Abstract
The interactions between the tumor suppressor protein p21WAF1 and the cyclin-dependent kinase (CDK) complexes and with proliferating cell nuclear antigen (PCNA) regulate and coordinate the processes of cell-cycle progression and DNA replication. We present the x-ray crystal structure of PCNA complexed with a 16-mer peptide related to p21 that binds with a Kd of 100 nM. Two additional crystal structures of native PCNA provide previously undescribed structures of uncomplexed human PCNA and show that significant changes on ligand binding include rigidification of a number of flexible regions on the surface of PCNA. In the competitive binding experiments described here, we show that a 20-mer sequence from p21 can be associated simultaneously with PCNA and CDK/cyclin complexes. A structural model for this quaternary complex is presented in which the C-terminal sequence of p21 acts like double-sided tape and docks to both the PCNA and cyclin molecules. The quaternary complex shows little direct interaction between PCNA and cyclin, giving p21 the role of an adaptor molecule. Taken together, the biochemical and structural results delineate a druggable inhibitor site on the surface of PCNA that may be exploited in the design of peptidomimetics, which will act independently of cyclin-groove inhibitors.
Keywords: p21, x-ray, structure, protein, interaction
Proliferating cell nuclear antigen (PCNA) is an essential auxiliary protein for the processes of both DNA replication and repair. It stimulates the activity of DNA polymerase δ and increases its processivity by acting as a clamp platform that slides along the DNA template (1–3). Apart from polymerase δ, PCNA associates with a host of other proteins, either involved directly in DNA replication and repair or in the regulation of these processes (4). The presence of a common PCNA-binding motif in such proteins suggests that regulation may depend largely on PCNA partner proteins competing with one another for access to PCNA.
Deregulation of PCNA expression is a hallmark of many proliferative diseases, and in the clinic PCNA serves as a general proliferative marker, especially in the prognosis of tumor development (5). In fact, PCNA expression levels are directly related to the malignancy of various tumors, and antisense oligonucleotide-mediated suppression of PCNA expression was demonstrated to selectively inhibit gastric cancer cell proliferation in vitro and in vivo (6). Antisense strategies targeting PCNA mRNA also have shown promise in models of other proliferative diseases, including glomerular nephritis (7) and rheumatoid arthritis (8). The fact that PCNA is required absolutely for cell proliferation indicates that pharmacological modulation of PCNA function should not be able to be circumvented by compensatory pathways. Furthermore, the ablation of PCNA expression or function in cells under proliferative stimuli appears to constitute an apoptotic trigger (5), suggesting that effective elimination of hyperproliferative cells should be possible in a therapeutic setting.
The tumor suppressor protein p21 modulates cell-cycle progression by direct binding to PCNA and also by inhibiting cyclin-dependent kinases (CDKs) (9). The CDK-binding site in its N-terminal region (residues 53–58) is distinct from two cyclin-binding sites located in the N- and C-terminal regions, respectively (10, 11). The PCNA-binding motif present in the C terminus of p21 has been characterized extensively (12, 13) and is conserved in many other PCNA protein partners (4). At the cellular level the competition between p21 and DNA replication factors for binding to PCNA is believed to be the mechanism through which DNA synthesis is inhibited. It is known that in cells PCNA and p21 can participate in quaternary complexes with CDK/cyclin pairs, particularly CDK4/cyclin D1 (14, 15), probably contributing to the coordination of cell-cycle progression and DNA replication (16). Synthetic peptides derived from the C terminus of p21 are capable of arresting and killing cancer cells (17–20). Interestingly, the C terminus of p21(141–160) (Fig. 1) harbors overlapping recognition sites for both cyclins and PCNA (21–23). Truncation of p21(141–160) peptides from either terminus was not tolerated without a serious diminution of the binding affinity for PCNA. However, based on the sequences of two particular PCNA binding proteins, the Pogo DNA transposase and DNA ligase I (Fig. 1), we were able to design a number of peptides (23), one of which was found to bind to PCNA with an affinity very similar to that of the p21(141–160) peptide. This peptide, which we designate here as Pogo-Ligase (PL) peptide, also was an efficient inhibitor of PCNA-dependent DNA replication in vitro.
Fig. 1.
Design of the PL hybrid peptide [previously designated as CM1 (23)]. The N-terminal 12 amino acids (bold) are from Pogo, and the remaining 4 (italic) are from DNA ligase (DNA-l). The red box highlights the residues binding in the PCNA binding pocket. The cyan box highlights the region of p21 that acts like double-sided tape, sticking to the linker strand of PCNA and the peptide binding groove on cyclin.
In this study, we provide results that suggest the formation of a quaternary complex between PCNA, p21, and CDK/cyclin in which a 20-mer peptide is sufficient to mimic the assembly role of full-length p21. We also present a structure of PCNA complexed with the PL peptide as well as previously undescribed x-ray structures of unliganded human PCNA and discuss how these can be used for the design of inhibitors of PCNA-dependent DNA replication.
Materials and Methods
For further experimental details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Protein Production. PCNA. Recombinant human PCNA was expressed in Escherichia coli BL21(DE3) from a pT7-PCNA expression vector. The protein was purified as described in ref. 23.
CDK4. The N-terminally His-6-tagged human recombinant protein was expressed in Sf9 insect cells by using a baculovirus construct. GST-pRb(773–928). The hyperphosphorylation domain of retinoblastoma protein (pRb; residues 773–982) with a N-terminal GST tag was expressed in E. coli BL21(DE3) and was purified on a glutathione-Sepharose column (Amersham Pharmacia). For the 96-well format in vitro kinase assay, GST-pRb was used immobilized on glutathione-Sepharose beads.
CDK4/cyclin D1 kinase assay. The reaction mixture consisted of 1 μM CDK4 and cyclin D1, 5 μg of GST-pRb, 100 μM ATP, and 0.2 μCi of [32P]ATP (1 Ci = 37 GBq). The kinase reaction was carried out for 10 min at 30°C.
Peptides were assembled by using standard solid-phase chemistry based on the fluorenylmethoxycarbonyl protecting group (24).
X-Ray Crystallography. PCNA monoclinic form. Crystals of PCNA were grown by the hanging-drop vapor-diffusion method. A 2-μl solution of PCNA (8–10 mg/ml) in a buffer consisting of 25 mM Tris and 2 mM DTT (pH 7.5) was added to a 2-μl well solution comprising 20% polyethylene glycol 3,350 and 0.2 M magnesium acetate. Crystals grew after 7–10 days at 18°C. A crystal of ≈0.05 mm in length was collected in a 0.05- to 0.1-mm Cryo-loop (Hampton Research, Riverside, CA), dipped briefly in immersion oil (Type B, Cargille, Cedar Grove, NJ), and frozen by plunging into liquid nitrogen. Diffraction data were collected on a charge-coupled device detector by using the synchrotron source in Daresbury station 9.6 (Table 1). X-ray data were processed by using the program denzo (25). Molecular replacement was carried out by using molrep (26) and refined by using the program refmac (27). PCNA trigonal form. The crystal of the trigonal form of PCNA was grown by the vapor-diffusion method. One microliter of PCNA complexed with P21 [6 mg/ml in PCNA storage buffer (25 mM Tris·HCl, pH 7.5/1 mM EDTA/0.01% Nonidet P-40/10% glycerol/2 mM benzamidine/1 mM PMSF/1 mM DTT/25 mM NaCl)] was added to 1 μl of well solution containing 30–40% polyethylene glycol–monomethyl ether 2,000, 0.1 M Na acetate (pH 4.6), and 0.2 M ammonium sulfate. Crystals grew after 3–5 days at 18°C.
Table 1. X-ray data and refinement statistics.
| hPCNA monoclinic | hPCNA trigonal | hPCNA_PL | |
|---|---|---|---|
| Space group | C2 | P3 | P3121 |
| a, Å | 136.6 | 82.89 | 119.1 |
| b, Å | 83.26 | 82.89 | 119.1 |
| c, Å | 71.63 | 70.86 | 305.82 |
| β(0) | 117.49 | 120 | 120 |
| Z, monomers per unit cell | 12 | 6 | 6 |
| Resolution range, Å | 35-2.3 | 35-3.1 | 30-2.78 |
| Synchrotron/station* | SRS/9.6 | SRS/14.1 | DESY/BW7B |
| λ, Å | 0.870 | 1.488 | 0.830 |
| No. of reflections measured | 220,606 | 9,268 | 241,549 |
| Unique reflections | 31,758 | 6,026 | 64,087 |
| Completeness,† % | 99.4 | 98.0 (98.4) | 99.6 (99.7) |
| Rmerge‡ (outer shell), % | 5.8 (45.7) | 8.8 (28) | 14 (67) |
| I/σ (I),§ (outer shell) | 15.5 (2.7) | 12.9 (4.8) | 2.8 (1.0) |
| Multiplicity | 6.95 | 1.5 | 3.8 |
| R factor | 18.7 | 18.3 | 17.9 |
| Rfree | 27.9 | 27.3 | 25.8 |
| % data used to calculate | 4.1 | 4.8 | 3.1 |
| Rfree | |||
| No. of atoms | |||
| Protein | 5,889 | 3,926 | 11,919 |
| Peptide | — | — | 810 |
| Solvent | 148 | 58 | 322 |
| rms bonds, Å | 0.014 | 0.012 | 0.015 |
| rms angles, ° | 1.68 | 1.47 | 2.04 |
| Average B factors, Å2 | |||
| Protein | 51.2 | 50.4 | 57.4 |
| Peptide | — | — | 74.5 |
| Solvent | 51.0 | 46.8 | 53.4 |
SRS, Daresbury Synchrotron Radiation Source; DESY, Deutches Elektronen Synchrotron.
The numbers in parentheses are statistics for the highest resolution shell.
Rmerge = Σh| I - 〈I〉|/Σh| I|, where 〈I〉 is the mean intensity of all observation of reflection h = hkl.
σ (I) is the SD of the measured intensity.
PCNA–PL peptide complex. Crystals were grown by the hanging-drop method. A 6-μl solution of PCNA (6–8 mg/ml) and 0.4 mM PL in a buffer consisting of 5 mM Hepes (pH 7.5), 5 mM NaCl, and 1.2% (vol/vol) DMSO was added to a 2-μl well solution comprising 2.7 M ammonium sulfate and Hepes (pH 8.0). Crystals grew after 3–4 days at 18°C.
Results
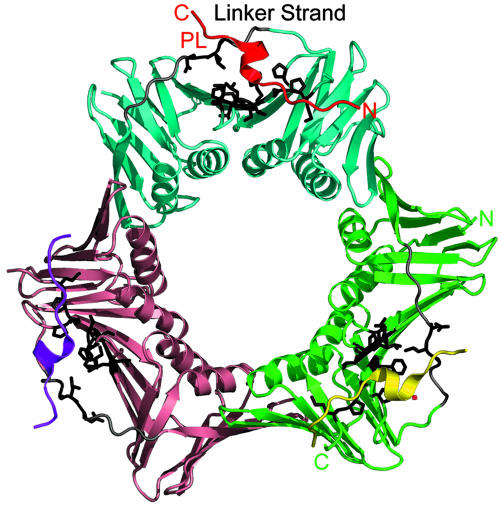
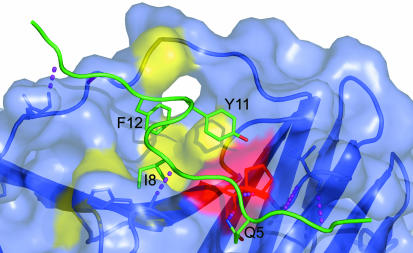
X-Ray Structures of a PL Peptide–PCNA Complex and Two Forms of Unliganded Human PCNA. Details of data collection and refinement for a PL peptide–PCNA complex and two forms of unliganded human PCNA are given in Table 1. Together they provide structural information on 11 crystallographically independent PCNA molecules. The PCNA–PL peptide complex structure (Fig. 2) has two independent trimers in the asymmetric unit with each of the six molecules showing clear electron density for all of the complete 16-mer PL peptide ligands. The PL peptide is held in place by a total of six main-chain to backbone H-bonds, as well as by a further two H-bonds involving side-chain atoms (Fig. 3). The N-terminal residues of the PL peptide form a short stretch of antiparallel sheet with the C-terminal residues of PCNA with the H-bonds L4(N)..I255(O) and L4(O)..I255(N) conserved in most of the six copies of the complex. The C-terminal residues 255–261 are not visible in the unliganded PCNA structures, and the ligand thus must play a role in tying down this disorder. A similar effect was noted in the complex of Archaeoglobus fulgidus PCNA with a 12-mer FEN-1 peptide in which a short β-sheet is formed with the C-terminal PCNA residues providing a putative control mechanism for mismatch repair (28). A prominent feature of the PCNA molecule is the “linker-strand” consisting of residues 121–132, which tethers together the N and C domains (Fig. 2) and which was shown to be important in the recognition of p21 (12). The N terminus of this strand is involved in binding to the PL peptide with the formation of two H-bonds, H13(O)... G127(N) and K15(N)... Q125(O), conserved in all six copies of the complex and that again result in a rigidification of this region of the molecule. The overall binding picture (Fig. 3) shows the N and C termini of the PL peptide in a rather extended conformation forming pairs of H-bonds with the rather mobile C terminus and the linker strand, respectively, whereas the central helical residues of PL peptide fit into a more ordered grooved surface and are pinned in place by only an additional three H-bonds: Q5(NE2)... A252(O), K6(N)..P253(O), and I8(N)... H44(O).
Fig. 2.
Surface features of the PCNA trimer relevant to ligand binding. The three PCNA molecules are shown in cyan, green, and salmon, and the corresponding PL-16-mer peptides are in red, yellow, and purple. PCNA residues involved in binding to the PL peptide are highlighted in black. N and C termini for the red PL peptide and green PCNA monomer are shown.
Fig. 3.
The PCNA binding pocket for PL peptide. A view of the interactions between the PL peptide (green) and PCNA (shown with transparent molecular surface) is shown. The four residues in the PL peptide making direct contact with PCNA are shown with side chains and are labeled. The PCNA binding pocket is delineated by the colored surface; the residues making hydrophobic contacts with I8 and F12 of the PL peptide are shown in yellow, and those involved in polar interactions with Y11 and Q5 are in red. The six intermolecular main-chain H-bonds are shown as purple dashed lines. The conserved intermolecular H-bonds important for recognition of the PL peptide are as follows: L4(N)..I255(O), L4(O)..I255(N), Q5(OE1)..W, Q5(NE2)... A252(O), K6(N)..P253(O), I8(N)... H44(O), H13(O)... G127(N), and K15(N)... Q125(O). There are five conserved intramolecular H-bonds (not shown) as follows: Q5(NE2)..K6(O), K7(O)..D10(N), I8(O)... Y11(N), I8(O)..F12(N), and D10(N)..D10(OD1).
The two native PCNA crystal structures (Table 1) offer the previously undescribed information on the conformations of unliganded human PCNA. These unliganded crystals belong to space groups C2 and P3, and together they contain a total of five crystallographically independent PCNA monomer structures: The C2 form has one trimer in the asymmetric unit, whereas the P3form has two independent (crystallographically exact) trimers in the unit cell. A rms fit of 0.16 Å for the P3 trimer Cα atoms shows that they are essentially identical. The overall fit of the P3 and C2 trimer rings, however, shows a buckling of the C2 trimer compared with the crystallographically constrained planar rings in the P3 structure: A fit of Cα atoms from any one subunit of the C2 structure onto the P3 structure gives a rms fit for the 218 Cα atoms of ≈0.7 Å. However, the average rms fit of Cα atoms of the other two subunits are near 1 Å and 2 Å, respectively. The individual atomic isotropic temperature factors for each of the 11 PCNA monomers show a very similar pattern, which is independent of crystal packing. Regions with high temperature factors correspond to exposed and inherently flexible loops on the PCNA surface (residues 62–66, 92–95, 120–130, 161–165, and 184–189). Ligand binding serves to lower temperature factors and rigidify the linker strand (121–132) and the C-terminal tail (residues 251–261).
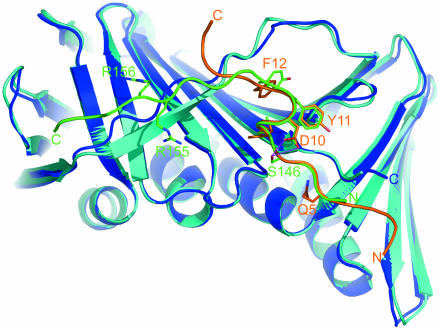
Despite substantial differences in amino acid composition, the N-terminal octapeptide helical sequence of PL peptide and the PCNA-bound 22-mer fragment of p21 (12) show similar conformations and interactions with PCNA (Fig. 4). The conserved glutamine of the QXXhXXaa PCNA-recognition motif (Fig. 1) (4) plays critical roles both in determining peptide conformation by forming the intramolecular H-bond Q5(NE2)... K6(O) and also in recognition and binding of PCNA by forming two intermolecular H-bonds (Fig. 3). The glutamine side chain refines to similar positions in both the p21 complex (12) and PL peptide complex (Fig. 4). At the base of a hydrophobic pocket formed by L251, A252, and F207, one H-bond is formed between the carbonyl oxygen of A252 and the amide nitrogen of the glutamine side chain; a structural water molecule anchors the carbonyl oxygen of the side chain with a second H-bond. Residues I8–T9–D10–Y11 adopt a 310-helical conformation, which is stabilized by three intramolecular H-bonds, K7(O)–D10(N), I8(O)–Y11(N), and I8(O)–F12(N). The conformation of the D10 side chain is determined by a conserved intraresidue D10(OD2)... D10(N) H-bond. There is also a favorable electrostatic interaction with the PL peptide side-chain amine of K6. Although D10 makes no contacts to PCNA, the presence of these intramolecular contacts are nonetheless influential on peptide inhibition as shown by the marked decrease in activity when replaced by alanine or serine (23). This finding suggests that D10 (along with Q5) is important in stabilizing the bound helical conformation of the peptide ligands. The local helical conformation places the side chain of I8 into a deep hydrophobic pocket formed by P234, L126, M40, and H44 (Fig. 3). On the same side of the helix, F12 fits into an extension of the same deeply grooved binding pocket, which is formed by the flexible strand (residues 122–132) wrapping across an antiparallel sheet (principally involving residues 232–236, 249–253, and 45–49).
Fig. 4.
Alignment of the PCNA structures in complex with the p21-derived peptide 139GRKRRQTSMTDFYHSKRRLIFS160 (green) with PCNA (cyan) (Protein Data Bank ID code 1AXC) and the PL peptide 1SAVLQKKITDYFHPKK16 (orange) with PCNA (blue). Key interacting residues are labeled. The phosphorylation site (S146) is highlighted, and the interaction with D149 (D10) is shown (purple).
The conformations of the bound PL and p21 peptide structures diverge significantly at the C terminus of the helix; H13 (H152) adopt different conformations in the two complexes, with the side chains pointing in different directions. The C-terminal eight residues of the p21 peptide fold down onto the extended linker residues (L126–M129), forming a stretch of antiparallel β-sheet. In contrast, the PKK terminal residues of the PL peptide straddle the linker strand with P14, providing a bridge clamped by two main chain H-bonds, H13(O)–G127(N) and K15(N)–Q125(O). The conformation of the linker sequence (residues 119–134) is clearly different in the p21 22-mer and PL 16-mer peptide complexes (Fig. 4).
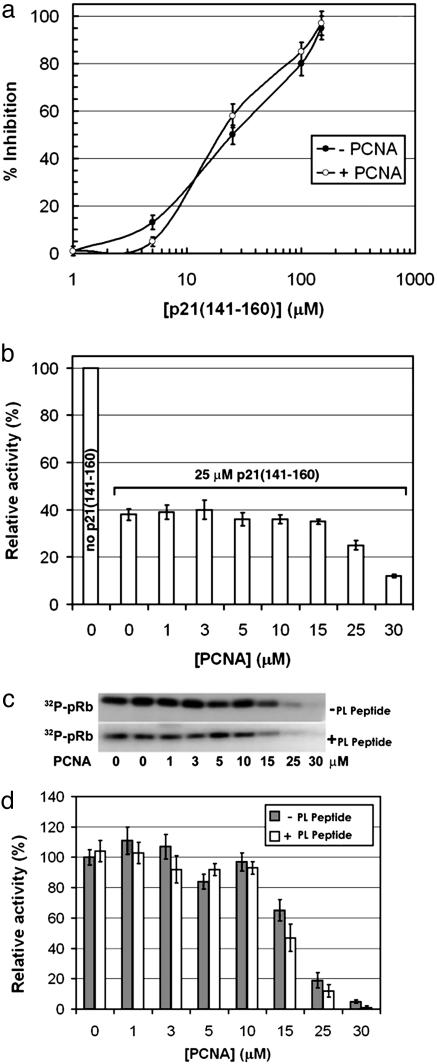
A 20-mer Peptide Mimics the Assembly Role of Full-Length p21. p21 interacts with both cyclin/CDK complex and also with PCNA, and results presented here suggest that a region of p21 can act like double-sided tape to glue cyclin/CDK and PCNA together to form a quaternary complex. We have shown previously that both the PL peptide and a C-terminal p21 peptide (residues 141–160) bind PCNA with nanomolar affinity (23). Furthermore, the PL peptide inhibits in vitro simian virus 40 DNA replication with a potency very similar to that of p21(141–160). However, whereas the PL peptide is devoid of CDK/cyclin inhibitory activity (29), the p21(141–160) peptide inhibits CDK4/cyclin D1 kinase activity with an IC50 of 24 μM (Fig. 5a). We hypothesized that because of competitive binding, the addition of PCNA to kinase assay mixtures should reverse the inhibitory effect of the p21 peptide. Surprisingly, the addition of 10 μM PCNA to kinase assays containing 1 μM CDK4/cyclin D1 did not change significantly the effect of the p21 peptide on pRb phosphorylation (Fig. 5 a and b). In fact, PCNA concentrations up to 15 μM did not diminish the CDK4-inhibitory potency of p21(141–160), although higher concentrations of PCNA did result in decreased pRb phosphorylation (Fig. 5b). The Kd values of binding of p21 peptide to PCNA and CDK4/cyclin are 80 and 24,000 nM, respectively, which means that with the concentrations of PCNA, p21 peptide, and CDK4/cyclin used in the assay, up to 50% of the available p21 peptide should be bound to PCNA. The loss of this bound peptide would give an expected (but unobserved) enhancement in the phosphorylation assay of ≈20%. One explanation for the lack of effect on addition of PCNA in this concentration range is that the p21(141–160) peptide is capable of binding PCNA and the CDK4/cyclin D1 complex simultaneously while maintaining its inhibitory effect on CDK4/cyclin D1.
Fig. 5.
Effect of PCNA and its complexes with p21 (141–160) or PL peptide on CDK4/cyclin D1 kinase activity in vitro. (a) Inhibition of in vitro pRb phosphorylation by CDK4/cyclin D1 in the presence or absence of 10 μM PCNA. The IC50 values were 25 ± 3 μM in the absence (–) and 24 ± 2 μM in the presence (+) of PCNA. (b) PCNA was titrated into the CDK4/cyclin D1 kinase assay reactions in the presence or absence of 25 μM p21(141–160) peptide, and relative activities were determined. (c) CDK4/cyclin D1 kinase activity (autoradiography image of 32P-pRB) in the presence of increasing concentrations of PCNA and 0 or 25 μM PL. (d) The autoradiography images from above scanned and quantified by using quickscan software.
We next studied the direct effect of PCNA on CDK4/cyclin D1 kinase activity. At up to 10 μM PCNA, the kinase activity of 1 μM CDK4/cyclin D1 was undiminished (Fig. 5 c and d). At higher concentrations kinase activity was affected, however, and at 30 μM PCNA the phosphorylation of pRb was abolished almost completely. The IC50 value determined for the inhibition of CDK4/cyclin D1 kinase activity by PCNA was 18 ± 2.4 μM. A possible explanation for the PCNA-induced inhibition of CDK4/cyclin D1 kinase activity is direct binding of PCNA to cyclin D1, leading to disruption of the CDK4/cyclin D1 complex when PCNA is present in excess. It has been demonstrated that PCNA forms complexes with D-type cyclins in vitro. Analysis of a set of deletion mutants of PCNA revealed that either the N (residues 2–64) or the C (residues 197–228) terminus is necessary for the direct association of PCNA with D-type cyclins, i.e., sites that are distinct from the p21-binding site of PCNA (30). However, it is still controversial whether trimeric PCNA does actually bind to cyclin D in vivo and, even if it did, how an increase of free cyclin D would lead to growth arrest (31). The in vitro inhibitory effect of high concentrations of PCNA on CDK4/cyclin D1 kinase activity is in any case unlikely to be relevant in vivo because the cellular levels of PCNA are comparatively lower (≈5 μM). These results suggest that the increase of the p21(141–160)-induced kinase inhibition in the presence of high concentrations of PCNA is due to direct effects of PCNA rather than a modulation of the peptide inhibitory activity (Fig. 5b). In the presence of 25 μM PL peptide, which binds to PCNA at the same site as p21(141–160) but does not directly inhibit CDK function, the inhibition of CDK4/cyclin D1 function by PCNA was practically unchanged (IC50 = 15 ± 1.8 μM) (Fig. 5 c and d). This finding suggests that there is a second, lower-affinity cyclin D1 binding site that is distinct from the p21-dependent site.
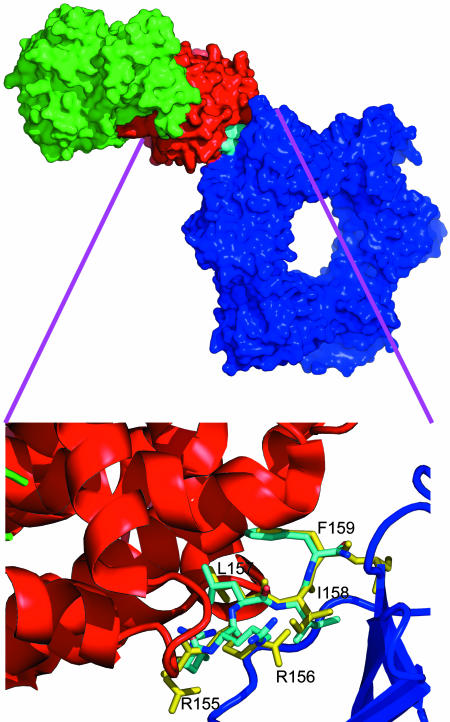
Model for Quaternary PCNA/p21/Cyclin/CDK Complexes. X-ray crystal structures of CDK/cyclin complexes with inhibitory peptides bound in the so-called cyclin binding groove have been described (21). The cyclin binding motif present in substrates and other protein partners of A-, D-, and E-type cyclin/CDK complexes has been defined as the sequence ZRXLYY′, where Z and X are basic residues, and Y and Y′ are hydrophobic (32). The minimal sequence in p21 that binds cyclins is 155RRLIF159. Intriguingly, this pentapeptide corresponds to the C-terminal extension in the PCNA/p21 fragment complex that forms an exposed antiparallel sheet with a section of the interdomain linker (PCNA residues 122–132). As discussed above, the PL peptide does not inhibit CDK function, whereas the p21 peptide with the additional 5-aa cyclinbinding motif sequence is an effective inhibitor. It is also striking that the conformation of the RRLIF sequence in the PCNA x-ray structure (12) is very similar to that found in our CDK2/cyclin A/RRLIF peptide complex (21). Furthermore, the side chains protrude in a way that allows the p21 peptide to act like double-sided sticky tape, with one face forming contacts with PCNA and the other face forming complementary contacts with the cyclin groove. It was possible to simply combine the two available x-ray structures and overlay the backbone of the RRLIF structure to give the large quaternary complex of PCNA/p21/CDK2/cyclin A shown in Fig. 6.
Fig. 6.
Quaternary complex of CDK (green), cyclin (red), and PCNA (blue). The model was produced by fitting together the “RRLIF sequences” from the two crystal complexes [PCNA/p21 (Protein Data Bank ID code 1AXC) with the labeled peptide in yellow] and CDK/cyclin/RRLIF (Protein Data Bank ID code 1OKV) with the peptide colored cyan. The exploded view shows that the RRLIF conformations in the cyclin A- and PCNA-bound structures are very similar.
The rms fit of backbone atoms in the RRLIF peptide for this docked structure is <0.5 Å. Surprisingly, even without further modeling or refinement and despite the tight complementary fit between RRLIF and the two proteins, the docked configuration only introduces <10 direct nonbonded contacts <3.5 Å between PCNA and cyclin, and most of theses involve the side chains D283 and I213 on cyclin A interacting with side chains from N95, D120, D122, and Q125 on PCNA. Restrained molecular mechanics keeping all main-chain atoms fixed was used to optimize side chains contacts to give a model with no unacceptably short contacts. By using this model, the calculated buried surface areas for cyclin (in the context of RRLIF and PCNA) and PCNA (in the context of RRLIF and cyclin) are 440 and 340 Å2, respectively, giving a total buried surface of 780 Å2. The total buried surface calculated by using a similar model of cyclin docked onto PCNA but without the RRLIF peptide present is only 270 Å.2
Discussion
Definition of a Compact Binding Pocket in PCNA. In our initial binding experiments, we were unable to truncate either terminus of the p21(141–160) peptide without significant loss of affinity (23), and it was only the use of different amino acid sequences less related to p21 that led to shorter peptides with appreciable binding affinity to PCNA. The PL peptide structure presented here shows that the contacts formed by the nine C-terminal peptide residues 152HSKRRLIFS160 in the p21 peptide/PCNA complex (12) with the shortand long-range electrostatic interactions involving 155RR156 and 140RKRR143 at the C and N termini of the peptide do not seem to be a requirement for tight binding. The solvent-accessible surface on PCNA that is buried when a complex is formed with p21(139–160) has an area of 960 Å2. This characteristic compares with a buried surface area of 680 Å2 on complex formation with PL peptide. The x-ray structures show that the p21(139–160) complex forms ≈15 direct H-bonds with PCNA. This result compares with only 8 direct H-bonds between the PL peptide and PCNA It is surprising, therefore, that the PL peptide has an affinity for PCNA (Kd = 100 nM) comparable with that of a closely related p21 peptide (residues 141–160; Kd = 88 nM) (23). The near doubling of both H-bonds and buried surface area of p21(139–160) compared with PL peptide should give a significantly larger enthalpic contribution to binding energy. The fact that the free energy of binding is similar in both complexes, despite the much larger enthalpic contribution from p21(141–160), suggests that there is an important entropic penalty for this longer, more flexible peptide, and this hypothesis also is supported by isothermal calorimetry data (23).
Unfavorable entropic terms can be reduced if the peptide is preorganized in the helical binding conformation. There is good evidence for this observation from isothermal calorimetry experiments for two peptides: p21(141–160) and the site point mutant p21(141–160)D149A (23). The structures of both PL peptide and p21(139–160) (Fig. 4) show that the aspartate side chain of D10 (or D149) can form two intramolecular H-bonds that may help stabilize the 310-helical conformation. For the D149A mutant, the isothermal calorimetry results show an unfavorable entropy term with a drop in Ka from 1.1 × 107 for p21(141–160) down to 7.8 × 105 for p21D149A. The x-ray structures suggest that this must be a purely entropic effect because there is no direct contact between the aspartate side chain and PCNA. The long-range calculated electrostatic binding energy contribution is also negative (see below), confirming that the major role for D149 is to hold the peptide in a favored binding conformation.
This binding model also can help explain how phosphorylation of p21 can be used to uncouple p21-regulated CDK activity and PCNA-mediated DNA synthesis. Phosphorylation of S146 was shown to prevent the binding of p21 to PCNA in insect cells (33), and a subsequent study showed that the protein kinase Akt specifically phosphorylates T145, which abrogates PCNA binding to p21 in endothelial cells (36). Both of these observations now can be explained as the result of a helix-to-coil conformational switch of p21 induced by the introduction of a phosphate group. Phosphorylation of side chains of either T145 or S146 (Fig. 4) place a bulky negatively charged phosphate group in close proximity (<4 Å) to the side chain of D149. Thus, phosphorylation of T145, S146, or (indeed) T148 will prevent the side chain of D149 from adopting a conformation promoting the required helical conformation for PCNA binding.
Inspection of the PL peptide binding pocket showed that the major interactions with PCNA involve the side chains Q5, I8, Y11, and F12 (Fig. 3). Together, these amino acids contribute 450 Å2, or 65% of the total buried surface for the PL peptide. This buried surface area is very similar to the value of 490 Å2 calculated for the equivalent residues in the p21(143–160) structure. To further quantify the peptide–PCNA interaction, affinity scores for the individual side chains were calculated using coordinates from the respective x-ray structures (Table 2). These values give a measure of the combined van der Waals, H-bonding, and electrostatic contributions to the binding energy. The total calculated nonbonded intermolecular energies are –469 and –478 kcal/mol for p21(143–160) and PL peptide, respectively, with positively charged side chains of both peptides providing the major coulombic contribution to intermolecular energies. Interestingly, there is a stronger interaction energy of M147 of the p21 peptide compared with the corresponding I8 side chain of the PL peptide, and this finding suggests that further changes in the PL peptide sequence could achieve even better binding. The delineation of this binding pocket provides a good starting point for the design and testing of smaller hybrid peptides and peptidomimetic inhibitors.
Table 2. Comparison of binding energies of p21WAF1 and PL peptides with PCNA.
| Peptide* | Affinity score, kcal/mol |
|---|---|
| p21 (residues 143-160) | |
| Q144 | -12.2 |
| T145 | -7.79 |
| S146 | -4.94 |
| M147 | -6.71 |
| T148 | -2.45 |
| D149 | 76.8 |
| F150 | -7.95 |
| Y151 | -7.19 |
| H152 | -1.97 |
| PL peptide (residues 1-16) | |
| Q5 | -10.3 |
| K6 | -77.1 |
| K7 | -84.8 |
| I8 | -3.55 |
| T9 | -2.23 |
| D10 | 76.0 |
| Y11 | -9.54 |
| F12 | -11.7 |
| H13 | -4.17 |
p21WAF1, Protein Data Bank ID code 1AXC.
Core residues are residues 5-13 in PL peptide and 144-153 in p21.
Comparison of the lipophilic pocket (formed in part by the linker strand) of the PL peptide structure with that of p21-bound PCNA reveals that the overall volume of the PL peptide cleft is larger and more concave (see Fig. 7, which is published as supporting information on the PNAS web site). These changes largely appear to result from movement of the linker strand to accommodate F12. Of the pocket-forming residues, side-chain atoms from M40, L126, I128, and Q131 all move between 1 and 3 Å relative to the p21 bound structure. Despite the lack of H-bonding contacts of the Y151 hydroxyl with Q131, the induced changes in the pocket result in more effective contact with F12 (Table 2). The larger volume of the PL peptide pocket indicates that bulkier side chains replacing F12 would potentially improve ligand binding. Indeed, the deep groove occupied by I8, Y11, and F12 covers ≈50% of the total buried surface of the PL peptide and provides a site well suited to the design of small-molecule inhibitors of PCNA and the development of novel peptidomimetic drugs.
The PCNA/p21/CDK/Cyclin Complex. In normal, untransformed cells, quaternary complexes are formed between cyclins (A, B, D, and E), CDKs, p21, and PCNA. Furthermore, subunit rearrangement of these CDK complexes is associated with cellular transformation (14). Upon cell transformation, the expression of p21 is frequently depressed and CDKs dissociate from PCNA. This finding suggests that p21 may participate in the coordination of cellular DNA replication and cell-cycle progression and that upon transformation these processes become uncoupled, permitting escape from the G1 DNA-damage checkpoint. Quaternary complexes of CDK/cyclin pairs with p21 and PCNA exist in multiple cell cycle phases, including G1, S, and even G2/M, where CDK1/cyclin B is implicated (34). The quaternary complex (Fig. 6) provides a structural basis to the finding that p21 can both block access to PCNA for other proteins involved in DNA replication and also act as an adaptor for various complexes of PCNA with kinases. This model also fits with the suggestion that PCNA acts as an adaptor protein bringing various kinases to substrate proteins involved in DNA replication (35).
The identification of a well defined “druggable” site on PCNA adjacent to (and potentially independent of) the cyclin-binding site identified in the model structure opens up the possibility of using these sites to probe the biological function of PCNA. In particular, it will be interesting to study the effects of small and specific “cyclin-groove” inhibitors that are now available (21) along with smaller PL-related peptides to examine a possible synergy of action. Such complementary effects could have important consequences in developing inhibitor cocktails to manipulate cell-cycle events.
Supplementary Material
Acknowledgments
We thank the Edinburgh Protein Interaction Centre for use of facilities and the European Synchrotron Radiation Facility, Deutsches Elektronen Synchrotron, and the Daresbury Synchrotron Radiation Source for synchrotron beam time.
Author contributions: D.P.L., P.M.F., and M.D.W. designed research; G.K., S.-Y.W., D.I.Z., P.T., and M.D.W. performed research; C.M., D.P.L., P.M.F., and M.D.W. analyzed data; and C.M., D.P.L., P.M.F., and M.D.W. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CDK, cyclin-dependent kinase; PCNA, proliferating cell nuclear antigen; PL, Pogo-Ligase.
Data deposition: The three x-ray structures have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 1VYM (hPCNA native monoclinic form), 1W60 (hPCNA native trigonal form), and 1VYJ (hPCNA-PL complex)].
References
- 1.Prelich, G., Kostura, M., Marshak, D. R., Mathews, M. B. & Stillman, B. (1987) Nature 326, 471–475. [DOI] [PubMed] [Google Scholar]
- 2.Krishna, T. S. R., Kong, X. P., Gary, S., Burgers, P. M. & Kuriyan, J. (1994) Cell 79, 1233–1243. [DOI] [PubMed] [Google Scholar]
- 3.Bowman, G. D., O'Donnell, M. & Kuriyan, J. (2004) Nature 429, 724–730. [DOI] [PubMed] [Google Scholar]
- 4.Warbrick, E. (2000) BioEssays 22, 997–1006. [DOI] [PubMed] [Google Scholar]
- 5.Paunesku, T., Mittal, S., Protic, M., Oryhon, J., Korolev, S. V., Joachimiak, A. & Woloschak, G. E. (2001) Int. J Radiat. Biol. 77, 1007–1021. [DOI] [PubMed] [Google Scholar]
- 6.Sakakura, C., Hagiwara, A., Tsujimoto, H., Ozaki, K., Sakakibara, T., Oyama, T., Ogaki, M. & Takahashi, T. (1994) Br. J. Cancer 70, 1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeshima, Y., Kashihara, N., Sugiyama, H., Makino, H. & Ota, Z. (1996) J. Am. Soc. Nephrol. 7, 2219–2229. [DOI] [PubMed] [Google Scholar]
- 8.Morita, Y., Kashihara, N., Yamamura, M., Okamoto, H., Harada, S., Maeshima, Y., Okamoto, K. & Makino, H. (1997) Arthritis Rheum. 40, 1292–1297. [DOI] [PubMed] [Google Scholar]
- 9.Gartel, A. L. & Tyner, A. L. (2002) Mol. Cancer Ther. 1, 639–649. [PubMed] [Google Scholar]
- 10.Chen, J. J., Peters, R., Saha, P., Lee, P., Theodoras, A., Pagano, M., Wagner, G. & Dutta, A. (1996) Nucleic Acids Res. 24, 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotedar, R., Fitzgerald, P., Rousselle, T., Cannella, D., Doree, M., Messier, H. & Fotedar, A. (1996) Oncogene 12, 2155–2164. [PubMed] [Google Scholar]
- 12.Gulbis, J. M., Kelman, Z., Hurwitz, J., O'Donnell, M. & Kuriyan, J. (1996) Cell 87, 297–306. [DOI] [PubMed] [Google Scholar]
- 13.Goubin, F. & Ducommun, B. (1995) Oncogene 10, 2281–2287. [PubMed] [Google Scholar]
- 14.Xiong, Y., Zhang, H. & Beach, D. (1993) Genes Dev. 7, 1572–1583. [DOI] [PubMed] [Google Scholar]
- 15.Xiong, Y., Zhang, H. & Beach, D. (1992) Cell 71, 505–514. [DOI] [PubMed] [Google Scholar]
- 16.Tsurimoto, T. (1998) Biochim. Biophys. Acta 1443, 23–39. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, P. M., Krausz, E. & Lane, D. P. (2001) Bioconjugate Chem. 12, 825–841. [DOI] [PubMed] [Google Scholar]
- 18.Warbrick, E., Lane, D. P., Glover, D. M. & Cox, L. S. (1995) Curr. Biol. 5, 275–282. [DOI] [PubMed] [Google Scholar]
- 19.Ball, K. L., Lain, S., Fahraeus, R., Smythe, C. & Lane, D. P. (1997) Curr. Biol. 7, 71–80. [DOI] [PubMed] [Google Scholar]
- 20.Fahraeus, R., Paramio, J. M., Ball, K. L., Lain, S. & Lane, D. P. (1996) Curr. Biol. 6, 84–91. [DOI] [PubMed] [Google Scholar]
- 21.Kontopidis, G., Andrews, M. J. I., McInnes, C., Cowan, A., Powers, H., Innes, L., Plater, A., Griffiths, G., Paterson, D., Zheleva, D. I., et al. (2003) Structure (London) 11, 1537–1546. [DOI] [PubMed] [Google Scholar]
- 22.Zheleva, D. I., McInnes, C., Gavine, A. L., Zhelev, N. Z., Fischer, P. M. & Lane, D. P. (2002) J. Pept. Res. 60, 257–270. [DOI] [PubMed] [Google Scholar]
- 23.Zheleva, D. I., Zhelev, N. Z., Fischer, P. M., Duff, S. V., Warbrick, E., Blake, D. G. & Lane, D. P. (2000) Biochemistry 39, 7388–7397. [DOI] [PubMed] [Google Scholar]
- 24.Chan, A. W. E. & White, A., eds. (2000) Fmoc Solid Phase Peptide Synthesis: A Practical Approach (Oxford Univ. Press, Oxford).
- 25.Otwinowski, Z. & Minor, W. (1997) Methods Enzymol. 276, 307–326. [DOI] [PubMed] [Google Scholar]
- 26.Vagin, A. & Teplyakov, A. (1997) J. Appl. Crystallogr. 30, 1022–1025. [Google Scholar]
- 27.Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997) Acta Crystallogr. D 53, 240–255. [DOI] [PubMed] [Google Scholar]
- 28.Chapados, B. R., Hosfield, D. J., Han, S., Qiu, J. Z., Yelent, B., Shen, B. H. & Tainer, J. A. (2004) Cell 116, 39–50. [DOI] [PubMed] [Google Scholar]
- 29.Fischer, P. M., Zheleva, D. I., McInnes, C., Gavine, A., Zhelev, N. Z. & Lane, D. P. (2001) Clin. Cancer Res. 7, 824.11309329 [Google Scholar]
- 30.Matsuoka, S., Yamaguchi, M. & Matsukage, A. (1994) J. Biol. Chem. 269, 11030–11036. [PubMed] [Google Scholar]
- 31.Cox, L. S. (1997) Trends Cell Biol. 7, 493–498. [DOI] [PubMed] [Google Scholar]
- 32.McInnes, C., Andrews, M. J., Zheleva, D. I., Lane, D. P. & Fischer, P. M. (2003) Curr. Med. Chem. Anti-Cancer Agents 3, 57–69. [DOI] [PubMed] [Google Scholar]
- 33.Scott, M. T., Morrice, N. & Ball, K. L. (2000) J. Biol. Chem. 275, 11529–11537. [DOI] [PubMed] [Google Scholar]
- 34.Ando, T., Kawabe, T., Ohara, H., Ducommun, B., Itoh, M. & Okamoto, T. (2001) J. Biol. Chem. 276, 42971–42977. [DOI] [PubMed] [Google Scholar]
- 35.Koundrioukoff, S., Jonsson, Z. O., Hasan, S., de Jong, R. N., van der Vliet, P. C., Hottiger, M. O. & Hubscher, U. (2000) J. Biol. Chem. 275, 22882–22887. [DOI] [PubMed] [Google Scholar]
- 36.Rossig, L., Jadidi, A. S., Urbich, C., Badorff, C., Zeiher, A. M. & Dimmeler, S. (2001) Mol. Cell. Biol. 21, 5644–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.