Abstract
The cytokine erythropoietin (EPO) protects the heart from ischemic injury, in part by preventing apoptosis. However, EPO administration can also raise the hemoglobin concentration, which, by increasing oxygen delivery, confounds assignment of cause and effect. The availability of EPO analogs that do not bind to the dimeric EPO receptor and lack erythropoietic activity, e.g., carbamylated EPO (CEPO), provides an opportunity to determine whether EPO possesses direct cardioprotective activity. In vivo, cardiomyocyte loss after experimental myocardial infarction (MI) of rats (40 min of occlusion with reperfusion) was reduced from ≈57% in MI-control to ≈45% in animals that were administered CEPO daily for 1 week (50 μg/kg of body weight s.c.) with the first dose administered intravenously 5 min before reperfusion. CEPO did not increase the hematocrit, yet it prevented increases in left ventricular (LV) end-diastolic pressure, reduced LV wall stress in systole and diastole, and improved LV response to dobutamine infusion compared with vehicle-treated animals. In agreement with the cardioprotective effect observed in vivo, staurosporine-induced apoptosis of adult rat or mouse cardiomyocytes in vitro was also significantly attenuated (≈35%) by CEPO, which is comparable with the effect of EPO. These data indicate that prevention of cardiomyocyte apoptosis, in the absence of an increase in hemoglobin concentration, explains EPO's cardioprotection. Nonerythropoietic derivatives such as CEPO, devoid of the undesirable effects of EPO, e.g., thrombogenesis, could represent safer and more effective alternatives for treatment of cardiovascular diseases, such as MI and heart failure. Furthermore, these findings expand the activity spectrum of CEPO to tissues outside the nervous system.
Keywords: apoptosis, cardioprotection, cytokine, tissue injury
Erythropoietin (EPO) protects the brain and the spinal cord from ischemic and traumatic injury (1, 2), the peripheral nerve from diabetic damage (3), the kidney from ischemic (4, 5) or toxic insults (6), and the heart from acute ischemia, either permanent (7–9) or with reperfusion (10). Current data suggest that the observed protective effects of EPO depend on an antiapoptotic effect of this cytokine (7, 10). In the brain, EPO also greatly reduces the inflammatory response after ischemia–reperfusion (11). It is notable that in several acute models, e.g., brain ischemia, a single dose of EPO that does not increase the Hb concentration nevertheless confers neuroprotection. However, in other in vivo injury models, including cardiac ischemia with reperfusion and diabetic neuropathy, injury develops gradually, and multiple doses of EPO appear superior but also increase the Hb concentration. The possibility that part of the benefit obtained with EPO in these models may depend on the increased oxygen-carrying capacity of the blood cannot be excluded. However, cardiac protection has clearly been demonstrated after only a single dose (8, 10) when evaluated 1–3 days after infarction before any increase in hematocrit was measurable. Furthermore, the fact that EPO is cytoprotective for cardiomyocytes in vitro (7) indicates that it has some direct cardioprotective effects independent of changes in Hb concentration.
Recently, a second receptor for EPO that mediates EPO's tissue protection has been identified as consisting of the EPO receptor and β-common (CD131) receptor, which is present in the myocardium (12). The EPO molecule can be modified, such as by carbamylation [carbamylated EPO (CEPO)], so that it signals only through this receptor and not the homodimeric EPO receptor yet is neuroprotective (13). By using CEPO, a definitive proof of concept that cardiac protection by EPO does not depend on, increases in red blood cell number can now be accomplished.
In the current study, we determined whether CEPO protects cardiac myocytes from ischemia–reperfusion injury-related apoptosis in vivo and in vitro. A CEPO-mediated reduced cell loss was observed in vitro, as expected, and was also obtained in vivo. Furthermore, a favorable outcome [left ventricular (LV) function, basal and under stress] occurred in vivo after 1 week of daily dosing, which did not affect Hb concentration. Taken together, these data confirm a direct effect of EPO/CEPO on the myocardium.
Materials and Methods
All experiments were performed in a blinded fashion and in accordance with all laws and regulations pertaining to animal research.
Cardiomyocytes in Primary Culture. LV myocytes were isolated from adult Sprague–Dawley rats and C57/BL6 mice as described (14). Briefly, hearts were perfused through the aorta with collagenase buffer (Selected Type II, Worthington Biochemical) and gassed in an atmosphere of 85% O2 and 15% N2 at 37°C. LV myocytes were then isolated by mechanical dissociation, separated by differential centrifugation, and plated on 35-mm laminin-coated polystyrene tissue culture dishes in MEM supplemented with Hanks' salts and l-glutamine (GIBCO). After 1 h of plating, the medium was changed, and CEPO [100 ng/ml, prepared as described (13)] was added to the myocytes 30 min before induction of apoptosis by staurosporine (2 μM, Sigma-Aldrich). After 16 h of incubation, cardiomyocytes were fixed and processed for the assessment of apoptosis. Identification of apoptotic cell death was determined by the presence of double-stranded DNA cleavage, using a terminal deoxynucleotidyltransferase (TdT) assay, and confirmed by evaluation using interference contrast microscopy of myocyte morphological features characteristic of apoptosis.
In Vivo Experimental Protocol. Male Sprague–Dawley rats (253 ± 4 g) were anesthetized with isoflurane 3% (O2, 0.25 liters/min; N2, 0.4 liters/min) and were ventilated (70 breaths per min, tidal volume of 1.2 ml per 100 g of body weight) through an endotracheal cannula. The left anterior descending coronary artery was ligated with a 7-O silk suture after exteriorization of the heart through a 15-mm opening at the fourth-intercostal space. An overhand knot was tied over two pieces of suture to arrest blood flow and was removed after 40 min to initiate reperfusion. Ischemia was confirmed by the appearance of ventricular ectopy and blanching of the myocardium. The chest was then closed under negative pressure, and the rat was weaned from mechanical ventilation under continuous electrocardiographic monitoring. Successful reperfusion was indicated by a restoration of normal cardiac rubor. Sham-operated rats underwent identical surgical procedures, but without coronary artery ligation. CEPO dissolved in saline was administered a dose of 50 μg/kg of body weight i.v. 5 min before reperfusion, then 50 μg/kg of body weight s.c. every day for 6 days. Hematocrit was assessed in duplicate from tail blood on day 7. Blood was collected 3–4 and 24 h after the last CEPO administration. CEPO in plasma was measured with an ELISA by using CEPO as a standard, a monoclonal anti-rhEPO (MAB287, R & D Systems) as capture antibody, and a polyclonal anti-rhEPO (AB-286-NA, R & D Systems), biotinylated according to standard procedures, as detection antibody. The sensitivity of the assay was 0.5 ng/ml.
Echocardiography. Rats surviving a 40-min left anterior descending coronary artery occlusion underwent transthoracic echocardiography (Aloka SSD-5500, Aloka, Tokyo) on day 14 under light sedation (diazepam and xylazine i.p.) by using a 13-MHz linear transducer at high frame rate imaging (57 Hz). Short- and long-axis 2D views and M-mode at the level of infarction were analyzed in real-time and recorded on a magnetooptic disk for off-line analysis by a sonographer who was blinded to study treatments. Anterior and posterior end-diastolic and end-systolic wall thicknesses and LV internal dimensions were measured, as recommended by the American Society of Echocardiography (15, 16). Shortening fraction (SF) was calculated from the composite LV internal, diastolic (LVIDd) and LV internal, systolic (LVIDs) dimensions as
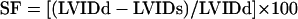 |
from M-mode short-axis views. LV end-diastolic volume, LV end-systolic volume, and LV ejection fraction were calculated by modified Simpson's single-plane rule from a long-axis view. The 2D echocardiographic images were divided into 16 segments that were counted as abnormal at any degree of wall motion abnormality, e.g., akinesis, dyskinesis, and hypokinesis. LV wall dyssynergy (akinetic, dyskinetic, and severely hypokinetic segments) was considered as an in vivo index of the extent of damage caused by infarction to the ventricular wall. Systolic and diastolic wall stress was calculated from LV systolic and diastolic pressure measured with a Millar catheter (see below) and echocardiographic dimensions by the formula
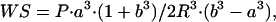 |
where P = intraventricular pressure, R = LV radial coordinate, a = LV inner radius, and b = LV outer radius (17, 18). After echocardiographic examination at rest, stepwise i.v. (22-gauge heparin lock percutaneously inserted into the tail vein) infusion of dobutamine (echo stress) was performed to evaluate myocardial viability and recovery of global LV function (19, 20).
Hemodynamics. At least 24 h after completion of echocardiographic exams (i.e., day 15), a microtip pressure transducer (SPC-320, Millar Instruments, Houston) was inserted into the right carotid artery to measure systolic and diastolic blood pressure and heart rate (Windowgraph, Gould Electronics, Valley View, OH) under pentobarbital anesthesia (50 mg/kg i.p.). The pressure transducer was then advanced into the LV to measure systolic and end-diastolic pressure, the first derivatives (positive and negative) of LV pressure over time.
Histomorphometry. Upon completion of hemodynamic measurements, the heart was arrested in diastole by i.v. injection of a 2.5-M solution of KCl, quickly removed from the chest, blotted dry, and weighted. The atria were trimmed, and the free right ventricle wall and LV inclusive of the septum were weighed individually. LV internal length was measured by use of a probe. Two slices just below the arterial ligation, basal and apical, were fixed in formaldehyde. Two 5-μm sections from each paraffin-embedded slice (i.e., basal and apical) were obtained (one just below the ligature and the other from the basal side of the apical part of LV) and were stained with hematoxylin/eosin. Scar and spared myocardium area were assessed by counting the number of intersection points of an ocular grid with 121 points overlying scar (infarct) or spared myocardium of the LV. Infarct area was calculated as the ratio between scar area and the area of the whole-LV section and expressed in percentages. The cardiomyocyte cross-sectional area was measured by manually tracing the cell contour on images acquired on the image analyzer ibas 2.0 (Kontron–Zeiss image analysis system) at ×400 magnification and averaged >50 cardiomyocytes in each section (21). The total number and volume of myocytes were determined by quantitative morphometric methods on hematoxylin/eosin-stained 5-μm sections (22). Measurements obtained in the two representative sections of each heart were averaged.
In Situ TdT Assay for Detection of Apoptosis. Myocytes obtained by isolation from different rat or mouse hearts of each experimental groups were incubated with TdT (23). Positive controls (myocytes treated with DNase I) and negative controls (omission of biotin-16-dUTP or TdT) were also included. Nuclei were visualized with propidium iodide, and interference contrast microscopy was used to exclude apoptotic nuclei of non-cardiomyocyte origin. The number of TdT-labeled cells was determined by two different operators blinded to the experimental groups by counting an average of >450 isolated myocytes in each culture dish. Immunofluorescent images were obtained by using an Olympus FV500 laser-scanning confocal microscope.
Calculations and Statistical Analysis. All data are presented as mean ± SEM. Mean changes versus baseline in echocardiographic variables during dobutamine infusion were calculated by area under effect versus dose–response curve truncated at 15 μg of dobutamine (i.e., the plateau) and used to estimate inotropic reserve. Maximal values of heart rate and SF on dobutamine were also calculated. Experimental groups were compared by one-way ANOVA, followed by post hoc Dunnett's test, using myocardial infarction (MI)-control as reference group (prism 4.0 for Windows).
Results
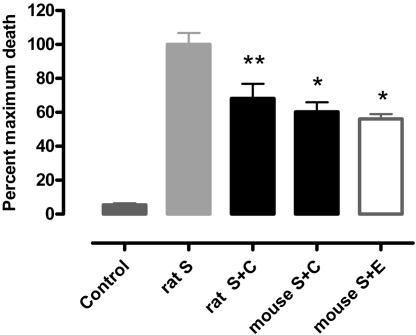
In Vitro Study. In contrast to cells cultured in serum-free medium for 16 h (Fig. 5 A and B, which is published as supporting information on the PNAS web site), myocytes in the presence of staurosporine showed cytoplasm shrinkage, nuclear condensation, and fragmentation (Fig. 5 C and D) associated with 3′-OH DNA ends identified by TdT assay (Fig. 5D). Only nuclei of myocytes that retained the typical rod-shape morphology of uninjured cells were negative for the TdT staining (Fig. 5 B–D). After 16 h of culture in the absence of staurosporine, rat myocytes exhibited a low basal percentage of TdT-labeled nuclei (3.0 ± 0.5%) that markedly increased 18.3-fold (55.0 ± 3.7%; P < 0.01, Fig. 1) after staurosporine incubation (S, 2 μM) to the medium. The presence of CEPO (S plus C) was associated with a significant reduction of staurosporine-induced apoptosis to 37.5 ± 4.8% (P < 0.01), confirming a direct effect of CEPO on survival. A similar degree of protection was observed for EPO (S plus E). Cells from either rats or mice behaved similarly.
Fig. 1.
Mean percentage of myocytes positive for TdT assay counted on 450 cells in each dish obtained by isolation from different rat or mouse hearts. Shown are data for rat control (n = 9), S (Staurosporine, n = 10), rat S plus C (S plus CEPO, n = 10), mouse S plus C (n = 4), and mouse S plus E (S plus EPO; n = 4). *, P < 0.05; **, P < 0.01 versus staurosporine.
In Vivo Study. A total of 38 male CD rats underwent surgery, and 8 rats (21%) died within 6 h after surgery. There were no differences in the distribution of perioperative mortality between the MI groups. Postmortem examination revealed extensive MIs. Ten infarcted rats received CEPO in saline 0.9% (MI-CEPO group), 13 rats used as controls received saline 0.9% (MI-control group), and 8 rats served as Sham-operated controls (Sham group). All were included in final analysis, except for three MI-control rats who died during followup. CEPO was measurable in all treated rats, at both 3–4 h and 24 h after s.c. administration; concentrations averaged 55.5 ± 8.7 ng/ml and 15.2 ± 1.4 ng/ml, respectively. CEPO was always below the detection limit in untreated rats. As anticipated, CEPO did not affect the hematocrit on day 7 after surgery: MI-CEPO, 43.6 ± 1.7%; MI-control, 41.6 ± 2.0% (P = not significant).
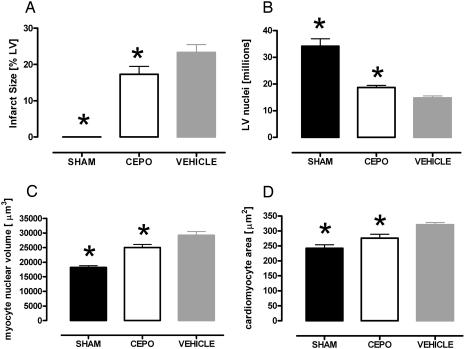
Histomorphometric Analysis. Infarct size, calculated as the fractional area of the scar, was smaller in MI-CEPO group than in the MI-control group: 17% versus 23% (Fig. 2A; P = 0.065). Calculated number of cardiac myocyte nuclei in the whole LV was decreased significantly in both MI groups (Fig. 2B). Cardiac myocyte volume per nucleus was smaller in CEPO-treated animals (Fig. 2C; P < 0.05). Consistent with this result, myocyte cross-sectional area in MI-CEPO group was 14% lower than in the MI-control group (Fig. 2D; P < 0.05).
Fig. 2.
Histomorphometric evaluation of infarct size (A), number of cardiac myocyte nuclei in left ventricle (B), cardiac myocyte volume per nucleus (C), and cross-sectional area of cardiac myocytes in the spared left ventricle of Sham-operated rats and in coronary-ligated rats untreated or treated with CEPO (D). MI-control group; CEPO, MI-CEPO group. *, P < 0.01 versus vehicle.
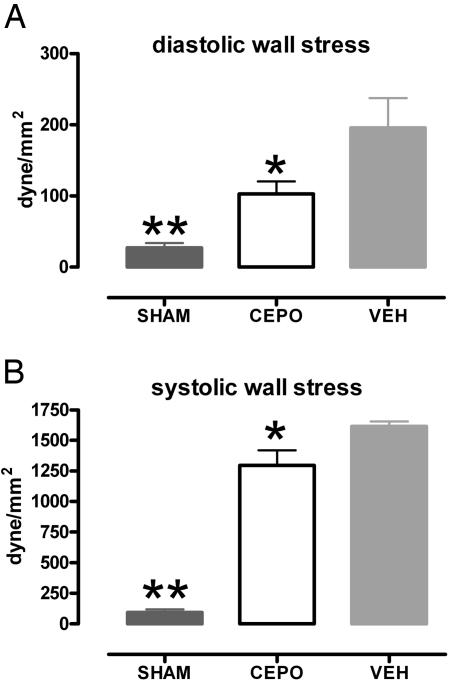
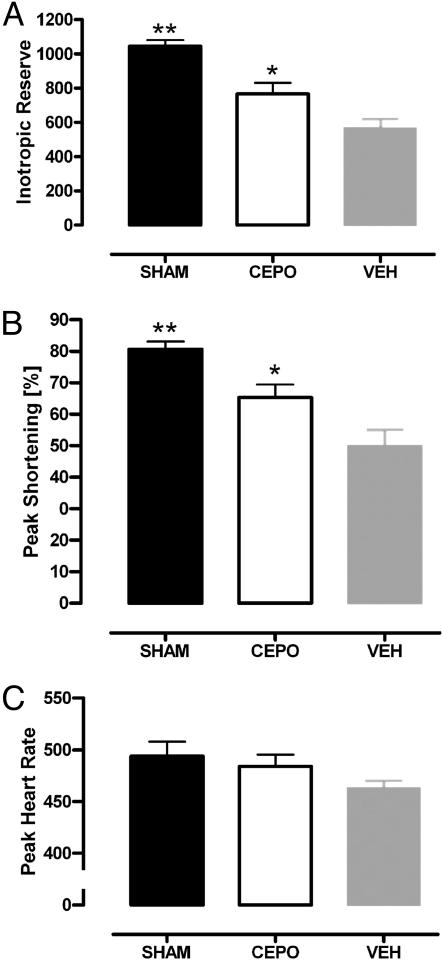
Echocardiography. Cardiac anatomical and functional variables (LV wall thickness, internal chamber diameter, SF, LV volumes, and ejection fraction, and LV wall motion) measured at rest showed a dilated and dysfunctional left ventricle in infarcted animals when compared with Sham-operated animals (Table 1). CEPO treatment was associated with a nonsignificant improvement in other variables except for LV wall stress in diastole. Although LV wall stress increased in both systole and diastole for infarcted animals, CEPO treatment significantly reduced the deterioration compared with vehicle-treated controls (Fig. 3). After dobutamine infusions from 0 to 40 μg/kg per min, Inotropic reserve (Fig. 4A) and peak (maximum) SF were greater in Sham-operated rats than in infarcted rats (P < 0.01), MI-CEPO rats responded to dobutamine better (P < 0.05) than did MI-control rats (Fig. 4 A and B). These effects on SF were observed in the absence of differences in peak heart rate (Fig. 4C).
Table 1. Baseline echocardiographic variables measured 14 days after ischemia and reperfusion in rats Sham-operated, with infarction treated with CEPO or untreated (VEH).
| CEPO (n = 10) | VEH (n = 10) | Sham (n = 7) | |
|---|---|---|---|
| Heart rate, bpm | 403 ± 20 | 346 ± 18 | 354 ± 25 |
| LVIDd, mm | 7.52 ± 0.30 | 7.79 ± 0.31 | 6.31 ± 0.10* |
| LVIDs, mm | 4.65 ± 0.41 | 5.34 ± 0.43 | 2.83 ± 0.32* |
| SE, % | 39.1 ± 3.7 | 31.8 ± 3.8 | 49.1 ± 2.2* |
| IVSThd, mm | 1.97 ± 0.05 | 1.91 ± 0.05 | 1.99 ± 0.04 |
| AWThd, mm | 1.22 ± 0.08 | 1.16 ± 0.12 | 1.94 ± 0.03* |
| EDV, ml | 0.35 ± 0.03 | 0.41 ± 0.03 | 0.30 ± 0.03 |
| ESV, ml | 0.15 ± 0.02 | 0.19 ± 0.02 | 0.06 ± 0.03* |
| EF, % | 54.7 ± 4.7 | 51.1 ± 12.1 | 81.1 ± 2.0* |
| A/D/H extension, % | 22.80 ± 1.87 | 26.67 ± 1.67 | — |
Dunnett comparison with the VEH group (reference group). *, P < 0.01. LVIDd, LV internal diastolic diameter; LVIDs, LV internal systolic diameter; IVSThd, diastolic interventricular septum thickness; AWThd, diastolic anterior wall thickness; EDV, end-diastolic volume; ESV, end-systolic volume; A/D/H, akinesis/dyskinesis/hypokinesis.
Fig. 3.
CEPO administration prevents full increase in LV wall stress in diastole (A) and in systole (B). *, P < 0.05 or **, P < 0.01 versus MI-control (VEH).
Fig. 4.
Echo dobutamine stress test for the assessment of myocardial viability in lightly sedated rats. Inotropic reserve (A), Maximum (peak) SF (B), and maximum heart rate (C) on dobutamine in Sham-operated rats and in coronary ligated rats untreated or treated with CEPO are shown. VEH, MI-control group; CEPO, MI-CEPO group. *, P < 0.05; **, P < 0.01 versus VEH group.
Hemodynamics. Systemic arterial and LV pressures, their derivative and heart rate, did not show significant changes attributable to MI, except for LV end-diastolic pressure that was significantly increased: 2.1 ± 0.5 mmHg in Sham versus 7.1 ± 1.0 mmHg in MI-control (P < 0.01). CEPO attenuated the increase in LV end-diastolic pressure observed in MI-controls: MI-CEPO, 4.3 ± 0.6 mmHg; MI-control, 7.1 ± 1.0 mmHg (P = 0.023).
Discussion
The results obtained from the isolated adult rat ventricular cardiomyocytes show that CEPO, a nonerythropoietic derivative of EPO, possesses the same antiapoptotic effect as previously observed for EPO (7). Positive results obtained by using cells from two species (rat and mouse) confirm that the findings are general in nature. Because CEPO has no affinity for the classical EPO receptor (i.e., the erythropoietic dimeric form), cytoprotection by CEPO is mediated by another receptor type. Prior study has shown that this tissue protective receptor uses the β-receptor common to IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor (12) Extension of the protective effects of CEPO from the nervous system to the heart supports the concept of EPO and CEPO as general tissue-protective molecules.
In the cardiomyocyte model, exposure to staurosporine induced the majority of myocytes to undergo apoptosis similar to that observed for previous studies using 12–24 h of hypoxia (7, 8). Staurosporine, an inhibitor of protein kinase C, has been shown to induce apoptosis in a wide variety of cell types, including cardiomyocytes (24). An advantage of using staurosporine to induce apoptosis is that the morphological and molecular characteristics of staurosporine-induced injury, such as cytoplasm shrinkage, nuclear condensation (Fig. 5) and fragmentation, loss of mitochondrial transmembrane potential, Bax translocation, cytochrome c release, and caspase-3 activation (25), closely mimic apoptotic processes during hypoxia and reoxygenation of isolated adult cardiomyocytes (26).
The positive results obtained in vitro encouraged testing cardiac protection in vivo by using a rat model of myocardial infarction with reperfusion. Treatment with CEPO, beginning just before the release of coronary occlusion, and then continued once daily for 6 additional days, resulted in an attenuated loss of cardiac myocytes and prevented compensatory hypertrophy, as assessed on day 15 after infarction. These findings, similar to those observed for EPO (7), can be explained by the antiapoptotic action observed for both EPO (7) and CEPO, particularly as expected for in the LV free wall (27, 28) and the region adjacent to the infarcted LV free wall (29, 30). Although assessment of MI size on day 15 by calculation of fractional area of the scar is potentially complicated by scar shrinkage and compensatory hypertrophy of spared myocardium, a trend toward smaller scars in CEPO-treated rats was observed, which is consistent with the observed 21% reduction in cardiomyocyte loss. The attenuation of cell loss and hypertrophy by CEPO was also accompanied by other evidence of more favorable changes in LV function under both basal and stressed conditions. Specifically, the small but significant increase in LV end-diastolic pressure observed in MI-control was normalized by CEPO. This effect, together with the strong trend for smaller LV volumes in the CEPO group, explains the significant reduction in LV diastolic wall stress by 47%. Wall stress is a major determinant of the LV progressive dilatation and the associated hemodynamic deterioration that begins in the first days after MI, and it becomes more evident over the following months.
Because 40 min of ischemia followed by reperfusion led to a mild-to-moderate structural and functional impairment over a period of 15 days, we reasoned that LV functional reserves, as assessed by dobutamine infusion, might amplify differences attributable to treatment with CEPO (19, 20). Indeed, although the SF under baseline conditions was comparable at 39 ± 4% in the CEPO group and 32 ± 4% in the MI-control group (P = NS), the CEPO-treated group showed a better response than vehicle to dobutamine (Fig. 4A), with a greater peak SF, without significant differences in the maximum heart rate (Fig. 4 B and C).
Cardioprotection by EPO has been shown to involve both acute and delayed components, which is consistent with a pharmacological form of preconditioning (reviewed by Bogoyevitch in ref. 31). In our experiments, we administered CEPO at the time of reperfusion in a manner consistent with timing for many clinical interventions, e.g., percutaneous angioplasty. This procedure likely provides both acute and delayed effects. However, it is currently unknown what schedule of dosing will result in maximum tissue salvage. In a recent limited time-window study using EPO, administration at the time of cardiac reperfusion was associated with a better outcome than when given 2 h before (32). However, given the prolonged time course of ventricular remodeling and development of heart failure and its association with continued cardiomyocyte apoptosis (reviewed in refs. 30 and 33), it is most likely that multiple doses of tissue-protective cytokines will be required to produce maximum protection. Another critical but unexplored question concerning intervention with tissue protective cytokines in myocardial ischemia is information on the optimum dose. This issue is especially critical because EPO has been shown to exhibit an inverted U-shaped dose–response curve in a number of experimental systems, e.g., EPO-mediated endothelial cell nitric oxide (NO) production (34), such that high doses of EPO lose biological activity. Furthermore, because EPO has been shown in the nervous system to require only a brief presence to trigger protective functions (2), further study will be needed to determine whether peak serum concentrations or area under the concentration curve is the important variable.
The larger fraction of myocardium recruitable for contraction in the CEPO group and the structural and functional benefits obtained with CEPO definitively demonstrate that cardioprotection can be separated from the erythropoietic action of EPO. The dissociation of myocardial protection from erythropoiesis is highly desirable from the perspective of a therapeutic use of CEPO. Notably, in mice, EPO-mediated increases in hematocrit causes vasoconstriction and cardiac dysfunction through NO depletion and endothelin activation (35, 36). Furthermore, in humans, recombinant human EPO (rhEPO) administration can lead to hypertension (37–39). It is also well known that EPO can cause thrombosis and therefore tissue injury (40). Even a single exposure of normal humans to EPO causes a dose-dependent increase in E-selectin within 2 days, which is consistent with significant endothelial cell activation (41). Similar responses have also been documented for other markers associated with thrombosis, e.g., P-selectin, and in vitro studies confirm direct and rapid prothrombotic effects of EPO on the endothelium (42). Clearly, an increased potential for thrombosis is not desirable in vascular occlusive myocardial disease.
These considerations suggest that the possible beneficial effects of rhEPO therapy might be masked or even reversed by these adverse effects of EPO. The availability of nonerythropoietic derivatives such as CEPO, retaining tissue protection but without the undesired effects of rhEPO, could be safer and more effective therapeutics for cardiovascular diseases such as myocardial infarction and heart failure. Finally, we expect that the protective effects of molecules like CEPO will extend to other tissues and organs for which EPO has been shown in experimental models to provide protection from injury.
Supplementary Material
Acknowledgments
We thank Ingrid Seinen (Aloka, Assago, Italy) for setting up echocardiography for the dobutamine stress test. This work was partially supported by a grant from Fondazione CARIPLO Bando 2003 and European Genomic Network Contract LSHM-CT-2003 503254.
Author contributions: S.C., L.S., A.B., P.G., T.C., M.B., A.C.C., and R.L. designed research; F.F., S.C., L.S., A.B., E.C., I.C., M.D., M.M., R.T., and T.C. performed research; F.F., L.S., and M.B. contributed new reagents/analytic tools; F.F., S.C., L.S., A.B., E.C., I.C., M.D., M.M., M.B., and R.L. analyzed data; and F.F., S.C., L.S., A.B., E.C., I.C., M.D., M.M., R.T., P.G., T.C., M.B., A.C.C., and R.L. wrote the paper.
Abbreviations: EPO, erythropoietin; rhEPO, recombinant human EPO; CEPO, carbamylated EPO; MI, myocardial infarction; LV, left ventricular; SF, shortening fraction; TdT, terminal deoxynucleotidyltransferase.
References
- 1.Brines, M. L., Ghezzi, P., Keenan, S., Agnello, D., de Lanerolle, N. C., Cerami, C., Itri, L. M. & Cerami, A. (2000) Proc. Natl. Acad. Sci. USA 97, 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erbayraktar, S., Grasso, G., Sfacteria, A., Xie, Q. W., Coleman, T., Kreilgaard, M., Torup, L., Sager, T., Erbayraktar, Z., Gokmen, N., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi, R., Buyukakilli, B., Brines, M., Savino, C., Cavaletti, G., Oggioni, N., Lauria, G., Borgna, M., Lombardi, R., Cimen, B., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong, H., Wang, W., Kwon, T. H., Jonassen, T., Li, C., Ring, T., Froki, A. J. & Nielsen, S. (2004) Kidney Int. 66, 683–695. [DOI] [PubMed] [Google Scholar]
- 5.Yang, C. W., Li, C., Jung, J. Y., Shin, S. J., Choi, B. S., Lim, S. W., Sun, B. K., Kim, Y. S., Kim, J., Chang, Y. S. & Bang, B. K. (2003) FASEB J. 17, 1754–1755. [DOI] [PubMed] [Google Scholar]
- 6.Yalcin, S., Muftuoglu, S., Cetin, E., Sarer, B., Yildirim, B. A., Zeybek, D. & Orhan, B. (2003) Med. Oncol. 20, 169–174. [DOI] [PubMed] [Google Scholar]
- 7.Calvillo, L., Latini, R., Kajstura, J., Leri, A., Anversa, P., Ghezzi, P., Salio, M., Cerami, A. & Brines, M. (2003) Proc. Natl. Acad. Sci. USA 100, 4802–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsa, C. J., Matsumoto, A., Kim, J., Riel, R. U., Pascal, L. S., Walton, G. B., Thompson, R. B., Petrofski, J. A., Annex, B. H., Stamler, J. S. & Koch, W. J. (2003) J. Clin. Invest. 112, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsa, C. J., Kim, J., Riel, R. U., Pascal, L. S., Thompson, R. B., Petrofski, J. A., Matsumoto, A., Stamler, J. S. & Koch, W. J. (2004) J. Biol. Chem. 279, 20655–20662. [DOI] [PubMed] [Google Scholar]
- 10.Moon, C., Krawczyk, M., Ahn, D., Ahmet, I., Paik, D., Lakatta, E. G. & Talan, M. I. (2003) Proc. Natl. Acad. Sci. USA 100, 11612–11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villa, P., Bigini, P., Mennini, T., Agnello, D., Laragione, T., Cagnotto, A., Viviani, B., Marinovich, M., Cerami, A., Coleman, T. R., et al. (2003) J. Exp. Med. 198, 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brines, M., Grasso, G., Fiordaliso, F., Sfacteria, A., Ghezzi, P., Fratelli, M., Latini, R., Xie, Q. W., Smart, J., Su-Rick, C. J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leist, M., Ghezzi, P., Grasso, G., Bianchi, R., Villa, P., Fratelli, M., Savino, C., Bianchi, M., Nielsen, J., Gerwien, J., et al. (2004) Science 305, 239–242. [DOI] [PubMed] [Google Scholar]
- 14.Kajstura, J., Fiordaliso, F., Andreoli, A. M., Li, B., Chimenti, S., Medow, M. S., Limana, F., Nadal-Ginard, B., Leri, A. & Anversa, P. (2001) Diabetes 50, 1414–1424. [DOI] [PubMed] [Google Scholar]
- 15.Sahn, D. J., DeMaria, A., Kisslo, J. & Weyman, A. (1978) Circulation 58, 1072–1083. [DOI] [PubMed] [Google Scholar]
- 16.Schiller, N. B., Shah, P. M., Crawford, M., DeMaria, A., Devereux, R., Feigenbaum, H., Gutgesell, H., Reichek, N., Sahn, D., Schnittger, I., et al. (1989) J. Am. Soc. Echocardiogr. 2, 358–367. [DOI] [PubMed] [Google Scholar]
- 17.Mirsky, I. (1969) Biophys. J. 9, 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anversa, P., Li, P., Malhotra, A., Zhang, X., Herman, M. V. & Capasso, J. M. (1993) Am. J. Physiol. 265, H713–H724. [DOI] [PubMed] [Google Scholar]
- 19.Taylor, A. L., Kieso, R. A., Melton, J., Hite, P., Pandian, N. G. & Kerber, R. E. (1985) Circulation 71, 1292–1300. [DOI] [PubMed] [Google Scholar]
- 20.Geleijnse, M. L., Fioretti, P. M. & Roelandt, J. R. (1997) J. Am. Coll. Cardiol. 30, 595–606. [DOI] [PubMed] [Google Scholar]
- 21.Masson, S., Arosio, B., Luvara, G., Gagliano, N., Fiordaliso, F., Santambrogio, D., Vergani, C., Latini, R. & Annoni, G. (1998) J. Mol. Cell. Cardiol. 30, 1505–1514. [DOI] [PubMed] [Google Scholar]
- 22.Anversa, P., Beghi, C., Kikkawa, Y. & Olivetti, G. (1985) Am. J. Pathol. 118, 484–492. [PMC free article] [PubMed] [Google Scholar]
- 23.Fiordaliso, F., Leri, A., Cesselli, D., Limana, F., Safai, B., Nadal-Ginard, B., Anversa, P. & Kajstura, J. (2001) Diabetes 50, 2363–2375. [DOI] [PubMed] [Google Scholar]
- 24.Yue, T. L., Wang, C., Romanic, A. M., Kikly, K., Keller, P., DeWolf, W. E., Jr., Hart, T. K., Thomas, H. C., Storer, B., Gu, J. L., et al. (1998) J. Mol. Cell. Cardiol. 30, 495–507. [DOI] [PubMed] [Google Scholar]
- 25.Shiraishi, J., Tatsumi, T., Keira, N., Akashi, K., Mano, A., Yamanaka, S., Matoba, S., Asayama, J., Yaoi, T., Fushiki, S., et al. (2001) Am. J. Physiol. 281, H1637–H1647. [DOI] [PubMed] [Google Scholar]
- 26.Kang, P. M., Haunstetter, A., Aoki, H., Usheva, A. & Izumo, S. (2000) Circ. Res. 87, 118–125. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb, R. A., Burleson, K. O., Kloner, R. A., Babior, B. M. & Engler, R. L. (1994) J. Clin. Invest. 94, 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajstura, J., Cheng, W., Reiss, K., Clark, W. A., Sonnenblick, E. H., Krajewski, S., Reed, J. C., Olivetti, G. & Anversa, P. (1996) Lab. Invest 74, 86–107. [PubMed] [Google Scholar]
- 29.Cheng, W., Kajstura, J., Nitahara, J. A., Li, B., Reiss, K., Liu, Y., Clark, W. A., Krajewski, S., Reed, J. C., Olivetti, G. & Anversa, P. (1996) Exp. Cell Res. 226, 316–327. [DOI] [PubMed] [Google Scholar]
- 30.Anversa, P., Cheng, W., Liu, Y., Leri, A., Redaelli, G. & Kajstura, J. (1998) Basic Res. Cardiol. 93, Suppl. 3, 8–12. [DOI] [PubMed] [Google Scholar]
- 31.Bogoyevitch, M. A. (2004) Cardiovasc. Res. 63, 208–216. [DOI] [PubMed] [Google Scholar]
- 32.Lipsic, E., van der Meer, P., Henning, R. H., Suurmeijer, A. J., Boddeus, K. M., van Veldhuisen, D. J., van Gilst, W. H. & Schoemaker, R. G. (2004) J. Cardiovasc. Pharmacol. 44, 473–479. [DOI] [PubMed] [Google Scholar]
- 33.Takemura, G. & Fujiwara, H. (2004) Pharmacol. Ther. 104, 1–16. [DOI] [PubMed] [Google Scholar]
- 34.Beleslin-Cokic, B. B., Cokic, V. P., Yu, X., Weksler, B. B., Schechter, A. N. & Noguchi, C. T. (2004) Blood 104, 2073–2080. [DOI] [PubMed] [Google Scholar]
- 35.Quaschning, T., Ruschitzka, F., Stallmach, T., Shaw, S., Morawietz, H., Goettsch, W., Hermann, M., Slowinski, T., Theuring, F., Hocher, B., Luscher, T. F. & Gassmann, M. (2003) FASEB J. 17, 259–261. [DOI] [PubMed] [Google Scholar]
- 36.Ruschitzka, F. T., Wenger, R. H., Stallmach, T., Quaschning, T., de Wit, C., Wagner, K., Labugger, R., Kelm, M., Noll, G., Rulicke, T., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 11609–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anonymous (1991) Am. J. Kidney Dis. 18, 50–59.2063855 [Google Scholar]
- 38.Lim, V. S. (1991) Am. J. Kidney Dis. 18, 34–37. [PubMed] [Google Scholar]
- 39.Varet, B., Casadevall, N., Lacombe, C. & Nayeaux, P. (1990) Semin. Hematol. 27, 25–31. [PubMed] [Google Scholar]
- 40.Wagner, K. F., Katschinski, D. M., Hasegawa, J., Schumacher, D., Meller, B., Gembruch, U., Schramm, U., Jelkmann, W., Gassmann, M. & Fandrey, J. (2001) Blood 97, 536–542. [DOI] [PubMed] [Google Scholar]
- 41.Stohlawetz, P. J., Dzirlo, L., Hergovich, N., Lackner, E., Mensik, C., Eichler, H. G., Kabrna, E., Geissler, K. & Jilma, B. (2000) Blood 95, 2983–2989. [PubMed] [Google Scholar]
- 42.Fuste, B., Serradell, M., Escolar, G., Cases, A., Mazzara, R., Castillo, R., Ordinas, A. & Diaz-Ricart, M. (2002) Thromb. Haemostasis 88, 678–685. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.