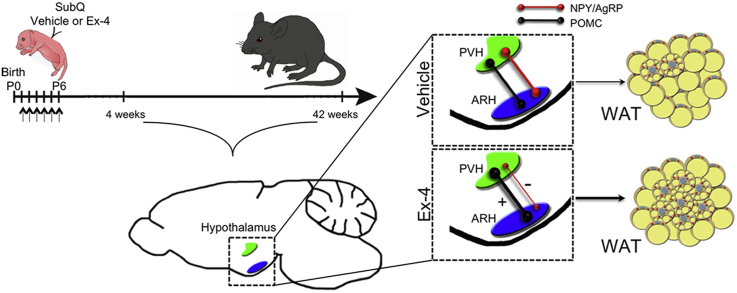
Abstract
Objective
Adult obesity risk is influenced by alterations to fetal and neonatal environments. Modifying neonatal gut or neurohormone signaling pathways can have negative metabolic consequences in adulthood. Here we characterize the effect of neonatal activation of glucagon like peptide-1 (GLP-1) receptor (GLP1R) signaling on adult adiposity and metabolism.
Methods
Wild type C57BL/6 mice were injected with 1 nmol/kg Exendin-4 (Ex-4), a GLP1R agonist, for 6 consecutive days after birth. Growth, body composition, serum analysis, energy expenditure, food intake, and brain and fat pad histology and gene expression were assessed at multiple time points through 42 weeks. Similar analyses were conducted in a Glp1r conditional allele crossed with a Sim1Cre deleter strain to produce Sim1Cre;Glp1rloxP/loxP mice and control littermates.
Results
Neonatal administration of Ex-4 reduced adult body weight and fat mass, increased energy expenditure, and conferred protection from diet-induced obesity in female mice. This was associated with induction of brown adipose genes and increased noradrenergic fiber density in parametrial white adipose tissue (WAT). We further observed durable alterations in orexigenic and anorexigenic projections to the paraventricular hypothalamic nucleus (PVH). Genetic deletion of Glp1r in the PVH by Sim1-Cre abrogated the impact of neonatal Ex-4 on adult body weight, WAT browning, and hypothalamic architecture.
Conclusion
These observations suggest that the acute activation of GLP1R in neonates durably alters hypothalamic architecture to limit adult weight gain and adiposity, identifying GLP1R as a therapeutic target for obesity prevention.
Keywords: Obesity, Metabolism, Incretin, Beige fat, Hypothalamic architecture
Graphical abstract
Highlights
-
•
Neonatal Ex-4 attenuates diet-induced obesity in adult female mice.
-
•
Neonatal Ex-4 enhances adult energy expenditure and beiging of parametrial WAT.
-
•
Neonatal Ex-4 durably alters hypothalamic architecture.
-
•
The impact of neonatal Ex-4 was abrogated in Sim1Cre;Glp1rloxP/loxP mice.
-
•
GLP1R may be a therapeutic target for obesity prevention.
1. Introduction
Central regulation of energy balance in response to feeding cues and environmental factors is regulated by a complex signaling network that integrates multiple brain regions as well as peripheral signals from adipose tissue and the gastrointestinal tract, resulting in physiological responses and altered energy balance specific to nutrient status [1]. Dysregulation of these essential homeostatic mechanisms can lead to obesity and metabolic disorders, including type 2 diabetes [1]. Many of the central circuits that regulate energy balance, specifically those of hypothalamic origin, originate during the first few weeks of life in rodents [2], making this a critical developmental window susceptible to environmental interference. Concurrently, as hypothalamic architecture is developing, white adipose tissues (WAT) depots, which are non-existent at birth, experience rapid expansion [3]. Brown adipose tissue (BAT) depots are also rapidly expanding and responding to environmental changes during this period [4], [5].
The risk of health disorders, including metabolic syndrome, is influenced and exacerbated by negative fetal and neonatal environmental exposures [6], [7]. This phenomenon, referred to as the ‘Barker Hypothesis’ or the ‘developmental origins of adult disease,’ intimates that challenges that occur during early development can permanently alter the physiology of the body in adulthood and result in increased susceptibility to disease [8]. In humans, low birth weight is a risk factor for the development of adult obesity and cardiovascular disease [8], [9], [10]. In rodents, intrauterine growth restriction (IUGR) has adverse effects on β-cell mass and the development of insulin resistance and diabetes in adulthood [11], [12], [13].
Physiological glucagon like peptide-1 (GLP-1) signaling acts both peripherally and centrally to stimulate gastric emptying, promote satiety and glucose-induced insulin secretion, and inhibit glucagon release [14]. In adult rodents, central activation of hypothalamic GLP-1 receptor (GLP1R) can transiently repress food intake and weight gain [15], and targeted activation of GLP1R specifically within the ventromedial hypothalamus (VMH) can activate BAT thermogenesis and induce WAT browning [15]. In humans, GLP1R agonists are in clinical use for improving glycemic control in patients with diabetes and have been approved recently for use in weight management [16]. In addition to central expression, GLP1R is widely expressed in other tissues, including in the pancreatic ß-cell, heart, lungs, and kidneys [17], [18]. While these studies support the utility of GLP-1 mimetics in the treatment of health disorders in adults, there is almost no appreciation for their biological action during early postnatal life. We have shown that IUGR in pregnant rats results in low birth weight and the development of obesity and overt diabetes in adult rats [11], [12], [13]. Administration of the GLP1R agonist Exendin-4 (Ex-4) during the neonatal period prevents the development of adult-onset diabetes in IUGR rats and attenuates age related weight gain in both IUGR and sham controls [11], [13].
The role of hormonal signaling during neonatal development in the context of susceptibility to adult disease is not a new concept nor is it restricted to GLP-1. The gut hormone ghrelin, a well characterized appetite stimulator in adult mammals [19], has been shown to play an inhibitory role in hypothalamic circuitry development, with temporal changes in expression during neonatal development having negative metabolic consequences that persist through adulthood [20]. Similarly, manipulation of the adipose-derived hormone leptin, which acts as a signal of stored energy status and an appetite suppressant [19], during the ‘leptin surge’ of neonatal development [21] alters adult metabolic profiles depending on the timing of manipulation [22]. Recently, maternal high fat diet (HFD) exposure during lactation was shown to impair POMC and AgRP hypothalamic projection development and increase risk of development of hyperinsulinemia, glucose intolerance, and adiposity in the offspring [23]. Together, these findings show that hormones relevant to metabolic regulation in adults can play pivotal roles during neonatal development and, when altered, can permanently change adult health and disease susceptibility.
These studies highlight the biological action of appetite-regulating hormones expressed during the neonatal period and help to identify critical developmental windows susceptible to modulation that could eventually provide a more targeted approach for the treatment and prevention of adult obesity and associated disorders. Here we show that administration of Ex-4 during the first week of life is protective against both age-related and diet-induced obesity. Neonatal Ex-4 leads to browning of parametrial adipose tissue, enhances energy expenditure, and durably alters hypothalamic architecture in female mice. Many of these effects are abrogated by the deletion of GLP1R in the Sim1 expression domain that includes the paraventricular hypothalamic nucleus (PVH), suggesting that this novel role of neonatal GLP1R activation is mediated, in part, through a central mechanism involving the PVH.
2. Materials and methods
2.1. Animal studies
2.1.1. Breeding and housing
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Mice were housed under standard conditions (average 22 °C) and allowed free access to food and water. Animals spontaneously delivered, and, at birth, the litters were randomly culled or fostered to eight. Animals were weaned at 21 days of age then allowed free access to either chow diet, 10% LFD, or 45% HFD (Research Diets, New Brunswick, NJ) and maintained with 12-hour light and dark cycles.
2.1.2. Ex-4 preparation
Ex-4 was prepared as a 1 μmol/l stock in 0.9% sodium chloride, and stored at −80 °C in single use aliquots. Just before injection, aliquots were thawed and diluted in 1% BSA 0.9% sodium chloride.
2.1.3. Experimental conditions
Experiments involving neonatal Ex-4 (Bachem [H-8730] King of Prussia) administration were performed using wild type C57BL/6N mice (Charles River) or mice with the conditional loss of Glp1r [24] generated by breeding Glp1r floxed mice with Sim1Cre deleter strain mice, obtained from B. Kublaoui (Children's Hospital of Philadelphia) [25], resulting in littermate control Glp1rloxP/loxP and experimental Sim1Cre;Glp1rloxP/loxP mice. Injections were as follows: Vehicle treated (1% BSA in 0.9% saline) and Ex-4 treated (1 nmol/kg initial body weight). Vehicle or Ex-4 was injected subcutaneously daily for 6 days, beginning 16–20 h after birth. Animal weights were monitored regularly.
2.1.4. Body composition
Body composition was determined by NMR (Echo Medical Systems, Houston, TX), as described previously [26].
2.1.5. Indirect calorimetry
For indirect calorimetry measurements, animals were individually housed for 72 h. Raw values were normalized to lean mass and data represent values over a 24 h period. Energy expenditure was measured as previously described [27] and calculated according to the following formula. Total energy expenditure (heat) = Calorific value (CV) × VO2, where CV = 3.815 + 1.232 × RER.
2.1.6. Intraperitoneal glucose tolerance
For glucose tolerance tests, 28 day old female and male mice were fasted overnight and given 1 g/kg glucose via intra-peritoneal (IP) injection; blood glucose was measured by handheld glucometer at 0, 15, 30, 60, and 120 min after injection.
2.1.7. Core body temperature
Mice were anesthetized with isofluorane and implanted with subcutaneous transponder devices longitudinally parallel but to one side of the spine (Animal Identification Transponder IPTT-300, Bio Medic Data Systems [BMDS]) below the intrascapular region. Mice were allowed to recover for 7 days prior to starting data collection of core body temperature using a compatible BMDS Smart Probe scanning device. Temperature data were collected for 5 days at the end of the dark cycle (6 am) and the end of the light cycle (6 pm).
2.2. Histology
Antisera were as follows: UCP1 (ab10983, Abcam, Cambridge, UK), TH (AB3036) and NPY (AB1583) (Millipore, Temecula, CA), AgRP (H-003-57), and POMC (H-029-30) (Phoenix Pharmaceuticals, INC, Burlingame, CA). Brains were fixed for 24 h at 4 °C in 4% paraformaldehyde (PFA), cryoprotected in 30% sucrose for 24 h, embedded and frozen on dry ice, and stored at −80 °C. 14 μm thick serial coronal cryosections were cut and mounted on glass slides prior to staining. For in situ analysis of Glp1r expression (ACD RNAscope®, Newark, CA), brains were dissected onto dry ice and stored at −80 °C until processing using the manufacturer's protocol (Probe-Mm-Glp1r-E7-E13, RNAscope® 2.0 HD Reagent Kit). Fat pads were dissected and fixed in 4% PFA overnight at 4 °C. Paraffin embedded sections were incubated with anti-UCP1 (1:1000) antibody followed by detection using the ABC Vectastain-Elite kit (Vector Laboratories, INC., Burlingame, CA) or for immunofluorescence UCP1 (1:500) and TH (1:500).
2.3. Quantitative RT-PCR (QPCR)
For isolation of total RNA from frozen adipose tissue, samples were homogenized in Trizol (Invitrogen) and processed (Maxwell, Promega). RNA was reverse-transcribed to cDNA with Super Script II (Invitrogen). QPCR was performed on a Bio-Rad CFX384. Primer sequences can be found in Table S1.
2.4. Ex vivo oxygen consumption
Six 5 mg portions of each parametrial fat pad were dissected from female mice. Three pieces were incubated in vehicle (n = 3), and three pieces were incubated in 10 uM isoproterenol for 1 h in DMEM-F12 containing fetal bovine serum prior to analysis using a Clark-type electrode as previously described with the following minor modifications [28]. The superfusion medium was maintained at 30 °C using a water bath. The composition of the perfusion solution was 114 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM NAHPO4, 2.2 mM CaCl2, 10 mM HEPES, 5 mM glucose (pH 7.4).
2.5. Statistics
All data are represented as mean +/− SEM. Statistical significance was assessed by two-tailed Student's t-test, unpaired heteroscedastic t-test, One-way or Two-way ANOVA as indicated in figure legends. Differences were considered significant if p < 0.05.
3. Results
3.1. Decreased adiposity and increased energy expenditure in adult mice treated with Ex-4 during the neonatal period
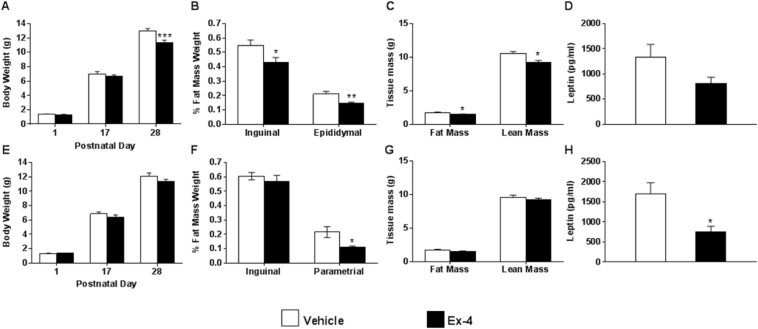
To determine the effect of neonatal Ex-4 administration on adult adiposity and metabolism, C57BL/6N mice were injected with 1 nmol/kg Ex-4 between 16 and 20 h after birth and then daily for a total of 6 consecutive days. By postnatal day 28 (P28), Ex-4 attenuated weight gain in males by 14.6% (Figure 1A) and reduced both inguinal and epididymal fat pad weights by 21% and 32%, respectively (Figure 1B). The apparent reductions in male adiposity were confirmed by nuclear magnetic resonance (NMR) spectroscopy (Figure 1C) and were consistent with a trend toward reduced leptin serum levels (Figure 1D). Neonatal Ex-4 did not have an effect on weight gain in female mice (Figure 1E) but did significantly reduce parametrial fat pad weight by P28 (Figure 1F). There was no difference in body composition in female mice by NMR (Figure 1G); however, there was a significant reduction in serum leptin (Figure 1H). To determine whether the observed fat loss had negative metabolic consequences [29], we assessed glucose homeostasis (Figure S1A–D). Nonfasting blood glucose was transiently reduced in both females and males at P13 but indistinguishable from vehicle treated mice by P28 (Figure S1A and B). Ex-4 had no effect on glucose tolerance in females or males at P28 (Figure S1C–D). A survey of additional serum markers revealed no significant effect of neonatal Ex-4 treatment on nonesterified free fatty acids, triglycerides, or adiponectin in either gender at this age (Figure S1E–G). Serum resistin was reduced by 25% in females and unaffected in males (Figure S1H). Serum insulin at P28 was also reduced by 58% in males treated with neonatal Ex-4 (Figure S1I).
Figure 1.
Neonatal Ex-4 decreases adiposity in male and female mice. (A–H) Analysis of C57BL/6N male (A–D) and female (E–H) mice given daily injections of vehicle (saline) or Ex-4 (1 nmol/kg birth weight). Mean body weight of male (A) and female (E) mice at postnatal days 1, 17, and 28. n = 4–9 mice per gender and treatment group. P28 fat pad weights from male (B) and female (F) mice normalized to body weight. n = 4–9 mice per gender and treatment group. NMR analysis of 25 day old male (C) and female (G) mice. n = 6–9 mice/group. Plasma leptin measured in P28 male (D) and female (H) mice. n = 4–9 mice per gender and treatment group. All data are expressed as mean ± SEM; *, p < 0.05, relative to corresponding vehicle (Two-tailed Student's t test).
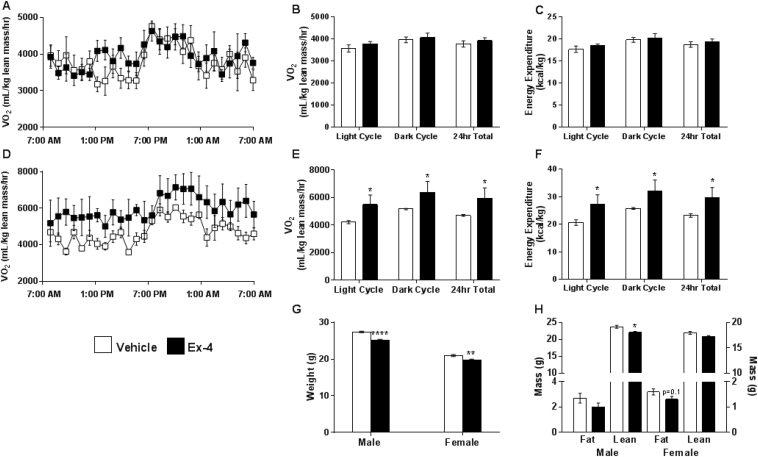
Despite the reduction in male body weight, we observed no differences in VO2 or energy expenditure at 8–10 weeks of age (Figure 2A–C). By contrast, neonatal administration of Ex-4 to female mice resulted in increased VO2 and energy expenditure during both light and dark cycles (Figure 2D–F). While body weight was significantly reduced in both genders at this age (Figure 2G), fat mass was no longer reduced in Ex-4 treated male mice, suggesting a more transient effect of Ex-4, whereas Ex-4 treated female mice began to display a trend in lower fat mass (Figure 2H). Cumulatively, these data suggest that activation of GLP1R during early development is protective against weight gain and adiposity and increases energy expenditure in a gender-specific manner.
Figure 2.
Neonatal Ex-4 enhances energy expenditure and protects against weight gain in adulthood. (A–F) Indirect calorimetry assessed in 8–10 week old male (A–C) and female (D–F) mice. Oxygen consumption for male (A–B) and female (D–E) mice normalized to lean mass. Energy expenditure for male (C) and female (F) mice calculated and normalized to lean mass. n = 4–5 mice/group. (G) Body weight of 8–10 week old male and female mice. n = 5 mice/group. (H) NMR analysis of 8–10 week old male and female mice. n = 5 mice/group. (A–F) *, p < 0.05, relative to corresponding vehicle (Two-way ANOVA with Bonferonni Posthoc test). (G–H). *, p < 0.05, relative to corresponding vehicle (Two-tailed Student's t test).
3.2. Neonatal Ex-4 protects adult female mice from diet-induced obesity through enhanced energy expenditure
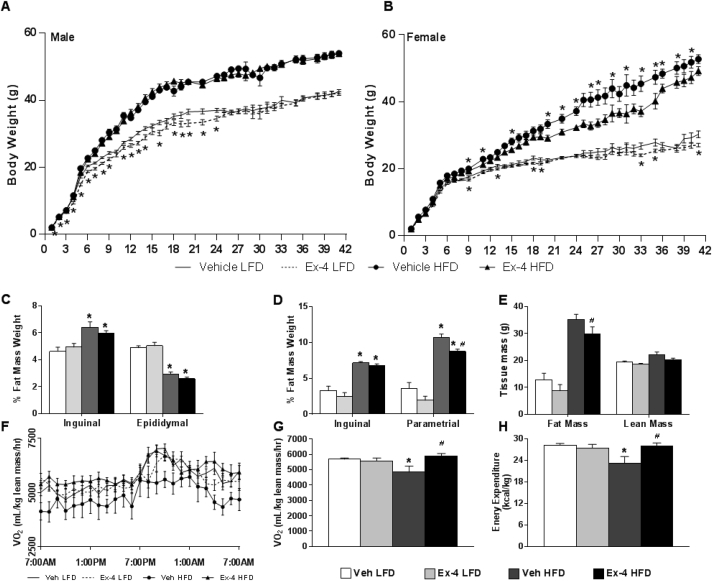
HFD can exacerbate the phenotype resulting from alterations in fetal and neonatal environments [30]. To assess the long-term protective effect of neonatal Ex-4 on body weight regulation, mice treated with neonatal Ex-4 or vehicle were weaned onto a 10% low fat diet (LFD) or 45% HFD for 42 weeks. In LFD-fed males, Ex-4 attenuated weight gain for the first 24 weeks (Figure 3A), associated with reduced fat mass by NMR assessed at postnatal week 9 (Figure S2A); however, there was no difference in weight in HFD-fed males, and weight was not different on either diet after 25 weeks. Neonatal Ex-4 treated LFD-fed females achieved a modest reduction in weight gain by postnatal week 9 (Figure 3B), which corresponded with a significant reduction in fat mass by NMR (Figure S2B). In striking contrast to males, however, neonatal Ex-4 protected adult females from diet-induced obesity, an effect that persisted through 42 weeks (Figure 3B). This was accompanied by a reduction in parametrial fat pad weight after HFD (Figure 3D) and a reduction in total fat mass by NMR (Figure 3E). We observed no apparent change in inguinal depots (Figure 3D). Despite altered body weight, treatment had no impact on food or fluid intake in female mice (Figure S2C–D).
Figure 3.
Neonatal Ex-4 protects adult female mice from diet-induced obesity through enhanced energy expenditure. (A–E) After neonatal vehicle or Ex-4 administration, mice were weaned onto a 10% LFD or 45% HFD. Body weight of male (A) and female (B) mice monitored for 42 weeks. Data expressed as mean ± SEM; n = 10–15 mice/group. *, p < 0.05, relative to corresponding vehicle. (C–E) Mice sacrificed at postnatal week 42 for dissection of male (C) and female (D) inguinal and perigonadal fat depots. Tissue weights normalized to body weight and data represented as means ± SEM; n = 3–9 mice/group. *, p < 0.05 relative to vehicle LFD, #, p < 0.05 relative to vehicle HFD. (E) NMR analysis of 42 week old females. Values represented as means ± SEM; n = 4 mice/group. *, p < 0.05 relative to vehicle LFD, #, p < 0.05 relative to vehicle HFD (Two-tailed Student's t test). (F–H) Indirect calorimetry performed on 42 week old female mice. Oxygen consumption (F–G) shown over 24 h period (F) or as a 24 h average (G). Energy expenditure shown as a 24 h average. All raw values normalized to lean mass. Data expressed as mean ± SEM; n = 4–8 mice/group. *, p < 0.05, relative to corresponding vehicle LFD, #, p < 0.05 relative to vehicle HFD. (Two-way ANOVA with Tukey Posthoc test).
Strikingly, in HFD-fed female mice, Ex-4 was able to reverse the negative effect of HFD on oxygen consumption (Figure 3F,G), carbon dioxide output (Figure S3A and B), and energy expenditure (Figure 3H), restoring these metabolic measures to levels similar to those of LFD-fed mice. Both LFD- and HFD-fed Ex-4 treated females displayed a lower respiratory exchange ratio (RER), suggesting that fat is more readily available for oxidation (Figure S3C and D). Altogether, these findings indicate that neonatal Ex-4 can protect against the deleterious effects of HFD on body weight, adiposity, and energy expenditure in adult animals in a gender specific manner.
3.3. Neonatal Ex-4 promotes browning of female perigonadal WAT
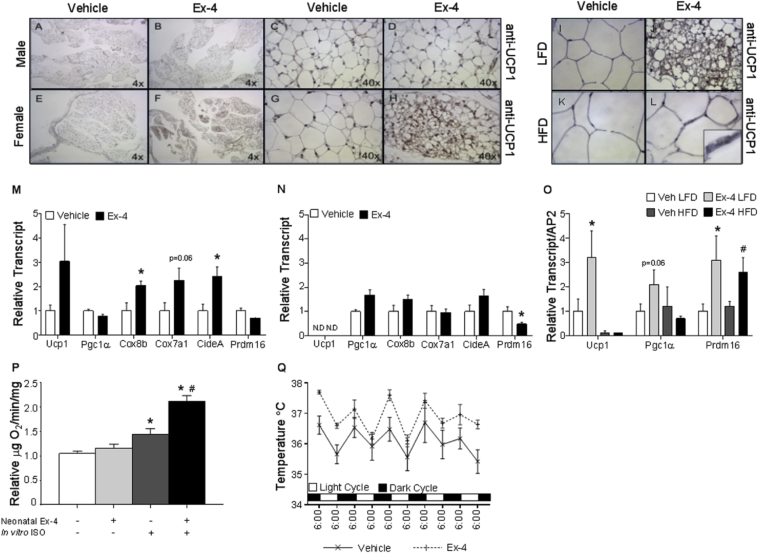
The persistent effect of short-term neonatal Ex-4 administration on body weight and adiposity during a critical window of white adipose tissue development prompted a histological analysis of the WAT. In P28 male mice, the thermogenic protein UCP1 was undetectable in epididymal fat pads (Figure 4A–D). By contrast, in P28 females, neonatal Ex-4 enhanced the appearance of UCP1 positive multilocular cells in the parametrial fat pad (Figure 4E–H). In both genders, analysis of inguinal depots revealed an abundance of multilocular cells with similar UCP1 levels and distribution regardless of treatment at P28 (Figure S4A). Together, these observations highlight an architectural change specific to adult female parametrial adipose morphology, resulting in increased beige adipocytes after neonatal Ex-4 administration.
Figure 4.
Neonatal Ex-4 promotes browning of female perigondal WAT. Histological analysis of perigonadal adipose tissue from P28 male (A–D) and female (E–H) mice stained with anti-UCP1 (n = 4). Images captured at 4× (A, B, E, F) and 40× (C, D, G, H) magnification. (I–L) Parametrial fat pads dissected from 42 week old females and immunostained with anti-UCP1: LFD 40X (I–J) or HFD 60X (K–L) magnification; n = 6–10 mice/group. RT-qPCR of perigonadal fat pads isolated from P28 female (M) and male (N) mice treated with neonatal Ex-4 or Vehicle. Values are means ± SEM for 4–9 mice per gender and treatment group. *, p < 0.05, relative to corresponding vehicle (Unpaired heteroscedastic t test). ND, transcript not detectable. (O) RT-qPCR of parametrial fat from female mice at 42 weeks. Data expressed as means ± SEM, n = 10–15 mice/group. *, p < 0.05, relative to vehicle LFD, #, p < 0.05 relative to vehicle HFD, Unpaired heteroscedastic t test). (P) Parametrial adipose isolated from P28 females. Explants were treated with vehicle or 10 μM ISO in vitro for 1 h prior to measurement of oxygen consumption with a Clark-type electrode (n = 4). Values expressed as means ± SEM; n = 4 mice/group. *, p < 0.05, relative to neonatal Ex-4- and ISO-, #, p < 0.05 relative to Neonatal Ex-4 – and ISO + (Two-tailed Student's t test). (Q) Core body temperature monitored twice daily for 5 days. Values expressed as means ± SEM; n = 5 mice/treatment. *, p < 0.05 (Two-way ANOVA).
To assess the durability of neonatal Ex-4 in the context of a HFD, we analyzed fat depots after LFD or HFD feeding (Figure 4I–L). The enhanced browning effect of parametrial fat pads was maintained at 42 weeks of age in LFD-fed female mice (Figure 4I vs J). Though to a lesser degree, multilocular UCP1 expressing cells were also present in the parametrial fat pads of neonatal Ex-4 treated HFD-fed females (Figure 4L). We observed similar distribution and expression of UCP1 in inguinal depots at both P28 and at 42 weeks on a LFD (Figure S4A and B). However, HFD induced a loss of UCP1 expression in the inguinal fat pads at 42 weeks; this was partially preserved by neonatal exposure to Ex-4 (Figure S4B).
Consistent with adipose morphology, we observed enhanced expression of the brown fat genes Cox8b and CideA with trending increases in both Ucp1 and Cox7a1 in P28 neonatal Ex-4 treated female mice (Figure 4M). Similarly, at 42 weeks, neonatal Ex4 elevated Ucp1, Pgc1a, and Prdm16 transcript in parametrial fat from LFD-fed females (Figure 4O). In males however, no changes in the expression of brown fat marker genes in epididymal pads were observed at P28 or at 42 weeks (Figure 4N, S4C). Gene expression analysis of inguinal depots isolated from both genders also revealed no effect of neonatal Ex-4 on brown fat marker genes (data not shown).
In contrast to the increased expression of genes associated with thermogenesis in females, neonatal Ex-4 was associated with a downward trend in expression of genes involved in adipogenesis and lipid synthesis, an effect that was enhanced under HFD-fed conditions (Figure S5A). mRNA levels of Pparγ2, C/ebpα, Srebp1c, HSL, Dgat1, and Leptin were decreased in HFD-fed Ex-4 mice as compared with HFD-fed vehicle controls, whereas expression of Fas was reduced by HFD alone and not further altered by neonatal Ex4 (Figure S5A). Expression of these genes in male epidydimal fat pads was affected by diet but not specifically by neonatal Ex4 (Figure S5B). Additionally, no difference was detected in the expression of brown fat markers or adipogenic/lipogenic/lipolytic factors in the inguinal fat pads regardless of diet or gender (data not shown). These data indicate that neonatal Ex-4 promotes the brown fat gene program specifically in female parametrial WAT.
To determine whether the appearance of UCP1 in parametrial pads of neonatal Ex-4 treated mice translated into a functional consequence on the thermogenic capacity of this WAT depot, we measured oxygen consumption of parametrial explants from neonatal vehicle or Ex-4 treated female P28 mice (Figure 4P). Neonatal Ex-4 did not influence basal oxygen consumption but did enhance isoproterenol (ISO)-stimulated oxygen consumption by 47% (Figure 4P). Together, these data revealed an enhanced appearance of UCP1 positive cells in the parametrial depot of neonatal Ex-4 treated mice that is associated with a functional increase in uncoupled respiration after beta-agonist stimulation. Further, an assessment of core body temperature to determine whether neonatal Ex-4 affects adaptive thermogenesis revealed a significant increase in core body temperature in Ex-4 treated female mice (Figure 4Q) at 8–10 weeks of age that corresponded with the increased oxygen consumption and energy expenditure (Figure 2D–F). In contrast, neonatal Ex-4 treatment did not raise the core body temperature of male mice (Figure S6). Altogether, these changes in core body temperature in concert with increased oxygen consumption and carbon dioxide output prior to changes in body weight suggest that thermogenic changes could contribute to weight loss and to the persistent elevation in energy expenditure during adulthood. These findings suggest that an acute intervention with Ex-4 during the neonatal period in female mice increases browning potential in parametrial fat depots and highlights a critical neonatal window during which adipose tissue may be susceptible to re-programming.
3.4. Neonatal Ex-4 durably alters NPY projections to the PVH
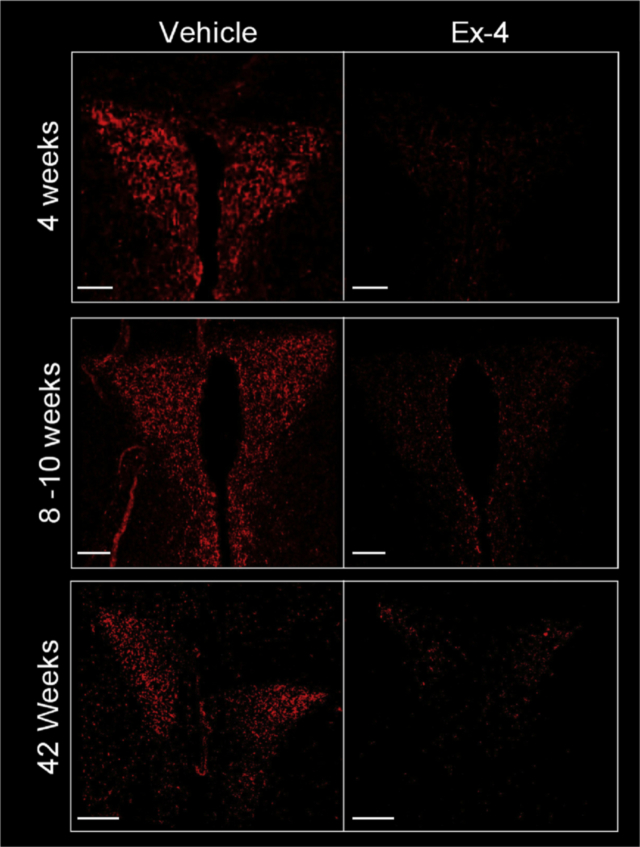
Neonatal Ex-4 treatment is protective against weight gain and adiposity during adulthood, in part through enhanced energy expenditure and browning of parametrial WAT. The PVH acts as a coordinating center for endocrine, autonomic, and behavioral signals [25], [31], [32], [33], [34], [35], [36]. The response to these signals is integrated in the PVH and ultimately regulates energy balance [33], [35]. Additionally, sympathetic input to BAT and WAT depots, which contribute to the regulation of energy homeostasis, originates in the PVH [35], [36]. Therefore, we evaluated NPY projections to the PVH to determine whether neonatal Ex-4 affected these adult neuronal projections. We found a robust reduction in NPY-positive projections to the PVH at 4 weeks of age in neonatal Ex-4 treated female mice that persisted to at least 42 weeks (Figure 5). In line with a previous report [37], vehicle treated HFD-fed mice displayed a significant reduction in NPY neuronal projections when compared to LFD-fed controls (Figure S7). Wei et al. report that HFD exposure results in a physical loss of projections originating in the arcuate nucleus of the hypothalamus (ARH) that target the PVH [37]. Interestingly, our findings show that neonatal Ex-4 was able to reverse the HFD-induced decrease in NPY fiber density in the PVH (Figure S7), possibly indicating a restoration of projections to the PVH.
Figure 5.
Neonatal Ex-4 durably reduces NPY fiber density in the PVH. Histological analysis of NPY-IR in the PVH at 4, 8–10, and 42 weeks in neonatal vehicle or Ex-4 treated female mice. Images representative of n = 3–4. Mice weaned at P21 on to standard chow (4 and 8–10 weeks) or LFD (42 weeks). Scale Bar 150 μm.
3.5. Sim1Cre deletion of Glp1r attenuates the impact of neonatal Ex-4 on adult body weight and WAT browning
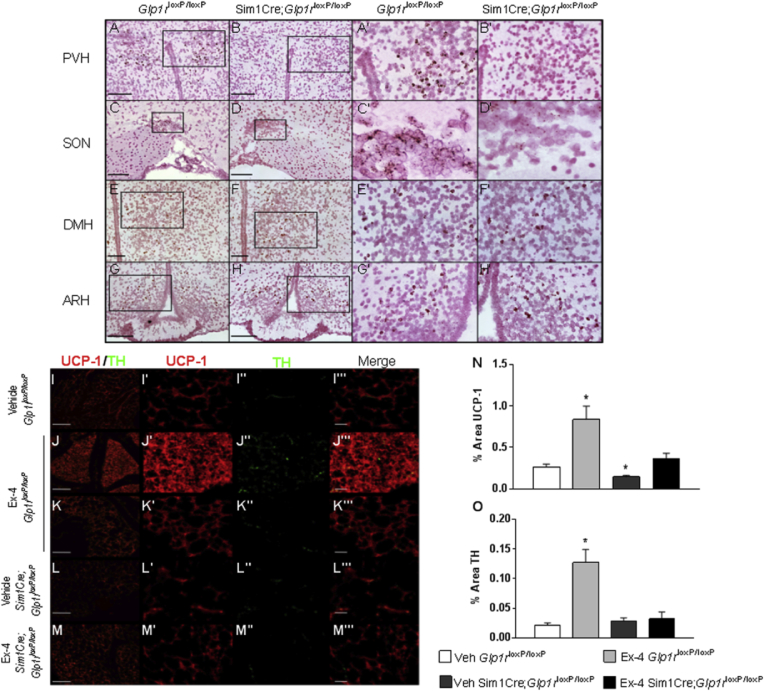
Given the importance of the PVH in the regulation of energy balance and our observations of persistently altered NPY projections in the PVH after neonatal Ex-4 (Figure 5), we postulated a role for early PVH specific GLP1R signaling in mediating the protective effects of Ex-4. GLP1R is expressed throughout the hypothalamus (Figure 6 A, C, E, G) and is particularly abundant within the PVH (Figure 6A, A′). By crossing Glp1r floxed mice with the Sim1Cre deleter strain that specifically targets the PVH, supraoptic nucleus (SON) within the hypothalamus, as well as parts of the amygdala, we were able to conditionally and efficiently delete Glp1r from the Sim1 expression domain (Figure 6B, B′, D, D′) with no effect on expression in other hypothalamic regions (Figure 6F, H). Similar to our initial findings, neonatal Ex-4 treated 8 week old Glp1rloxP/loxP (control) female mice weighed 9% less than vehicle treated control mice (Figure S8A). However, when Glp1r was deleted from the Sim1 expression domain (Sim1Cre;Glp1rloxP/loxP), neonatal Ex-4 treatment had no effect on body weight (Figure S8A). Similarly, neonatal Ex-4 treatment resulted in a 32% reduction in parametrial fat pad weight in Ex-4 treated Glp1rloxP/loxP mice. Protection from weight gain (Figure S8A) and adiposity (Figure S8B) were attenuated in Ex-4 treated Sim1Cre;Glp1rloxP/loxP female mice (Figure S8).
Figure 6.
Sim1Cre deletion of Glp1r attenuates impact of neonatal Ex-4 on adult body weight and WAT browning.Glp1r expression in the hypothalamus in P28 female mice by in situ in Glp1rloxP/loxP (control) and Sim1Cre;Glp1rloxP/loxP mice (A–H). PVH: paraventricular nucleus of the hypothalamus, SON: supraoptic nucleus, DMH: dorsal medial hypothalamus, ARH: arcuate nucleus of the hypothalamus. (Scale Bar 150 μm PVH and ARH, 100 μm SON and DMH). (I–O) Histological analysis of parametrial adipose tissue. Parametrial fat pads from P28 Glp1rloxP/loxP (control) and Sim1Cre;Glp1rloxP/loxP female mice stained with anti-UCP1 and anti-TH (I–M UCP1/TH merge Scale Bar: 10 μm, I′–M′ UCP1, I″–M″ TH, I‴-M‴ merge Scale Bar 2.5 μm). IR of UCP1 and TH in parametrial WAT quantified using ImageJ (N–O). Values are means ± SEM, n = 3–5 mice/group. *p < 0.05 relative to Veh Glp1rloxP/loxP (Two-tailed Student's t test).
Neonatal Ex-4 treatment durably altered parametrial fat pads resulting in the browning of this fat pad in adult female mice (Figure 4F,H, J, L). In order to determine whether central neonatal GLP1R signaling contributes to WAT browning, we assessed the parametrial fats pads of female Glp1rloxP/loxP and Sim1Cre;Glp1rloxP/loxP mice. We observed enhanced multilocular UCP1 positive adipocytes that also stained positively for tyrosine hydroxylase (TH), a marker for noradrenergic parenchymal fibers [40] (Figure 6J, N, O). The appearance of multilocular UCP1 positive adipocytes was region specific (Figure 6J) while other parts of the parametrial fat pad maintained a more normal WAT morphology, similar to vehicle treated counterparts (Figure 6I, K). Despite neonatal exposure to Ex-4, Sim1Cre;Glp1rloxP/loxP mice displayed UCP1 and TH levels comparable to vehicle treated Glp1rloxP/loxP mice (Figure 6M, N, O). Interestingly, UCP1 expression in vehicle treated Sim1Cre;Glp1rloxP/loxP mice was significantly reduced compared to vehicle treated Glp1rloxP/loxP mice (Figure 6L, N), suggesting an endogenous role of central GLP1R signaling in the development and/or maintenance of resident beige adipocytes in the parametrial fat pads of female mice. Altogether, these findings support a role for neonatal GLP1R signaling in the Sim1 expression domain in Ex-4 mediated protection from weight gain, adiposity, and browning of parametrial fat pads.
3.6. Neonatal Ex-4 effect on NPY and POMC fiber density in the PVH requires intact GLP1R signaling in the Sim1 expression domain
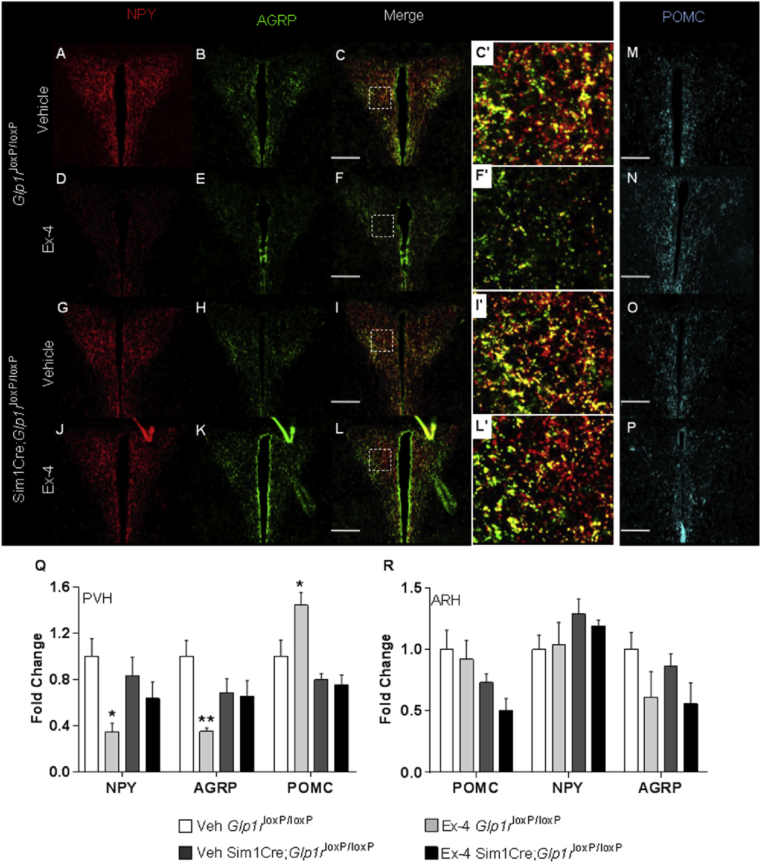
Neonatal Ex-4 treatment is protective against weight gain and adiposity during adulthood, in part through enhanced energy expenditure and browning of parametrial WAT. The central regulation of these processes [1], [25], [36], [38], [40], [41], [42] prompted the evaluation of orexigenic and anorexigenic factors that are responsible for regulating energy expenditure and for the response to appetite triggering and suppressive signals. We observed a dramatic ∼65% reduction of NPY and AgRP fiber densities in the PVH of 4-week old neonatal Ex-4 Glp1rloxP/loxP mice (Figure 7A–F, Q) with a corresponding increase in POMC fiber density (Figure 7M–N, Q). This effect was abrogated by deletion of Glp1r from the Sim1 expression domain (Figure 7J–L, P, Q). We considered the possibility that Ex-4 mediated alterations in fiber densities result from changes in the content of these neurohormones in the ARH; however, no differences in ARH content were observed (Figure 7R). As in females, neonatal Ex-4 treatment in male mice markedly reduced NPY fiber density in the PVH (Figure S9A, C). Further, the deletion of Glp1r from the Sim1 expression domain reversed the effect of neonatal Ex-4 treatment in 4 week old male mice (Figure S9B, D). These findings could suggest a sexual dimorphic difference downstream of the PVH or working directly at the level of WAT. These findings also leave open the possibility of additional areas, both central and peripheral, that could be contributing to the gender differences observed with neonatal Ex-4 exposure. Cumulatively, these findings demonstrate that neonatal Ex-4 offers protection from weight gain and adiposity through browning of fat depots and altered hypothalamic projection patterning. The data further suggest that Ex-4 mediated browning of parametrial adipose tissue and alterations in hypothalamic architecture both require intact GLP1R signaling within the Sim1 expression domain.
Figure 7.
Neonatal Ex-4 effect on NPY and POMC fiber density in the PVH is reversed by Sim1Cre Glp1r deletion. Histological analysis of orexigenic neuropeptides NPY (A, D, G, J) and AgRP (B, E, H, K) and the anorexigenic neuropeptide POMC (M−P) evaluated in the hypothalamus of 4 week old Glp1rloxP/loxP (control) and Sim1Cre;Glp1rloxP/loxP female mice. IR of NPY, AgRP and POMC quantified using ImageJ (Q–R). Values are means ± SEM n = 4–6 mice/group. Scale Bar 150 μm. *, p < 0.05, **, p < 0.01 relative to Veh Glp1rloxP/loxP by (Two-way ANOVA with Tukey Posthoc test).
4. Discussion
Alterations to fetal and neonatal environments can result in adult predisposition to obesity, metabolic syndrome, and diabetes. The concept that the neonatal environment can influence hypothalamic projection development was recently suggested by the elegant observation that modification of maternal diet during gestation and lactation can alter hypothalamic axonal development and adult body weight regulation [23]. Here, we define a discrete neonatal window and a specific signaling pathway, the manipulation of which has a long-term impact on body weight regulation. Short-term treatment of neonatal mice with a GLP1R agonist, Ex-4, durably alters hypothalamic architecture and results in resistance to diet-induced weight gain and adiposity through a permanent browning of parametrial WAT depots and increased energy dissipation in adult female mice. The period of Ex-4 administration represents an important window for the development of hypothalamic circuitry relevant to the regulation of energy expenditure and feeding behavior as well as for the development of WAT. A permanent remodeling of hypothalamic circuitry is observed in mice after neonatal Ex-4 exposure through 42 weeks of age. Within the PVH, the diminished NPY and AgRP fiber density and the antagonistic increase in POMC fiber density was lost with the genetic disruption of GLP1R signaling in the Sim1 expression domain. These findings implicate the GLP1R signaling pathway as a target for developmental intervention to prevent adult obesity.
Our observations suggest that neonatal Ex-4 affects multiple components contributing to daily energy expenditure, including basal metabolic rate, diet-induced thermogenesis, and physical activity [43]. Ex-4 enhanced respiration as well as other metabolic parameters during both light and dark cycles. During the dark (active) cycle, Ex-4 enhanced locomotor activity (data not shown), which may, in part, explain increases in energy expenditure during this phase; however, energy expenditure was also elevated during the light (resting) phase when energy dissipation occurs through alterations in basal metabolic rate. We postulate that neonatal Ex-4 modulates basal metabolic rate by enhancing the thermogenic capacity of parametrial WAT. This is supported by the appearance of multilocular adipocytes exhibiting enhanced expression of UCP1, an essential component of the high mitochondrial content of BAT and beige fat. Gene expression analyses coincided with these observations, with increases in multiple brown fat marker genes in parametrial fat pads from Ex-4 treated female mice at P28. These observations were consistent with morphological changes found in 42-week old parametrial fat pads, in which UCP1 positive adipocytes were observed, regardless of diet. Further supporting fundamental alterations in metabolic rate, we observed a higher capacity for oxygen consumption after β-adrenergic stimulation of parametrial adipose explants dissected from neonatal Ex-4 treated female mice, illustrating functional changes in this fat depot.
We observe a gender selective effect of neonatal administration of Ex-4, which could relate to previously described sexual dimorphisms. Development, expansion, and metabolic function of adipose are influenced by sex hormones [44], [45] and X chromosome dosage [46]. The origin and distribution of depot-specific progenitors and preadipocytes vary by gender and affects the capacity for growth, proliferation, and differentiation [47], [48]. Female rodents have a higher temperature threshold for thermogenic response [49] and are thus more sensitive to ambient colony temperature than males. Additionally, female rodents are more susceptible to persistent metabolic disturbances in response to alterations in maternal diet during fetal and neonatal development [50], [51]. In these reports, and consistent with our findings, while males were similarly affected by changes in maternal diet, male rodents were resistant to permanent or long-term effects [50], [51]. Interestingly, many of these observations translate to humans. Per kg of tissue, WAT depots in women are more metabolically active than complementary depots in men and exhibit higher expression of genes involved in thermogenesis and mitochondrial function, including Ucp1 [52]. With these points in mind, it is possible that alternative conditions will be required to reveal the role of neonatal GLP1R signaling in adult metabolism in males.
Many regions within and outside of the hypothalamus are necessary for the regulation of energy balance; however, the PVH is particularly relevant as a pivotal integration point in the central regulation of energy homeostasis, in addition to acting as a major source of sympathetic input to both WAT and BAT [36], [38], [40], [41], [53]. We postulate that Ex-4 permanently alters the projections that are established during a critical developmental window. We observed a 65% reduction in NPY and AgRP fibers within the PVH of neonatal Ex-4 treated mice, which coincided with a modest but significant increase in POMC fibers. Conditional Cre-mediated deletion of Glp1r from the Sim1 expression domain, which contains the PVH, abrogates Ex-4 mediated alterations of both POMC and NPY projections to the PVH. ARH populations of NPY and POMC neurons begin to form during fetal development [54]; however, circuitry connecting these defining regions of the hypothalamus does not begin to form until the early neonatal period in rodents [2]. Genetic ablation of Glp1r abrogated Ex-4 mediated alterations in fiber densities within the PVH; however, ARH content of NPY, POMC, and AgRP was unaffected by either Ex-4 treatment or the loss of Glp1r.
The strong evidence supporting a role for the PVH in regulating energy expenditure [25], [36], [42], [55], sympathetic outflow [36], [38], [40], as well as our observations of abrogated Ex-4 effect with the loss of GLP1R in the PVH suggests that the PVH is a likely target of neonatal Ex-4. It is, however, important to note that the Sim1 expression domain directing Cre recombinase expression includes neuronal populations located within the supraoptic nucleus and the amygdala [25], [56], both of which express Glp1r. Glp1r is expressed in other central regions that could also contribute to these observations, including the hindbrain, known for its involvement in the regulation of energy balance and for endogenous GLP-1 production, as well as in the ventral tegmental area, nucleus accumbens, and lateral parabrachial nucleus [57], [58], [59], [60], [61], [62]. Interestingly, we did not observe altered food intake in adult mice after Ex-4 treatment, which could suggest that our treatment window is specific to the establishment of networks regulating energy expenditure. Circuitry regulating feeding behavior may develop later and fall outside our treatment window, which may suggest specific and distinct periods available for intervention.
The incidence of obesity and its related disorders is affecting humans at an alarming rate. Even modest reductions in body weight, as low as 5% of initial weight, have been shown to improve blood pressure, lipid profile, insulin sensitivity, and glucose tolerance as well as reduce or eliminate comorbidities associated with obesity [63], [64], [65]. Thus, there is great interest in reducing overall adiposity while simultaneously enhancing the longevity of beige cells and facilitating their expression in WAT for the prevention of or protection from diet and aged-induced obesity. Our findings in rodents illustrate that a short-term intervention during a specific developmental window with a therapy already in clinical use for human type 2 diabetes can exert a long-term impact on body weight regulation, energy expenditure, and browning of visceral white adipose. This is a critical finding in our ongoing understanding of adipose development and obesity.
Funding
This work was supported by the National Institutes of Health R01 DK062965 (D.A.S), a gift fund from the Welsh family. (D.A.S), NRSA F32 DK085939 (D.A.B), and NIH T32 DK007314 (A.V.R).
Author contributions
A.V.R., D.A.B, and D.A.S conceptualized, planned, and designed the experiments. A.V.R., D.A.B., P.A.S., and D.N.G. performed the experiments. A.V.R., D.A.B., and D.A.S. analyzed data and wrote original manuscript draft. R.J.S and P.S provided resources. A.V.R., D.A.S., D.A.B., R.J.S., R.A.S., P.S, and R.S.A. reviewed and edited the manuscript.
Acknowledgements
We thank M. Lazar, K. Bence, L. Zeltser, M. Meyers, S. Mullican, S. Soleimanpour, and J. Raum for technical support and for helpful discussions. This work was supported by the Raymond and Joanna Welsh gift, the Cox Medical Institute, pilot funding from the University of Pennsylvania Perelman School of Medicine Penn Medicine Translational Neuroscience Center, and the Institute for Diabetes, Obesity and Metabolism of the University of Pennsylvania, and by the National Institutes of Health R01 DK062965 (D.A.S), NIH T32 DK007314 (A.V.R), and NRSA F32 DK085939 (D.A.B). We thank B. Kublaoui for gifting us the Sim1Cre mouse line. We acknowledge the University of Pennsylvania Diabetes Research Center for the use of the Mouse Phenotyping, Physiology and Metabolism Core, the Islet Cell Biology Core, the Radioimmunoassay/Biomarkers Core (P30DK19525) and the Molecular Pathology and Imaging Core of the Digestive Disease Center (MPIC, P30DK050306).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.05.006.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Stanley S., Wynne K., McGowan B., Bloom S. Hormonal regulation of food intake. Physiological Reviews. 2005;85(4):1131–1158. doi: 10.1152/physrev.00015.2004. [DOI] [PubMed] [Google Scholar]
- 2.Bouret S.G., Draper S.J., Simerly R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(11):2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geloen A., Collet A.J., Guay G., Bukowiecki L.J. Insulin stimulates in vivo cell proliferation in white adipose tissue. The American Journal of Physiology. 1989;256(1 Pt 1):C190–C196. doi: 10.1152/ajpcell.1989.256.1.C190. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 5.Symonds M.E., Mostyn A., Pearce S., Budge H., Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. The Journal of Endocrinology. 2003;179(3):293–299. doi: 10.1677/joe.0.1790293. [DOI] [PubMed] [Google Scholar]
- 6.Lau C., Rogers J.M., Desai M., Ross M.G. Fetal programming of adult disease: implications for prenatal care. Obstetrics and Gynecology. 2011;117(4):978–985. doi: 10.1097/AOG.0b013e318212140e. [DOI] [PubMed] [Google Scholar]
- 7.Simmons R.A. Developmental origins of adult disease. Pediatric Clinics of North America. 2009;56(3):449–466. doi: 10.1016/j.pcl.2009.03.004. [Table of Contents] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker D.J. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 9.Novak D.A., Desai M., Ross M.G. Gestational programming of offspring obesity/hypertension. Journal of Maternal-Fetal & Neonatal Medicine. 2006;19(10):591–599. doi: 10.1080/14767050600708233. [DOI] [PubMed] [Google Scholar]
- 10.Susser M., Stein Z. Timing in prenatal nutrition: a reprise of the Dutch Famine study. Nutrition Reviews. 1994;52(3):84–94. doi: 10.1111/j.1753-4887.1994.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 11.Ham J.N., Crutchlow M.F., Desai B.M., Simmons R.A., Stoffers D.A. Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: potential role of VEGF. Pediatric Research. 2009;66(1):42–46. doi: 10.1203/PDR.0b013e3181a282a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons R.A., Templeton L.J., Gertz S.J. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50(10):2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 13.Stoffers D.A., Desai B.M., DeLeon D.D., Simmons R.A. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52(3):734–740. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- 14.Drucker D.J. Glucagon-like peptides. Diabetes. 1998;47(2):159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 15.Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villarroya F. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 16.Halford J.C. Obesity drugs in clinical development. Current Opinion in Investigational Drugs. 2006;7(4):312–318. [PubMed] [Google Scholar]
- 17.Campos R.V., Lee Y.C., Drucker D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134(5):2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 18.Bullock B.P., Heller R.S., Habener J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 19.Klok M.D., Jakobsdottir S., Drent M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Reviews. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 20.Steculorum S.M., Collden G., Coupe B., Croizier S., Lockie S., Andrews Z.B. Neonatal ghrelin programs development of hypothalamic feeding circuits. The Journal of Clinical Investigation. 2015;125(2):846–858. doi: 10.1172/JCI73688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahima R.S., Prabakaran D., Flier J.S. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. The Journal of Clinical Investigation. 1998;101(5):1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granado M., Garcia-Caceres C., Fuente-Martin E., Diaz F., Mela V., Viveros M.P. Effects of acute changes in neonatal leptin levels on food intake and long-term metabolic profiles in rats. Endocrinology. 2011;152(11):4116–4126. doi: 10.1210/en.2011-1233. [DOI] [PubMed] [Google Scholar]
- 23.Vogt M.C., Paeger L., Hess S., Steculorum S.M., Awazawa M., Hampel B. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156(3):495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson-Perez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D.A., Stoffers D. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Varela G.M., Antwi D.A., Dhir R., Yin X., Singhal N.S., Graham M.J. Inhibition of ADRP prevents diet-induced insulin resistance. American Journal of Physiology–Gastrointestinal and Liver Physiology. 2008;295(3):G621–G628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong T., Hildebrandt M.A., Thrasher S.M., Appleton J.A., Ahima R.S., Wu G.D. Divergent metabolic adaptations to intestinal parasitic nematode infection in mice susceptible or resistant to obesity. Gastroenterology. 2007;133(6):1979–1988. doi: 10.1053/j.gastro.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doliba N.M., Vatamaniuk M.Z., Buettger C.W., Qin W., Collins H.W., Wehrli S.L. Differential effects of glucose and glyburide on energetics and Na+ levels of betaHC9 cells: nuclear magnetic resonance spectroscopy and respirometry studies. Diabetes. 2003;52(2):394–402. doi: 10.2337/diabetes.52.2.394. [DOI] [PubMed] [Google Scholar]
- 29.Savage D.B. Mouse models of inherited lipodystrophy. Disease Models & Mechanisms. 2009;2(11–12):554–562. doi: 10.1242/dmm.002907. [DOI] [PubMed] [Google Scholar]
- 30.Glavas M.M., Kirigiti M.A., Xiao X.Q., Enriori P.J., Fisher S.K., Evans A.E. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology. 2010;151(4):1598–1610. doi: 10.1210/en.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biag J., Huang Y., Gou L., Hintiryan H., Askarinam A., Hahn J.D. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. The Journal of Comparative Neurology. 2012;520(1):6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler A.A., Cone R.D. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36(2–3):77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 33.Cowley M.A., Pronchuk N., Fan W., Dinulescu D.M., Colmers W.F., Cone R.D. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 34.Elmquist J.K., Coppari R., Balthasar N., Ichinose M., Lowell B.B. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. The Journal of Comparative Neurology. 2005;493(1):63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson A.V., Latchford K.J., Samson W.K. The paraventricular nucleus of the hypothalamus – a potential target for integrative treatment of autonomic dysfunction. Expert Opinion on Therapeutic Targets. 2008;12(6):717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster M.T., Song C.K., Bartness T.J. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 2010;18(4):682–689. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labbe S.M., Caron A., Lanfray D., Monge-Rofarello B., Bartness T.J., Richard D. Hypothalamic control of brown adipose tissue thermogenesis. Frontiers in Systems Neuroscience. 2015;9:150. doi: 10.3389/fnsys.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bamshad M., Aoki V.T., Adkison M.G., Warren W.S., Bartness T.J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. The American Journal of Physiology. 1998;275(1 Pt 2):R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 40.Murano I., Barbatelli G., Giordano A., Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. Journal of Anatomy. 2009;214(1):171–178. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartness T.J., Song C.K. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. Journal of Lipid Research. 2007;48(8):1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Myers M.G., Jr., Munzberg H., Leinninger G.M., Leshan R.L. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metabolism. 2009;9(2):117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westerterp K.R. Diet induced thermogenesis. Nutrition & Metabolism (London) 2004;1(1):5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi H., Seeley R.J., Clegg D.J. Sexual differences in the control of energy homeostasis. Frontiers in Neuroendocrinology. 2009;30(3):396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quarta C., Mazza R., Pasquali R., Pagotto U. Role of sex hormones in modulation of brown adipose tissue activity. Journal of Molecular Endocrinology. 2012;49(1):R1–R7. doi: 10.1530/JME-12-0043. [DOI] [PubMed] [Google Scholar]
- 46.Chen X., McClusky R., Chen J., Beaven S.W., Tontonoz P., Arnold A.P. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genetics. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fried S.K., Lee M.J., Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23(7):1345–1352. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majka S.M., Fox K.E., Psilas J.C., Helm K.M., Childs C.R., Acosta A.S. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quevedo S., Roca P., Pico C., Palou A. Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue. Pflügers Archiv European Journal of Physiology. 1998;436(5):689–695. doi: 10.1007/s004240050690. [DOI] [PubMed] [Google Scholar]
- 50.Dearden L., Balthasar N. Sexual dimorphism in offspring glucose-sensitive hypothalamic gene expression and physiological responses to maternal high-fat diet feeding. Endocrinology. 2014;155(6):2144–2154. doi: 10.1210/en.2014-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samuelsson A.M., Matthews P.A., Jansen E., Taylor P.D., Poston L. Sucrose feeding in mouse pregnancy leads to hypertension, and sex-linked obesity and insulin resistance in female offspring. Frontiers in Physiology. 2013;4:14. doi: 10.3389/fphys.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuente-Martin E., Argente-Arizon P., Ros P., Argente J., Chowen J.A. Sex differences in adipose tissue: it is not only a question of quantity and distribution. Adipocyte. 2013;2(3):128–134. doi: 10.4161/adip.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song C.K., Enquist L.W., Bartness T.J. New developments in tracing neural circuits with herpesviruses. Virus Research. 2005;111(2):235–249. doi: 10.1016/j.virusres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Padilla S.L., Carmody J.S., Zeltser L.M. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Medicine. 2010;16(4):403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouret S.G., Bates S.H., Chen S., Myers M.G., Jr., Simerly R.B. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(4):1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michaud J.L., Rosenquist T., May N.R., Fan C.M. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes & Development. 1998;12(20):3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alhadeff A.L., Baird J.P., Swick J.C., Hayes M.R., Grill H.J. Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014;39(9):2233–2243. doi: 10.1038/npp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alhadeff A.L., Grill H.J. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2014;307(4):R465–R470. doi: 10.1152/ajpregu.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alhadeff A.L., Rupprecht L.E., Hayes M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mietlicki-Baase E.G., Ortinski P.I., Reiner D.J., Sinon C.G., McCutcheon J.E., Pierce R.C. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(20):6985–6992. doi: 10.1523/JNEUROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mietlicki-Baase E.G., Ortinski P.I., Rupprecht L.E., Olivos D.R., Alhadeff A.L., Pierce R.C. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American Journal of Physiology, Endocrinology and Metabolism. 2013;305(11):E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richard J.E., Farkas I., Anesten F., Anderberg R.H., Dickson S.L., Gribble F.M. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology. 2014;155(11):4356–4367. doi: 10.1210/en.2014-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blackburn G. Effect of degree of weight loss on health benefits. Obesity Research. 1995;3(Suppl. 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 64.Valsamakis G., McTernan P.G., Chetty R., Al Daghri N., Field A., Hanif W. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipocytokines. Metabolism. 2004;53(4):430–434. doi: 10.1016/j.metabol.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 65.Wing R.R., Lang W., Wadden T.A., Safford M., Knowler W.C., Bertoni A.G. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.