Abstract
Understanding the ultimate causes of population declines and extinction is vital in our quest to stop the currently rampant biodiversity loss. Comparison of ecological characteristics between threatened and nonthreatened species may reveal these ultimate causes. Here, we report an analysis of ecological characteristics of 23 threatened and 72 nonthreatened butterfly species. Our analysis reveals that threatened butterflies are characterized by narrow niche breadth, restricted resource distribution, poor dispersal ability, and short flight period. Based on the characteristics, we constructed an ecological extinction risk rank and predicted which of the currently nonthreatened species are at the highest risk of extinction. Our analysis reveals that two species currently classified as nonthreatened are, in fact, at high risk of extinction, and that the status of a further five species should be reconsidered.
Keywords: conservation biology, threatened species, biodiversity, Lepidoptera, World Conservation Union Red List
The Convention on Biological Diversity in 1992 marks a historic political commitment by 157 nations to conserve biological diversity (1, 2). Yet today, over a decade later, biological diversity is still declining at an accelerating rate (3–7). The immediate causes of the worldwide decline of biological diversity are anthropogenic disturbance, habitat loss, and climate change (3, 5, 6, 8–15). The immediate causes being recognized, one of the most important challenges for contemporary ecologists is to identify any shared ecological or life history characteristics of species that are the ultimate causes of population declines and extinctions. Understanding the ultimate causes of the population declines and extinctions is of vital importance in our quest to stop biodiversity loss.
Species that are classified as threatened in the World Conservation Union (IUCN) Red Lists face the highest risk of extinction (16). Comparisons of the ecological characteristics of threatened and nonthreatened species may reveal the causes of risk of extinction and help to predict future extinction risk. Here, we analyze the ecological characteristics of the butterfly fauna of Finland.
A total of 116 species of butterflies have been recorded from Finland (17). In our analysis, we included the 95 resident species (18), 23 of which are classified as threatened and 72 as non-threatened (16). In our analysis, we first compare the distribution change and density between threatened and nonthreatened species and then proceed by comparing six ecological characteristics: niche breadth measured as larval specificity, resource distribution, dispersal ability, adult habitat breadth, length of the flight period, and body size. Finally, we construct an ecological extinction risk rank to predict which species face the highest risk of becoming extinct. Our data are predominantly based on published literature (18–22).
Methods
Red List Classification. We used the Finnish Red List (16), which is based on the categories and criteria developed and approved by the IUCN in 1994 (23). Species that are classified as near-threatened (NT), vulnerable (VU), endangered (EN), or critically endangered (CR) were classified as threatened and all others as nonthreatened. The principal determinant of the IUCN Red List classification for butterflies in Finland is the area of occupancy or distribution of species (criteria B1-B2, D2) (16). Area of occupancy has been measured as the number of occupied 10 km × 10 km grid cells. In addition, the number of occupied 1 km × 1 km grid cells within each 10 km × 10 km grid cell was estimated (16). By using this information, Rassi et al. (16) calculated the actual area of occupancy based on models by Virkkala (24), Thomas and Avery (25), and Kunin (26). Given the interrelatedness of the area of occupancy and Red List classification, the strong relationship between Red List classification and distribution of species is not surprising (logistic regression, Nagelkerke R2 = 0.62, χ2 = 50.79, df = 1, n = 95, P < 0.001).
Distribution and Distribution Change. Distribution of butterflies is based on the Atlas of Finnish Macrolepidoptera (18). This atlas contains extensive and detailed distribution data of butterflies in Finland, covering all reliable records and observations of butterflies from 1747 to 1997 (18). The data are compiled from many different sources, including ≈1,500 literature references and information extracted from many large museum and private collections (18). The distribution data in the atlas are divided into old (before 1988) and new (1988–1997) observations. Distribution change is calculated by the difference between the old and new records corrected by the number of observations made during the respective time periods. Correction is made simply by dividing the number of old records (224,239) by the number of new records (173,357) and multiplying the difference between the old records and new records with the quotient (18).
Density. The National Butterfly Recording Scheme in Finland is a formal monitoring study that was established in 1991 for the purpose of monitoring the population trends of the butterflies across the country (20, 27). Monitoring studies may be liable to sampling biases (28). Therefore, explicit instructions were drafted to avoid any bias in sampling or reporting the abundance of butterflies (27). The study provides quantitative abundance data, including the 10 km × 10 km grid square of the Finnish national coordinate system in which the observation was made, the year, the number of individuals of each species observed, and the number of observation days (27). During the first 10-year period (1991–2000) of the study, 432 lepidopterologists provided data on 1,523,989 individuals representing a total of 106 butterfly species.
To obtain density for each butterfly species per 10 km × 10 km grid square, we divided the total number of individuals of each butterfly species by the number of 10 km × 10 km squares occupied by the species. Some rare butterfly species with known occurrence sites may face proportionally higher sampling effort than common species (28). To remove this effect of sampling effort on density, we divided the density by the number of observation days. Note that observation days are the days when the observer was observing at a given square and thus include also days when a given species was not observed. In total, the data comprise 52,066 observation days.
Larval Specificity and Resource Distribution. From this analysis, we excluded the species that occur only in northern Finland (n = 14). This exclusion was done because their larval host plants are either unknown or unconfirmed. The data on larval host-plant specificity in Finland are based on Huldén et al. (18) and Wahlberg (29). We classified the larval host-plant specificity into two classes: monophages (i.e., species that feed only on a single plant species) and polyphages (i.e., species that feed on more than one species of food plants).
To analyze the effects of resource distribution, we restricted our data set only to monophagous butterfly species (n = 23). Our plant distribution data are based on the national floristic database, the Atlas of the Distribution of Vascular Plants in Finland (21). The distribution maps in the atlas are based on 10 km × 10 km grid square distribution data but do not report the numerical data on distribution of plants (number of occupied squares). The authors of the atlas kindly provided us with the numerical data.
Dispersal Ability. To describe the relative dispersal ability of butterfly species, we adopted the method described in Cowley et al. (30). We sent a questionnaire to experienced lepidopterists in Finland and asked them to give a “dispersal ability index” (0–10) for each butterfly species. In the questionnaire, value 0 indicates that a given butterfly species is extremely sedentary, and value 10 means that it is extremely mobile. To obtain a relative dispersal ability for each butterfly species, we calculated the average dispersal ability from returned questionnaires (n = 13).
To ensure our measure of dispersal ability is reliable, we tested how our measure corresponds with four mobility indices estimated previously (30–33). The correlations between our measure and the four previous measures were all strongly positive and significant. For details of these analyses, see Komonen et al. (22).
Adult Habitat Breadth. The habitats that Finnish butterflies occupy have been categorized into four main habitat types (19): (i) uncultivable lands (e.g., edge zones beside industrial area, harbor and storage areas, loading places, uncropped fields, and other unbuilt areas that have been exposed to human impact), (ii) meadows (many kinds of noncultivated open grasslands), (iii) forest edges (e.g., roadsides), and (iv) bogs. We describe the adult niche breadth as the number of habitat types in which the adults typically are found. As there were only two species (Green-veined White, Pieris napi, and Brimstone, Gonepteryx rhamni) that occupied all four main habitat types, habitat types iii and iv were combined. Value 1 represents specialist species (i.e., butterfly species that are limited to one habitat type), value 2 represents intermediate species (i.e., those that occur in two habitat types), and value 3 represents generalist species (i.e., those that occur in three or four habitat types). From this analysis, we excluded the species that occur only in northern Finland (n = 14). This exclusion was done because the habitat types in northern Finland do not correspond the division of habitat types in the rest of Finland.
Flight Period. The average length of the flight period (days) for each butterfly species is based on Marttila et al. (19). When the flight period of a given species was different in northern and southern Finland, we used the flight period in southern Finland. For butterfly species with two generations per year, we used the length of the flight period of the first generation because, in most cases, the second generation is facultative. To get the length of the flight period for species with overwintering adults (n = 5), we summed the flight periods of autumn and spring.
Body Size. We used female wing span (mm) as a measure of butterfly body size, because there is a very strong positive correlation between female and male body size (22). Wing-span measurements for both sexes are based on Marttila et al. (19), in which the mean of a sample of 20 individuals was given, with an exception of some rare species with fewer individuals measured.
Results
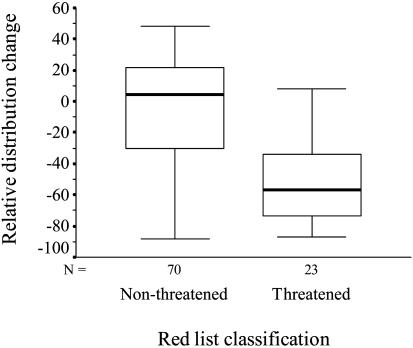
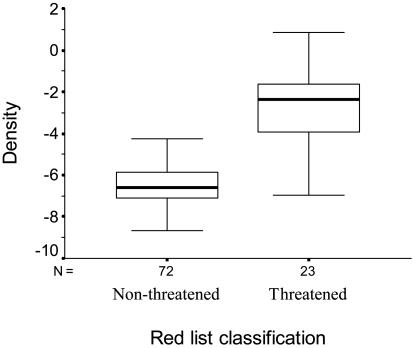
The distribution of Finnish butterflies has declined over the past decade, and this decline is due to threatened species: the distribution of threatened species has remarkably declined on average of 51.5% (one-sample t test against 0, t = –8.42, df = 22, P < 0.001), whereas the distribution of nonthreatened species has not changed (one-sample t test against 0, t =–0.98, df = 69, P = 0.328) (Fig. 1). Surprisingly, the density of threatened species is not lower than that of nonthreatened species; on the contrary, the density of threatened species is significantly higher than that of nonthreatened species (two-sample t test, t =–7.82, df = 93, P < 0.001) (Fig. 2).
Fig. 1.
Relative distribution change (%) of butterflies in relation to their Red List classification (median and quartiles).
Fig. 2.
Density (ln-transformed) of butterflies in relation to their Red List classification (median and quartiles).
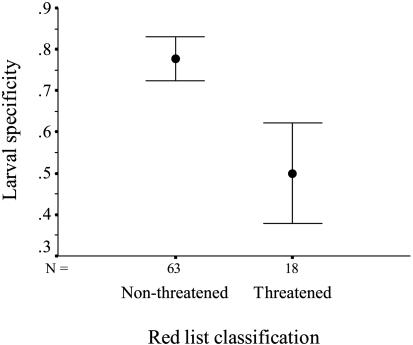
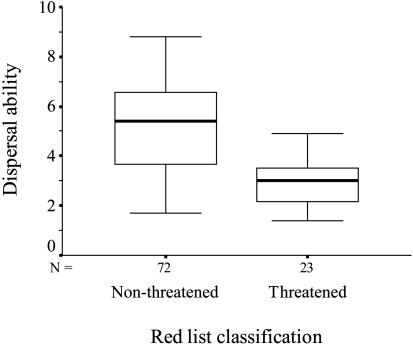
When we analyzed the relationship between species-specific ecological characteristics and their IUCN Red List status, we found that species that have monophagous larvae are more likely to be threatened than species that have polyphagous larvae (logistic regression, χ2 = 4.96, df = 1, n = 81, P = 0.026) (Fig. 3). Furthermore, the distribution of the larval food plant, i.e., the resource distribution of the monophagous butterfly species, is more restricted in threatened species, compared with one of the nonthreatened species (two-sample t test, t = 5.98, df = 21, P < 0.001). Interestingly, we also found that threatened species have poorer dispersal ability than non-threatened species (two-sample t test, degrees of freedom adjusted for unequal variances, t = 6.33, df = 57.8, n = 95, P < 0.001) (Fig. 4). In addition, threatened species have more restricted habitat breadth as adults (two-sample t test, degrees of freedom adjusted for unequal variances, t = 5.53, df = 69.9, n = 81, P < 0.001), and shorter flight period (two-sample t test, t = 2.81, df = 93, P = 0.006) than do nonthreatened species. Finally, we found no evidence that body size would be an important determinant of IUCN Red List status (two-sample t test, t = 0.589, df = 93, P = 0.557).
Fig. 3.
Larval specificity for threatened and nonthreatened species. Original data are coded as 0 for monophagous and 1 for polyphagous species. Bars represent mean and 1 SEM.
Fig. 4.
Dispersal ability of butterflies in relation to their Red List classification (median and quartiles).
By comparing the ecological similarity of threatened and nonthreatened species, we may be able to predict which of the nonthreatened species face the highest risk of extinction. To do this, we constructed an ecological extinction risk rank based on four ecological characteristics: dispersal ability, larval specificity, adult habitat breath, and length of the flight period. First, we ranked the species based on the above four ecological characteristics. Species were ranked independently for each of the characteristics by assigning the smallest rank for the species that had the smallest value of the given ecological characteristic. As an example, Baton Blue, Scolitantides vicrama, had the poorest dispersal ability and thus was assigned value 1 for this characteristic. Next, we summed the ranks of each of the four ecological characteristics for each species to form the ecological extinction risk rank of the species. To analyze how well our ecological extinction risk rank is able to predict the current IUCN Red List classification, we ran a logistic regression between the IUCN Red List classification (nonthreatened vs. threatened) and the ecological extinction risk rank. The ecological extinction risk rank was able to explain 37.3% (Nagelkerke R2) of the variation in the IUCN Red List classification (logistic regression, χ2 = 22.64, df = 1, n = 81, P < 0.001) (Table 1).
Table 1. Ecological extinction risk rank of Finnish butterfly species in order of decreasing risk of extinction.
| Species | EERR | RL |
|---|---|---|
| Melitaea diamina | 1 | EN |
| Scolitantides vicrama | 2 | CR |
| Euphydryas aurinia | 3 | VU |
| Boloria frigga | 4 | |
| Glaucopsyche arion | 5 | CR |
| Lycaena helle | 6 | VU |
| Scolitantides orion | 7 | VU |
| Pyrgus centaureae | 8 | |
| Satyrium w-album | 9 | NT |
| Favonius quercus | 10 | |
| Boloria freija | 11 | |
| Lopinga achine | 12 | NT |
| Boloria titania | 13 | VU |
| Aricia nicias | 14 | |
| Boloria thore | 15 | |
| Melitaea cinxia | 16 | VU |
| Lycaena hippothoe | 17 | |
| Parnassius mnemosyne | 18 | VU |
| Erebia embla | 19 | |
| Satyrium pruni | 20 | |
| Thecla betulae | 21 | |
| Aricia eumedon | 22 | |
| Hipparchia semele | 23 | |
| Parnassius apollo | 24 | NT |
| Carterocephalus silvicola | 25 | |
| Coenonympha tullia | 26 | |
| Carterocephalus palaemon | 27 | |
| Cupido minimus | 28 | EN |
| Pararge aegeria | 29 | |
| Hesperia comma | 30 | NT |
| Coenonympha glycerion | 31 | |
| Oeneis jutta | 32 | |
| Araschnia levana | 33 | |
| Maniola jurtina | 34 | NT |
| Euphydryas maturna | 35 | |
| Colias palaeno | 36 | |
| Glaucopsyche alexis | 37 | VU |
| Aporia crataegi | 38 | |
| Pyrgus alveus | 39 | |
| Apatura iris | 40 | NT |
| Boloria aquilonaris | 41 | |
| Lasiommata maera | 42 | |
| Argynnis niobe | 43 | |
| Melitaea athalia | 44 | |
| Boloria eunomia | 45 | |
| Albulina optilete | 46 | |
| Limenitis populi | 47 | |
| Aricia artaxerxes | 48 | |
| Lycaena phlaeas | 49 | |
| Pyrgus malvae | 50 | |
| Leptidea sinapis | 51 | |
| Polyommatus icarus | 52 | |
| Lasiommata petropolitana | 53 | |
| Lycaena virgaureae | 54 | |
| Erebia ligea | 55 | |
| Argynnis aglaja | 56 | |
| Coenonympha pamphilus | 57 | |
| Polyommatus semiargus | 58 | |
| Issoria lathonia | 59 | NT |
| Boloria euphrosyne | 60 | |
| Argynnis laodice | 61 | |
| Argynnis paphia | 62 | |
| Thymelicus lineola | 63 | |
| Papilio machaon | 64 | |
| Aphantopus hyperantus | 65 | |
| Celastrina argiolus | 66 | |
| Boloria selene | 67 | |
| Plebejus idas | 68 | |
| Brenthis ino | 69 | |
| Callophrys rubi | 70 | |
| Plebejus argus | 71 | |
| Argynnis adippe | 72 | |
| Aglais io | 73 | |
| Aglais urticae | 74 | |
| Polyommatus amandus | 75 | |
| Anthocharis cardamines | 76 | |
| Ochlodes faunus | 77 | |
| Nymphalis antiopa | 78 | |
| Pieris napi | 79 | |
| Polygonia c-album | 80 | |
| Gonepteryx rhamni | 81 |
EERR, ecological extinction risk rank; RL, current World Conservation Union Red List classification; EN, endangered; CR, critically endangered; VU, vulnerable; NT, near threatened.
Discussion
Somewhat surprisingly, threatened species had higher density than did nonthreatened species. This result may be good news for current conservation planning that focuses on species listed as threatened in the IUCN Red Lists (34). Recently, Possingham et al. (35) questioned the focusing of limited resources on the most threatened species, but our results suggest that despite being under threat, the few populations that still persist are relatively numerous and thus likely to be viable.
Our data show that the risk of extinction of butterflies can be predicted from a few key ecological characteristics: threatened species are specialists in both larval resource requirements and adult habitat requirements, they are poor dispersers, and they have a short flight period. Based on our ecological extinction risk rank, we were able to predict the risk of extinction of butterflies with an accuracy of nearly 40%. Although not perfect, the predictive value of this rank is high enough to allow us to use it to predict which of the currently nonthreatened species are at the highest risk of becoming threatened. There are two nonthreatened species that closely share the ecological characteristics of the threatened species: Frigga's Fritillary, Boloria frigga, and Grizzled Skipper, Pyrgus centaureae (Table 1). Over the past decade, both of these species have declined drastically in southern Finland (distribution decline 42% and 32%, respectively), where they primarily occur in natural pine bogs. This decline is likely the result of habitat loss due to extensive draining of the bogs during the latter half of the 20th century. There are a further three to five species that share many of the ecological characteristics of the threatened species, and thus their status should be investigated further (Table 1). If we consider the other end of the ecological extinction risk rank, there seem to be two species, Purple Emperor, Apatura iris, and Queen of Spain Fritillary, Issoria lathonia, that possibly should not be listed as threatened (Table 1). Both are strongly migratory species, having their main distribution in lower latitudes. According to Huldén et al. (18), both species are resident, but in fact, the more stable populations in the lower latitudes are likely to act as sources from where they are able to migrate to Finland. Thus, their residency is likely to be heavily dependent on regular migrations and therefore these species should not be included in the Red List classification of Finnish butterflies.
Here, we illustrated how basic ecological knowledge of an assemblage of species can be utilized to predict the risk of extinction of species. Unfortunately, even the basic ecology of species is not readily available for most taxa. The lack of such data is a serious concern illustrating the fact that, in our scientific society, the detailed observations and often tedious work of the traditional naturalists (36–38) are no longer respected. Nevertheless, our results show that this basic ecological information plays a vital role in our attempts to understand the ultimate causes of population declines and extinctions. Only when we understand the causes of extinction can we successfully plan management that is effective in stopping the loss of biodiversity.
Acknowledgments
We thank Kimmo Saarinen for generously providing us the data on the number of observation days; Raino Lampinen and Arto Kurtto for the numerical data for the distribution of plants; and Mikael Puurtinen for valuable comments on the manuscript. J.S.K., V.K., and A.K. were supported by the Academy of Finland.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: IUCN, World Conservation Union.
References
- 1.Anonymous (1992) Report of the United Nations Conference on Environment and Development, Rio de Janeiro, June 3–14 (United Nations, New York).
- 2.Glowka, L., Burhenne-Guilmin, F., Synge, H., McNeely, J. & Gündling, L. (1994) A Guide to the Convention on Biological Diversity (IUCN, Cambridge, U.K.).
- 3.Novacek, M. & Cleland, E. (2001) Proc. Natl. Acad. Sci. USA 98, 5466–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous (2002) United Nations Report of the World Summit on Sustainable Development, Johannesburg, South Africa, Aug. 26–Sept. 4 (United Nations, New York).
- 5.Brook, B., Sodhi, N. & Ng, P. (2003) Nature 424, 420–423. [DOI] [PubMed] [Google Scholar]
- 6.Thomas, C., Cameron, A., Green, R., Bakkenes, M., Beaumont, L., Collingham, Y., Erasmus, B., Ferreira de Siqueira, M., Grainger, A., Hannah, L., et al. (2004) Nature 427, 145–148. [DOI] [PubMed] [Google Scholar]
- 7.Thomas, J. A., Telfer, M. G., Roy, D. B., Preston, C. D., Greenwood, J. J. D., Asher, J., Fox, R., Clarke, R. T. & Lawton, J. H. (2004) Science 303, 1879–1881. [DOI] [PubMed] [Google Scholar]
- 8.Chapin, F., III, Zavaleta, E., Eviner, V., Naylor, R., Vitousek, P., Reynolds, H., Hooper, D., Lavorel, S., Sala, O., Hobbie, S., et al. (2000) Nature 405, 234–242. [DOI] [PubMed] [Google Scholar]
- 9.Wilson, E. (1985) Bioscience 35, 700–706. [Google Scholar]
- 10.Pimm, S., Russell, G., Gittleman, J. & Brooks, T. (1995) Science 269, 347–350. [DOI] [PubMed] [Google Scholar]
- 11.Pounds, J., Fogden, M. & Campbell, J. (1999) Nature 398, 611–615. [Google Scholar]
- 12.Warren, M., Hill, J., Thomas, J., Asher, J., Fox, R., Huntley, B., Roy, D., Telfer, M., Jeffcoate, S., Harding, P., et al. (2001) Nature 414, 65–68. [DOI] [PubMed] [Google Scholar]
- 13.Fahrig, L. (2003) Annu. Rev. Ecol. Syst. 34, 487–515. [Google Scholar]
- 14.Gaston, K., Blackburn, T. & Goldewijk, K. (2003) Proc. R. Soc. London Ser. B 270, 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.du Toit, J., Walker, B. & Campbell, B. (2004) Trends Ecol. Evol. 19, 12–17. [DOI] [PubMed] [Google Scholar]
- 16.Rassi, P., Alanen, A., Kanerva, T. & Mannerkoski, I. (2001) The 2000 Red List of Finnish Species (Ministry of the Environment and Finnish Environment Institute, Helsinki).
- 17.Kullberg, J., Albrecht, A., Kaila, L. & Varis, V. (2002) Sahlbergia 6, 45–190. [Google Scholar]
- 18.Huldén, L., Albrecht, A., Itämies, J., Malinen, P. & Wettenhovi, J. (2000) Atlas of Finnish Macrolepidoptera (Lepidopterological Society of Finland, Finnish Museum of Natural History, Helsinki).
- 19.Marttila, O., Haahtela, T., Aarnio, H. & Ojalainen, P. (1990) Suomen Päiväperhoset (Kirjayhtymä, Helsinki).
- 20.Marttila, O., Saarinen, K. & Lahti, T. (2001) Baptria 26, 29–61. [Google Scholar]
- 21.Lahti, T., Lampinen, R. & Kurtto, A. (1995) Atlas of the Distribution of Vascular Plants in Finland (University of Helsinki, Finnish Museum of Natural History, Botanical Museum, Helsinki).
- 22.Komonen, A., Kaitala, V., Kotiaho, J. S. & Päivinen, J. (2004) Oikos 105, 41–54. [Google Scholar]
- 23.Anonymous (1994) IUCN Red List Categories (IUCN, Gland, Switzerland).
- 24.Virkkala, R. (1993) Oikos 67, 218–226. [Google Scholar]
- 25.Thomas, C. D. & Avery, J. C. G. (1995) Biol. Conserv. 73, 59–65. [Google Scholar]
- 26.Kunin, W. E. (1998) Science 281, 151–155. [DOI] [PubMed] [Google Scholar]
- 27.Saarinen, K., Lahti, T. & Marttila, O. (2003) Biodiversity Conserv. 12, 2147–2159. [Google Scholar]
- 28.Dennis, R. L. H., Sparks, T. H. & Hardy, P. B. (1999) J. Insect Conserv. 3, 33–42. [Google Scholar]
- 29.Wahlberg, N. (2000) Entomol. Fenn. 11, 167–174. [Google Scholar]
- 30.Cowley, M. J. R., Thomas, C. D., Roy, D. B., Wilson, R. J., León-Cortés, J. L., Gutiérrez, D., Bulman, C. R., Quinn, R. M., Moss, D. & Gaston, K. J. (2001) J. Anim. Ecol. 70, 410–425. [Google Scholar]
- 31.Bink, F. A. (1992) Ecologische Atlas van de Dagvlinders van Nordwest-Europa (Schuyt, Haarlem, The Netherlands).
- 32.Pollard, E. & Yates, T. J. (1993) Monitoring Butterflies for Ecology and Conservation (Chapman & Hall, London).
- 33.Cook, L. M., Dennis, R. L. H. & Hardy, P. B. (2001) Ecography 24, 497–504. [Google Scholar]
- 34.Lamoreux, J., Akçakaya, H. R., Bennun, L., Collar, N. J., Boitani, L., Brackett, D., Bräutigam, A., Brooks, T. M., da Fonseca, G. A. B., Mittermeier, R. A., et al. (2003) Trends Ecol. Evol. 18, 214–215. [Google Scholar]
- 35.Possingham, H. P., Andelman, S. J., Burgman, M. A., Medellín, R. A., Master, L. L. & Keith, D. A. (2002) Trends Ecol. Evol. 17, 503–507. [Google Scholar]
- 36.Darwin, C. (1884) The Origin of Species by Means of Natural Selection (John Murray, London).
- 37.Fabre, J. H. (1918) The Sacred Beetle (Hodder & Stoughton, London).
- 38.Huxley, T. H. (1880) The Crayfish: An Introduction to the Study of Zoology (Kegan Paul, London).