Abstract
Objective
Type 1 diabetes is characterized by autoimmune destruction of β-cells leading to severe insulin deficiency. Although many improvements have been made in recent years, exogenous insulin therapy is still imperfect; new therapeutic approaches, focusing on preserving/expanding β-cell mass and/or blocking the autoimmune process that destroys islets, should be developed. The main objective of this work was to test in non-obese diabetic (NOD) mice, which spontaneously develop autoimmune diabetes, the effects of local expression of Insulin-like growth factor 1 (IGF1), a potent mitogenic and pro-survival factor for β-cells with immunomodulatory properties.
Methods
Transgenic NOD mice overexpressing IGF1 specifically in β-cells (NOD-IGF1) were generated and phenotyped. In addition, miRT-containing, IGF1-encoding adeno-associated viruses (AAV) of serotype 8 (AAV8-IGF1-dmiRT) were produced and administered to 4- or 11-week-old non-transgenic NOD females through intraductal delivery. Several histological, immunological, and metabolic parameters were measured to monitor disease over a period of 28–30 weeks.
Results
In transgenic mice, local IGF1 expression led to long-term suppression of diabetes onset and robust protection of β-cell mass from the autoimmune insult. AAV-mediated pancreatic-specific overexpression of IGF1 in adult animals also dramatically reduced diabetes incidence, both when vectors were delivered before pathology onset or once insulitis was established. Transgenic NOD-IGF1 and AAV8-IGF1-dmiRT-treated NOD animals had much less islet infiltration than controls, preserved β-cell mass, and normal insulinemia. Transgenic and AAV-treated islets showed less expression of antigen-presenting molecules, inflammatory cytokines, and chemokines important for tissue-specific homing of effector T cells, suggesting IGF1 modulated islet autoimmunity in NOD mice.
Conclusions
Local expression of Igf1 by AAV-mediated gene transfer counteracts progression to diabetes in NOD mice. This study suggests a therapeutic strategy for autoimmune diabetes in humans.
Keywords: Autoimmune diabetes, NOD, IGF1, Pancreas, AAV
Highlights
-
•
Local pancreatic IGF1 expression prevents spontaneous autoimmune diabetes.
-
•
Protection achieved after one-time local administration of IGF1-encoding AAV vectors.
-
•
Efficacious in animals treated early or once autoimmunity is already established.
-
•
Protection through maintenance of β-cell mass and endogenous insulin secretion.
-
•
Treatment leads to reduced infiltration and expression of immunity genes in islets.
1. Introduction
Type 1 diabetes (T1D) results from the progressive autoimmune destruction of β-cells that ultimately leads to insulin deficiency and overt hyperglycemia. Patients with T1D require lifelong insulin replacement therapy to survive. Despite the improvements in insulin formulation and delivery, glycemic control is imperfect with this treatment, and patients are at high risk of suffering potentially life-threatening hypoglycemia episodes or, due to chronic exposure to hyperglycemia, developing serious microvascular, macrovascular, and neurological complications with high morbidity and mortality. Islet transplantation has also shown therapeutic benefit in humans. The broad application of this approach, however, is limited by the shortage of donors and the eventual loss of most transplanted islets after recurrent autoimmune destruction [1]. Therefore, there is a need to develop novel, more efficacious treatments. Gene therapy can circumvent these limitations and help maintain the endogenous β-cell mass and, therefore, physiologic insulin secretion, by enabling the expression of key factors that protect β-cells from the autoimmune attack.
Insulin-like growth factor 1 (IGF1) is a β-cell mitogen and pro-survival factor that has also been reported to play important roles in β-cell maturation and function [2]. Additionally, IGF1 regulates immune functions and is one of the main players in the crosstalk between the endocrine and immune systems [3], [4]. Pre-clinical therapeutic strategies based on multiple administrations of recombinant IGF1 [5], [6], [7] or plasmid-mediated hepatic overexpression of IGF1 [8] reduce the incidence of the disease in mouse models of T1D. In addition, administration of recombinant IGF1 to humans improves glycemic control both in type 1 [9], [10], [11], [12] and type 2 diabetic patients [13], [14]. However, therapeutic efficacy was not sustained after withdrawal of IGF1 treatment [10], [11].
Previously, we demonstrated that IGF1 overexpression in β-cells of transgenic mice counteracts the cytotoxic effects of streptozotocin (STZ) and promotes β-cell regeneration in a genetic background-independent manner [15], [16]. Moreover, IGF1 overexpression in β-cells also prevented islet infiltration and immune cell-mediated β-cell death in transgenic mice that overexpress human interferon-β (IFN-β) in β-cells [17]. IFN-β mice have chronic insulitis and develop autoimmune diabetes upon administration of very low doses of STZ [17]. Despite this solid evidence supporting the protective role of IGF1 on diabetes induction, the fair argument was raised that the effects of IGF1 had not been tested in the context of spontaneous autoimmune diabetes. In addition, it remained to be demonstrated if the protection against diabetes mediated by IGF1 was due to the expression of the growth factor during embryonic development and early postnatal life and if the same protection would be achieved if IGF1 was expressed in an adult animal.
In vivo gene transfer of therapeutic candidate genes through adeno-associated viral (AAV) vectors may offer the possibility of lifelong beneficial effects after a one-time treatment, as the production of therapeutic proteins for extended periods of time after a single administration of these vectors has repeatedly been demonstrated in several animal models and in humans [18], [19]. AAV vectors are predominantly non-integrative vectors that efficiently transduce dividing and non-dividing cells in vivo in a wide range of animal and human tissues with low toxicity and immunogenicity [18]. Several naturally-occurring and engineered serotypes exist which exhibit differential tissue tropism, and we and others have previously demonstrated the feasibility of efficacious gene transfer to the pancreas of small animals with AAV vectors of serotypes 8 and 9 [20], [21], [22], [23], [24], [25]. Moreover, incorporation of microRNA target sequences (miRTs) in the AAV expression cassette has recently been shown to enable tissue-specific transgene expression [26], [27], opening the door to sophisticated ways of regulation of vector tropism.
In this work, we have tested the effects of local expression of IGF1 in non-obese diabetic (NOD) mice that spontaneously develop the disease and share many genetic and immunopathogenic features with human T1D [28]. First, we generated transgenic NOD mice overexpressing IGF1 in β-cells and demonstrated long-term suppression of diabetes onset and robust protection of β-cell mass from the autoimmune insult. Then we used miRT-containing, IGF1-encoding, AAV8 vectors to show that pancreatic IGF1 expression in adult mice was sufficient to protect against diabetes onset in non-transgenic NOD mice through blockage of β-cell-directed autoimmune attack. Our results highlight the potential that a therapeutic strategy based on IGF1 gene transfer to the pancreas may hold for the treatment of autoimmune diabetes in humans.
2. Material and methods
2.1. Animals
RIP-1/IGF1 transgenic mice of ICR genetic background [15] were successively backcrossed with NOD/LtJ mice (originally from Charles River) to generate a NOD-IGF1 transgenic colony. Heterozygous female NOD-IGF1 mice of the N15 generation onwards (>99.99% NOD background) were used to perform studies. Non-transgenic littermates were used as controls. For AAV-mediated gene transfer studies, wild type female NOD/Ltj mice were used. Mice were housed in specific pathogen-free conditions under 12-h light–dark cycle and standard diet (Harlan) ad libitum feeding. Mice were considered diabetic after two consecutive blood glucose readings >250 mg/dL. AAV retrograde pancreatic intraductal delivery was performed as previously [20]. All experimental procedures were approved by the Ethics Committee for Animal and Human Experimentation of Universitat Autònoma de Barcelona.
2.2. AAV vector production
Single-stranded AAV vectors were generated by cloning the Green Fluorescent Protein (GFP) or murine IGF1Ea propeptide (IGF1) coding sequences under control of the ubiquitous CAG hybrid promoter (CMV enhancer, chicken β-actin promoter) into AAV backbone plasmids. When indicated, miRT-122a and miRT-1 sequences [27] were cloned in the 3′ untranslated region (UTR). AAV8 were produced by triple transfection of HEK293 and purified by an optimized CsCl-based gradient method that renders high purity vectors preps [29]. Vectors were titered by quantitative PCR (qPCR).
2.3. Islet isolation and culture
Pancreata were intraductally perfused (Collagenase I/II (0.1 mg/mL) and thermolysin) (Roche), diluted in M199 media (Thermo Scientific), excised, and digested for 19 min at 37 °C. Islets were purified by gradient centrifugation on Histopaque-1077 (Sigma–Aldrich) and hand-picked under a stereomicroscope (Leica). When indicated, islets were cultured at 37 °C for 40 h in RPMI 1640 medium (7 mM glucose), supplemented with 1% BSA, 2 mM glutamine, and penicillin/streptomycin in an atmosphere of 95% humidified air, 5% CO2. After the incubation period, islets were handpicked, and infiltrating immune cells extruded from the islets were recovered after pelleting the culture media and filtering through a 30 μm PARTEC Cell Trics filter (#04-0042-2316).
2.4. Hormone and metabolite assays
Glycemia was determined with Elite™ Glucometers (Bayer) after tail-tip bleeding. To perform glucose tolerance test, awake mice were fasted overnight (16 h) and administered with an intraperitoneal injection of glucose (1 g/kg body weight). Insulin and IGF1 were measured by ELISA (Crystal Chemical and Immunodiagnostic Systems, respectively). Serum triglycerides and total cholesterol were quantified spectrophotometrically using an enzymatic assay kit (Horiba-ABX). Serum free fatty acids were measured by the acyl-CoA synthase and acyl-CoA oxidase methods (Wako Chemicals GmbH). Serum β-hydroxybutyrate was quantified by an enzymatic assay (Randox Lab). All biochemical parameters were determined using Pentra 400 Analyzer (Horiba-ABX).
2.5. Immunohistochemistry
Tissues were fixed in 4% formalin, embedded in paraffin, sectioned, and incubated with the corresponding primary and secondary antibodies (Supplemental Table 1). Hoechst (Sigma–Aldrich) was used for fluorescent nuclear counterstaining. For brightfield images, signal was visualized with ABC-Peroxidase (Thermo Scientific) and 3,3-diaminobenzidina (DAB) (Sigma) and counterstained in Mayer's hematoxylin (Merk). TUNEL staining was performed following manufacturer's instructions (Roche 1.684817). Images were obtained with a Nikon Eclipse 90i microscope (Nikon). β-Cell mass, β-cell replication, and insulitis score were determined as previously described [20]. For histological islet size determination, the number of islets found per pancreas (examined in three sections per individual, 150 μm apart from each other) was distributed according to their insulin-positive area. The β-cell apoptosis rate was calculated as the number of cells positive for TUNEL and negative for glucagon, somatostatin and pancreatic polypeptide per islet (at least 300 islets per group were analyzed). The number of Foxp3 positive cells/islet area was quantified in all islets of two different pancreatic sections per mouse (at least 224 islets per group were analyzed).
2.6. Gene expression analysis
Total RNA was extracted from pancreas, liver, and heart using Tripure Isolation Reagent (Roche) and Rneasy Mini Kit (Qiagen) and from isolated islets with Rneasy Micro Kit (Qiagen). Total RNA was reverse-transcribed with Transcriptor First Strand cDNA Synthesis Kit (Roche). qPCR was performed in a LightCycler® 480 II (Roche) using LightCycler® 480 SYBR Green I Master mix (Roche). Primer sequences are listed in Supplemental Table 2. Results were analyzed using the Pfaffl's mathematical model [30] and values were normalized to murine Rplp0 expression. miRNA-enriched RNA was extracted using miRVana™ miRNA Isolation Kit (Life Technologies). Ten nanograms of RNA were reverse-transcribed with miRCURY LNA™ Universal RT microRNA PCR – Universal cDNA synthesis kit II (Exiqon). Expression was quantified by qPCR using ExiLENT SYBR® Green Master mix (Exiqon) relative to U6snRNA expression.
2.7. Vector genome copy number
Tissues were digested overnight in Proteinase K (0.2 mg/mL), and total DNA was isolated with MasterPureDNA Purification Kit (Epicentre Biotechnologies). Vector genome copy numbers were determined in 20 ng of DNA by qPCR with primers and probe specific for vector-borne polyA (Supplemental Table 2). A standard curve was built by serial dilution of linearized plasmid bearing the target sequence spiked into 20 ng of non-transduced mouse genomic DNA.
2.8. Statistics
All values are expressed as mean ± SEM. Statistical comparisons were made using Student's t-test or one-way analysis of variance (ANOVA) and Tukey post-test. Statistical significance was considered if P < 0.05.
3. Results
3.1. β-Cell overexpression of IGF1 suppresses diabetes in NOD mice
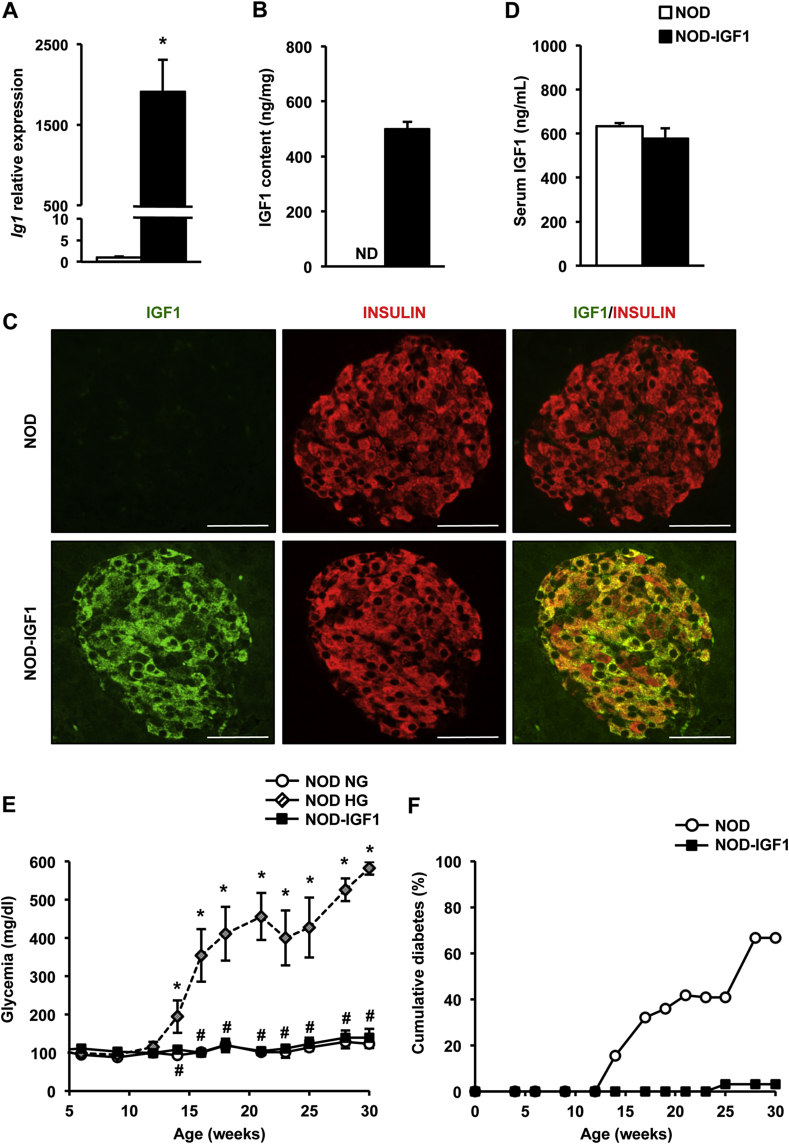
We generated NOD transgenic mice overexpressing IGF1 in β-cells (NOD-IGF1) by successive backcrossings (N = 15) of transgenic mice of ICR genetic background overexpressing IGF1 specifically in β-cells [15] with NOD mice. NOD-IGF1 islets showed high levels of expression and content of IGF1 (Figure 1A,B), which was limited to β-cells due to the use of the Rat Insulin Promoter I (RIP-I) (Figure 1C). Despite the high levels of expression of the transgene, NOD-IGF1 mice did not show increased circulating levels of IGF1 (Figure 1D), in agreement with previous observations of the same transgenic in other genetic backgrounds [15], [16].
Figure 1.
NOD-IGF1 mice that overexpress IGF1 in β-cells are protected against diabetes development. (A)Igf1 gene expression in islets (n = 9–10/group). (B) IGF1 content in islet extracts (n = 5–6/group). (C) Representative images of pancreatic sections immunostained for IGF1 (green) and insulin (red) (n = 3/group). Original magnification ×400 (scale bar: 50 μm). (D) Serum IGF1 levels (n = 5/group). All determinations (A–D) were performed at the pre-diabetic stage (4–8 weeks of age). (E) Monitoring of glycemia from 4 to 30 weeks of age in NOD (n = 34) and NOD-IGF1 (n = 32) mice. NG: normoglycemic. HG: hyperglycemic. (F) Cumulative incidence of diabetes over a period of 30 weeks in NOD (n = 34) and NOD-IGF1 (n = 32) mice. Results are expressed as mean ± SEM. *P < 0.05 vs. NOD. ND: Non-detected.
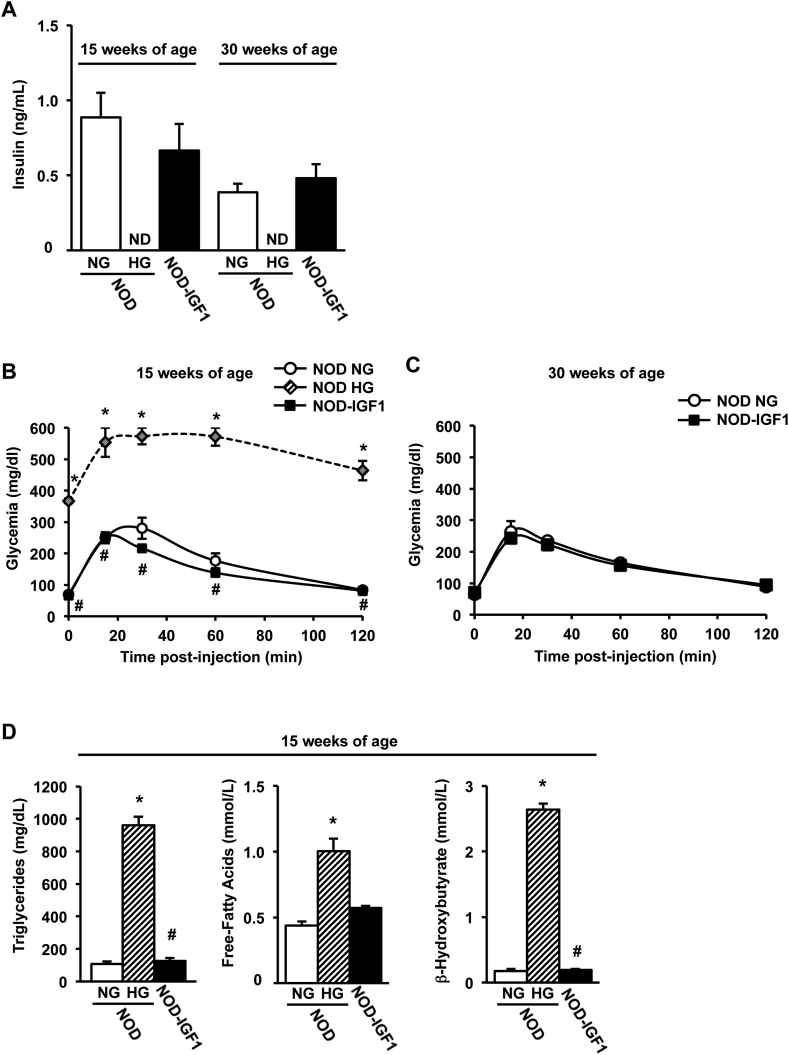
To assess the effect of IGF1 overexpression on diabetes development in the NOD model, blood glucose was monitored biweekly in female transgenic and non-transgenic NOD mice up to 30 weeks of age (Figure 1E). Animals were considered diabetic when two consecutive blood glucose readings were >250 mg/dL. Only 3% of transgenic NOD-IGF1 mice (1/32) developed hyperglycemia, as opposed to the 70% of wild type NOD littermates (23/34) that became diabetic over the same period of time (Figure 1F), indicating a clear protection from diabetes mediated by local IGF1. Approximately 30% of wild-type NODs never became diabetic, as previously described [31]. Normoglycemia in NOD-IGF1 mice was sustained by normal levels of circulating insulin, which was undetectable at either 15 or 30 weeks of age in NOD mice that had become hyperglycemic (Figure 2A). A glucose tolerance test performed at 15 weeks of age, i.e. at the time of onset of hyperglycemia in the NOD colony, showed that NOD-IGF1 mice and the NOD mice that had remained normoglycemic (NG) presented indistinguishable fasting blood glucose levels and responded similarly to a glucose overload, whereas hyperglycemic (HG) NOD mice were glucose intolerant (Figure 2B). Similar observations were made at 30 weeks of age (Figure 2C), indicating that IGF1 helped maintain long-term glucose tolerance. Moreover, secondary to diabetes development, as soon as 15 weeks of age, HG NOD mice had highly increased levels of serum triglycerides, free-fatty acids and β-hydroxybutyrate. In contrast, NOD-IGF1 mice displayed normal levels of these metabolites (Figure 2D).
Figure 2.
NOD-IGF1 mice have normal insulinemia and are glucose tolerant. (A) Serum insulin levels in fed conditions in normoglycemic (NG) and hyperglycemic (HG) NOD mice and in NOD-IGF1 mice at 15 and 30 weeks of age (n = 4–8/group). (B) Glucose tolerance test performed in 15-week-old NOD NG mice (n = 5), in NOD HG mice (n = 2) and in NOD-IGF1 mice (n = 8). (C) Glucose tolerance test performed in 30-week-old NOD NG mice (n = 2) and NOD-IGF1 mice (n = 9). (D) Serum concentrations of triglycerides, free-fatty acids, and β-hydroxybutyrate in fed conditions at 15 weeks of age in all experimental groups (n = 5/group). Results are expressed as mean ± SEM. *P < 0.05 vs. NOD NG; #P < 0.05 vs. NOD HG. ND: Non-detected.
3.2. Preservation of β-cell mass in transgenic NOD-IGF1 mice through reduced apoptosis
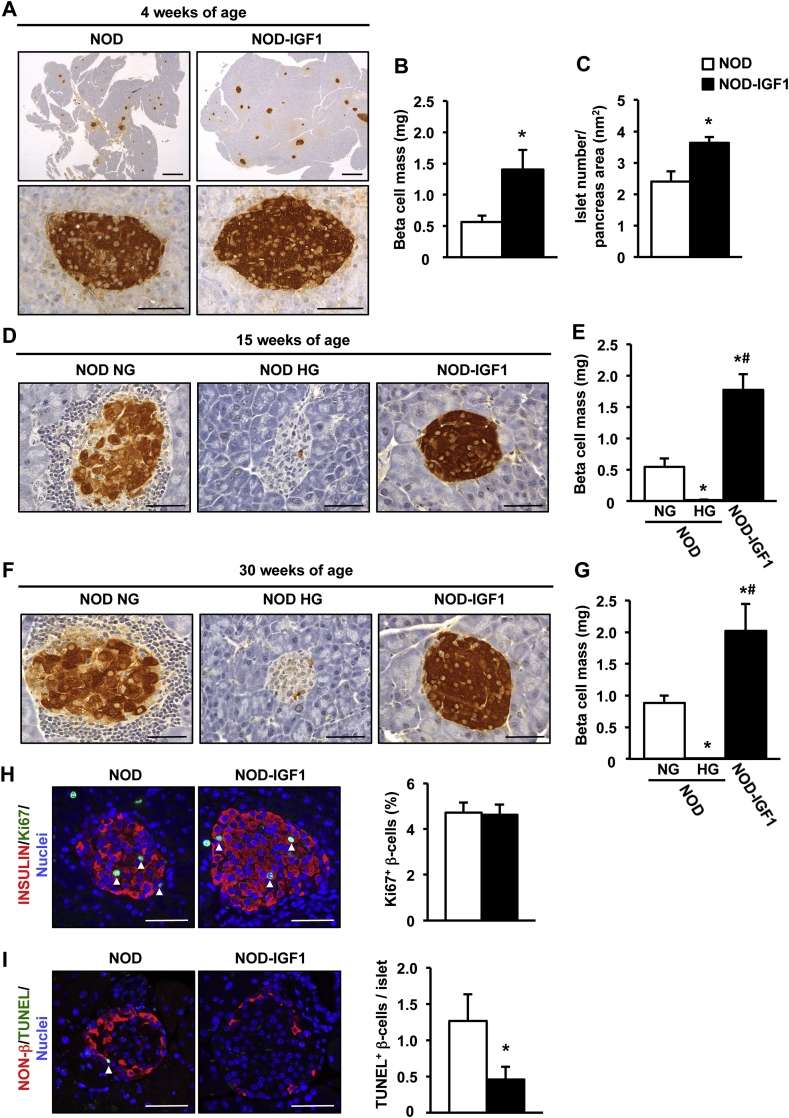
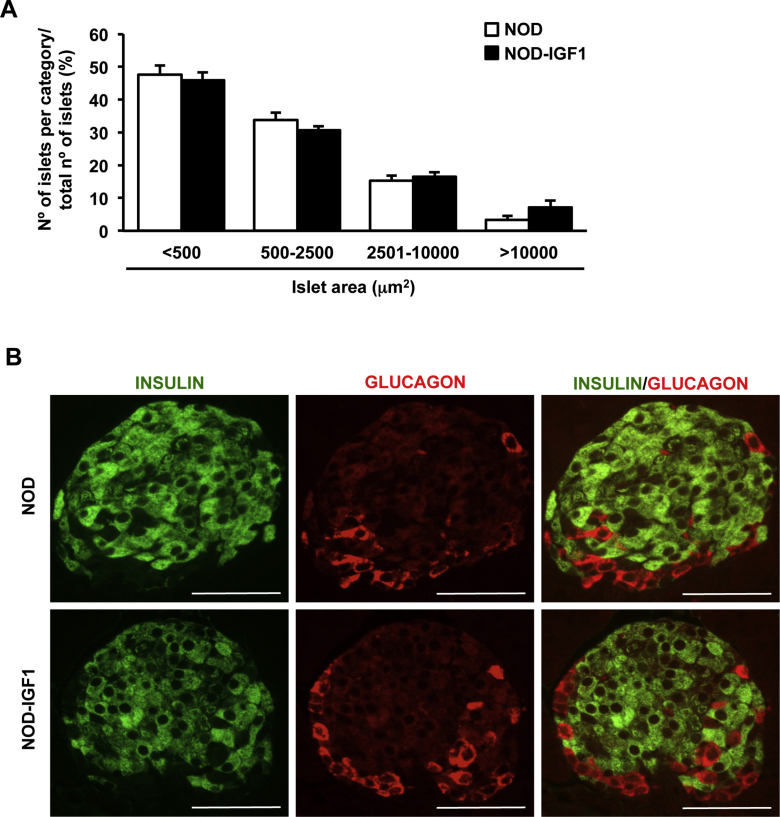
Pancreata from NOD and NOD-IGF1 mice were isolated and analyzed at several ages to evaluate IGF1-mediated effects on β-cell mass. At 4 weeks of age, NOD-IGF1 mice had ∼2.5-fold greater β-cell mass compared with NOD mice (Figure 3A,B). Such gain in β-cell mass was due to an increased number of islets (Figure 3C) rather than an increase in islet size (Supplemental Figure 1A). Double glucagon and insulin immunostaining of pancreas sections showed NOD-IGF1 islets had normal distribution of α and β endocrine cells, with glucagon-expressing cells located in the periphery and insulin-expressing in the core of the islet (Supplemental Figure 1B).
Figure 3.
β-Cell mass is preserved in NOD-IGF1 transgenic mice. (A) Immunohistochemical detection of insulin (brown) in pancreas from 4-week-old NOD and NOD-IGF1 mice (n = 6/group). Original magnification: ×20 (pancreas, scale bar: 500 μm) and ×400 (islets, scale bar: 50 μm). (B–C) Quantification of β-cell mass (B) and number of islets/pancreatic area (C) in 4-week-old NOD and NOD-IGF1 mice (n = 6/group). (D–G) Immunohistochemical detection of insulin (D, F) and β-cell mass quantification (E, G) in normoglycemic NOD (NOD NG), hyperglycemic NOD (NOD HG), and NOD-IGF1 mice aged 15 and 30 weeks, respectively (n = 3–7/group). Original magnification ×400 (scale bar: 50 μm). (H) Double immunostaining for insulin (red) and Ki67 (green) of islets from 4-week-old NOD and NOD-IGF1 mice (n = 5/group). Blue, nuclei. Arrowheads indicate Ki67+ β-cells. Original magnification ×400 (scale bar: 50 μm). The histogram depicts the quantification of the % of Ki67 positive β-cells. (I) TUNEL staining (green) of islets from 8-week-old NOD and NOD-IGF1 mice (n = 6/group). Non-β cells were immunostained with anti-glucagon, anti-somatostatin, and anti-pancreatic polypeptide cocktail (red). Blue, nuclei. Arrowheads indicate TUNEL+ β cells. Original magnification ×400 (scale bar: 50 μm). The histogram depicts the quantification of the number of TUNEL positive β-cells in these islets (n = 6/group). Results are expressed as mean ± SEM. *P < 0.05 vs. NOD NG; #P < 0.05 vs. NOD HG.
By 15 weeks of age, ∼20% of NOD mice had become hyperglycemic (Figure 1F). In these animals, the β-cell mass was almost undetectable (Figure 3D,E). In normoglycemic NOD mice, the β-cell mass was preserved; islets, however, showed considerable infiltration (Figure 3D,E). In contrast, islets from 15-week-old NOD-IGF1 mice showed preserved β-cell mass and were mostly free of insulitis (Figure 3D,E). Similar observations were made at 30 weeks of age (Figure 3F,G), an age at which 70% of NOD mice had become diabetic (Figure 1F), indicating that local overexpression of IGF1 mediated long-term preservation of the β-cell mass also in the context of the autoimmune NOD model.
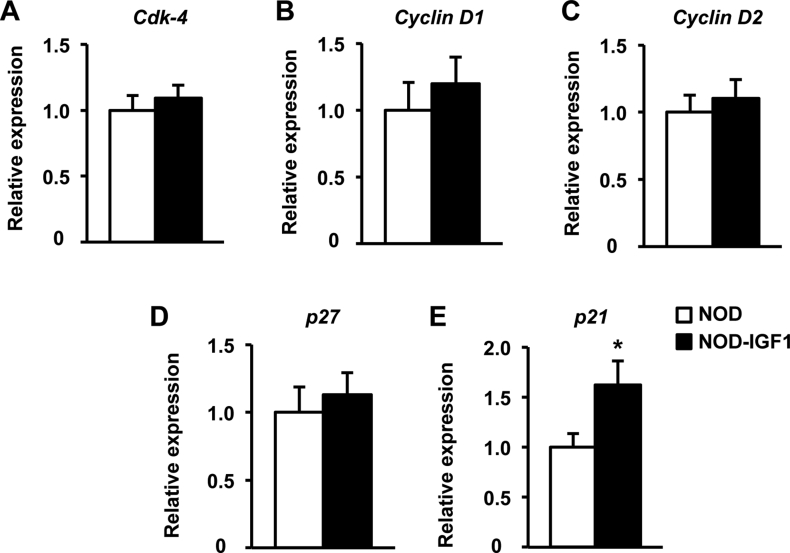
In order to characterize which mechanism/s were responsible for the maintenance of β-cell mass in NOD-IGF1 mice, β-cell replication, and apoptosis were assessed at the prediabetic stage. In islets from 8-week-old mice, we detected no differences in the expression of several key regulators of the β-cell cycle, such as cyclin-dependent kinase 4 (Cdk-4), cyclins D1 and D2, and the cyclin-dependent kinase inhibitor 1B (p27) (Supplemental Figure 2A–D). In addition, we found a moderate increase in the expression of cyclin-dependent kinase inhibitor 1A (p21) (Supplemental Figure 2E), which is believed to act as a molecular brake to mitogenic stimuli [32]. Consistent with this expression data, no differences were found in the % of replicating β-cells, as determined by double Insulin-Ki67 immunostaining (Figure 3H). However, the TUNEL assay evidenced a marked decrease in β-cell apoptosis in transgenic NOD-IGF1 islets compared with wild type NOD islets (Figure 3I). These results indicated that while NOD mice lost their β-cells, became glucose intolerant, and developed hyperglycemia over time, IGF1 overexpression in the β-cells of transgenic NOD-IGF1 mice was able to preserve β-cell mass and functionality and protect from diabetes, mainly through a reduction in the number of apoptotic β-cells.
3.3. Overexpression of IGF1 in β-cells halts the autoimmune attack
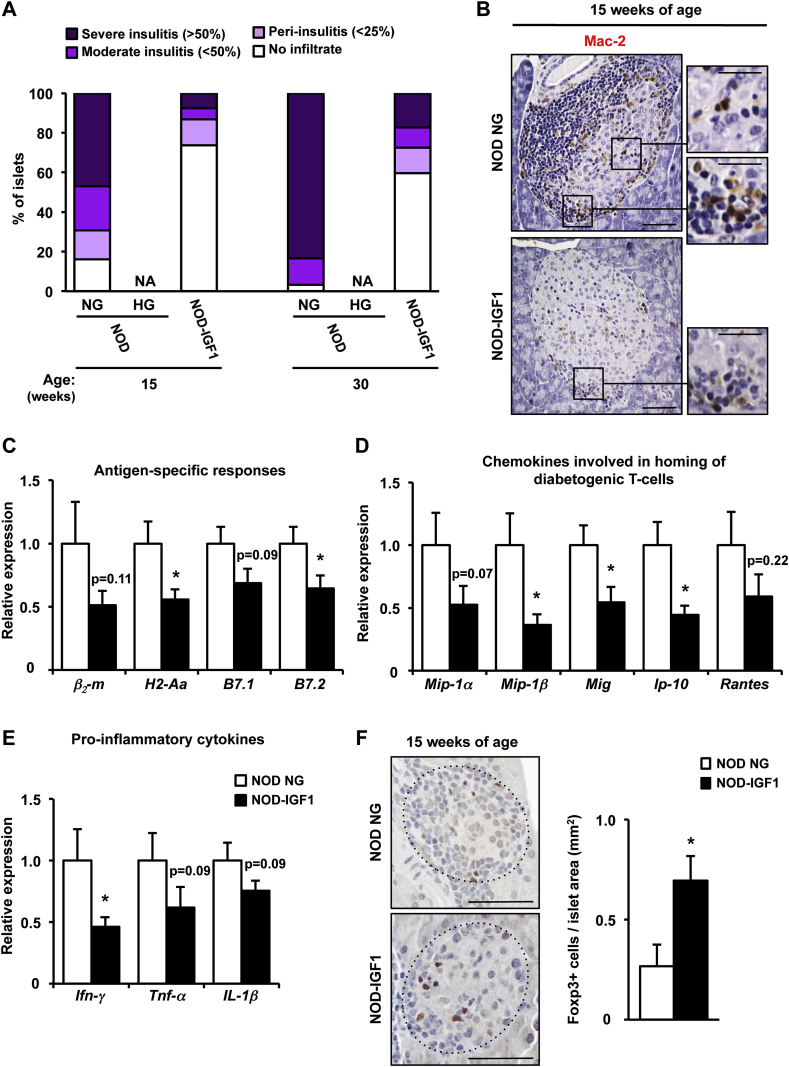
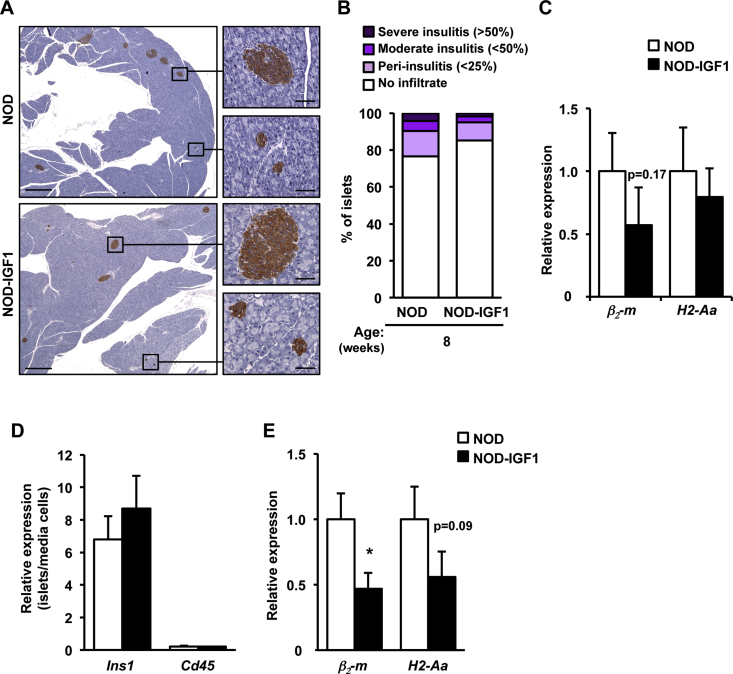
In NOD mice, the extent of intra-islet mononuclear cell infiltrates (insulitis) correlates with the progression to autoimmune diabetes [33]. We then investigated whether IGF1-mediated modulation of islet infiltration may contribute to the phenotype of transgenic NOD-IGF1 islets. To this end, the insulitis score was determined in pancreatic histological sections at 15 and 30 weeks of age. At both ages, NOD mice that had become diabetic (HG NOD) had lost all of their islets; β-cells could barely be detected and the infiltrate surrounding islets had mostly disappeared (Figure 3D,F), which precluded the quantification of the insulitis score in these animals (Figure 4A). Nevertheless, NOD mice that had not developed overt hyperglycemia (NG NOD) had extensive islet infiltration (Figure 3D,F), with over 50% (15 weeks of age) or 80% (30 weeks of age) of islets showing severe insulitis (Figure 4A). In contrast, at least 60% of the islets from 15- and, remarkably, 30-week-old NOD-IGF1 mice had no sign of infiltration (Figure 3, Figure 4A). Moreover, Mac-2 immunostaining revealed decreased infiltration by macrophages in the islets of NOD-IGF1 transgenic mice compared to normoglycemic NOD mice at 15 weeks of age (Figure 4B).
Figure 4.
Overexpression of IGF1 in β-cells prevents immune infiltration of NOD islets. (A) Insulitis score in 15 and 30-week-old NOD and NOD-IGF1 mice (n = 3–6/group). (B) Immunohistochemical detection of Mac-2 (brown) in islets from 15-week-old normoglycemic NOD (NOD NG) and NOD-IGF1 mice (n = 5/group). Original magnification ×200 (scale bar: 50 μm), insets ×1000 (scale bar: 25 μm). (C–E) Gene expression in islets from 15-week-old NOD NG and NOD-IGF1 mice. (C) Relative expression of molecules that participate in antigen presentation and activation of immune cells. (D) Relative gene expression of chemokines known to be involved in homing of diabetogenic T-cell to the pancreas or (E) in promoting inflammation. β2-m: β2-microglobulin; H2-Aa: histocompatibility 2, class II antigen A, alpha; B7.1: CD80 antigen; and B7.2: CD86 antigen; Mip-1α: macrophage inflammatory protein 1α; Mip-1β: macrophage inflammatory protein 1β; Mig: monokine induced by interferon-γ; Ip-10: interferon-γ-induced protein 10; Rantes: regulated on activation normal T-cell expressed and secreted; Ifn-γ: interferon-γ; Tnf-α: tumor necrosis factor α; Il-1β: interleukin 1β. Results are expressed as mean ± SEM. (F) Immunohistochemical detection of Foxp3 (brown) in islets from 15-week-old NOD NG (n = 5) and NOD-IGF1 mice (n = 8). Original magnification ×400 (scale bar: 50 μm). Histogram depicts the quantification of the number of Foxp3 positive cells/islet area. The dotted line defines islet contour. *P < 0.05 vs. NOD NG. NA, Non-available.
Subsequently, using qPCR, we evaluated the expression of genes involved in the inflammatory process leading to β-cell damage. As it was not feasible to isolate islets from diabetic NOD mice (vide supra), the analysis was performed in normoglycemic (NG NOD) animals; as these islets were severely infiltrated by 15 weeks of age (Figure 3, Figure 4A). In comparison to NG NOD mice, NOD-IGF1 animals showed diminished expression of the β2-microglobulin (β2-m) and H2-Aa genes (Figure 4C), which encode for the major histocompatibility complex (MHC) class I and class II antigens, respectively. NOD-IGF1 also showed reduced expression of the costimulatory receptors B7.1 (CD80) and B7.2 (CD86) (Figure 4C). To evaluate the possible contribution of infiltrating immune cells to the observed changes in the profile of islet gene expression, several approaches were undertaken. First, we analyzed the expression of MHC molecules in islets obtained from 8-week-old animals. At this age, the degree of infiltration is low and very similar in NOD and NOD-IGF1 mice (Supplemental Figure 3A and B), and differences in gene expression can be attributed mostly to phenotypic differences in β-cells. The expression of β2-m was lower in NOD-IGF1 islets than in non-transgenic NOD islets, although the decrease did not reach statistical significance (Supplemental Figure 3C). On the other hand, there was no apparent difference in the expression of H2-Aa (Supplemental Figure 3C). As a second approach, we cultivated islets obtained from NOD and NOD-IGF1 animals for 40 h to deplete them of infiltrating cells by spontaneous extrusion of leukocytes [34], [35], [36], [37]. Confirming this separation, Ins1 expression was detected preferentially in islets and Cd45 preferentially in cells present in the culture media (Supplemental Figure 3D). When β2-m and H2-Aa were analyzed in the immune cell-depleted islets, a statistically significant decrease in the expression of β2-m was observed in the islets obtained from NOD-IGF1 mice, and a non-statistically significant decrease (P = 0.09) was recorded in the expression of H2-Aa (Supplemental Figure 3E). In addition, in NOD-IGF1 transgenic animals, we documented a decrease of at least 50% in the expression of several genes that encode chemokines key for the homing of effector T cells to islets, such as macrophage inflammatory protein 1α (Mip-1α) and 1β (Mip-1β), a monokine induced by interferon-γ (Mig), interferon-γ-induced protein 10 (Ip-10) and regulated on activation normal T-cell expressed and secreted (Rantes) (Figure 4D). Similarly, the expression of the main inflammatory cytokines secreted by effector T-cells, interferon-γ (Ifn-γ), tumor necrosis factor α (Tnf-α), and interleukin 1β (Il-1β) was also lower in NOD-IGF1 islets (Figure 4E). At 15 weeks of age, Foxp3 immunostaining revealed increased infiltration of NOD-IGF1 transgenic islets by regulatory T cells (Figure 4F). Altogether, these results suggest that local overexpression of IGF1 manages to halt the immune process that mediates destruction of β-cells in the NOD model.
3.4. AAV8-mediated specific expression of IGF1 in the pancreas
The results obtained in transgenic NOD mice provided solid proof-of-concept that IGF1 expression specifically in β-cells could protect from autoimmune diabetes development, underscoring the therapeutic potential of IGF1. Therefore, we evaluated if IGF1 was also capable of protecting against diabetes following gene transfer of the IGF1 coding sequence to the pancreas of juvenile and adult NOD mice by means of AAV vectors.
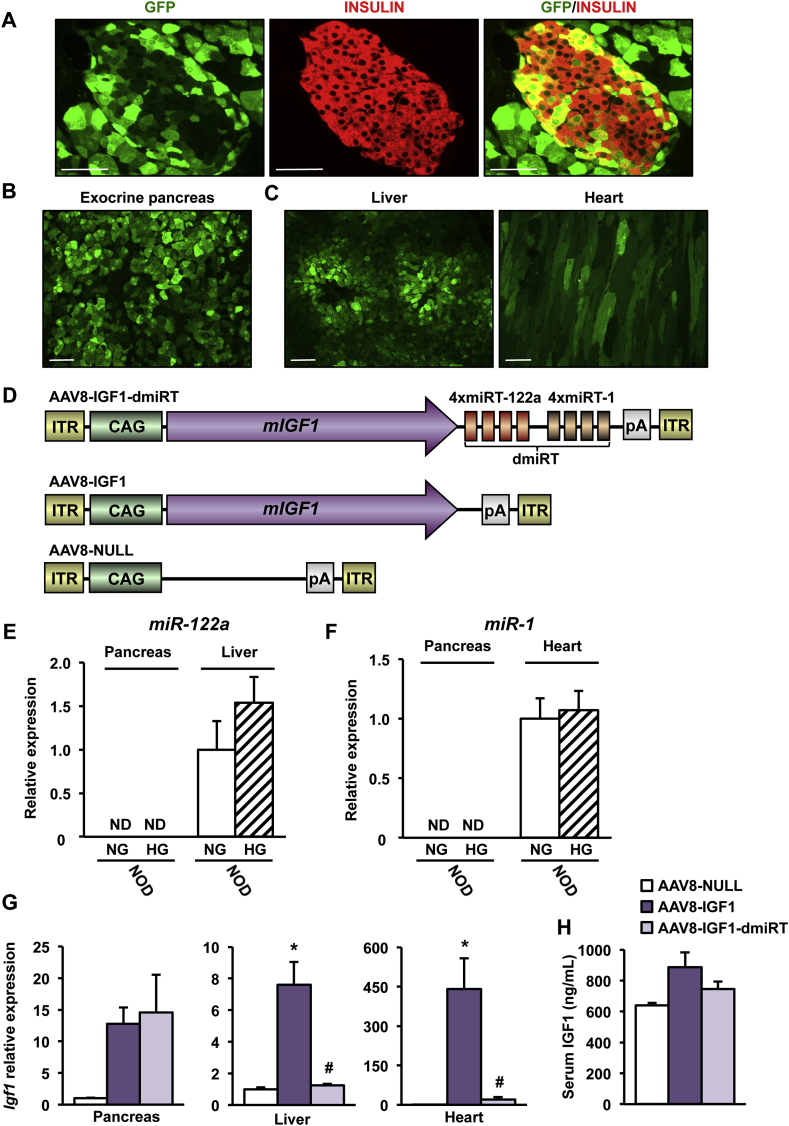
To maximize the number of cells that would supply IGF1 to islets in a paracrine/autocrine manner, our gene therapy approach requires the use of a strong ubiquitous promoter to drive IGF1 expression as well as an AAV serotype that can deliver the therapeutic construct to both endocrine and acinar cells after intraductal administration. This strategy is of great interest considering that β-cells are selectively destroyed by the immune system in T1D. To assess in vivo transduction efficiency in NOD mouse pancreas, 1012 vector genomes (vg) of AAV8 vectors encoding for GFP under control of the ubiquitous CAG promoter (AAV8-CAG-GFP) were administered to the pancreatic duct of 4-week-old female NOD mice. Robust transduction of both endocrine and exocrine pancreas was observed 2 weeks post-injection (Figure 5A,B). However, the use of the ubiquitous CAG promoter also led to production of GFP in the liver and heart (Figure 5C), in agreement with previous reports in other mouse strains [20], [22], [23].
Figure 5.
AAV vectors can specifically transduce the pancreas. (A) Immunohistochemical detection of GFP (green) and insulin (red) in pancreas 2 weeks after intraductal administration of AAV8-CAG-GFP (1012 vg) to 4-week-old NOD mice (n = 4/group). Original magnification ×400 (scale bar: 50 μm). (B, C) GFP immunostaining (green) in exocrine pancreas (B) and liver and heart (C) in the same cohort of mice. Original magnification ×200 (scale bar: 50 μm). (D) Schematic representation (not to scale) of the different AAV genomes. ITR: Inverted Terminal Repeats; CAG: hybrid cytomegalovirus enhancer/chicken β-actin promoter; mIGF1: murine Igf1 cDNA corresponding to IGF1Ea propeptide; miRT-122a: microRNA-122a target sequence (4 copies); miRT-1: microRNA-1 target sequence (4 copies); pA: polyadenylation signal. dmiRT refers to incorporation of both miRNA-122a and miRNA-1target sequences. The schematic representation is not to scale. (E, F) Expression of miR-122a (E) and miR-1 (F) was quantified in either normoglycemic (NG) (8-week-old) or hyperglycemic (HG) (24 week-old) NOD females. ND, Non-Detected; (n = 4–6/group). (G)Igf1 gene expression in pancreas, liver and heart of NOD mice 1 month after intraductal administration of 1012 vg of AAV8-IGF1-dmiRT, AAV8-IGF1 or AAV8-NULL to 4-week-old NOD mice (n = 4–6/group). (H) Serum IGF1 levels in the same cohort than in G (n = 3–5/group). Results are expressed as mean ± SEM. *P < 0.05 vs. AAV8-NULL; #P < 0.05 vs. AAV8-IGF1.
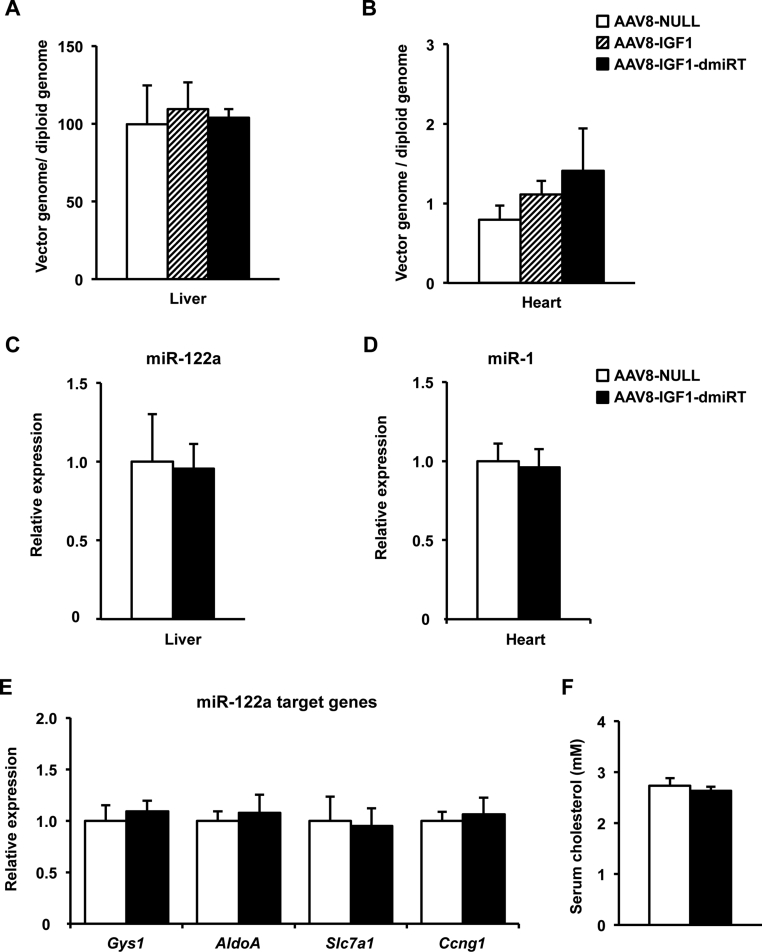
To prevent transgene expression in liver and heart and restrict AAV8-mediated IGF1 overexpression to pancreas, we took advantage of microRNAs (miRs) [26]. Target sequences for liver-specific miR-122a and heart-specific miR-1, which selectively detarget transgene expression from liver and heart when included into AAV vectors [27], were added in tandem repeats of 4 copies to the 3′-UTR of the IGF1 expression cassette (AAV8-IGF1-dmiRT) (Figure 5D). Despite the metabolic alterations, miR-122a and miR-1 expression levels were normal in liver (Figure 5E) or heart (Figure 5F) of diabetic NOD females, and the disease did not induce expression of these miRs in pancreas (Figure 5E,F). To validate our detargeting strategy, 1012 vg of IGF1-encoding AAV8 vectors containing or not miRNA target sequences (AAV8-IGF1-dmiRT and AAV8-IGF1, respectively) were intraductally delivered to 4-week-old NOD females. Animals injected with the same dose of non-coding AAV8 (AAV8-NULL, Figure 5D) served as controls. One month after vector administration, pancreatic Igf1 mRNA levels were similar in those animals that received AAV8-IGF1-dmiRT or AAV8-IGF1 (Figure 5G). In liver and heart, however, IGF1 expression differed considerably between both groups. While in the AAV8-IGF1-treated group Igf1 expression was high in both organs, the inclusion of miR-122a and miR-1 sequences in the transgene cassette abolished expression in liver and heart (Figure 5G). Indeed, animals treated with AAV8-IGF1 vectors showed a tendency towards an increase in IGF1 levels in serum which was not present in the AAV8-IGF1-dmiRT treated group (Figure 5H). All mice showed similar vector genome copy numbers in all tissues (Supplemental Figure 4A and B), further confirming that the observed differences in Igf1 levels were due to miRT-mediated specific silencing of transgene expression.
Six months after AAV delivery, miR-122a and miR-1 levels were similar in liver and heart of NOD mice injected with AAV8-IGF1-dmiRT or AAV8-NULL (Supplemental Figure 4C and D). Accordingly, hepatic expression of several of miR-122a-target genes was unaffected by administration of vectors containing miRT sequences (Supplemental Figure 4E), nor was serum cholesterol (Supplemental Figure 4F), allegedly regulated by miR-122a [38]. Altogether, these results suggested that long-term prevention of IGF1 overexpression in off-target tissues by inclusion of miRT sequences in the AAV expression cassette did not repress endogenous microRNAs by competitive inhibition.
3.5. AAV-mediated pancreatic Igf1 expression protects against autoimmune diabetes in NOD mice
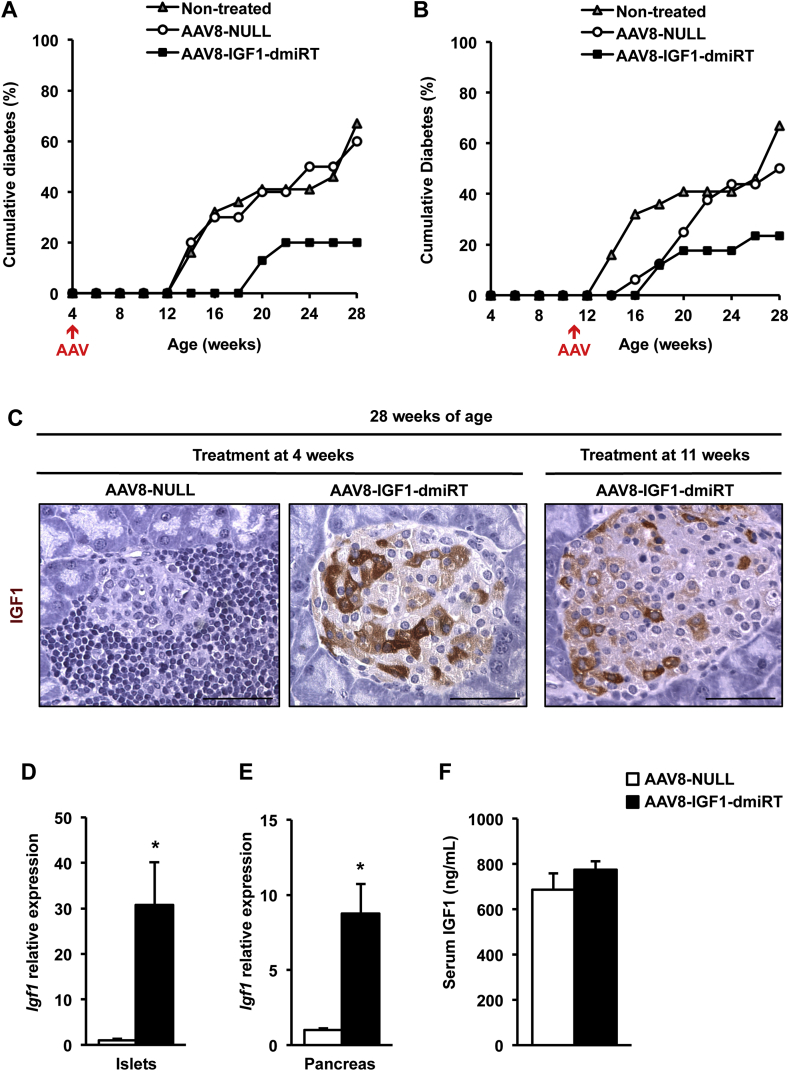
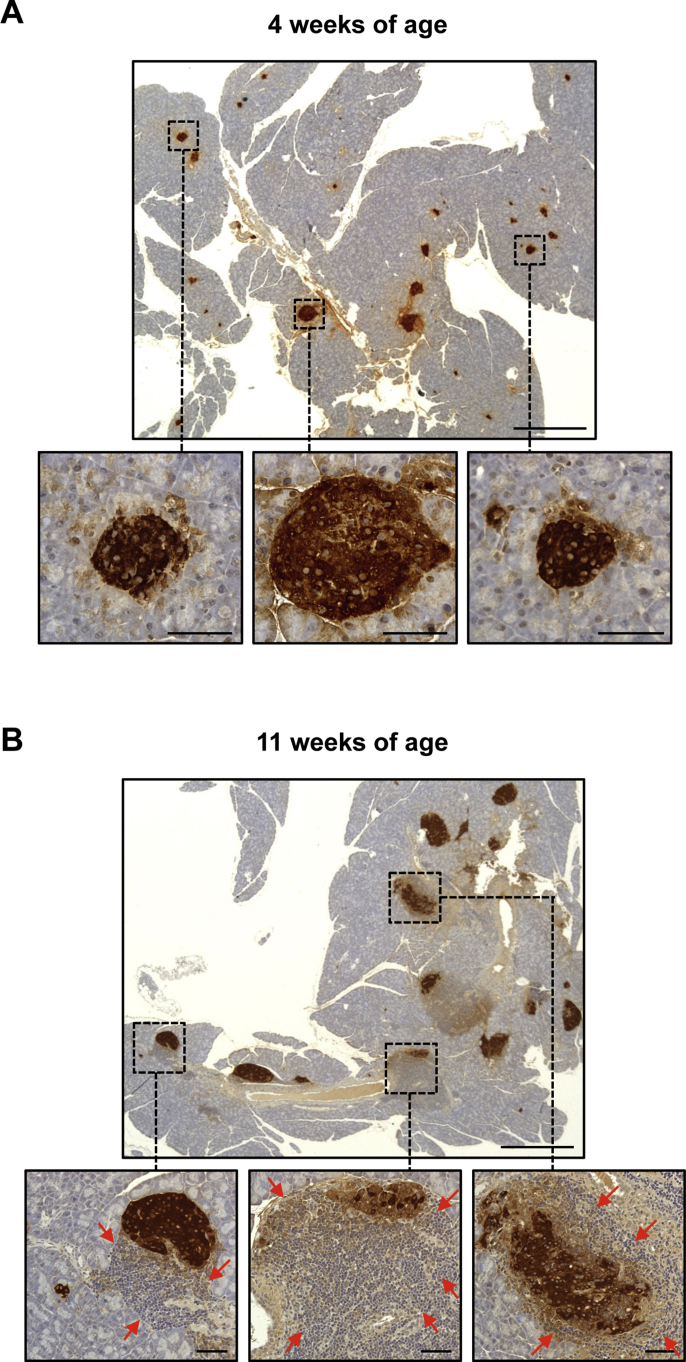
To determine whether pancreatic Igf1 expression could prevent diabetes development in NOD mice, 1012 vg of AAV8-IGF1-dmiRT or AAV8-NULL were intraductally delivered to 4-week-old pre-diabetic NOD females. At this age, islet infiltration by mononuclear cells (insulitis) was negligible (Supplemental Figure 5A). Following vector administration, glycemia was monitored biweekly in all treatment groups up to 28 weeks of age. By week 28, 80% of NOD mice treated with AAV8-IGF1-dmiRT vectors remained normoglycemic (Figure 6A), indicating they had been protected from disease development by therapeutic vector administration. Moreover, in the remaining 20% of AAV8-IGF1-dmiRT-treated mice that did develop hyperglycemia, diabetes onset was delayed by 6–8 weeks (Figure 6A). In contrast, AAV8-NULL-injected NOD females behaved similarly to untreated NOD animals, showing 60–65% accumulative incidence of diabetes by week 28 (Figure 6A).
Figure 6.
Intraductal delivery of AAV8-IGF1-dmiRT vectors protects against autoimmune diabetes in NOD mice. (A) Cumulative incidence of diabetes over a period of 28 weeks in non-treated NOD mice (n = 34) or NOD mice treated at 4 weeks of age with AAV8-NULL (n = 10) or AAV8-IGF1-dmiRT (n = 15). (B) Cumulative incidence of diabetes over a period of 28 weeks in non-treated NOD mice (n = 34) or NOD mice treated at 11 weeks of age with either AAV8-NULL (n = 16) or AAV8-IGF1-dmiRT (n = 17). (C) Immunohistochemical analysis of pancreas sections to detect IGF1 in 28-week-old NOD mice treated at 4 weeks or 11 weeks with either AAV8-NULL or AAV8-IGF1-dmiRT (n = 4–5/group). Original magnification ×400 (scale bar: 50 μm). (D, E)Igf1 gene expression analysis in islets (D) and total pancreas (E) from 28-week-old mice treated at 4 weeks of age (n = 5–8/group). (F) IGF1 levels in serum from normoglycemic 28-week-old mice treated at 4 weeks of age with AAV8-NULL (n = 4) or AAV8-IGF1-dmiRT (n = 10). Results are expressed as mean ± SEM. *P < 0.05 vs. AAV8-NULL.
After having tested Igf1 delivery at very early ages and getting positive results, treatment efficacy in stopping diabetes progression was evaluated at a more advanced stage of the pathology by intraductally delivering 1012 vg of AAV8-IGF1-dmiRT to 11-week-old NOD mice. Diabetes onset typically occurs close to this age in NOD females [33], whose pancreas showed infiltration and β-cell destruction in >50% of their islets (Supplemental Figure 5B and vide infra). Despite the advanced disease progression, treatment at 11 weeks also resulted in marked reduction of diabetes incidence. More than 75% of AAV8-IGF1-dmiRT-treated mice remained normoglycemic at 28 weeks of age while 50% of mice that received Null vectors became hyperglycemic (Figure 6B). Overall, these results indicate that AAV-mediated pancreatic overexpression of IGF1 protected NOD mice against diabetes development even when the pathogenic mechanisms responsible for disease onset and progression were already established.
3.6. Long-term pancreatic IGF1 expression after AAV-mediated gene transfer
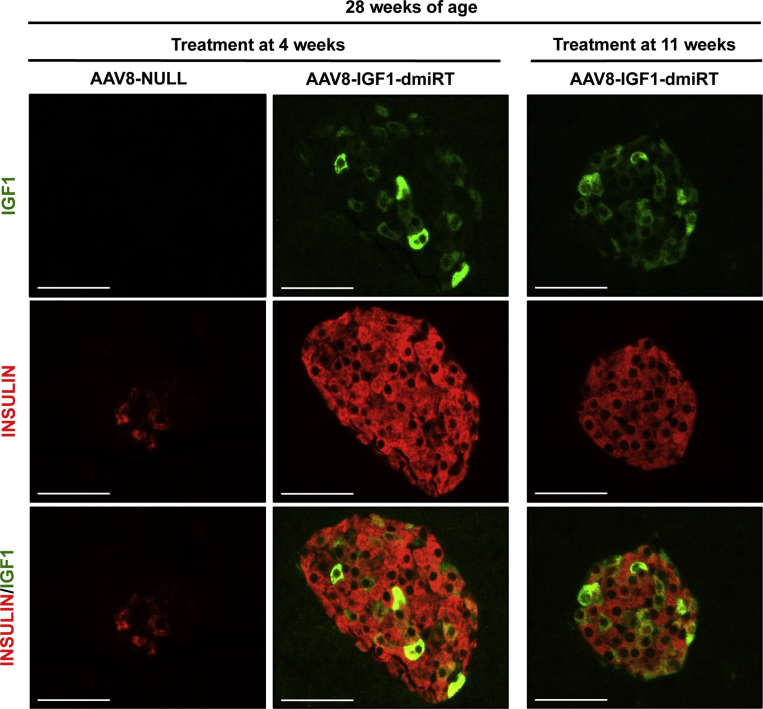
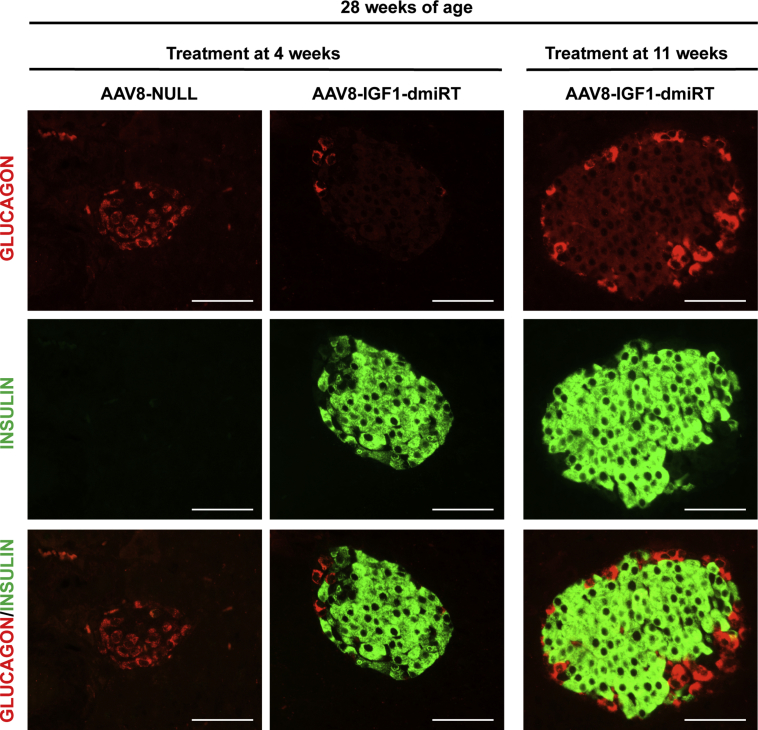
Long-term IGF1 expression was evaluated at the end of the follow-up period, i.e. in 28-week-old mice. Histological analysis suggested the majority of cells that overexpressed IGF1 within the islets of AAV8-IGF1-dmiRT-treated mice at either 4 or 11 weeks of age were β-cells (Figure 6C). Double immunostaining confirmed the identity of most IGF1-producing cells as insulin positive β-cells (Supplemental Figure 6). IGF1 production was not easily detected in exocrine cells (Figure 6C and Supplemental Figure 6), probably due to rapid excretion of the factor from these cells, as opposed to its accumulation in the secretory granules of β-cells. As expected, AAV8-NULL-injected animals had very few insulin positive cells and showed no IGF1 production in either the endocrine or exocrine pancreas (Figure 6C and Supplemental Figure 6). Moreover, double glucagon and insulin immunostaining of AAV8-IGF1-dmiRT-treated pancreas showed islets with normal distribution of different endocrine cells, with insulin-expressing cells located in the core of the islet and glucagon-expressing cells in the periphery (Supplemental Figure 7). In contrast, AAV8-NULL-treated mice showed islet destruction and lack of insulin-expressing cells (Supplemental Figures 6 and 7). Quantification of IGF1 mRNA levels in islets obtained from 28-week-old NOD mice treated with AAV8-IGF1-dmiRT at the age of 4 weeks showed 30-fold increase in Igf1 mRNA levels over the values obtained in islets of Null-injected animals (Figure 6D). When the quantification was performed on RNA isolated from whole pancreas, a 10-fold increase was documented (Figure 6E). These levels of expression were very similar to those observed 1 month after vector administration (Figure 5G), suggesting that there had been no loss of transgene expression over time. Similar to the observations made in transgenic NOD mice overexpressing IGF1 specifically in β-cells (Figure 1D), no differences were observed between groups in the levels of circulating IGF1 (Figure 6F). Altogether, these results suggested that prevention of diabetes in AAV8-IGF1-dmiRT-treated NOD mice was mediated by autocrine/paracrine secretion of IGF1 in the pancreas.
3.7. AAV-mediated pancreatic expression of IGF1 preserves pancreatic β-cell mass and insulinemia
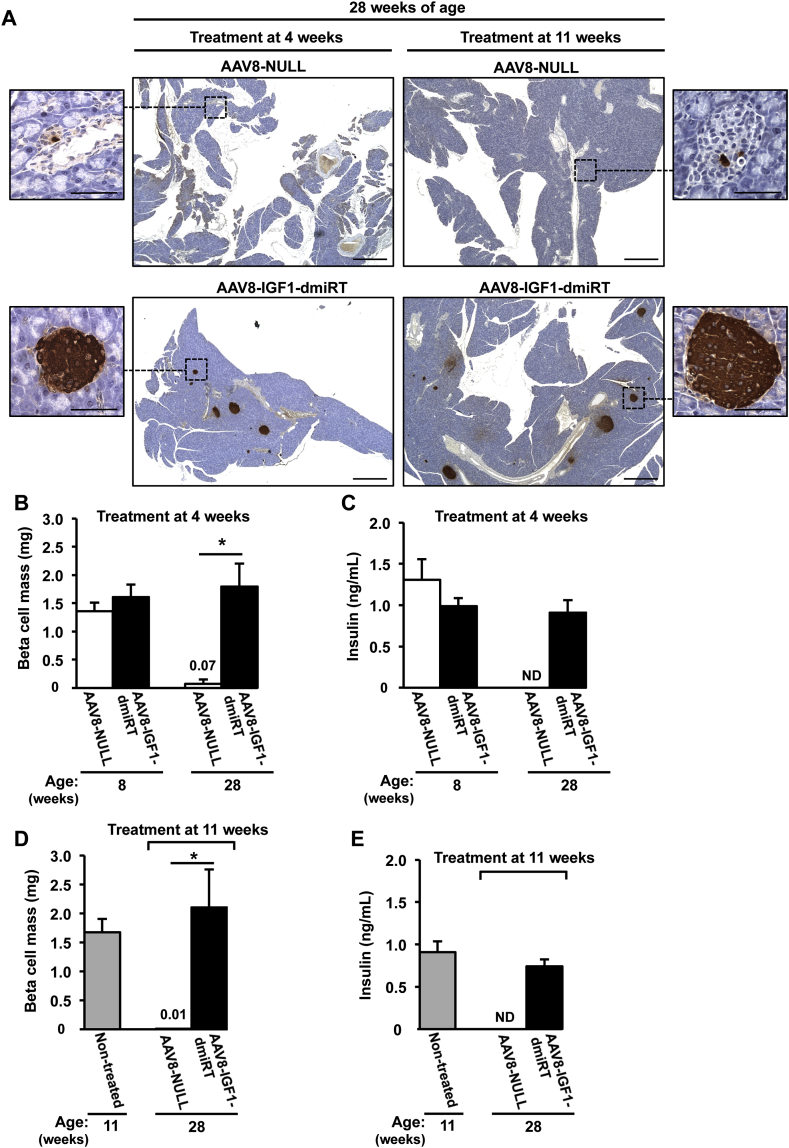
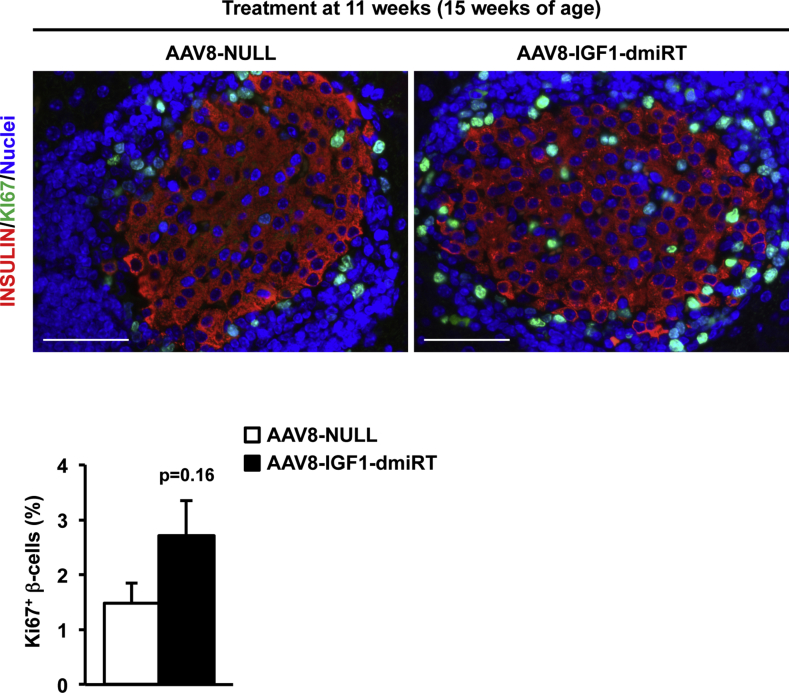
Insulin immunostaining was performed to evaluate β-cell mass at 28 weeks of age in different cohorts of NOD mice (Figure 7A). At 4 weeks, untreated pre-diabetic NOD mice had islets with normal appearance, and there was no sign of islet infiltration (Figure 3A and Supplemental Figure 5A). At 8 weeks of age, when insulitis is already detectable in the NOD model (vide infra), β-cell mass showed no differences between AAV8-NULL-injected or AAV8-IGF1-dmiRT-treated NOD animals (Figure 7B). Accordingly, both cohorts had comparable levels of circulating insulin (Figure 7C). Similar values of β-cell mass and insulinemia were documented in untreated 11-week-old NOD mice (Figure 7D,E). Secondary to diabetes development, however, 28-week-old NOD mice injected with AAV8-NULL vectors at either 4 or 11 weeks of age showed almost complete β-cell loss (Figure 7B,D); very few islets and insulin positive β-cells were observed in histological sections (Figure 7A), and insulin was undetectable in serum (Figure 7C,E). In contrast, 28-week-old NOD mice treated at either 4 or 11 weeks of age with therapeutic AAV8-IGF1-dmiRT vectors showed preservation of β-cells (Figure 7A,B and D). This maintenance of the β-cell mass did not seem to be sustained by a significant increase in β-cell replication in AAV8-IGF1-dmiRT-treated NOD animals, as evidenced by the quantification of Ki67 positive cells 4 weeks after vector delivery (Supplemental Figure 8). The β-cell mass documented in IGF1-treated animals was similar to that previously reported for immunodeficient NOD-SCID mice of similar age in which the immune-mediated destruction of β-cells does not occur [39]. Accordingly, AAV8-IGF1-dmiRT-treated NOD mice had preserved insulinemia (Figure 7C,E).
Figure 7.
Pancreatic IGF1 expression preserves β-cell mass and circulating insulin levels. (A) Immunohistochemical detection of insulin (brown) in pancreas from 28-week-old mice treated at 4 weeks or 11 weeks of age with either AAV8-NULL (n = 3–4) or AAV8-IGF1-dmiRT (n = 4–5). Insulin positive β-cells could barely be detected in AAV8-NULL-treated mice. Red arrowheads indicate islets. Original magnification ×20 (scale bar: 500 μm), insets ×400 (scale bar: 50 μm). (B) Quantification of the β-cell mass in 8 or 28-week-old NOD mice treated with AAV8-NULL (n = 3–4) or AAV8-IGF1-dmiRT (n = 5) vectors at 4 weeks of age. (C) Serum insulin levels in fed conditions in the same cohorts of animals as in B. AAV8-NULL (n = 3–4); AAV8-IGF1-dmiRT (n = 5). (D) Quantification of β-cell mass in non-treated 11-week-old NOD mice (n = 4) or 28-week-old NOD mice treated with AAV8-NULL (n = 3) or AAV8-IGF1-dmiRT (n = 4) at 11 weeks of age. (E) Serum insulin levels in fed conditions in the same experimental cohorts as in D. Non-treated (n = 7); AAV8-NULL (n = 4); AAV8-IGF1-dmiRT (n = 10). Results are expressed as mean ± SEM. *P < 0.05 vs. AAV8-NULL. ND, Non-detected.
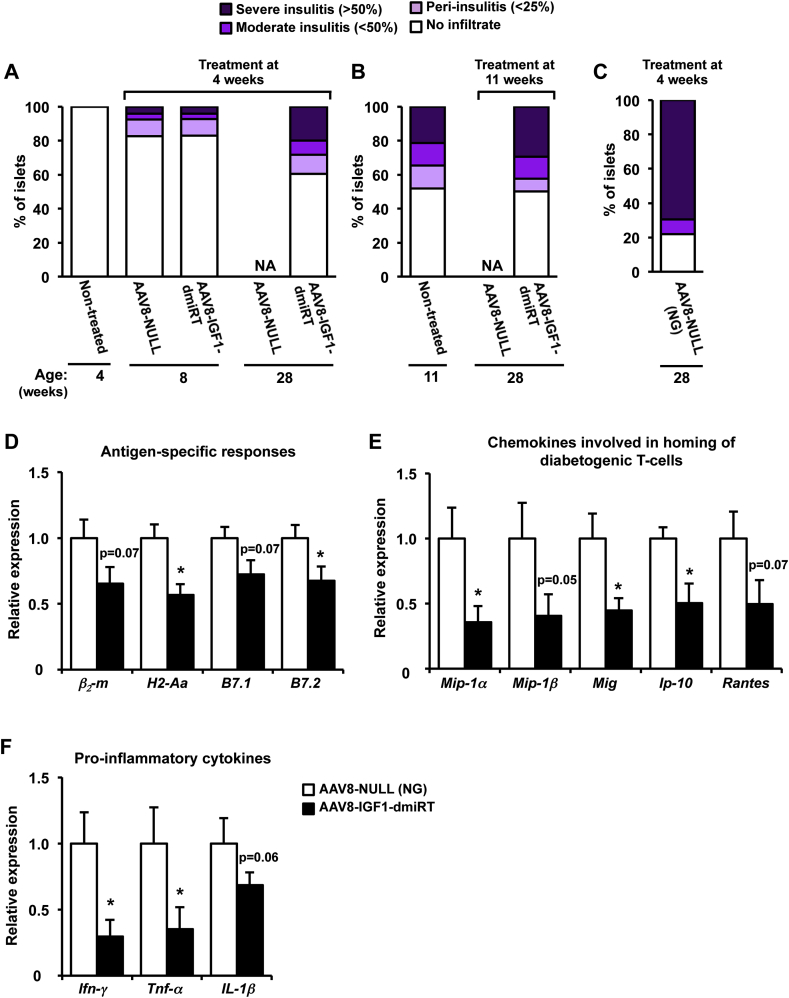
3.8. AAV-mediated pancreatic expression of IGF1 blocks immune-mediated β-cell destruction
At 4 weeks of age, no sign of infiltration was observed in any of the islets analyzed, and NOD mice were considered insulitits-free (Figure 8A and Supplemental Figure 5A). However, by 8 weeks, a similar extent of islet infiltration become apparent in NOD mice administered with either NULL or AAV8-IGF1-dmiRT vectors: ∼20% of islets showed some degree of insulitis at this age (Figure 8A). At 11 weeks, non-treated NOD mice had ∼50% of islets infiltrated, ∼20% of which showed severe insulitis (Figure 8B and Supplemental Figure 5B). By 28 weeks, animals injected at either 4 or 11 weeks with AAV8-NULL vectors that became diabetic had lost all their islets: β-cells were barely detectable and the infiltrate surrounding islets had mostly disappeared (Figure 7A), precluding insulitis scoring (Figure 8A,B). AAV8-NULL-injected NOD mice that had not developed overt hyperglycemia by 28 weeks had, nevertheless, extensive (>80%) islet infiltration, with ∼70% of islets showing severe insulitis (Figure 8C). In contrast, 28-week-old NOD mice that received IGF1-encoding vectors at 11 weeks had at least 50% of insulitis-free islets; the percentage was slightly higher (∼60%) in animals receiving the therapeutic vector at 4 weeks (Figure 8A,B). In animals treated with AAV8-IGF1-dmiRT at 11 weeks, the insulitis score at 28 weeks resembled that observed pre-treatment, suggesting that AAV-mediated local expression of IGF1 had managed to halt the immune process that leads to β-cell loss.
Figure 8.
Pancreatic overexpression of IGF1 prevents immune infiltration of islets. (A) Insulitis score in non-treated 4-week-old NOD mice (n = 6), and 8 or 28-week-old NOD mice treated at 4 weeks with either AAV8-NULL (n = 4) or AAV8-IGF1-dmiRT (n = 5). (B) Insulitis score in non-treated 11-week-old NOD mice (n = 6) and 28-week-old NOD mice treated at 11 weeks with AAV8-NULL (n = 4) and AAV8-IGF1-dmiRT (n = 4). (C) Insulitis score in 28-week-old NOD mice treated with AAV8-NULL (n = 3) at 4 weeks that remained normoglycemic (NG). (D–F) Gene expression in islets from normoglycemic 28-week-old NOD mice treated with AAV8-NULL or AAV8-IGF1-dmiRT at 4 weeks. (D) Relative expression of molecules that participate in antigen presentation and immune cell activation. (E) Relative gene expression of chemokines known to be involved in homing of diabetogenic T-cell to the pancreas or (F) promoting inflammation. β2-m: β2-microglobulin; H2-Aa: histocompatibility 2, class II antigen A, alpha; B7.1: CD80 antigen; and B7.2: CD86 antigen; Mip-1α: macrophage inflammatory protein 1α; Mip-1β: macrophage inflammatory protein 1β; Mig: monokine induced by interferon-γ; Ip-10: interferon-γ-induced protein 10; Rantes: regulated on activation normal T-cell expressed and secreted; Ifn-γ: interferon-γ; Tnf-α: tumor necrosis factor α; Il-1β: interleukin 1β. AAV8-NULL (n = 4); AAV8-IGF1-dmiRT (n = 6). Results are expressed as mean ± SEM. *P < 0.05 vs. AAV8-NULL. NA, Non-available.
Then, we evaluated by qPCR the expression of genes involved in the immune process leading to β-cell death. As it is not feasible to isolate islets from 28-week-old diabetic AAV8-NULL-injected NOD mice, the analysis was performed in normoglycemic (NG) animals administered with NULL vectors at 4 weeks; as previously demonstrated the islets of these mice were severely infiltrated by 28 weeks (Figure 8C). In comparison to NG AAV8-NULL NOD mice, NG NOD mice that received the therapeutic AAV8-IGF1-dmiRT vector showed diminished expression of the β2-m and H2-Aa genes, as well as reduced expression of the costimulatory receptors B7.1 and B7.2 (Figure 8D). In addition, we documented a decrease of at least 50% in the expression of Mip-1α and Mip-1β, Mig, IP-10, and Rantes (Figure 8E). Similarly, expression of the main inflammatory cytokines secreted by effector T-cells, Ifn-γ, Tnf-α, and Il-1β was also lower in islets from AAV8-IGF1-dmiRT-treated NOD mice (Figure 8F). Altogether, these observations indicate that AAV-mediated IGF1 pancreatic overexpression protected from diabetes development by limiting the autoimmune attack against β-cells. Furthermore, observations made following treatment of adult animals suggest that the expression of IGF1 during embryonic development is not a requirement to achieve this protection.
4. Discussion
Here we show, first in a transgenic animal model and then through AAV-mediated pancreatic gene transfer, that local IGF1 production protects NOD mice against spontaneous immune-mediated β-cell loss and development of hyperglycemia. Thus, we demonstrate the therapeutic potential of a gene therapy that specifically overexpresses IGF1 in the pancreas to modify disease progression in autoimmune diabetes.
The overexpression of IGF1 in NOD islets completely protected from development of spontaneous autoimmune diabetes. Transgenic NOD-IGF1 mice had normal levels of insulin in circulation and were glucose tolerant. Also, and in contrast to their diabetic NOD counterparts, NOD-IGF1 animals had normal levels of serum triglycerides, free-fatty acids, and β-hydroxybutyrate, indicative of normal energy metabolism. IGF1 overexpression clearly protected β-cells. While non-transgenic NOD mice that became hyperglycemic had lost almost all their β-cells by 15 weeks of age, NOD-IGF1 mice showed preserved β-cell mass up to the end of the follow-up period (30 weeks). By this age, >70% of NOD animals had overt diabetes. Noticeably, 4-week-old NOD-IGF1 mice already showed greater β-cell mass than non-transgenic NOD littermates. This early-age increase in the β-cell mass was associated with an increased number of islets, which were otherwise of normal size and structure, showing normal distribution of the different endocrine cells. Such an increase in β-cell mass was not observed in RIP-IGF1 transgenic mice with other genetic backgrounds prior to STZ administration [15], [16], [17], suggesting that the increase is relative to the NOD mouse. Indeed, it has been described that NOD mice have decreased β-cell mass (∼70%) before diabetes onset when compared to NOD/Scid in which autoimmune diabetes does not occur [40]. Thus, the overexpression of IGF1 from birth in NOD-IGF1 transgenic mice may be compensating for this reduction. Moreover, despite IGF1 overexpression in these mice, we could not find any indication of increased rate of replication in transgenic β-cells. In fact, of all the controllers of the cell cycle whose expression was analyzed, only p21 expression was increased in NOD-IGF1 islets. p21 is an inhibitor of cyclin-dependent kinases, and hence a negative regulator of the cell cycle [32]. Likely, this increase in p21 compensated for the chronic promitogenic stimuli provided by IGF1, resulting in a normal rate of β-cell replication in NOD-IGF1 mice. We did, however, observed evidence of reduced apoptosis in NOD-IGF1 islets. Thus, our current hypothesis is that this early-age increase in β-cell mass is a consequence of IGF1 expression at the early postnatal period in which the β-cell mass is reshaped through physiological waves of apoptosis [41].
Several clinical studies have provided evidence of therapeutic benefit, such as reductions in glycated hemoglobin or insulin requirements, following administration of high doses of recombinant human IGF1 to diabetic patients [10], [11], [12]. IGF1 systemic treatment however, was associated with clinically significant adverse events attributed to increased circulating IGF1, such as edema, jaw ache, headache, tachycardia, and fatigue [42]. In addition, multiple product administrations were required and therapeutic efficacy was transient by nature, limited by the short half-life of IGF1 in circulation. To overcome these limitations, we designed a novel therapeutic strategy based on AAV-mediated gene transfer to achieve long-lasting, local production of IGF1 following a single administration of therapeutic product. AAVs are not associated with any human disease and are hence regarded as safe vectors for gene therapy, which has encouraged their use in an increasing number of clinical trials [18]. Indeed, in 2012 the European Commission granted marketing authorization to Glybera®, an AAV-based therapeutic for the treatment of Lipoprotein Lipase deficiency [43], and other market authorizations are underway. AAV8 was chosen, because we previously demonstrated this serotype is very efficient at transducing exocrine and endocrine pancreas [20]. AAV8 also has significant tropism for liver and heart, and transduction of these organs occurs even if the vector is administered locally to other organs. Given that clinical studies have highlighted the potential toxicity associated with increased circulating IGF1, we detargeted expression of IGF1 from these organs by incorporation to our expression cassette of miRTs for miR-122a and miR-1-miRNAs highly expressed in liver and heart, respectively. This enabled us to conveniently overexpress IGF1 in pancreas while efficiently silencing transgene expression in liver and heart. Importantly, administration of miRT-containing vectors proved very safe. Six months after AAV8 administration, endogenous miR-1 and miR-122a expression was not altered, nor was de-repression of miR-122a target genes triggered. This observation agreed with other studies, indicating that the miR-122 pathway is not affected by AAV vectors containing several copies of miR-122 miRT [44]. A marked reduction in serum cholesterol has also been reported when antisense oligonucleotides were used to sequester miR-122, indicating that cholesterol metabolism is a target of this miRNA [45]. Six months after delivering our vectors to NOD mice, however, animals showed normal levels of cholesterol in circulation, further demonstrating that the delivery of miR-122a-containing AAV vectors did not affect miR-122 physiological functions.
AAV-mediated Igf1 gene transfer to pancreas protected NOD mice against development of autoimmune diabetes, as evidenced by the significant reduction in the incidence of spontaneous diabetes in the animals that received therapeutic vector. IGF1-treated mice also showed preservation of β-cell mass and normal levels of circulating insulin. Noticeably, as in all previous models, serum IGF1 remained unaltered. Besides confirming previous observations made in transgenic mice, this study also discarded the possibility that the phenotype described in transgenic models was due to expression of the transgene during embryonic development and early postnatal life. Also, although RIP-IGF1 transgenic mice have been backcrossed more than 15 times onto the NOD background, studies with the AAV platform rule out that protection from diabetes is related to some resistance gene that has been carried forward with the transgene from the original background strain. Although therapeutic benefit following gene transfer was slightly greater in animals treated at 4 weeks, before the immune process commences in this model, an unexpected finding of our study was the ability of AAV8-IGF1-dmiRT to halt disease progression even when animals were treated at 11 weeks, an age at which NOD mice treated with NULL vectors begin to turn hyperglycemic. In NOD mice treated with AAV8-IGF1-dmiRT at 11 weeks, diabetes incidence at 28 weeks was 23%, as opposed to ∼70% or ∼50% registered in the untreated or Null-injected groups, respectively.
The mechanisms by which IGF1 exerts its protective action on transgenic islets or after AAV-mediated gene delivery have not been fully elucidated. The observations made after gene transfer argue against the possibility that the protection against diabetes documented in transgenic animals was due to an initially greater number of β-cells, as NOD mice that received AAV8-IGF1-dmiRT vectors had the same β-cell mass at treatment than untreated NOD mice that became diabetic. Protection against diabetes cannot be attributed either to systemic effects of IGF1 on metabolism. NOD-IGF1 mice had normal circulating levels of IGF1, similar to all the previous transgenic lines in which IGF1 was overexpressed specifically in β-cells [15], [16], [17]. Likewise, AAV8-IGF1-dmiRT-treated NOD mice had the same IGF1 levels as untreated controls.
Protection against diabetes, in theory, could result from a combination of enhancement of β-cell survival, stimulation of β-cell replication, and promotion of an immunoprotective milieu within the islet that allows survival of functional insulin-producing β-cells. IGF1 protects against apoptosis [46] and has positive effects on β-cell mitogenesis and islet growth [2], [47]. IGF1 stimulates β-cell replication, in particular under conditions of β-cell damage. We previously demonstrated in RIP-IGF1 transgenic mice with C57Bl/SJL genetic background that β-cell-specific IGF1 overexpression protects the β-cell mass through activation of cyclins that promote mitosis [16]. Noticeably, activation of these cyclins only occurs upon STZ-induced damage [16]. An obvious increase in β-cell replication, however, was not detected in the current study, either in NOD transgenic islets or following gene transfer of Igf1 to the NOD pancreas. This could argue against a major role of enhanced β-cell replication in the maintenance of the β-cell mass in the context of autoimmune diabetes or could reflect technical limitations associated with the detection of replication at specific time-points. We did, however, detect reduced apoptosis in the islets of transgenic NOD-IGF1.
Autoimmunity plays a key pathogenic role in the development of diabetes in the NOD model; immunodeficient SCID-NOD mice do not develop overt diabetes [48]. The most striking finding of our study was the documentation of a dramatic reduction in the degree of insulitis in NOD-IGF1 transgenic islets or after Igf1 gene transfer to adult animals. More than 60% of NOD-IGF1 islets showed no sign of infiltration at an age (30 weeks) at which 80% of non-transgenic NOD islets had severe insulitis and no remaining β-cells. Similarly, NOD mice that received IGF1-encoding AAV vectors at either 4 or 11 weeks had at least half of their islets free of insulitis by 28 weeks of age. In animals treated with AAV8-IGF1-dmiRT at 11 weeks, the insulitis score at 28 weeks resembled that observed pre-treatment, suggesting that local expression of IGF1 had managed to halt the immune process.
In addition, in NOD-IGF1 transgenic mice and in non-transgenic NOD animals following AAV8-IGF1-dmiRT treatment, we observed a reduction in the expression of antigen-presenting molecules and chemokines known to be involved in homing of diabetogenic T-cells to the pancreas [49], [50]. The presence of class I antigen-presenting molecules on β-cells is key to the activation of diabetogenic T-cells and initiation of β-cell autoimmune destruction [51], [52], [53]. Thus, the documented reduction in β2-microglobulin and B7 expression could provide an explanation to the lesser degree of β-cell destruction. This is consistent with other reports suggesting that IGF1 plays a role in immune evasion in a variety of cells by down-regulating MHC-I and B7 [54], [55]. Expression of genes associated with antigen presentation through MHC-II, such as H2-Aa, was also reduced. However, there is controversy regarding whether β-cells express MHC-II molecules [56]; hence, our observations may result from reduction in professional APCs, such as islet-infiltrating macrophages. Besides preventing activation of antigen-specific immune responses or recruitment of immune cells to pancreatic islets, it is also possible that pancreatic IGF1 overexpression acted by modulating the function of the infiltrate. IGF1 receptors are expressed on T-cells, and regulatory T-cell function and number may be modulated by IGF1 promoting anti-inflammatory effects [7], [57]. Although a statistically significant increase in the number of Foxp3 positive cells was documented in the islets of NOD-IGF1 transgenic mice, no differences were observed 4 weeks post vector delivery (data not shown). This apparent discrepancy is likely the consequence of the difficulty in selecting the proper time-point of quantification.
Several preclinical and clinical trials have shown potential efficacy of immunotherapies in counteracting T1D. For example, administration of anti-CD3 or anti-CD20 monoclonal antibodies targeting T and B-cells, respectively, to patients with recent-onset diabetes has shown beneficial effects on preservation of β-cell function [58], [59]. Combined therapies with Rapamycin and IL-2 to suppress T-cell activation and enhance tolerance have also effectively treated diabetes in NOD mice [60], yet clinical studies have yielded conflictive results with transient worsening of β-cell function [61]. Through its immune-modulating, anti-apoptotic and trophic effects on β-cells, local IGF1 gene transfer offers an attractive alternative to the chronic and systemic modulation of the immune system, potentially associated with life-threatening risks.
Finally, an important aspect for the clinical translation of the approach is that the technique used to administer vectors to the pancreas, the retrograde pancreatic intraductal injection, can be scaled to large animals and humans [62] through a nonsurgical procedure. Indeed, endoscopic retrograde cholangiopancreatography (ERCP) [63] is commonly used in the clinical setting to study and treat problems of bile and pancreatic ducts. The existence of an established procedure to reach the human pancreas in a minimally-invasive manner opens the possibility of safe gene transfer to the pancreas of diabetic patients.
Although a considerable number of therapeutic approaches have been developed for T1D, efficacy has been limited by systemic toxicity or limited magnitude and/or duration of therapeutic benefits. Our study demonstrates that AAV-mediated Igf1 gene transfer, in combination with miRNA technology, may be an effective way to protect β-cells from the immune attack in vivo, and highlights the therapeutic potential of this approach to treat autoimmune diabetes in humans.
Author contributions
C.M. and F.B. designed experiments, wrote and edited the manuscript. C.M, E.C, V.J., A.C., C.J., V.S., M.M, J.A., and L.V. generated reagents and performed experiments. V.H., V.J., and A.C. analyzed data, contributed to discussion, and reviewed/edited manuscript.
Acknowledgements
This work was supported by grants from Ministerio de Economía y Competitividad (MINECO), Plan Nacional I+D+I (SAF2011–24698 and SAF2014-54866R) and Generalitat de Catalunya (2014 SGR 1669 and ICREA Academia award 2012 to F.B.), Spain, and from Juvenile Diabetes Research Foundation (2-SRA-2015-59-Q-R), USA. C.M., E.C., and V.S. received predoctoral fellowships from Ministerio de Educación, Cultura y Deporte, Spain. L.V. was recipient of a post-doctoral fellowship from Ministerio de Ciencia e Innovación, Spain. The authors thank Marta Moya, Maria Molas, and Xavier León for technical assistance.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.05.007.
Conflict of interest
The authors have declared that no conflict of interest exists.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Figure 1Number, size, and architecture of islets in NOD-IGF1 mice. (A) Distribution of islets according to their size in 4-week-old NOD and NOD-IGF1 mice (n = 6/group). Histograms depict the % of islets corresponding to each size category, and demonstrate that NOD-IGF1 islets had the same size as non-transgenic NOD islets. (B) Double immunostaining for glucagon (red) and insulin (green) in 4-week-old NOD and NOD-IGF1 mice showing normal islet architecture (n = 4). Original magnification ×400 (scale bar: 50 μm). Results are expressed as mean ± SEM.
Supplemental Figure 2Expression of key regulators of the β-cell cycle. Analysis of gene expression in islets isolated from NOD and NOD-IGF1 mice at 8 weeks of age (n = 9–10/group). Relative expression of molecules that participate in β-cell replication: (A) Cdk-4: cyclin-dependent kinase 4, (B)Cyclin D1, (C)Cyclin D, (D)p27: cyclin kinase inhibitor p27, and (E)p21: cyclin kinase inhibitor p21. Results are expressed as mean ± SEM. *P < 0.05 vs. NOD.
Supplemental Figure 3Expression of MHC molecules in islets at 8 weeks of age. (A) Representative images of the immunohistochemical detection of insulin (brown) in pancreas from 8-week-old NOD and NOD-IGF1 mice (n = 6–7/group). Original magnification ×20 (scale bar: 500 μm), insets ×200 (scale bar: 50 μm). (B) Insulitis score in the same cohorts as in A, showing a low degree of infiltration at this age. (C) Relative expression of the molecules that participate in antigen presentation β2-microglobulin (β2-m) and histocompatibility 2, class II antigen A (H2-Aa) in islets isolated from 8-week-old NOD and NOD-IGF1 mice (n = 5–6 animals/group). (D) Relative gene expression of insulin (Ins1) and Cd45 in pancreatic islets isolated from 8-week-old NOD and NOD-IGF1 mice and cultured for 40 h to allow spontaneous extrusion of leucocytes as indicated in Material and Methods. Histograms depict the ratio of qPCR data obtained for each gene in islets over the value obtained in cells pelleted from the culture media, demonstrating efficient depletion of islet leukocytes. (E) Relative expression of β2-m and H2-Aa in cultured, immune cell-depleted islets from NOD and NOD-IGF1 mice. Results are expressed as mean of 6 NOD and 10 NOD-IGF1 mice ± SEM. *P < 0.05 vs. NOD.
Supplemental Figure 4Absence of miR dysregulation following intraductal administration of AAV8 vectors bearing miRT sequences. (A–B) Vector genome quantification in liver (A) and heart (B) 1 month after intraductal administration of 1012 vg of AAV8-NULL, AAV8-IGF1, or AAV8-IGF1-dmiRT vectors to NOD mice (n = 4–6/group). (C–D) Relative expression of miR-122a in liver (C) and miR-1 in heart (D) of 28-week-old NOD mice treated with AAV8-NULL (n = 5) or AAV8-IGF1-dmiRT (n = 7) at 4 weeks of age. (E) Relative expression of miR-122a-regulated genes in the liver of 28-week-old NOD mice treated with AAV8-NULL (n = 5) or AAV8-IGF1-dmiRT (n = 7) at 4 weeks of age. Gys1, glycogen synthase 1; AldoA, aldolase A/fructose-biphosphate; Slc7a1, solute carrier family 7; Ccng1, cyclin G1. (F) Fed serum cholesterol levels in 28-week-old NOD mice treated with AAV8-NULL or AAV8-IGF1-dmiRT at 4 weeks of age (n = 9–10/group). Results are expressed as mean ± SEM.
Supplemental Figure 5Islet morphology at the age of treatment with AAV vectors. (A) Immunohistochemical detection of insulin (brown) in islets of NOD mice at 4 weeks of age (pre-treatment) (n = 6). Original magnification ×20 (scale bar: 500 μm), insets ×400 (scale bar: 50 μm). (B) Immunohistochemical detection of insulin (brown) in islets of NOD mice at 11 weeks of age (n = 4). Original magnification ×20 (scale bar: 500 μm), insets ×200 (scale bar: 50 μm). Red arrowheads indicate insulitis.
Supplemental Figure 6IGF1 expression in β-cells from NOD mice injected with AAV vectors. Double immunostaining for IGF1 (green) and insulin (red) in pancreas from 28-week-old mice treated at 4 weeks or 11 weeks of age with either AAV8-NULL or AAV8-IGF1-dmiRT (n = 3/group). Original magnification ×400 (scale bar: 50 μm).
Supplemental Figure 7Distribution of endocrine cells in NOD mice injected with AAV vectors. Double immunostaining for glucagon (red) and insulin (green) in 28-week-old NOD mice treated at 4 weeks or 11 weeks with either AAV8-NULL or AAV8-IGF1-dmiRT (n = 3/group). Original magnification ×400 (scale bar: 50 μm).
Supplemental Figure 8Assessment of β-cell replication. Double immunostaining for insulin (red) and Ki67 (green) in 15-week-old NOD mice treated at 11 weeks with either AAV8-NULL or AAV8-IGF1-dmiRT (n = 4/group). Blue, nuclei. Original magnification ×400 (scale bar: 50 μm). The histogram below depicts the quantification of the % of insulin positive β-cells that are Ki67 positive in the same cohort of mice. Results are expressed as mean ± SEM.
List of the antibodies used for immunohistochemistry.
List of primer sequences used for RT-PCR.
References
- 1.O'Sullivan E.S., Vegas A., Anderson D.G., Weir G.C. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocrine Reviews. 2011;32(6):827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill D.J., Hogg J. Expression of insulin-like growth factors (IGFs) and their binding proteins (IGF BPs) during pancreatic development in rat, and modulation of IGF actions on rat islet DNA synthesis by IGF BPs. Advances in Experimental Medicine and Biology. 1992;321:113–120. doi: 10.1007/978-1-4615-3448-8_12. 2. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor J.C., McCusker R.H., Strle K., Johnson R.W., Dantzer R., Kelley K.W. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cellular Immunology. 2008;252(1–2):91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith T.J. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacological Reviews. 2010;62(2):199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaino Y., Hirai H., Ito T., Kida K. Insulin-like growth factor I (IGF-I) delays the onset of diabetes in non-obese diabetic (NOD) mice. Diabetes Research and Clinical Practice. 1996;34(1):7–11. doi: 10.1016/s0168-8227(96)01326-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen W., Salojin K.V., Mi Q.-S., Grattan M., Meagher T.C., Zucker P. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology. 2004;145(2):627–638. doi: 10.1210/en.2003-1274. [DOI] [PubMed] [Google Scholar]
- 7.Bilbao D., Luciani L., Johannesson B., Piszczek A., Rosenthal N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Molecular Medicine. 2014;6(11):1423–1435. doi: 10.15252/emmm.201303376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anguela X.M., Tafuro S., Roca C., Callejas D., Agudo J., Obach M. Nonviral-mediated hepatic expression of IGF-I increases Treg levels and suppresses autoimmune diabetes in mice. Diabetes. 2013;62(2):551–560. doi: 10.2337/db11-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheetham T.D., Holly J.M., Clayton K., Cwyfan-Hughes S., Dunger D.B. The effects of repeated daily recombinant human insulin-like growth factor I administration in adolescents with type 1 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association. 1995;12(10):885–892. doi: 10.1111/j.1464-5491.1995.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 10.Acerini C.L., Patton C.M., Savage M.O., Kernell A., Westphal O., Dunger D.B. Randomised placebo-controlled trial of human recombinant insulin-like growth factor I plus intensive insulin therapy in adolescents with insulin-dependent diabetes mellitus. Lancet (London, England) 1997;350(9086):1199–1204. doi: 10.1016/S0140-6736(97)06467-2. [DOI] [PubMed] [Google Scholar]
- 11.Carroll P.V., Umpleby M., Alexander E.L., Egel V.A., Callison K.V., Sönksen P.H. Recombinant human insulin-like growth factor-I (rhIGF-I) therapy in adults with type 1 diabetes mellitus: effects on IGFs, IGF-binding proteins, glucose levels and insulin treatment. Clinical Endocrinology. 1998;49(6):739–746. doi: 10.1046/j.1365-2265.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Clemmons D.R., Moses A.C., McKay M.J., Sommer A., Rosen D.M., Ruckle J. The combination of insulin-like growth factor I and insulin-like growth factor-binding protein-3 reduces insulin requirements in insulin-dependent type 1 diabetes: evidence for in vivo biological activity. The Journal of Clinical Endocrinology and Metabolism. 2000;85(4):1518–1524. doi: 10.1210/jcem.85.4.6559. [DOI] [PubMed] [Google Scholar]
- 13.Schalch D.S., Turman N.J., Marcsisin V.S., Heffernan M., Guler H.P. Short-term effects of recombinant human insulin-like growth factor I on metabolic control of patients with type II diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism. 1993;77(6):1563–1568. doi: 10.1210/jcem.77.6.8263142. [DOI] [PubMed] [Google Scholar]
- 14.Moses A.C., Young S.C., Morrow L.A., O'Brien M., Clemmons D.R. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45(1):91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 15.George M., Ayuso E., Casellas A., Costa C., Devedjian J.C., Bosch F. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. The Journal of Clinical Investigation. 2002;109(9):1153–1163. doi: 10.1172/JCI12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agudo J., Ayuso E., Jimenez V., Salavert A., Casellas A., Tafuro S. IGF-I mediates regeneration of endocrine pancreas by increasing beta cell replication through cell cycle protein modulation in mice. Diabetologia. 2008;51(10):1862–1872. doi: 10.1007/s00125-008-1087-8. [DOI] [PubMed] [Google Scholar]
- 17.Casellas A., Salavert A., Agudo J., Ayuso E., Jimenez V., Moya M. Expression of IGF-I in pancreatic islets prevents lymphocytic infiltration and protects mice from type 1 diabetes. Diabetes. 2006;55(12):3246–3255. doi: 10.2337/db06-0328. [DOI] [PubMed] [Google Scholar]
- 18.Mingozzi F., High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nature Reviews Genetics. 2011;12(5):341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 19.Grieger J.C., Samulski R.J. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods in Enzymology. 2012;507:229–254. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez V., Ayuso E., Mallol C., Agudo J., Casellas A., Obach M. In vivo genetic engineering of murine pancreatic beta cells mediated by single-stranded adeno-associated viral vectors of serotypes 6, 8 and 9. Diabetologia. 2011;54(5):1075–1086. doi: 10.1007/s00125-011-2070-3. [DOI] [PubMed] [Google Scholar]
- 21.Loiler S.A., Tang Q., Clarke T., Campbell-Thompson M.L., Chiodo V., Hauswirth W. Localized gene expression following administration of adeno-associated viral vectors via pancreatic ducts. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2005;12(3):519–527. doi: 10.1016/j.ymthe.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55(4):875–884. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H., Wolfe S.H., Valencia V., Qian K., Shen L., Phillips M.I. Efficient and persistent transduction of exocrine and endocrine pancreas by adeno-associated virus type 8. Journal of Biomedical Science. 2007;14(5):585–594. doi: 10.1007/s11373-007-9159-1. [DOI] [PubMed] [Google Scholar]
- 24.Agudo J., Ayuso E., Jimenez V., Casellas A., Mallol C., Salavert A. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of β-cell mass. Diabetes. 2012;61(11):2851–2861. doi: 10.2337/db12-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacovetti C., Jimenez V., Ayuso E., Laybutt R., Peyot M.-L., Prentki M. Contribution of intronic miR-338-3p and its hosting gene AATK to compensatory β-cell mass expansion. Molecular Endocrinology (Baltimore, Md.) 2015;29(5):693–702. doi: 10.1210/me.2014-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown B.D., Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nature Reviews Genetics. 2009;10(8):578–585. doi: 10.1038/nrg2628. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez V., Muñoz S., Casana E., Mallol C., Elias I., Jambrina C. In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice. Diabetes. 2013;62(12):4012–4022. doi: 10.2337/db13-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayasimhan A., Mansour K.P., Slattery R.M. Advances in our understanding of the pathophysiology of Type 1 diabetes: lessons from the NOD mouse. Clinical Science (London, England: 1979) 2014;126(1):1–18. doi: 10.1042/CS20120627. [DOI] [PubMed] [Google Scholar]
- 29.Ayuso E., Mingozzi F., Bosch F. Production, purification and characterization of adeno-associated vectors. Current Gene Therapy. 2010;10(6):423–436. doi: 10.2174/156652310793797685. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu Experimental Animals. 1980;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 32.Cozar-Castellano I., Weinstock M., Haught M., Velázquez-Garcia S., Sipula D., Stewart A.F. Evaluation of beta-cell replication in mice transgenic for hepatocyte growth factor and placental lactogen: comprehensive characterization of the G1/S regulatory proteins reveals unique involvement of p21cip. Diabetes. 2006;55(1):70–77. [PubMed] [Google Scholar]
- 33.Anderson M.S., Bluestone J.A. The NOD mouse: a model of immune dysregulation. Annual Review of Immunology. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 34.Strandell E., Eizirik D.L., Sandler S. Reversal of beta-cell suppression in vitro in pancreatic islets isolated from nonobese diabetic mice during the phase preceding insulin-dependent diabetes mellitus. Journal of Clinical Investigation. 1990;85(6):1944–1950. doi: 10.1172/JCI114657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faideau B., Larger E., Lepault F., Carel J.C., Boitard C. Role of beta-cells in type 1 diabetes pathogenesis. Diabetes. 2005;54(Suppl. 2):S87–S96. doi: 10.2337/diabetes.54.suppl_2.s87. [DOI] [PubMed] [Google Scholar]
- 36.Jarchum I., Takaki T., DiLorenzo T.P. Efficient culture of CD8(+) T cells from the islets of NOD mice and their use for the study of autoreactive specificities. Journal of Immunological Methods. 2008;339(1):66–73. doi: 10.1016/j.jim.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roggli E., Gattesco S., Caille D., Briet C., Boitard C., Meda P. Changes in microRNA expression contribute to pancreatic-cell dysfunction in prediabetic NOD mice. Diabetes. 2012;61(7):1742–1751. doi: 10.2337/db11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai W.-C., Hsu S.-D., Hsu C.-S., Lai T.-C., Chen S.-J., Shen R. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. The Journal of Clinical Investigation. 2012;122(8):2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suarez-Pinzon W.L., Power R.F., Yan Y., Wasserfall C., Atkinson M., Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57(12):3281–3288. doi: 10.2337/db08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreenan S., Pick A.J., Levisetti M., Baldwin A.C., Pugh W., Polonsky K.S. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48(5):989–996. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 41.Finegood D.T., Scaglia L., Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44(3):249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 42.Jabri N., Schalch D.S., Schwartz S.L., Fischer J.S., Kipnes M.S., Radnik B.J. Adverse effects of recombinant human insulin-like growth factor I in obese insulin-resistant type II diabetic patients. Diabetes. 1994;43(3):369–374. doi: 10.2337/diab.43.3.369. [DOI] [PubMed] [Google Scholar]
- 43.Büning H. Gene therapy enters the pharma market: the short story of a long journey. EMBO Molecular Medicine. 2013;5(1):1–3. doi: 10.1002/emmm.201202291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geisler A., Jungmann A., Kurreck J., Poller W., Katus H.A., Vetter R. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Therapy. 2011;18(2):199–209. doi: 10.1038/gt.2010.141. [DOI] [PubMed] [Google Scholar]
- 45.Esau C., Davis S., Murray S.F., Yu X.X., Pandey S.K., Pear M. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metabolism. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine & Growth Factor Reviews. 2006;17(4):305–323. doi: 10.1016/j.cytogfr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Lingohr M.K., Dickson L.M., McCuaig J.F., Hugl S.R., Twardzik D.R., Rhodes C.J. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes. 2002;51(4):966–976. doi: 10.2337/diabetes.51.4.966. [DOI] [PubMed] [Google Scholar]
- 48.Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. Journal of Immunology (Baltimore, Md.: 1950) 1995;154(1):180–191. [PubMed] [Google Scholar]
- 49.Bradley L.M., Asensio V.C., Schioetz L.K., Harbertson J., Krahl T., Patstone G. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. Journal of Immunology (Baltimore, Md.: 1950) 1999;162(5):2511–2520. [PubMed] [Google Scholar]
- 50.Cameron M.J., Arreaza G.A., Grattan M., Meagher C., Sharif S., Burdick M.D. Differential expression of CC chemokines and the CCR5 receptor in the pancreas is associated with progression to type I diabetes. Journal of Immunology (Baltimore, Md.: 1950) 2000;165(2):1102–1110. doi: 10.4049/jimmunol.165.2.1102. [DOI] [PubMed] [Google Scholar]
- 51.Foulis A.K., Farquharson M.A. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986;35(11):1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- 52.Guerder S., Meyerhoff J., Flavell R. The role of the T cell costimulator B7-1 in autoimmunity and the induction and maintenance of tolerance to peripheral antigen. Immunity. 1994;1(2):155–166. doi: 10.1016/1074-7613(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton-Williams E.E., Serreze D.V., Charlton B., Johnson E.A., Marron M.P., Mullbacher A. Transgenic rescue implicates beta2-microglobulin as a diabetes susceptibility gene in nonobese diabetic (NOD) mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11533–11538. doi: 10.1073/pnas.191383798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ly A., Bouchaud C., Henin D., Sanson M., Delattre J.Y., Pan Y. Expression of insulin-like growth factor-I in rat glioma cells is associated with change in both immunogenicity and apoptosis. Neuroscience Letters. 2000;281(1):13–16. doi: 10.1016/s0304-3940(00)00758-8. [DOI] [PubMed] [Google Scholar]
- 55.Upegui-Gonzalez L.C., Duc H.T., Buisson Y., Arborio M., Lafarge-Frayssinet C., Jasmin C. Use of the IGF-I antisense strategy in the treatment of the hepatocarcinoma. Advances in Experimental Medicine and Biology. 1998;451:35–42. doi: 10.1007/978-1-4615-5357-1_6. [DOI] [PubMed] [Google Scholar]
- 56.Signore A., Cooke A., Pozzilli P., Butcher G., Simpson E., Beverley P.C. Class-II and IL2 receptor positive cells in the pancreas of NOD mice. Diabetologia. 1987;30(11):902–905. doi: 10.1007/BF00274802. [DOI] [PubMed] [Google Scholar]
- 57.Johannesson B., Sattler S., Semenova E., Pastore S., Kennedy-Lydon T.M., Sampson R.D. Insulin-like growth factor-1 induces regulatory T cell-mediated suppression of allergic contact dermatitis in mice. Disease Models & Mechanisms. 2014;7(8):977–985. doi: 10.1242/dmm.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pescovitz M.D., Greenbaum C.J., Bundy B., Becker D.J., Gitelman S.E., Goland R. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37(2):453–459. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagopian W., Ferry R.J., Sherry N., Carlin D., Bonvini E., Johnson S. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62(11):3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grinberg-Bleyer Y., Baeyens A., You S., Elhage R., Fourcade G., Gregoire S. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. The Journal of Experimental Medicine. 2010;207(9):1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Long S.A., Rieck M., Sanda S., Bollyky J.B., Samuels P.L., Goland R. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61(9):2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayuso E., Chillón M., García F., Agudo J., Andaluz A., Carretero A. In vivo gene transfer to healthy and diabetic canine pancreas. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2006;13(4):747–755. doi: 10.1016/j.ymthe.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Hendrick L.M., Harewood G.C., Patchett S.E., Murray F.E. Utilization of resource leveling to optimize ERCP efficiency. Irish Journal of Medical Science. 2011;180(1):143–148. doi: 10.1007/s11845-010-0570-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the antibodies used for immunohistochemistry.
List of primer sequences used for RT-PCR.