system, low levels of leptin in ABA and AN may have a role in decreased feeding and hyperactivity.
In vivo studies report various effects of CORT on DA, showing that it can increase, decrease, or not alter DA utilization and release in rodents; no conclusive statements can be made on this interaction with feeding.324–329 The interaction of estrogen with DA has been extensively studied, though not in direct relation to feeding. Further study of these interactions in the ABA model is imperative.
Serotonin’s Role in Feeding and Activity
Serotonin plays a critical role in animals’ adaptation to aversive events, in the inhibition of appetite, in anxious and obsessive behaviors, and in depression. The serotonergic neurons are predominantly clustered into two major anatomic groups: the dorsal raphe nucleus (DRN), which projects to the forebrain, and the caudal raphe nuclei, which innervate brain stem structures and the spinal cord. Virtually all brain nuclei implicated in energy-balance regulation receive serotonergic afferents, including the PVH, dorsomedial hypothalamus, and lateral hypothalamus.330–333 Food restriction decreases serotonin levels in the hypothalamus.241 In turn, serotonin decreases food intake in humans and rodents, whether it is given systemically or centrally.147 Microinjection of serotonin directly into the PVH or LH of rats reduces meal size and feeding rate, and in the ARC, serotonin stimulates POMC neurons and inhibits AgRP neurons, leading to reduced food intake.149–152 In the LH, MCH reduces the activity of serotonergic neurons of the DRN.334 A more complex relationship exists between serotonin in the DRN and orexin.335 Both are implicated in the regulation of sleep and in the depressive disorders, though there is no direct study of the effects on feeding.
Ghrelin inhibits serotonin release in the hypothalamus of rats, and systemic administration of leptin increases serotonin levels, specifically in the hypothalamus and hippocampus.153,154 Serotonin and stress are tightly linked, and the administration of serotonin agonists increases CORT levels in rats.336 The complex relationship between estrogen and serotonin is reviewed elsewhere and is outside the scope of this review.337
Levels of serotonin markers are lower in AN patients compared to healthy controls, and in ABA rats compared to ad lib fed active controls.33,242 Though low levels of serotonin would be expected to drive feeding, that does not occur in either ABA or AN—again pointing to the complexity of the systems.289,338
Reward in AN and ABA: Hyperactivity, Motivation, and Feeding Neuropeptides
Serotonin and DA neurons have been shown to exert stimulatory and inhibitory control, respectively, over pituitary release of the opioid beta-endorphins and are also modulated by the beta-endorphins.339,340 Feeding decreases plasma levels of endogenous opioids in patients with AN, suggesting that these decreases may alter the otherwise rewarding experience of eating.204 Anatomical and biochemical data reveal an interaction between opioids and DA actions on dopaminergic nerve terminals.340 Specifically, it has been shown that beta-endorphins effectively decrease DA neurotransmission in the hypothalamus.341 Furthermore, antagonizing the opioid system with naloxone, an opiate antagonist, in ad lib fed rats blocks palatable food intake but not running-wheel activity.312 Mice deficient in mu-opioid receptors (with reduced beta-endorphin signaling) display attenuated FAA during food restriction, indicating that opioid signaling may work together with DA signaling to effect reward salience.342
Orexin in the LH is another hormone involved in driving food intake and physical activity; activation of orexin receptors leads to an increase in feeding and physical activity.246,343,344 Orexin plays a central role in reward mechanisms and in the effects of drugs of abuse, most likely through LH orexin neurons projecting to VTA dopaminergic neurons.345–349 Since orexin is elevated in ABA, it may mediate the rewarding properties of hyperactivity by interacting with the mesolimbic pathway and amplifying DA release, thus providing another incentive for an animal to engage in running activity.
MCH neurons from the LH project to the reward system, predominantly to the NAc. Interestingly, MCH receptors in the NAc have been shown to be co-localized with DA receptors.224–226 Injection of MCH to the NAc activates release of DA and increases feeding in sated rats, whereas injection of an MCH receptor antagonist has the opposite effect.224 Blockade of MCH activity in the NAc shell reduces food intake.350 Furthermore, MCH-deficient mice do not become hyperphagic when presented with a palatable diet, suggesting that MCH dysfunction in these mice affects the processing of hedonic cues associated with feeding.225
FAA has been linked to the orexigenic neurons in the ARC. AgRP neurons have direct projections to areas implicated in the reward value of FAA, including the VTA and the LH. Ablating these neurons impairs the adaptation to restricted feeding in rodents, demonstrating their necessity to entrain FAA.351 Through these projections, AgRP and alpha-MSH may act directly on dopaminergic VTA neurons to affect (increase or decrease, respectively) hedonic feeding, whereas the injection of melanocortin receptor agonist into the VTA in rats decreases the consumption of a palatable sucrose solution.352 When food delivery to food-restricted mice is delayed, AgRP neurons increase their activity dramatically, which may indicate a function in reward valuation.303,353 Furthermore, AgRP activity was found to be an aversive signal outright, which may explain the role of FAA or exercise as a reinforcing behavior to counteract the discomfort of metabolic hunger signals.164
Taken together, in both AN and ABA, there is an increase in orexigenic signals, but these are compromised by simultaneously malfunctioning signals from the reward circuitry that manipulate or override the hypothalamic metabolic drive to eat.
DISCUSSION
Although the ABA rodent model was developed 50 years ago, it has not yet been fully embraced as a homologue to AN. Though no animal model can fully recapitulate the emotional and environmental stressors inherent in the human condition, important parallels make ABA a valuable model for understanding AN. These parallels include the following: severely restricted food intake; low body weight; excessive exercise and hyperactivity; increased susceptibility in adolescents, females, and those with a history of traumatic early-life events; and loss of normal estrous cycling. In this review, we have shown that both ABA and AN share common changes in neuropeptides of the LH, have elevated CRH and dysregulated HPA-axis signal response, and have dysregulated reward signaling related to DA, serotonin, and beta-endorphins. These changes—all of which differ from what would be expected in states of negative energy balance—would benefit from further study at the level of single cells, neuronal populations, or behavior. The goal would be to identify new targets for treatment and prevention of AN.
Notably, the ABA model shows similar changes to AN in homeostatic feeding-hormone and neuropeptide expression. Importantly, in the ARC these changes are not significantly different from what would be expected in a state of starvation. Yet somehow both the ABA animals and AN patients who progress from disease to death overcome the strong homeostatic drive to eat. In human studies, researchers are restricted to functional-imaging and biomarker changes, both of which are population-level studies in terms of bodily function. In preclinical studies, researchers have focused primarily on manipulating neuronal activity and studying neuropeptide expression and behavior. However, recent animal studies have shown significant changes, on multiple timescales, in the in vivo activity of AgRP and POMC neurons related to food availability, palatability, nutritional status, and time of day.163,164,172 Some of the reported changes are too fast to be induced by hormonal signals, indicating that a paradigm shift is required in order to understand the role of these neurons. It is highly likely that similar functions are at play in humans and that they may be tremendously important in the development of AN. Since no technology is yet available for this type of study in humans, the ABA rodent could provide a vast amount of information—with potential application to neuromodulatory treatments, which are currently being investigated for use in AN.354
The orexigenic neuropeptides of the LH show similar perturbation when studied in AN and ABA, but the expression levels are higher than would be expected in a state of negative energy balance alone. The LH is historically known as a feeding center of the brain, as lesions of the area lead to starvation due to lack of motivation.355 The orexigenic peptides and the LH in general are of considerable interest for better understanding AN, and early studies of electrical stimulation in the LH have shown promise in terms of driving food intake.356 Remarkably, few studies address the neuropeptide changes in ABA, and only one attempted invasive stimulation of the LH in ABA rats.178,357 Combining the study of neuropeptides with the study of neuronal population and single-neuron activity in the ABA model could enhance our knowledge of brain circuitry in AN, especially in the LH, potentially leading to the identification of new pharmacologic targets.
The LH is highly interconnected with stress and reward circuitry, two systems in the brain that are similarly dysfunctional in ABA and AN. The HPA axis shows a failure in negative feedback from elevated CORT, with sequelae related to both elevated systemic and central activity. The reward circuitry in both AN and ABA shows altered expression of the neuropeptides and receptors for serotonin and DA, with resultant changes in sensitivity to reward. In combination, these changes may amplify the incentive value of cues/behaviors previously experienced as rewarding or as stress modulating (e.g., food restriction and exercise). The result would be a DA-mediated bias of motivational processing toward reward-associated stimuli, thus causing a pathological drive for illness-related reward that magnifies anorectic psychopathology.320,358–360 To understand these systems, manipulations of chronicity and receptor type will be required, which the ABA model allows.
The alterations in serotonin and dopaminergic signaling in AN patients and ABA rodents may play a role in the elevated anxiety seen in patients with AN, underlying the fear of weight gain.33,34 AN patients are frequently treated with antipsychotics and antidepressants targeting DA and serotonin signaling to reduce agitation, obsessionality, and anxiety about refeeding.361,362 This treatment has been associated with reduced physical activity levels and increased body weight.363,364 By focusing on how the stress-related modulation of DA or serotonin in the NAc and VTA is accompanied by modulation in feeding, FAA, and hyperactivity in ABA rats, we will gain valuable information regarding the interplay between anxiety, reward, hyperactivity, and feeding in AN. This information may also be applicable to stress-related binge eating. Two other systems—for executive function and fear—are also well known to be dysfunctional in AN, and to modulate homeostatic feeding. Because those systems have been less well studied in ABA, they were not discussed in this review.
A better understanding of the biology of relevant systemic interactions is important for developing rational treatments for AN. ABA rodents and, in particular, ABA mice can provide the genetic and anatomic access needed to precisely focus on one hormone, peptide, or receptor at a time and to broadly determine behavioral and biological outcomes of a miniscule perturbation within a network. This reductionist approach is crucial for rational design of improved pharmacologic and neuromodulatory interventions for AN.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: shared genetic and environmental risk factors. Am J Psychiatry. 2000;157:469–71. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 3.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–31. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 4.Strober M, Freeman R, Lampert C, Diamond J, Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry. 2000:393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- 5.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol Med. 2001;31:737–40. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 6.Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63:305–12. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Tchanturia K, Campbell IC, Morris R, Treasure J. Neuropsychological studies in anorexia nervosa. Int J Eat Disord. 2005;37(suppl):S72–6. doi: 10.1002/eat.20119. [DOI] [PubMed] [Google Scholar]
- 8.Shroff H, Reba L, Thornton LM, et al. Features associated with excessive exercise in women with eating disorders. Int J Eat Disord. 2006;39:454–61. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- 9.Harrison A, O’Brien N, Lopez C, Treasure J. Sensitivity to reward and punishment in eating disorders. Psychiatry Res. 2010;177:1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. Eur Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 11.Jimerson DC, Wolfe BE. Neuropeptides in eating disorders. CNS Spectr. 2004;9:516–22. doi: 10.1017/s1092852900009603. [DOI] [PubMed] [Google Scholar]
- 12.Housova J, Anderlova K, Krizova J, et al. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90:1366–70. doi: 10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- 13.Terra X, Auguet T, Aguera Z, et al. Adipocytokine levels in women with anorexia nervosa. Relationship with weight restoration and disease duration. Int J Eat Disord. 2013;46:855–61. doi: 10.1002/eat.22166. [DOI] [PubMed] [Google Scholar]
- 14.Seeger G, Braus DF, Ruf M, Goldberger U, Schmidt MH. Body image distortion reveals amygdala activation in patients with anorexia nervosa—a functional magnetic resonance imaging study. Neurosci Lett. 2002;326:25–8. doi: 10.1016/s0304-3940(02)00312-9. [DOI] [PubMed] [Google Scholar]
- 15.Zastrow A, Kaiser S, Stippich C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–16. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 16.Wierenga CE, Bischoff-Grethe A, Melrose AJ, et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry. 2015;77:642–52. doi: 10.1016/j.biopsych.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kas MJ, Van Dijk G, Scheurink AJ, Adan RA. Agouti-related protein prevents self-starvation. Mol Psychiatry. 2003;8:235–40. doi: 10.1038/sj.mp.4001206. [DOI] [PubMed] [Google Scholar]
- 18.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–35. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 19.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–97. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 20.Avraham Y, Hao S, Mendelson S, Berry EM. Tyrosine improves appetite, cognition, and exercise tolerance in activity anorexia. Med Sci Sports Exerc. 2001;33:2104–10. doi: 10.1097/00005768-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA. Leptin treatment in activity-based anorexia. Biol Psychiatry. 2005;58:165–71. doi: 10.1016/j.biopsych.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–21. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- 23.Doerries LE, Stanley EZ, Aravich PF. Activity-based anorexia: relationship to gender and activity-stress ulcers. Physiol Behav. 1991;50:945–9. doi: 10.1016/0031-9384(91)90419-o. [DOI] [PubMed] [Google Scholar]
- 24.Pare WP. The influence of food consumption and running activity on the activity-stress ulcer in the rat. Am J Dig Dis. 1975;20:262–73. doi: 10.1007/BF01070729. [DOI] [PubMed] [Google Scholar]
- 25.Glavin GB, Pare WP. Early weaning predisposes rats to exacerbated activity-stress ulcer formation. Physiol Behav. 1985;34:907–9. doi: 10.1016/0031-9384(85)90012-5. [DOI] [PubMed] [Google Scholar]
- 26.Pare WP. Prior stress and susceptibility to stress ulcer. Physiol Behav. 1986;36:1155–9. doi: 10.1016/0031-9384(86)90493-2. [DOI] [PubMed] [Google Scholar]
- 27.Carrera O, Gutierrez E, Boakes RA. Early handling reduces vulnerability of rats to activity-based anorexia. Dev Psychobiol. 2006;48:520–7. doi: 10.1002/dev.20175. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez E, Baysari MT, Carrera O, Whitford TJ, Boakes RA. High ambient temperature reduces rate of body-weight loss produced by wheel running. Q J Exp Psychol. 2006;59:1196–211. doi: 10.1080/17470210500417688. [DOI] [PubMed] [Google Scholar]
- 29.Kim SF. Animal models of eating disorders. Neuroscience. 2012;211:2–12. doi: 10.1016/j.neuroscience.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K, Hara C, Ogawa N. Feeding conditions and estrous cycle of female rats under the activity-stress procedure from aspects of anorexia nervosa. Physiol Behav. 1992;51:827–32. doi: 10.1016/0031-9384(92)90122-i. [DOI] [PubMed] [Google Scholar]
- 31.Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407–14. doi: 10.1038/ncpendmet0872. [DOI] [PubMed] [Google Scholar]
- 32.Barbarich-Marsteller NC, Underwood MD, Foltin RW, et al. Identifying novel phenotypes of vulnerability and resistance to activity-based anorexia in adolescent female rats. Int J Eat Disord. 2013;46:737–46. doi: 10.1002/eat.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhagen LA, Luijendijk MC, Korte-Bouws GA, Korte SM, Adan RA. Dopamine and serotonin release in the nucleus accumbens during starvation-induced hyperactivity. Eur Neuropsychopharmacol. 2009;19:309–16. doi: 10.1016/j.euroneuro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 36.Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–7. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–98. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 39.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 40.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 41.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–4. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 43.Exner C, Hebebrand J, Remschmidt H, et al. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol Psychiatry. 2000;5:476–81. doi: 10.1038/sj.mp.4000771. [DOI] [PubMed] [Google Scholar]
- 44.Holtkamp K, Herpertz-Dahlmann B, Hebebrand K, Mika C, Kratzsch J, Hebebrand J. Physical activity and restlessness correlate with leptin levels in patients with adolescent anorexia nervosa. Biol Psychiatry. 2006;60:311–3. doi: 10.1016/j.biopsych.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Holtkamp K, Herpertz-Dahlmann B, Mika C, et al. Elevated physical activity and low leptin levels co-occur in patients with anorexia nervosa. J Clin Endocrinol Metab. 2003;88:5169–74. doi: 10.1210/jc.2003-030569. [DOI] [PubMed] [Google Scholar]
- 46.Otto B, Tschöp M, Frühauf E. Postprandial ghrelin release in anorectic patients before and after weight gain. Psychoneuroendocrinology. 2005;30:577–81. doi: 10.1016/j.psyneuen.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E373–81. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 48.Sakata I, Nakamura K, Yamazaki M. Ghrelin-producing cells exist as two types of cells, closed and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531–6. doi: 10.1016/s0196-9781(01)00633-7. [DOI] [PubMed] [Google Scholar]
- 49.Naleida AM, Graceb MK, Cummings EE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 51.Yin Y, Li Y, Zhang W. The growth hormone secretagogue receptor: its intracellular signaling and regulation. Int J Mol Sci. 2014;15:4837–55. doi: 10.3390/ijms15034837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135:1320–5. doi: 10.1093/jn/135.5.1320. [DOI] [PubMed] [Google Scholar]
- 53.Francois M, Barde S, Achamrah N, et al. The number of preproghrelin mRNA expressing cells is increased in mice with activity-based anorexia. Neuropeptides. 2015;51:17–23. doi: 10.1016/j.npep.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Nakahara T, Harada T, Yasuhara D, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008;64:252–5. doi: 10.1016/j.biopsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E347–56. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 56.Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol. 2011;21:384–92. doi: 10.1016/j.euroneuro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Peino R, Baldelli R, Rodriguez-Garcia J, et al. Ghrelin-induced growth hormone secretion in humans. Eur J Endocrinol. 2000;143:R11–4. doi: 10.1530/eje.0.143r011. [DOI] [PubMed] [Google Scholar]
- 58.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–7. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 59.Hohmann JG, Krasnow SM, Teklemichael DN, Clifton DK, Wynick D, Steiner RA. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology. 2003;77:354–66. doi: 10.1159/000071308. [DOI] [PubMed] [Google Scholar]
- 60.Boyar RM, Hellman LD, Roffwarg H, et al. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med. 1977;296:190–3. doi: 10.1056/NEJM197701272960403. [DOI] [PubMed] [Google Scholar]
- 61.Licinio J, Wong ML, Gold PW. The hypothalamic-pituitary-adrenal axis in anorexia nervosa. Psychiatry Res. 1996;62:75–83. doi: 10.1016/0165-1781(96)02991-5. [DOI] [PubMed] [Google Scholar]
- 62.Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology. 2013;38:312–30. doi: 10.1016/j.psyneuen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:10. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 64.Lobo MJ, Remesar X, Alemany M. Effect of chronic intravenous injection of steroid hormones on body weight and composition of female rats. Biochem Mol Biol Int. 1993;29:349–58. [PubMed] [Google Scholar]
- 65.Wingfield JC. Allostatic load and life cycles: implications for neuroendocrine control mechanisms. In: Schulkin J, editor. Allostasis, homeostasis, and the costs of physiological adaptation. Cambridge, UK: Cambridge University Press; 2004. pp. 302–42. [Google Scholar]
- 66.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Burden VR, White BD, Dean RG, Martin RJ. Activity of the hypothalamic-pituitary-adrenal axis is elevated in rats with activity-based anorexia. J Nutr. 1993;123:1217–25. doi: 10.1093/jn/123.7.1217. [DOI] [PubMed] [Google Scholar]
- 68.Racine SE, Culbert KM, Pamela K, et al. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord. 2012;45:333–44. doi: 10.1002/eat.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69:417–23. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- 70.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–38. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 73.Prevot V, Langlet F, Dehouck B. Flipping the tanycyte switch: how circulating signals gain direct access to the metabolic brain. Aging (Albany NY) 2013;5:332–4. doi: 10.18632/aging.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hillebrand JJ, Kas MJ, van Elburg AA, Hoek HW, Adan RA. Leptin’s effect on hyperactivity: potential downstream effector mechanisms. Physiol Behav. 2008;94:689–95. doi: 10.1016/j.physbeh.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 76.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Biol Cell. 2002;22:5027–35. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260:R321–7. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 80.Stanley BG, Totowa NJ. Neuropeptide Y in multiple hypothalamic sites controls eating behavior, endocrine and autonomic systems for body energy balance. In: Colmers WF, Wahlestedt C, editors. The biology of neuropeptide Yand related peptides. Totowa, NJ: Humana; 1993. pp. 457–509. [Google Scholar]
- 81.Semjonous NM, Smith KL, Parkinson JR, et al. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. Int J Obes (Lond) 2009;33:775–85. doi: 10.1038/ijo.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaiyala KJ, Woods SC, Schwartz MW. New model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. 1995;62(5 suppl):1123S–34S. doi: 10.1093/ajcn/62.5.1123S. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz MW, Baskin DG, Bukowski TR, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–5. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 86.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R941–8. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 87.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 88.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 89.Stanley BG, Lanthier D, Chin AS, Leibowitz SF. Suppression of neuropeptide Y–elicited eating by adrenalectomy or hypophysectomy: reversal with corticosterone. Brain Res. 1989;501:32–6. doi: 10.1016/0006-8993(89)91023-8. [DOI] [PubMed] [Google Scholar]
- 90.McKibbin PE, Cotton SJ, McCarthy HD, Williams G. The effect of dexamethasone on neuropeptide Y concentrations in specific hypothalamic regions. Life Sci. 1992;51:1301–7. doi: 10.1016/0024-3205(92)90020-p. [DOI] [PubMed] [Google Scholar]
- 91.Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS. Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology. 1999;140:2645–50. doi: 10.1210/endo.140.6.6829. [DOI] [PubMed] [Google Scholar]
- 92.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–62. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 93.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 94.Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143:3268–75. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- 95.Larsen PJ, Vrang N, Petersen PC, Kristensen P. Chronic intracerebroventricular administration of recombinant CART(42–89) peptide inhibits and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes Res. 2000;8:590–6. doi: 10.1038/oby.2000.76. [DOI] [PubMed] [Google Scholar]
- 96.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 97.Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–70. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 99.Graham A, Wakamatsu K, Hunt G, Ito S, Thody AJ. Agouti protein inhibits the production of eumelanin and phaeomelanin in the presence and absence of alpha-melanocyte stimulating hormone. Pigment Cell Res. 1997;10:298–303. doi: 10.1111/j.1600-0749.1997.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 100.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–85. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 102.Zhan C, Zhou J, Feng Q, et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–32. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–91. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 104.Vong L, Ye C, Yang Z, Choi B, Chua SJ, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 106.Seger MA, van Eekelen JA, Kiss JZ, Burbach JP, de Kloet ER. Stimulation of pro-opiomelanocortin gene expression by glucocorticoids in the denervated rat intermediate pituitary gland. Neuroendocrinology. 1988;47:350–7. doi: 10.1159/000124936. [DOI] [PubMed] [Google Scholar]
- 107.Appleyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–60. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- 108.Koch M, Varela L, Kim JG, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ogata R, Matsuzaki T, Iwasa T, et al. Hypothalamic ghrelin suppresses pulsatile secretion of luteinizing hormone via beta-endorphin in ovariectomized rats. Neuroendocrinology. 2009;90:364–70. doi: 10.1159/000257421. [DOI] [PubMed] [Google Scholar]
- 110.Abbott CR, Rossi M, Wren AM, Murphy KG, Kennedy AR, Stanley SA. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology. 2001;142:3457–63. doi: 10.1210/endo.142.8.8304. [DOI] [PubMed] [Google Scholar]
- 111.Wang C, Billington CJ, Levine AS, Kotz CM. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. Neuroreport. 2000;11:3251–5. doi: 10.1097/00001756-200009280-00040. [DOI] [PubMed] [Google Scholar]
- 112.Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- 113.Tang F, Hsieh AC, Lee CP, Baconshone J. Interaction of cold and starvation in the regulation of plasma corticosterone levels in the male rat. Horm Metab Res. 1984;16:445–8. doi: 10.1055/s-2007-1014812. [DOI] [PubMed] [Google Scholar]
- 114.Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 115.Baranowska B, Wolińska-Witort E, Martyńska L, Chmielowska M, Baranowska-Bik A. Effects of cocaine-amphetamine regulated transcript (CART) on hormone release. Regul Pept. 2004;122:55–9. doi: 10.1016/j.regpep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 116.Robson AJ, Rousseau K, Loudon AS, Ebling FJ. Cocaine and amphetamine-regulated transcript mRNA regulation in the hypothalamus in lean and obese rodents. J Neuroendocrinol. 2002;14:697–709. doi: 10.1046/j.1365-2826.2002.00830.x. [DOI] [PubMed] [Google Scholar]
- 117.Lartigue de G, Dimaline R, Varro A, Dockray GJ. Cocaine-and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–82. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 119.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 120.Mohammed M, Ootsuka Y, Yanagisawa M, Blessing W. Reduced brown adipose tissue thermogenesis during environmental interactions in transgenic rats with ataxin-3-mediated ablation of hypothalamic orexin neurons. Am J Physiol Regul Integr Comp Physiol. 2014;307:R978–89. doi: 10.1152/ajpregu.00260.2014. [DOI] [PubMed] [Google Scholar]
- 121.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 122.Haynes AC, Jackson B, Overend P, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 123.Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59:385–96. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- 124.Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–34. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- 125.Funato H, Tsai AL, Willie JT, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pirnik Z, Bundzikova J, Mikkelsen JD, Zelezna B, Maletinska L, Kiss A. Fos expression in hypocretinergic neurons in C57B1/6 male and female mice after long-term consumption of high fat diet. Endocr Regul. 2008;42:127–36. [PubMed] [Google Scholar]
- 127.Maletınska L, Maixnerova J, Matyskova R, Haugvicova R, Pirnık Z, Kiss A. Synergistic effect of CART (cocaine- and amphetamine-regulated transcript) peptide and cholecystokinin on food intake regulation in lean mice. BMC Neurosci. 2008;9:1–11. doi: 10.1186/1471-2202-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hakansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol. 1999;11:653–63. doi: 10.1046/j.1365-2826.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- 129.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–12. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 130.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 131.Mystkowski P, Seeley RJ, Hahn TM, et al. Hypothalamic melanin-concentrating hormone and estrogen-induced weight loss. J Neurosci. 2000;20:8637–42. doi: 10.1523/JNEUROSCI.20-22-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 133.Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neurondeficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–42. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 134.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 135.Qu D, Ludwig DS, Gammeltoft S, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 136.Rossi M, Choi SJ, O’Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–5. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 137.Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142:680–6. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- 138.Drazen DL, Coolen LM, Strader AD, Wortman MD, Woods SC, Seeley RJ. Differential effects of adrenalectomy on melanin-concentrating hormone and orexin A. Endocrinology. 2004;145:3404–12. doi: 10.1210/en.2003-1760. [DOI] [PubMed] [Google Scholar]
- 139.Presse F, Hervieu G, Imaki T, Sawchenko PE, Vale W, Nahon JL. Rat melanin-concentrating hormone messenger ribonucleic acid expression: marked changes during development and after stress and glucocorticoid stimuli. Endocrinology. 1992;131:1241–50. doi: 10.1210/endo.131.3.1505462. [DOI] [PubMed] [Google Scholar]
- 140.Jacobson L. Glucocorticoid replacement, but not corticotropin-releasing hormone deficiency, prevents adrenalectomy-induced anorexia in mice. Endocrinology. 1999;140:310–7. doi: 10.1210/endo.140.1.6416. [DOI] [PubMed] [Google Scholar]
- 141.Kotz CM, Wang C, Levine AS, Billington CJ. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R546–51. doi: 10.1152/ajpregu.00436.2001. [DOI] [PubMed] [Google Scholar]
- 142.Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–3. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 143.Morrison CD, Bouchard C, Katzmarzyk PT. Physical activity level and hypothalamic peptides. In: Bouchard C, Katzmarzyk PT, editors. Physical activity and obesity. 2nd. Champaign, IL: Human Kinetics; 2000. [Google Scholar]
- 144.Uehara Y, Shimizu H, Ohtani K, Sato N, Mori M. Hypothalamic corticotropin-releasing hormone is a mediator of the anorexigenic effect of leptin. Diabetes. 1998;47:890–3. doi: 10.2337/diabetes.47.6.890. [DOI] [PubMed] [Google Scholar]
- 145.Timofeeva E, Huang Q, Richard D. Effects of treadmill running on brain activation and the corticotropin-releasing hormone system. Neuroendocrinology. 2003;77:388–405. doi: 10.1159/000071311. [DOI] [PubMed] [Google Scholar]
- 146.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–63. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 147.Lucas JJ, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. Absence of fenfluramine-induced anorexia and reduced c-Fos induction in the hypothalamus and central amygdaloid complex of serotonin 1B receptor knock-out mice. J Neurosci. 1998;18:5537–44. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pollock JD, Rowland N. Peripherally administered serotonin decreases food intake in rats. Pharmacol Biochem Behav. 1981;15:179–83. doi: 10.1016/0091-3057(81)90174-x. [DOI] [PubMed] [Google Scholar]
- 149.Shor-Posner G, Grinker JA, Marinescu C, Brown O, Leibowitz SF. Hypothalamic serotonin in the control of meal patterns and macronutrient selection. Brain Res Bull. 1986;17:663–71. doi: 10.1016/0361-9230(86)90198-x. [DOI] [PubMed] [Google Scholar]
- 150.Hutson PH, Dourish CT, Curzon G. Evidence that the hyperphagic response to 8-OH-DPAT is mediated by 5-HT1A receptors. Eur J Pharmacol. 1988;150:361–6. doi: 10.1016/0014-2999(88)90019-2. [DOI] [PubMed] [Google Scholar]
- 151.Schwartz DH, McClane S, Hernandez L, Hoebel BG. Feeding increases extracellular serotonin in the lateral hypothalamus of the rat as measured by microdialysis. Brain Res. 1989;479:349–54. doi: 10.1016/0006-8993(89)91639-9. [DOI] [PubMed] [Google Scholar]
- 152.Paez X, Leibowitz SF. Changes in extracellular PVN monoamines and macronutrient intake after idazoxan or fluoxetine injection. Pharmacol Biochem Behav. 1993;46:933–41. doi: 10.1016/0091-3057(93)90225-i. [DOI] [PubMed] [Google Scholar]
- 153.Haleem DJ, Haque Z, Inam QU, Ikram H, Haleem MA. Behavioral, hormonal and central serotonin modulating effects of injected leptin. Peptides. 2015;74:1–8. doi: 10.1016/j.peptides.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 154.Brunetti L, Recinella L, Orlando G, Michelotto B, Di Nisio C, Vacca M. Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur J Pharmacol. 2002;454:189–92. doi: 10.1016/s0014-2999(02)02552-9. [DOI] [PubMed] [Google Scholar]
- 155.Szczypka MS, Rainey MA, Kim DS, et al. Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci U S A. 1999;96:12138–43. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verhagen LA, Luijendijk MC, Hillebrand JJ, Adan RA. Dopamine antagonism inhibits anorectic behavior in an animal model for anorexia nervosa. Eur Neuropsychopharmacol. 2009;19:153–60. doi: 10.1016/j.euroneuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 157.Opland DM, Leinninger GM, Myers MG., Jr Modulation of the mesolimbic dopamine system by leptin. Brain Res. 2010;1350:65–70. doi: 10.1016/j.brainres.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 159.Fulton S, Pissios P, Manchon RP, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 160.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–32. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 162.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–98. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 163.Chen Y, Lin Y-C, Kuo T-W, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–41. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Betley JN, Xu S, Cao ZF, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–5. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Diano S, Naftolin F, Goglia F, Csernus V, Horvath TL. Monosynaptic pathway between the arcuate nucleus expressing glial type II iodothyronine 5′-deiodinase mRNA and the median eminence-projective TRH cells of the rat paraventricular nucleus. J Neuroendocrinol. 1998;10:731–42. doi: 10.1046/j.1365-2826.1998.00204.x. [DOI] [PubMed] [Google Scholar]
- 166.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 167.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–2. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 168.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–7. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 169.Cowley MA, Cone R, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N YAcad Sci. 2003;994:175–86. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 170.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu T, Kong D, Shah Bhavik P, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–22. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015 Jul 10;:4. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leibowitz SF. Brain peptides and obesity: pharmacologic treatment. Obes Res. 1995;3(suppl 4):573S–589S. doi: 10.1002/j.1550-8528.1995.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 174.Kalra SP, Kalra PS. Neuropeptide Y: a physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine. 2003;22:49–56. doi: 10.1385/ENDO:22:1:49. [DOI] [PubMed] [Google Scholar]
- 175.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 176.Bannon AW, Seda J, Carmouche M, et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- 177.Patel HR, Qi Y, Hawkins EJ, et al. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesityprone mice. Diabetes. 2006;55:3091–8. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- 178.de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35:381–90. doi: 10.1677/jme.1.01808. [DOI] [PubMed] [Google Scholar]
- 179.Wang J, Leibowitz KL. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 1997;777:231–6. doi: 10.1016/s0006-8993(97)00963-3. [DOI] [PubMed] [Google Scholar]
- 180.Tempel DL, Leibowitz SF. Adrenal steroid receptors: interactions with brain neuropeptide systems in relation to nutrient intake and metabolism. J Neuroendocrinol. 1994;6:479–501. doi: 10.1111/j.1365-2826.1994.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 181.Olofsson LE, Pierce AA, Xua AW. Functional requirement of AgRP and NPY neurons in ovarian cycle–dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106:15932–7. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Krysiak R, Obuchowicz E, Herman ZS. Interactions between the neuropeptide Y system and the hypothalamic-pituitary-adrenal axis. Eur J Endocrinol. 1999;140:130–6. doi: 10.1530/eje.0.1400130. [DOI] [PubMed] [Google Scholar]
- 183.Hill JW, Urban JH, Xu M, Levine JE. Estrogen induces neuropeptide Y (NPY) Y1 receptor gene expression and responsiveness to NPY in gonadotrope-enriched pituitary cell cultures. Endocrinology. 2004;145:2283–90. doi: 10.1210/en.2003-1368. [DOI] [PubMed] [Google Scholar]
- 184.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Acuna-Goycolea C, van den Pol AN. Peptide YY(3-36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis. J Neurosci. 2005;25:10510–9. doi: 10.1523/JNEUROSCI.2552-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hasan TF, Hasan H. Anorexia nervosa: a unified neurological perspective. Int J Med Sci. 2011;8:679–703. doi: 10.7150/ijms.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–28. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 189.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–95. [PubMed] [Google Scholar]
- 190.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–20. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 191.Filliol D, Ghozland S, Chluba J, et al. Mice deficient for delta-and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 192.Morley JE, Blundell JE. The neurobiological basis of eating disorders: some formulations. Biol Psychiatry. 1988;23:53–78. doi: 10.1016/0006-3223(88)90106-0. [DOI] [PubMed] [Google Scholar]
- 193.Plotsky PM, Thrivikraman KV, Meaney MJ. Central and feedback regulation of hypothalamic corticotropin-releasing factor secretion. Ciba Found Symp. 1993;172:59–75. doi: 10.1002/9780470514368.ch4. discussion 75–84. [DOI] [PubMed] [Google Scholar]
- 194.Yanagita K, Shiraishi J, Fujita M, Bungo T. Effects of N-terminal fragments of beta-endorphin on feeding in chicks. Neurosci Lett. 2008;442:140–2. doi: 10.1016/j.neulet.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 195.Havel PJ, Hahn TM, Sindelar DK, Baskin DG, Dallman MF, Weigle DS. Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats. Diabetes. 2000;49:244–52. doi: 10.2337/diabetes.49.2.244. [DOI] [PubMed] [Google Scholar]
- 196.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–52. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hirosawa M, Minata M, Harada KH, Hitomi T, Krust A, Koizumi A. Ablation of estrogen receptor alpha (ERa) prevents upregulation of POMC by leptin and insulin. Biochem Biophys Res Commun. 2008;371:320–3. doi: 10.1016/j.bbrc.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 198.Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–33. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- 199.Wang C, Mullet MA, Glass MJ, Billington CJ, Levine AS, Kotz CM. Feeding inhibition by urocortin in the rat hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R473–80. doi: 10.1152/ajpregu.2001.280.2.R473. [DOI] [PubMed] [Google Scholar]
- 200.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–82. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu L, Song Z, Sheikhahmadi A, Jiao H, Lin H. Effect of corticosterone on gene expression of feed intake regulatory peptides in laying hens. Comp Biochem Physiol B Biochem Mol Biol. 2012;162:81–7. doi: 10.1016/j.cbpb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 202.Silva LE, Castro M, Amaral FC, Antunes-Rodrigues J, Elias LL. Estradiol-induced hypophagia is associated with the differential mRNA expression of hypothalamic neuropeptides. Braz J Med Biol Res. 2010;43:759–66. doi: 10.1590/s0100-879x2010007500059. [DOI] [PubMed] [Google Scholar]
- 203.Hillebrand JJ, Kas MJ, Scheurink A, Dijk van G, Adan RA. AgRP(83-132) and SHU9119 differently affect activity-based anorexia. Eur Neuropsychopharmacol. 2006;16:403–12. doi: 10.1016/j.euroneuro.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 204.Rigaud D, Verges B, Colas-Linhart N, et al. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;5:1623–9. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]
- 205.Goldfarb AH. Exercise and endogenous opiates. In: Constantini N, Hackney AC, editors. Endocrinology of physical activity and sport. 2nd. Totowa, NJ: Humana; 2013. [Google Scholar]
- 206.Aravich PF, Rieg TS, Lauterio TJ, Doerries LE. Betaendorphin and dynorphin abnormalities in rats subjected to exercise and restricted feeding: relationship to anorexia nervosa? Brain Res. 1993;622:1–8. doi: 10.1016/0006-8993(93)90794-n. [DOI] [PubMed] [Google Scholar]
- 207.Marrazzi MA, Luby ED. An auto-addiction opioid model of chronic anorexia nervosa. Int J Eat Dis. 1986;5:191–208. [Google Scholar]
- 208.Broberger C, de Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Yand agouti gene-related protein systems. J Comp Neurol. 1998;402:460–74. [PubMed] [Google Scholar]
- 209.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–59. [PubMed] [Google Scholar]
- 210.Peyron C, Tighe DK, Van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 212.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 213.Fu L-Y, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–51. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Karatayev O, Barson JR, Chang GQ, Leibowitz SF. Hypothalamic injection of non-opioid peptides increases gene expression of the opioid enkephalin in hypothalamic and mesolimbic nuclei: possible mechanism underlying their behavioral effects. Peptides. 2009;30:2423–31. doi: 10.1016/j.peptides.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dube MG, Horvath TL, Leranth C, Kalra PS, Kalra SP. Naloxone reduces the feeding evoked by intracerebroventricular galanin injection. Physiol Behav. 1994;56:811–3. doi: 10.1016/0031-9384(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 216.Sweet DC, Levine AS, Kotz CM. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides. 2004;25:307–14. doi: 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 217.Borgland SL, Chang SJ, Bowers MS, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res. 2010;1314:139–44. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 219.Horvath TL, Diano S, Van Den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19:1072–87. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Swart I, Overton JM, Houpt TA. The effect of food deprivation and experimental diabetes on orexin and NPY mRNA levels. Peptides. 2001;22:2175–9. doi: 10.1016/s0196-9781(01)00552-6. [DOI] [PubMed] [Google Scholar]
- 221.Kaye WH. Neuropeptide abnormalities in anorexia nervosa. Psychiatry Res. 1996;62:65–74. doi: 10.1016/0165-1781(96)02985-x. [DOI] [PubMed] [Google Scholar]
- 222.Bronsky J, Nedvidkova J, Krasnicanova H, et al. Changes of orexin A plasma levels in girls with anorexia nervosa during eight weeks of realimentation. Int J Eat Disord. 2011;44:547–52. doi: 10.1002/eat.20857. [DOI] [PubMed] [Google Scholar]
- 223.Barson JR, Morganstern I, Leibowitz SF. Complementary roles of orexin and melanin-concentrating hormone in feeding behavior. Int J Endocrinol. 2013;2013:983964. doi: 10.1155/2013/983964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: the multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–20. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 225.Pissios P, Frank L, Kennedy AR, et al. Dysregulation of the mesolimbic dopamine system and reward in MCH−/− mice. Biol Psychiatry. 2008;64:184–91. doi: 10.1016/j.biopsych.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 226.Georgescu D, Sears RM, Hommel JD, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–40. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Borowsky B, Durkin MM, Ogozalek K, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–30. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 228.Williamson-Hughes PS, Grove KL, Smith MS. Melanin concentrating hormone (MCH): a novel neural pathway for regulation of GnRH neurons. Brain Res. 2005;1041:117–24. doi: 10.1016/j.brainres.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 229.Shearman LP, Camacho RE, Sloan SD, Zhou D, Bednarek MA, Hreniuk DL. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- 230.Forray C. The MCH receptor family: feeding brain disorders? Curr Opin Pharmacol. 2003;3:85–9. doi: 10.1016/s1471-4892(02)00013-9. [DOI] [PubMed] [Google Scholar]
- 231.Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obes Res. 2004;12:716–24. doi: 10.1038/oby.2004.84. [DOI] [PubMed] [Google Scholar]
- 232.Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol. 2002;440:189–97. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- 233.Tizabi Y, Aguilera G. Desensitization of the hypothalamic-pituitary-adrenal axis following prolonged administration of corticotropin-releasing hormone or vasopressin. Neuroendocrinology. 1992;56:611–8. doi: 10.1159/000126283. [DOI] [PubMed] [Google Scholar]
- 234.Wong ML, Licinio J, Gold PW, Glowa J. Activity-induced anorexia in rats does not affect hypothalamic neuropeptide gene expression chronically. Int J Eat Disord. 1993;13:399–405. doi: 10.1002/1098-108x(199305)13:4<399::aid-eat2260130408>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 235.Battezzati A, Bertoli S. Is physical activity a meaningful treat in AN? In: Luzi L, editor. Cellular physiology and metabolism of physical exercise. New York: Springer; 2012. [Google Scholar]
- 236.Ehrlich S, Weiss D, Burghardt R, et al. Promoter specific DNA methylation and gene expression of POMC in acutely underweight and recovered patients with anorexia nervosa. J Psychiatr Res. 2010;44:827–33. doi: 10.1016/j.jpsychires.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 237.Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol. 1988;255:E255–9. doi: 10.1152/ajpendo.1988.255.3.E255. [DOI] [PubMed] [Google Scholar]
- 238.Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Ann N Y Acad Sci. 1990;579:183–91. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- 239.Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- 240.Klein DA, Mayer LES, Schebendach JE, Walsh BT. Physical activity and cortisol in anorexia nervosa. Psychoneuroendocrinology. 2007;32:539–47. doi: 10.1016/j.psyneuen.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 241.Haleem DJ, Haider S. Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport. 1996;7:1153–6. doi: 10.1097/00001756-199604260-00011. [DOI] [PubMed] [Google Scholar]
- 242.Gauthier C, Hassler C, Mattar L, et al. Symptoms of depression and anxiety in anorexia nervosa: links with plasma tryptophan and serotonin metabolism. Psychoneuroendocrinology. 2014;39:170–8. doi: 10.1016/j.psyneuen.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 243.Rollins BL, King BM. Amygdala-lesion obesity: what is the role of the various amygdaloid nuclei? Am J Physiol Regul Integr Comp Physiol. 2000;279:R1348–56. doi: 10.1152/ajpregu.2000.279.4.R1348. [DOI] [PubMed] [Google Scholar]
- 244.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. 2012;63:87–96. doi: 10.1016/j.neuropharm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides. 2008;29:1588–95. doi: 10.1016/j.peptides.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 247.Hebebrand J, Albayrak O, Adan R, et al. “Eating addiction,” rather than “food addiction,” better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 248.Hebebrand J, Exner C, Hebebrand K, et al. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav. 2003;79:25–37. doi: 10.1016/s0031-9384(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 249.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wahlestedt C, Skagerberg G, Ekman R, Heilig M, Sundler F, Hakanson R. Neuropeptide Y (NPY) in the area of the hypothalamic paraventricular nucleus activates the pituitary-adrenocortical axis in the rat. Brain Res. 1987;417:33–8. doi: 10.1016/0006-8993(87)90176-4. [DOI] [PubMed] [Google Scholar]
- 251.Dhillo WS, Small CJ, Seal LJ. The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology. 2002;75:209–16. doi: 10.1159/000054712. [DOI] [PubMed] [Google Scholar]
- 252.Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull. 1988;21:905–12. doi: 10.1016/0361-9230(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 253.Vrang N, Larsen PJ, Clausen JT, Kristensen P. Neurochemical characterization of hypothalamic cocaine- amphetamine-regulated transcript neurons. J Neurosci. 1999;(19):RC5. doi: 10.1523/JNEUROSCI.19-10-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Smith SM, Vaughan JM, Donaldson CJ. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor–dependent mechanism. Endocrinology. 2004;145:5202–9. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- 255.Rivest S, Richard D. Hypothalamic paraventricular nucleus lesions do not prevent anorectic effect of exercise in male rats. Am J Physiol. 1990;259:R579–84. doi: 10.1152/ajpregu.1990.259.3.R579. [DOI] [PubMed] [Google Scholar]
- 256.Rivest S, Richard D. Involvement of corticotropin-releasing factor in the anorexia induced by exercise. Brain Res Bull. 1990;1:69–72. doi: 10.1016/0361-9230(90)90270-a. [DOI] [PubMed] [Google Scholar]
- 257.Vergoni AV, Bertolini A, Wikberg JE, Schioth HB. Selective melanocortin MC4 receptor blockage reduces immobilization stress-induced anorexia in rats. Eur J Pharmacol. 1999;369:11–5. doi: 10.1016/s0014-2999(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 258.Ryan KK, Mul JD, Clemmensen C, et al. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. doi: 10.1016/j.psyneuen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 260.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.van der Doelen RH, Deschamps W, Dannibale C, et al. Early life adversity and serotonin transporter gene variation interact at the level of the adrenal gland to affect the adult hypothalamo-pituitary-adrenal axis. Transl Psychiatry. 2014;4:e409. doi: 10.1038/tp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hancock S, Grant V. Early maternal separation increases symptoms of activity-based anorexia in male and female rats. J Exp Psychol Anim Behav Process. 2009;35:394–406. doi: 10.1037/a0014736. [DOI] [PubMed] [Google Scholar]
- 263.Mastorakos G, Pavlatou M. Exercise as a stress model and the interplay between the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes. Horm Metab Res. 2005;37:577–84. doi: 10.1055/s-2005-870426. [DOI] [PubMed] [Google Scholar]
- 264.Broocks A, Liu J, Pirke KM. Semistarvation-induced hyperactivity compensates for decreased norepinephrine and dopamine turnover in the mediobasal hypothalamus of the rat. J Neural Transm Gen Sect. 1990;79:113–24. doi: 10.1007/BF01251006. [DOI] [PubMed] [Google Scholar]
- 265.Holsen L, Lawson E, Blum J, et al. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci. 2012;37:322–32. doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lawson EA, Holsen LM, DeSanti R. Increased hypothalamic-pituitary-adrenal drive is associated with decreased appetite and hypoactivation of food motivation neurocircuitry in anorexia nervosa. Eur J Endocrinol. 2013;169:639–47. doi: 10.1530/EJE-13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vocks S, Herpertz S, Rosenberger C, Senf W, Gizewski ER. Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: an fMRI study. J Psychiatr Res. 2011;45:395–403. doi: 10.1016/j.jpsychires.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 268.Yamada C, Saegusa Y, Nahata M, Sadakane C, Hattori T, Takeda H. Influence of aging and gender differences on feeding behavior and ghrelin-related factors during social isolation in mice. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.La Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–9. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 270.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–62. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 271.Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10-day-old rats. Behav Neurosci. 1989;103:1168–75. doi: 10.1037//0735-7044.103.6.1168. [DOI] [PubMed] [Google Scholar]
- 272.Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–8. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- 273.Ulrich-Lai YM, Christiansen AM, Ostrander MM, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ulrich-Lai YM, Ostrander MM, Thomas IM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–34. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- 276.Gorelick DA. Alcohol and cocaine. Clinical and pharmacological interactions. Recent Dev Alcohol. 1992;10:37–56. [PubMed] [Google Scholar]
- 277.Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behav Brain Res. 2002;133:301–8. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 278.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–44. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ferreira A, Lamarque S, Boyer P, Perez-Diaz F, Jouvent R, Cohen-Salmon C. Spontaneous appetence for wheel-running: a model of dependency on physical activity in rat. Eur Psychiatry. 2006;21:580–8. doi: 10.1016/j.eurpsy.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 282.Keating C. Theoretical perspective on anorexia nervosa: the conflict of reward. Neurosci Biobehav Rev. 2010;34:73–9. doi: 10.1016/j.neubiorev.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 283.Ramsey JJ, Hagopian K. Energy expenditure and restriction of energy intake: could energy restriction alter energy expenditure in companion animals? J Nutr. 2006;136(7 suppl):1958S–66S. doi: 10.1093/jn/136.7.1958S. [DOI] [PubMed] [Google Scholar]
- 284.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 285.Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci. 2009;30:1718–29. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 286.Bruch H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosom Med. 1962;24:187–94. doi: 10.1097/00006842-196203000-00009. [DOI] [PubMed] [Google Scholar]
- 287.Gull WW. Anorexia nervosa (apepsia hysterica, anorexia hysterica). 1868. Obes Res. 1997;5:498–502. doi: 10.1002/j.1550-8528.1997.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 288.Routtenberg A. “Self-starvation” of rats living in activity wheels: adaptation effects. J Comp Physiol Psychol. 1968;66:234–8. doi: 10.1037/h0025977. [DOI] [PubMed] [Google Scholar]
- 289.Bailer UF, Frank GK, Price JC, et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 2013;211:160–8. doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cohen JY, Amoroso MW, Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. Elife. 2015 Feb 25;:4. doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 292.Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Ann N Y Acad Sci. 2003;985:251–62. doi: 10.1111/j.1749-6632.2003.tb07086.x. [DOI] [PubMed] [Google Scholar]
- 293.Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–8. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 295.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 297.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kempadoo KA, Tourino C, Cho SL, et al. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33:7618–26. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–21. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 301.Jennings JH, Sparta DR, Stamatakis AM, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–8. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 305.Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- 306.Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. 2013;3:39–53. doi: 10.3390/brainsci3010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study. Brain Res. 1992;592:29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- 308.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–50. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- 310.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–63. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 312.Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–81. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- 313.Bagnol D, Lu XY, Kaelin CB, et al. Anatomy of an endogenous antagonist: relationship between agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;(19):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–44. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 315.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 316.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15:1330–5. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Harrison A, Treasure J, Smillie LD. Approach and avoidance motivation in eating disorders. Psychiatry Res. 2011;188:396–401. doi: 10.1016/j.psychres.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 318.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 319.Bailer UF, Kaye WH. Serotonin: imaging findings in eating disorders. Curr Top Behav Neurosci. 2011;6:59–79. doi: 10.1007/7854_2010_78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bergh C, Sodersten P. Anorexia nervosa, self-starvation and the reward of stress. Nat Med. 1996;2:21–2. doi: 10.1038/nm0196-21. [DOI] [PubMed] [Google Scholar]
- 321.Denis RG, Joly-Amado A, Webber E, et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 2015;22:646–57. doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Davis JF, Choi DL, Schurdak JD, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69:668–74. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Verhagen LA, Luijendijk MC, Adan RA. Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur Neuropsychopharmacol. 2011;21:274–81. doi: 10.1016/j.euroneuro.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 324.Dunn AJ. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem. 1988;51:406–12. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 325.Inoue T, Koyama T. Effects of acute and chronic administration of high-dose corticosterone and dexamethasone on regional brain dopamine and serotonin metabolism in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:147–56. doi: 10.1016/0278-5846(95)00299-5. [DOI] [PubMed] [Google Scholar]
- 326.Rothschild AJ, Langlais PJ, Schatzberg AF, et al. The effects of a single acute dose of dexamethasone on monoamine and metabolite levels in rat brain. Life Sci. 1985;36:2491–501. doi: 10.1016/0024-3205(85)90145-6. [DOI] [PubMed] [Google Scholar]
- 327.Tanganelli S, Fuxe K, von Euler G, Eneroth P, Agnati LF, Ungerstedt U. Changes in pituitary-adrenal activity affect the apomorphine- and cholecystokinin-8-induced changes in striatal dopamine release using microdialysis. J Neural Transm Gen Sect. 1990;81:183–94. doi: 10.1007/BF01245041. [DOI] [PubMed] [Google Scholar]
- 328.Wolkowitz O, Sutton M, Koulu M, et al. Chronic corticosterone administration in rats: behavioral and biochemical evidence of increased central dopaminergic activity. Eur J Pharmacol. 1986;122:329–38. doi: 10.1016/0014-2999(86)90413-9. [DOI] [PubMed] [Google Scholar]
- 329.Lindley SE, Bengoechea TG, Schatzberg AF, Wong DL. Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology. 1999;21:399–407. doi: 10.1016/S0893-133X(98)00103-1. [DOI] [PubMed] [Google Scholar]
- 330.Ma QP, Yin GF, Ai MK, Han JS. Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett. 1991;134:21–4. doi: 10.1016/0304-3940(91)90499-j. [DOI] [PubMed] [Google Scholar]
- 331.Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci. 1998;18:9529–38. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- 333.Steinbusch HW, Nieuwenhuys R. Localization of serotonin-like immunoreactivity in the central nervous system and pituitary of the rat, with special references to the innervation of the hypothalamus. Adv Exp Med Biol. 1981;133:7–35. doi: 10.1007/978-1-4684-3860-4_1. [DOI] [PubMed] [Google Scholar]
- 334.Devera A, Pascovich C, Lagos P, et al. Melanin-concentrating hormone (MCH) modulates the activity of dorsal raphe neurons. Brain Res. 2015;1598:114–28. doi: 10.1016/j.brainres.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 335.Jalewa J, Joshi A, McGinnity TM, Prasad G, Wong-Lin K, Holscher C. Neural circuit interactions between the dorsal raphe nucleus and the lateral hypothalamus: an experimental and computational study. PLoS One. 2014;9:e88003. doi: 10.1371/journal.pone.0088003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Bagdy G, Calogero AE, Murphy DL, Szemeredi K. Serotonin agonists cause parallel activation of the sympathoadrenomedullary system and the hypothalamo-pituitary-adrenocortical axis in conscious rats. Endocrinology. 1989;125:2664–9. doi: 10.1210/endo-125-5-2664. [DOI] [PubMed] [Google Scholar]
- 337.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1215–67. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110–20. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sapun-Malcolm D, Farah JM, Jr, Mueller GP. Serotonin and dopamine independently regulate pituitary beta-endorphin release in vivo. Neuroendocrinology. 1986;42:191–6. doi: 10.1159/000124439. [DOI] [PubMed] [Google Scholar]
- 340.Schmauss C, Emrich HM. Dopamine and the action of opiates: a reevaluation of the dopamine hypothesis of schizophrenia. With special consideration of the role of endogenous opioids in the pathogenesis of schizophrenia. Biol Psychiatry. 1985;20:1211–31. doi: 10.1016/0006-3223(85)90179-9. [DOI] [PubMed] [Google Scholar]
- 341.George SR, Van Loon GR. Beta-endorphin alters dopamine uptake by the dopamine neurons of the hypothalamus and striatum. Brain Res. 1982;248:293–303. doi: 10.1016/0006-8993(82)90587-x. [DOI] [PubMed] [Google Scholar]
- 342.Kas MJ, van den Bos R, Baars AM, et al. Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci. 2004;20:1624–32. doi: 10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- 343.Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Ann NY Acad Sci. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 345.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 346.Nakamura T, Uramura K, Nambu T, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 347.Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–84. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- 348.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 349.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 350.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 351.Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab. 2014;3:694–704. doi: 10.1016/j.molmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yen HH, Roseberry AG. Decreased consumption of rewarding sucrose solutions after injection of melanocortins into the ventral tegmental area of rats. Psychopharmacology (Berl) 2015;232:285–94. doi: 10.1007/s00213-014-3663-6. [DOI] [PubMed] [Google Scholar]
- 353.Cansell C, Denis RG, Joly-Amado A, Castel J, Luquet S. Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne) 2012;3:169. doi: 10.3389/fendo.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Cleary DR, Ozpinar A, Raslan AM, Ko AL. Deep brain stimulation for psychiatric disorders: where we are now. Neurosurg Focus. 2015;38:E2. doi: 10.3171/2015.3.FOCUS1546. [DOI] [PubMed] [Google Scholar]
- 355.Anand BK, Brobeck JR. Localization of a feeding center in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–4. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 356.Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172:162–8. doi: 10.1152/ajplegacy.1952.172.1.162. [DOI] [PubMed] [Google Scholar]
- 357.Welkenhuysen M, van Kuyck K, Das J, Sciot R, Nuttin B. Electrical stimulation in the lateral hypothalamus in rats in the activity-based anorexia model. Neurosurg Focus. 2008;25:E7. doi: 10.3171/FOC/2008/25/7/E7. [DOI] [PubMed] [Google Scholar]
- 358.Zink CF, Weinberger DR. Cracking the moody brain: the rewards of self starvation. Nat Med. 2010;16:1382–3. doi: 10.1038/nm1210-1382. [DOI] [PubMed] [Google Scholar]
- 359.Yacubian J, Sommer T, Schroeder K, et al. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–30. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jappe LM, Frank GK, Shott ME, et al. Heightened sensitivity to reward and punishment in anorexia nervosa. Int J Eat Disord. 2011;44:317–24. doi: 10.1002/eat.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Bissada H, Tasca GA, Barber AM, Bradwejn J. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2008;165:1281–8. doi: 10.1176/appi.ajp.2008.07121900. [DOI] [PubMed] [Google Scholar]
- 362.Malina A, Gaskill J, McConaha C, et al. Olanzapine treatment of anorexia nervosa: a retrospective study. Int J Eat Disord. 2003;33:234–7. doi: 10.1002/eat.10122. [DOI] [PubMed] [Google Scholar]
- 363.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(suppl 7):22–31. [PubMed] [Google Scholar]
- 364.Hillebrand JJ, van Elburg AA, Kas MJ, van Engeland H, Adan RA. Olanzapine reduces physical activity in rats exposed to activity-based anorexia: possible implications for treatment of anorexia nervosa? Biol Psychiatry. 2005;58:651–7. doi: 10.1016/j.biopsych.2005.04.008. [DOI] [PubMed] [Google Scholar]


