Abstract
Treatments based on antimonials to cutaneous leishmaniasis (CL) entail a range of toxic side effects. Propolis, a natural compound widely used in traditional medical applications, exhibits a range of biological effects, including activity against infectious agents. The aim of this study was to test the potential leishmanicidal effects of different propolis extracts against Leishmania (Viannia) braziliensis promastigotes and intracellular amastigotes in vitro. Stationary-phase L. (V) braziliensis promastigotes were incubated with medium alone or treated with dry, alcoholic, or glycolic propolis extract (10, 50, or 100 μg/mL) for 96 h. Our data showed that all extracts exhibited a dose-dependent effect on the viability of L. (V) braziliensis promastigotes, while controlling the parasite burden inside infected macrophages. Dry propolis extract significantly modified the inflammatory profile of murine macrophages by downmodulating TGF-β and IL-10 production, while upmodulating TNF-α. All three types of propolis extract were found to reduce nitric oxide and superoxide levels in activated L. braziliensis-infected macrophages. Altogether, our results showed that propolis extracts exhibited a leishmanicidal effect against both stages of L. (V) braziliensis. The low cell toxicity and efficient microbicidal effect of alcoholic or glycolic propolis extracts make them candidates to an additive treatment for cutaneous leishmaniasis.
1. Introduction
Leishmaniasis is a neglected vector-borne tropical disease caused by obligate protozoan parasites of the genus Leishmania [1–3]. Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis worldwide, representing 50–75% of all new cases. According to the World Health Organization (WHO), the number of CL cases is around 1–1.5 million annually [4, 5]. In Brazil, Leishmania (V) braziliensis is the etiological agent mainly responsible for CL [6]. The CL lesion then ulcerates and may become secondarily infected with bacteria. Secondary bacterial infections in CL lesions are responsible for pain, can prolong disease duration, increase tissue destruction, and result in increased scarring [7, 8].
Treatment of CL can be complex, and this disease may be chronic and latent in the human host. Chemotherapy to treat leishmaniasis has been based on the parenteral administration of pentavalent antimonials for more than 60 years [9]. These compounds are highly toxic and expensive and have been associated with drug resistance [1]. Amphotericin B and Paromomycin, two other currently available second-line antileishmanial treatments [10, 11], also present significant shortcomings with regard to toxicity, cost, and duration of treatment. In this context, the search for new, safer, and more effective formulations [12], including substances from natural sources, offering less expensive and less toxic treatment options is urgently needed [13].
Propolis, a natural compound produced by Apis mellifera honeybees, has been widely used in traditional applications [14]. This substance has shown promising results against different infectious agents and exhibits a broad spectrum of biological properties [15–18]. The chemical composition of propolis is dependent on the biodiversity of each area visited by bees, as well as the method of extraction. These can influence the quantity and makeup of the specific biologically active compounds present in each sample, potentially leading to a range of biological effects [19, 20].
Previous studies using alcoholic and glycolic EPP-AF® extracts showed a potential antibacterial and antifungal effect in in vitro and in vivo models against Staphylococcus aureus (ATCC 25923), Staphylococcus aureus (ATCC 43300), Staphylococcus epidermidis (ATCC 14990) [21], Saccharomyces cerevisiae [22], and Candida albicans [23]. Berretta et al. [24] also showed the ability of glycolic extract to improve skin wound healing, such as in rat models of burn injury. Furthermore, a recent study also showed the ability of alcoholic propolis extract to inhibit the inflammasome, a key function mediated by the innate immune system, by reducing IL-1β secretion in mouse macrophages and decreasing the activation of caspase-1 [25].
Previous reports have shown that propolis extracts, mainly alcoholic extract, exhibit prominent microbicidal effects in vitro against Leishmania parasites, as well as reduced lesion size during experimental infection [26–30]. The present study shows for the first time a comparison of the potential leishmanicidal effect among distinct presentations of green propolis extracts, against promastigotes and intracellular amastigotes of Leishmania (V.) braziliensis. Uncovering which type of excipient and chemical presentation produces the extract with most potent leishmanicidal effect is critical for development of more effective adjunct therapies to patients with poor response to conventional antimicrobial treatment.
2. Methodology
2.1. Material and Reagents
Purified water (Milli-Q), HPLC grade methanol (J. T. Baker, L. 9093-68), formic acid (Vetec, L.0804789), caffeic acid (Fluka, L. 43706045),ρ-coumaric acid (Fluka, L.3250759), cinnamic acid (Fluka, L.21907066), isosakuranetin (ChromaDex), Artepillin C (Wako, L. 016.19131), 3,4-dicaffeoylquinic acid (Phytolab; L. 13672938; ≥90,0% de purity), 3,5-dicaffeoylquinic acid (Phytolab; L. 13672946; ≥90,0% of purity), 4,5-dicaffeoylquinic acid (Phytolab; L. 13672903; ≥90,0% purity), gallic acid (Synth, L.109250), sodium bicarbonate (Vetec, L.0906112), and aromadendrin-4′O-methyl ether were previously isolated, identified, and [31] kindly provided by the authors. High performance liquid chromatography (HPLC) was performed using a Shimadzu chromatograph equipped with a CBM-20A controller, LC-20AT quaternary pump, an SPD-M diodes 20A array detector, a Shimadzu Shim-Pack column CLC-ODS (M) (4.6 mm × 250 mm, 5 mm particle diameter, pore diameter, 100 Å), and Shimadzu LC software version 1.21 SP1. Schneider's insect medium, lipopolysaccharide (LPS), Acridine Orange, Ethidium Bromide, IFN-γ, and hydroxylamine were obtained from SIGMA-Aldrich (St Louis, MO, USA). Inactive fetal bovine serum (FBS), RPMI medium, penicillin, and Amphotericin B were purchased from GIBCO (Carlsbad, CA, USA). Streptomycin, L-glutamine, and Alamar Blue® were obtained from Invitrogen (Carlsbad, CA, USA). In addition, macrophage colony stimulating factor (M-CSF) was purchased from PEPROTECH (Rocky Hill, NJ, USA), mouse TNF-alpha and TGF-beta 1 Quantikine ELISA kits were obtained from R&D Systems (Minneapolis, MN), and a superoxide dismutase activity assay kit was purchased from Cayman Chemical Company (Ann Arbor, Michigan).
2.2. Propolis Extract Preparation
Standardized alcoholic propolis extract (Batch 1402110) (PSE), glycolic propolis extract (Batch 1480210) (PGE), and water-soluble propolis dry extract (Batch 9050213) (PSDE) were produced by Apis Flora (Ribeirão Preto/SP-Brazil) by formulating a mixture of raw propolis materials [24]. These “blends” of raw propolis material were made to effectively standardize the qualitative and quantitative chemical composition of all batches, that is, ensuring reproducibility, since the compounds and concentrations of substances present in propolis vary in accordance with each geographical region of production (the states of Minas Gerais, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul) [24].
To prepare the three different extracts, a mixture of raw propolis materials was first kept in a freezer for 12 hours and then reduced to a fine powder under maceration. To obtain each type of extract, standardized crude propolis material was initially extracted using an alcohol solution (7 : 3) in a dynamic maceration process, followed by percolation and filtration. Propolis glycolic extract (PGE) was obtained from standardized alcoholic propolis extract after evaporation of the ethanol portion and the addition of propylene glycol. The alcoholic and glycolic extracts contained 11% w/v of propolis dry matter. The dry propolis extract was obtained via a concentration of alcoholic extract containing around 80% of propolis dry material, followed by alkaline hydrolysis and conversion in aqueous extract by slowly adding heated purified water. The hydrolysis process following the evaporation of the hydroalcoholic solvent resulted in the ionization of the compounds found in propolis, transforming them into water-soluble structures (aqueous extract) [32]. Maltodextrin, at a ratio of 7 : 3 (propolis : maltodextrin by dry weight), was added and mixed under stirring and then is dried using a spray drying process.
2.3. HPLC Chemical Characterization
The propolis extracts were quantitatively analyzed on high performance liquid chromatography (HPLC). The mobile phase consisted of a gradient of methanol and acidified water with formic acid (0.1% v/v) ranging from 20% to 95%, for a runtime of 77 minutes at a flow rate of 0.8 mL/min. The detection wavelength was set at 275 nm [24]. To assess the flavonoid content in the extracts, the aluminium chloride method was employed as previously described by Woiskya and Salantino [33]. All samples were prepared in accordance with the mass and dilutions necessary to quantification in the analytical curve.
2.4. Biological Assay
2.4.1. Ethics Statement
Male BALB/c mice aged 6–8 weeks were obtained from the animal care facility at CPqGM/FIOCRUZ, located in the city of Salvador, Bahia, Brazil. All animal experimentation was conducted in accordance with the Guidelines for Animal Experimentation as established by the Brazilian Council for Animal Experimentation Control (CONCEA). The present study received approval from the local institutional review board (CEUA) (protocol: CEUA-015/2015-CPqGM/FIOCRUZ).
2.4.2. Parasites
Leishmania Viannia braziliensis (MHOM/BR/01/BA788) parasites were cultured in Schneider's insect medium supplemented with 10% inactive fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM L-glutamine in 25 cm2 flasks at 24°C for seven days.
2.4.3. L. (V.) braziliensis Promastigote Viability Assay
Stationary-phase L. (V.) braziliensis promastigotes (2 × 105/mL) were cultivated in supplemented Schneider medium (as described above) alone or in the presence of three concentrations (10, 50, and 100 μg/mL) of dry, alcoholic, or glycolic propolis extract. Amphotericin B (0.25 μg/mL) was used as a positive control. All cultures were incubated for 120 hours at 24°C, after which the number of viable promastigotes was determined by direct counting performed daily in a Neubauer Chamber.
2.4.4. Fluorescence Microscopy
Stationary-phase L. (V.) braziliensis promastigotes (5 × 105/mL) were cultured in supplemented Schneider medium (as described above) alone or in the presence of dry, alcoholic, and glycolic propolis extract (50 μg/mL) for 96 h at 24°C. Treatment with Amphotericin B (0.5 μg/mL) was used as positive control. The samples were mounted on cytospin slides to fluorescence microscopy. A solution containing 100 μg/mL Acridine Orange and 100 μg/mL Ethidium Bromide was prepared as previously described and added to the slides containing promastigotes [34, 35]. Parasite staining was assessed using a fluorescence microscope (OLYMPUS, Japan). Parasite promastigotes were considered alive when positively stained by Acridine Orange, while Ethidium Bromide staining was used to detect dead cells.
2.4.5. Scanning Electron Microscopy
Stationary-phase L. (V.) braziliensis promastigotes (5 × 105/mL) were cultured in supplemented Schneider medium (as described above) with dry, alcoholic, and glycolic propolis extracts (50 μg/mL) for 96 h at 24°C. Supplemented Schneider medium was used as a control. The samples were then attached to coverslips and fixed in a solution containing 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH = 7.4). After fixation, cells were washed in cacodylate buffer and postfixed with 1% osmium tetroxide. All samples were then dehydrated in an ethanol series (70, 80, 90, and 100°GL). Cells were finally dried by the critical point method, mounted on stubs, coated with gold (20–30 nm), and observed in a Jeol JSM 6390LV scanning electron microscope.
2.4.6. Transmission Electron Microscopy
Stationary-phase L. (V.) braziliensis promastigotes (107/mL) were cultured in Schneider's medium with dry extract and alcoholic and glycolic propolis extracts (50 μg/mL) for 96 h. Supplemented Schneider medium was used as a control. All samples were fixed and postfixed as described above. Cells were then dehydrated in an acetone series (70, 80, 90, and 100°GL) and embedded in Polybed resin. Ultrathin sections were mounted on 300-mesh grids, stained with 5% uranyl acetate and lead citrate, and then observed using a Jeol Jem 1230 transmission electron microscope.
2.4.7. Macrophage Toxicity Assay
Human macrophages (3 × 105/well) were isolated from peripheral blood mononuclear cells (PBMC) of healthy donors by Ficoll gradient centrifugation and plastic adherence and then allowed to differentiate into macrophages in vitro (7 days), with RPMI medium supplemented with 10% FBS, 100 U/mL penicillin, 100 mmg/mL streptomycin, and 2 mM L-glutamine and 50 nM of M-CSF.
Bone marrow-derived murine (BMM) cells were harvested from BALB/c mice femurs and cultured at 37°C under 5% CO2 for 7 days in RPMI medium supplemented with 20% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM L-glutamine and 30% L929 cell culture supernatant as a source of macrophage colony stimulating factor. Next, differentiated BMMs were detached from the plate using cold saline solution. BMMs (105/well) were plated in 96-well plates and cultured at 37°C under 5% CO2 in RPMI-supplemented medium for 24 hours.
Human and BMMs uninfected macrophages were then treated with either dry extract and alcoholic or glycolic propolis extracts at varying concentrations (10, 50, and 100 μg/mL) at 37°C for 48 h. Next, the cells were reincubated for another 4 h with supplemented RPMI medium containing 10% Alamar Blue. The reagent absorbance was read at 570 nm and 600 nm using a spectrophotometer (SPECTRA Max 190). Hydrogen peroxide (H2O2) was used as positive control.
2.4.8. Macrophage Infection
Human and BMM monocytes were isolated as described above and 2 × 105/cells per well were seeded in 96-well plates. Macrophages were infected (10 : 1) with stationary-phase Leishmania (V.) braziliensis (MHOM/BR/01/BA788) promastigotes for 24 h and treated with varying concentrations (10, 50, and 100 μg/mL) of one of the three propolis extracts for 48 h. Next, the medium was replaced with 0.2 mL of supplemented Schneider medium. Cells were then cultured at 24°C for an additional five days and the number of viable parasites was determined by direct counting. Amphotericin B (0.25 μg/mL) was used as positive control.
2.4.9. Quantification of Inflammatory and Oxidative Stress Mediators
BMMs (106/well) were stimulated with IFN-γ (100 UI/mL) for 24 h and infected with L. (V) braziliensis stationary-phase promastigotes (107/well) for another 24 h. The macrophages were then washed to remove any noninternalized parasites, the RPMI cell medium was replaced, and IFN-γ stimulation was reapplied together with 50 μg/mL of dry extract and alcoholic or glycolic propolis extract for 48 h [13, 36]. Next, culture supernatants were collected. The Griess reaction was used to measure nitric oxide (NO) and superoxide (O2−) production. O2− production was assessed by adding hydroxylamine (0.5 mM) to infected macrophages, which converts superoxide into nitrite [37]. Background levels of nitrite generated by the release of NO were determined in parallel with O2−, without the addition of hydroxylamine [13]. Superoxide dismutase (SOD) activity was determined using an SOD activity assay kit. Production of IL-10, TGF-β, and TNF-α was evaluated using a Quantikine ELISA kit in accordance with manufacturer instructions.
2.4.10. Statistical and Data Analyses
Data are presented as the mean ± standard deviation (SD) from experiments performed in quintuplicate. GraphPad Prism Software 5.0 (GraphPad, San Diego, CA) was used for all data analyses. The Kruskal–Wallis nonparametric test with Dunn's posttest was used for multiple comparisons. Linear trend ad hoc analysis was used to evaluate statistical significance among the groups, considered when p < 0.05.
3. Results
3.1. Chemical Characterization of Standardized Propolis Extracts
The chemical compounds found in each type of extract, following normalization to 11% of propolis dry matter, are listed in Table 1. Alcoholic extract presented higher values for each compound, except Artepillin C, baccharin, and total flavonoids as quercetin (5.329, 0.500, and 5.794 mg/g resp.). Glycolic extract showed the highest values of 3,5-dicaffeoylquinic acid (1.703 mg/g) and total flavonoids (6.625 mg/g), while dry extract (dryness) showed markedly more caffeic acid (0.642 mg/g), Artepillin C (7.076 mg/g), and baccharin (0.907 mg/g).
Table 1.
Chemical characterization of propolis samples.
| Compounds | Alcoholic (mg/g) | Glycolic (mg/g) | Dry (mg/g) |
|---|---|---|---|
| Caffeic acid | 0.215 ± 0.001 | 0.200 ± 0.001 | 0,642 ± 0.032 |
| p-Coumaric acid | 1.315 ± 0.008 | 1.272 ± 0.006 | 1.037 ± 0.003 |
| 3,5-Dicaffeoylquinic acid | 1.617 ± 0.020 | 1.703 ± 0.017 | 0.696 ± 0.019 |
| 4,5-Dicaffeoylquinic acid | 3.732 ± 0.231 | 2.488 ± 0.031 | 1.422 ± 0.012 |
| Cinnamic acid | 0.306 ± 0.014 | ND | 0.029 ± 0.001 |
| Aromadendrin | 0.955 ± 0.028 | 0.479 ± 0.027 | 0.064 ± 0.007 |
| Drupanin | 3.254 ± 0.060 | 2,993 ± 0.148 | 2.167 ± 0.008 |
| Artepillin C | 5.329 ± 0.077 | 4.675 ± 0.182 | 7.076 ± 0.040 |
| Baccharin | 0.500 ± 0.026 | 0.452 ± 0.045 | 0.907 ± 0.007 |
| Total flavonoids | 5.794 ± 0.017 | 6.625 ± 0.026 | 4,744 ± 0.359 |
Alcoholic and glycolic extract contained 11% w/v of propolis dry matter (n = 3). Data shown represent mean and SD values. ND: not detected.
3.2. Exposure to Propolis Extract Reduces the Viability of Leishmania (V.) braziliensis Promastigotes
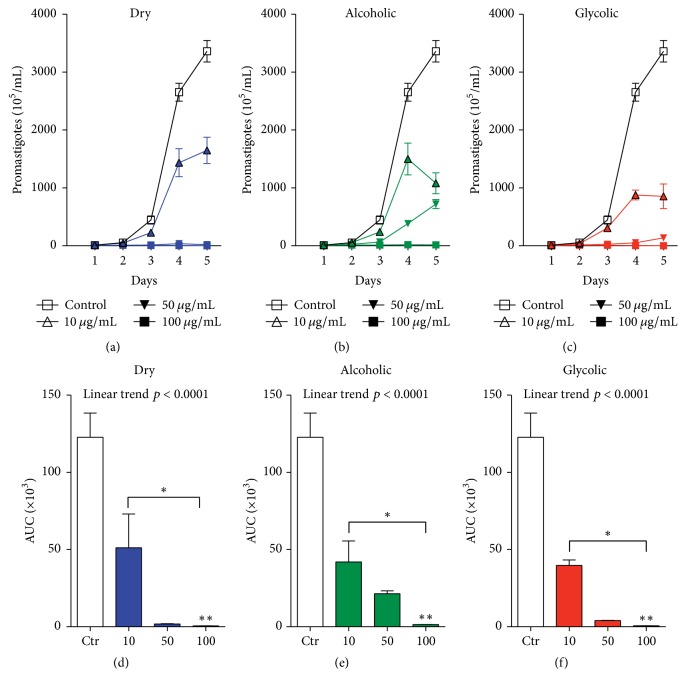
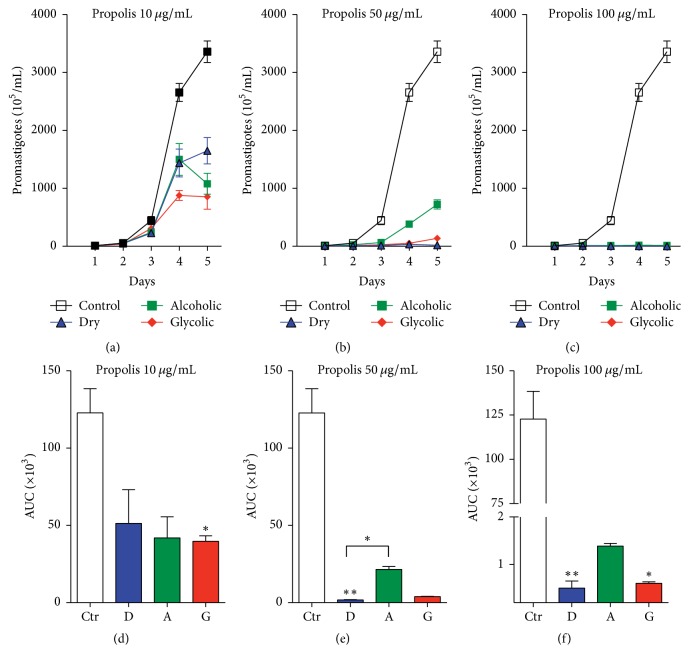
To analyze the direct effect of each type of propolis extract on parasite viability, stationary-phase L. (V.) braziliensis promastigotes were incubated with one of three extracts: dry, alcoholic, and glycolic extract at different concentrations (10, 50, and 100 μg/mL) for 96 h. The viability assay (Figures 1(a)–1(c)) showed a dose-dependent reduction in the number of viable promastigotes in comparison with untreated controls, as demonstrated by analysis of the Area under the Curve (Figures 1(d)–1(f)). The propolis extracts used at 50 μg/mL and 100 μg/mL concentrations demonstrated significant leishmanicidal effect in comparison to untreated controls (Figure 2). The following reductions in promastigotes were observed at treatment concentrations of 10, 50, and 100 μg/mL, respectively, in comparison to untreated parasites (123 ± 15.6): dry extract: 58.3% (58.3 ± 21.8), 98.5% (1.7 ± 0.19), and 99.5% (0.5 ± 0.14); alcoholic extract: 65.9% (41.9 ± 13.6), 82.6% (21.4 ± 1.8), and 98.8% (1.3 ± 0.05); glycolic extract: 67.7% (39.7 ± 3.5), 96.7% (3.96 ± 0.16), and 99.5% (0.6 ± 0.03). Treatment with Amphotericin B (0.25 μg/mL), used as positive control for leishmanicidal activity, completely eliminated all parasites after 24 h of treatment (data not shown).
Figure 1.
Dose-response effect of propolis extract on L (V). braziliensis promastigote viability. Parasites were incubated for 96 h with 10, 50, or 100 ug/mL of either (a and d) dry, (b and e) alcoholic, or (c and f) glycolic propolis extract. Bars represent means ± SD of two representative experiments performed in quadruplicate. AUC accounts for Area under the Curve and it corresponds to the area of the geometric figure made by the concentration curve of a drug as a function of time. The Kruskal–Wallis nonparametric test, followed by Dunn's posttest, was used to compare among experimental groups (∗p < 0.05, ∗∗p < 0.01, and, posttest for linear trend, p < 0.0001).
Figure 2.
Viability of L. (V) braziliensis promastigotes in response to each type of propolis extract. Parasites were incubated for 96 h with dry, alcoholic, and glycolic propolis extract at (a and d) 10 μg/mL, (b and e) 50 μg/mL, or (c and f) 100 μg/mL. Bars represent means ± SD of two representative experiments performed in quadruplicate. AUC accounts for Area under the Curve and it corresponds to the area of the geometric figure made by the concentration curve of a drug as a function of time. The Kruskal–Wallis nonparametric test, followed by Dunn's posttest, was used to compare among experimental groups (∗p < 0.05, ∗∗p < 0.01, and, posttest for linear trend, p < 0.0001).
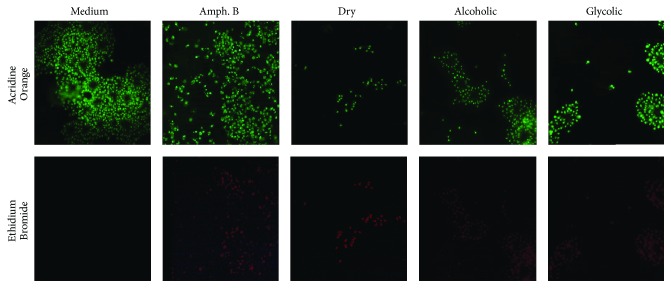
The propolis extract treatment concentration was standardized at 50 μg/mL for subsequent promastigote experimentation and analysis. Moreover, fluorescence microscopy employing double staining with Acridine Orange/Bromide Ethidium (OA/BE) (Figure 3) revealed that no significant cell death was observed in the untreated control group (medium), whereas all types of propolis extract (50 μg/mL), as well as the positive control (Amph. B), successfully killed L. (V) braziliensis in vitro (Figure 3).
Figure 3.
L. (V) braziliensis promastigote viability assessed by fluorescence microscopy. Live cells were stained with Acridine Orange (green color) and dying cells with Ethidium Bromide (red color). L. braziliensis promastigotes were treated with medium alone or with dry, alcoholic, or glycolic extracts of propolis (50 μg/mL) for 96 h. Amphotericin B treatment (0.5 μg/mL) for 24 h was used as a positive control for cell death. Magnification: 1000x.
3.3. Morphological and Ultrastructural Analyses of Treated Leishmania (V.) braziliensis Promastigotes
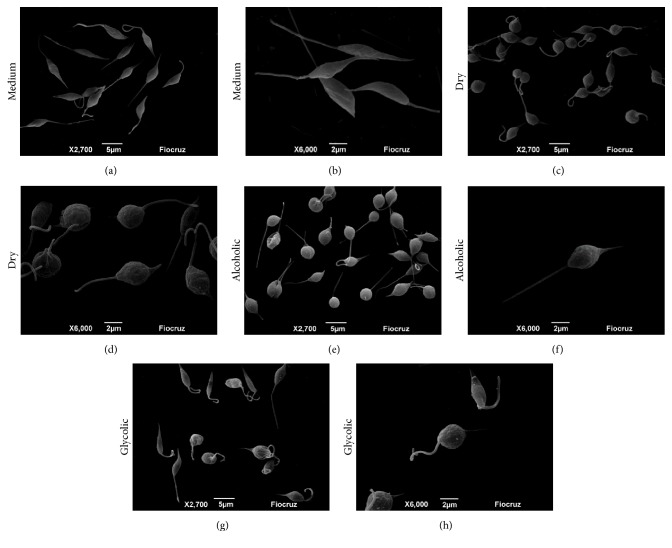
Scanning electron microscopy (SEM) was employed to document morphological changes induced by treatment with propolis extract. Normal morphology was preserved in untreated parasites (Figures 4(a) and 4(b)), for example, stable cell surfaces, typically elongated shapes, and longer flagella. On the other hand, parasites treated with each type of propolis extract (50 μg/mL) exhibited marked morphological changes, such as cell shrinkage and rounded forms, in addition to irregular surface protrusions and indentations in the plasma membrane (Figures 4(c)–4(h)).
Figure 4.
Scanning electron microscopy of morphological changes in L. (V) braziliensis promastigotes. Parasites were treated with either medium alone or dry, alcoholic, or glycolic extracts of propolis (50 μg/mL) for 96 h. Image amplifications show scale bar representative of 5 μm or 2 μm.
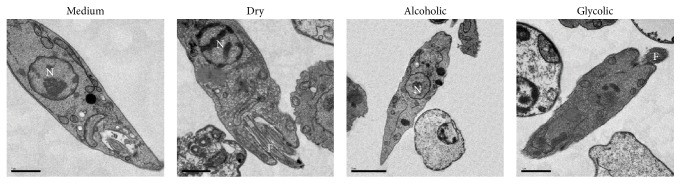
Transmission electron microscopy (TEM) was used to investigate ultrastructural changes in treated L. (V) braziliensis promastigotes (Figure 5). Analysis by TEM showed that control parasites retained normal morphology, with clearly defined membranes, preserved nuclei, Golgi complex, and flagellar pocket. In contrast, the parasites treated with alcoholic and glycolic extracts showed electron-dense granules in the cytoplasm without well-demarcated cytoplasmic organelles, suggestive of cell death. The parasites treated with dry extract showed less-marked morphological alterations, with irregular cell surfaces and an increased density of electron-dense granules in comparison to untreated promastigotes.
Figure 5.
Transmission electron microscopy of ultrastructural changes and cell death in L. (V) braziliensis promastigotes. Parasites were treated with either medium alone or dry, alcoholic, or glycolic extracts of propolis (50 μg/mL) for 96 h. Nucleus (N). Flagellum (F). Scale bars represent 1 μm.
3.4. Macrophage Viability and the Leishmanicidal Effects of Propolis Extract on Human and Murine Macrophages In Vitro
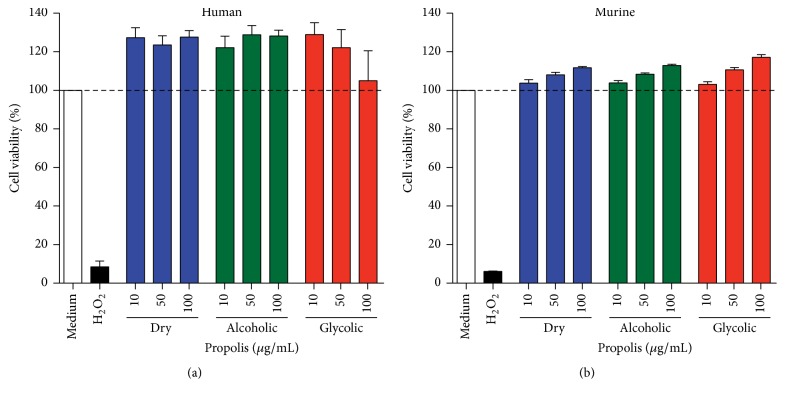
Cell viability was unaffected by each concentration propolis extract tested, as assayed by Alamar Blue, in human (Figure 6(a)) and BALB/c murine macrophages (Figure 6(b)).
Figure 6.
Cell cytotoxicity assessment by Alamar Blue. Data representative of viability of uninfected (a) human and (b) murine macrophages treated for 48 h with either medium alone or the dry, alcoholic, or glycolic (10, 50, and 100 μg/mL) extracts of propolis. Hydrogen peroxide (H2O2) was used as a positive control for cell death. Bars represent mean ± SD values of three representative experiments performed using cells from six healthy human donors or in quintuplicate for murine cells.
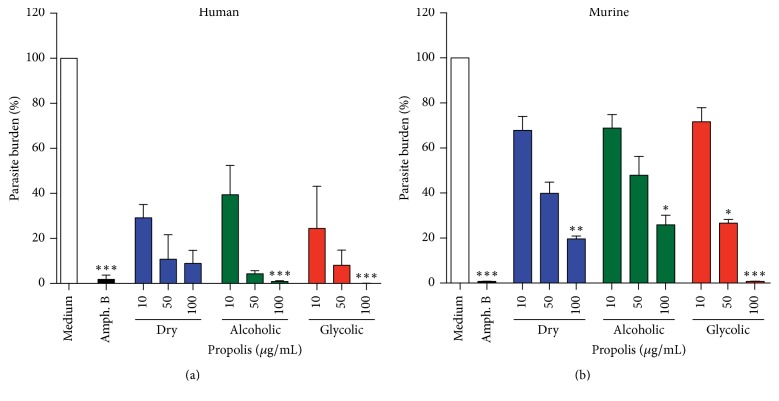
The leishmanicidal effect of varying concentrations (10, 50, and 100 μg/mL) of the three propolis extracts (dry, alcoholic, and glycolic extract) was evaluated in human and BALB/c macrophages infected with L. (V.) braziliensis (Figure 7). A significant reduction in the number of viable promastigotes recovered from infected cells was observed in human macrophages treated with alcoholic or glycolic extract, in comparison to untreated cells. The propolis extract treatment concentration was standardized at 50 μg/mL for all subsequent macrophage experimentation and analysis.
Figure 7.
Evaluation of viability of promastigotes recovered from L. (V) braziliensis-infected macrophages. Human (a) and murine (b) macrophages infected for 24 h with L. braziliensis, treated with medium alone or with dry, alcoholic, or glycolic extracts of propolis (10, 50, and 100 μg/mL) for 48 h. Amphotericin B (0.25 μg/mL) was used as a positive control. Bars represent means ± SD of two representative experiments performed using cells from six healthy human donors or in quintuplicate for murine cells. Kruskal–Wallis nonparametric test, followed by Dunn's posttest, was used to compare among experimental groups (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001).
3.5. Inflammatory Mediator Production in Response to Propolis Extract Treatment in L. (V) braziliensis-Infected Murine Macrophages
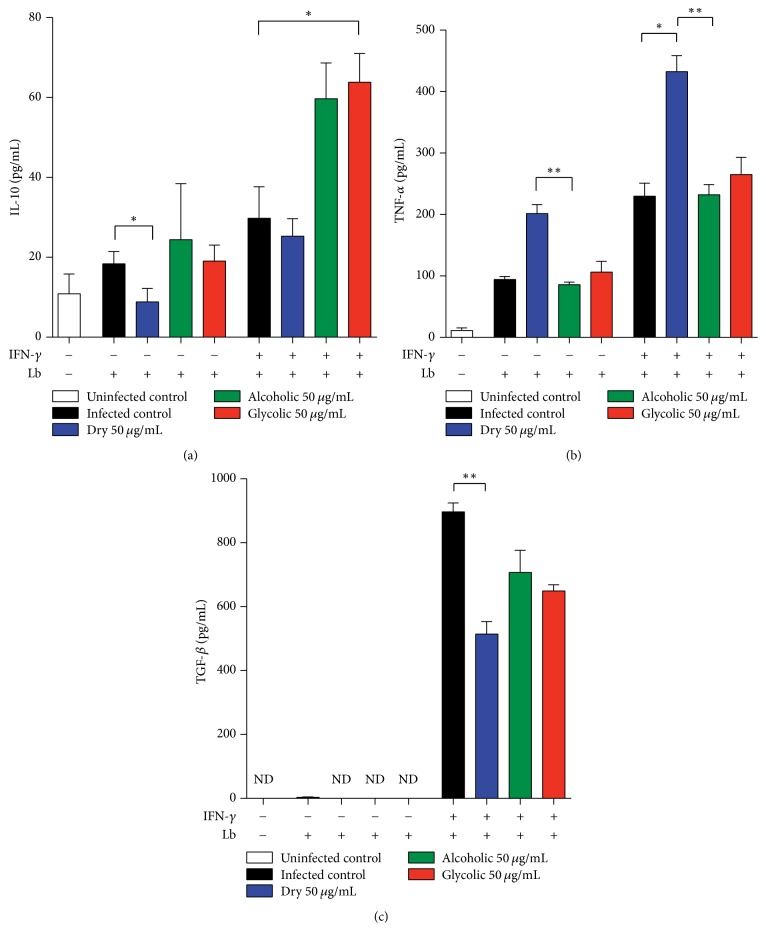
Cytokine production by unstimulated, or INF-γ-activated, infected murine macrophages was assessed in vitro (Figure 8). Similar levels of IL-10 and TNF-α were seen in unstimulated infected macrophages treated with alcoholic and glycolic extracts, without statistical significance in relation to untreated infected cells. In contrast, the unstimulated infected macrophages treated with dry extract exhibited downmodulated IL-10 but upregulated TNF-α production, when compared to untreated infected cells. Additionally, increased IL-10 production was observed in the INF-γ-activated L. (V) braziliensis-infected macrophages treated with glycolic extract in comparison to activated infected controls. Finally, dry extract was observed to downmodulate TGF-β, but upregulate TNF-α production, in INF-γ-activated infected macrophages, compared to activated infected controls (Figure 8).
Figure 8.
Modulation of cytokine production by propolis extract treatment. Quantification of inflammatory mediators secreted by propolis extract-treated L. (V) braziliensis-infected macrophages, stimulated by IFN-γ or not, and measured in cellular supernatant, as described in Materials and Methods: (a) interleukin-10 (IL-10), (b) Transforming Growth Factor (TGF-β), and (c) tumor necrosis factor (TNF-α). The Kruskal–Wallis nonparametric test, followed by Dunn's posttest, was used to compare among experimental groups (∗p < 0.05 and ∗∗p < 0.01).
3.6. Antioxidant Effects of Propolis Extract in L. (V) braziliensis-Infected Murine Macrophages
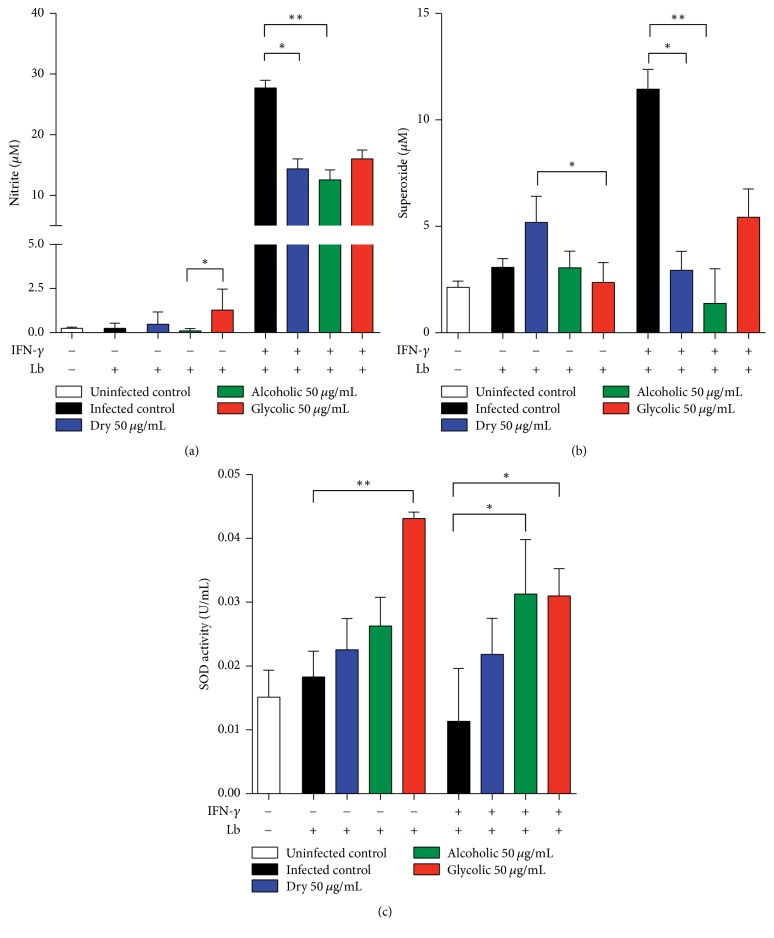
All three types of propolis extract were found to reduce nitric oxide and superoxide levels in activated L. (V) braziliensis-infected macrophages (Figure 9). Moreover, glycolic extract was observed to significantly increase SOD activity in both nonactivated and activated L. (V) braziliensis-infected macrophages, while alcoholic extract significantly increased SOD activity only in activated L. (V) braziliensis-infected macrophages, all in comparison to INF-γ-activated infected macrophages.
Figure 9.
Modulation of oxidative response by propolis extract treatment. Quantification of mediators of oxidative stress released by propolis extract-treated (V) braziliensis-infected macrophages, stimulated by IFN-γ or not, and measured in cellular supernatant, as described in Materials and Methods: (a) nitric oxide; (b) superoxide; (c) superoxide dismutase (SOD) activity. The Kruskal–Wallis nonparametric test, followed by Dunn's posttest, was used to compare among experimental groups (∗p < 0.05 and ∗∗p < 0.01).
4. Discussion
Propolis, a complex and resinous substance composed of variable vegetable sources and honeybee secretions [20, 38], has a broad spectrum of biological effects and, therefore, has attracted the attention of scientists as an alternative to traditional treatment in several diseases [15, 39]. Several studies have shown antileishmanial effects in vitro and in vivo in Leishmania parasites using murine models employing propolis extracts from different sources, including propolis from Paraná, red propolis from Alagoas, and green propolis from Minas Gerais in Brazil [14, 26, 27, 40–43].
The chemical composition of propolis varies greatly in accordance with the climatic conditions and local flora at the site of collection [15, 28–30], in addition to the method of extraction. Here, we used standardized green propolis presented in three different pharmaceutical preparations: dry, alcoholic, and glycolic extract. The most prevalent chemical components detected were phenolic acids (e.g., p-coumaric acid, drupanin, Artepillin C, caffeic acid, and baccharin), which is consistent with previous reports [21, 44–46]. Moreover, Artepillin C, a characteristic compound found in Brazilian green propolis, was the primary component found in the propolis extracts utilized herein. Phenolic compounds have been associated with antimicrobial, antioxidant, trypanocidal, and antitumoral activities [44, 47–52]. Previous studies have demonstrated the antileishmanial effects of p-coumaric acid and quercetin in in vitro and in vivo models [53, 54]. Herein, the levels of p-coumaric acid were found to be similar in all extracts evaluated, while total flavonoid content was higher for glycolic and alcoholic extracts (Table 1). Although it is extremely important to elucidate the chemical composition of propolis, its distinct pharmacological activities are considered complex and may be the result of synergistic interactions among various chemical compounds [55].
All propolis extracts demonstrated a direct effect against the proliferation of axenic promastigotes. The dosage of 50 μg/mL was shown to reduce parasite viability, verified by fluorescent double staining with Acridine Orange and Ethidium Bromide. Furthermore, the alcoholic and glycolic extracts were also shown to induce morphological and ultrastructural changes in parasites, whereas dry extract was only able to induce morphological changes. Similar morphological and ultrastructural changes consistent with decreased cell viability have been previously reported, suggestive of an apoptosis-like process induced by drugs traditionally used to treat leishmaniasis [56, 57].
None of the three propolis extracts exhibited any cytotoxic effects on BMMs or human cells after 48 h of treatment, as determined by the Alamar Blue assay. Importantly, in in vitro infection, all propolis extracts were shown to reduce L. (V) braziliensis burden in a dose-dependent manner. This finding is in agreement with previous reports that obtained propolis from different sources [19, 41, 58]. Moreover, treatment with alcoholic and glycolic extracts was shown to be more effective at reducing parasite numbers than dry extract, perhaps due to the distinct chemical compositions among these extracts.
The L. (V) braziliensis-infected macrophages treated with propolis extract were shown to modulate the production of inflammatory mediators, as well as markers of oxidative stress. Dry propolis extract significantly modified the inflammatory profile of murine macrophages by downmodulating TGF-β and IL-10 production, while upmodulating TNF-α. TGF-β and IL-10 are known to play an important role in macrophage deactivation, leading to increased parasite load in experimental models of L. (L) amazonensis, L. (V) braziliensis, and L. (L) major infection [59–62]. In addition, dos Santos Thomazelli et al. [63] reported an immunomodulatory effect by treatment with hydroalcoholic propolis extract on PBMCs from CL patients, as well as on PBMCs from healthy donors infected or not with Leishmania (V) braziliensis, in which the extract was shown to increase IL-4 and IL-17, while decreasing IL-10 levels.
The generation of reactive oxygen species (ROS), especially superoxide, and tumor necrosis factor-alpha (TNF-α) had both been shown to play an important role in the control of cutaneous leishmaniasis. Previous reports have demonstrated that increases in TNF-α and superoxide production effectively decrease parasite load, resulting in the elimination of L (V). braziliensis in vitro [64]. All of the extracts herein confirmed the antioxidant effects of propolis, as evidenced by significant decreases in nitric oxide and superoxide production [65–69]. In addition, our results showed increased superoxide dismutase (SOD) activity following treatment by alcoholic and glycolic extracts. Pronounced SOD-1 expression levels were also detected in biopsies from New World cutaneous leishmaniasis patients [70]. The results presented herein demonstrate that different presentations of propolis extract reacted differently against L. (V) braziliensis. Numbers of intracellular amastigotes were reduced following treatment of infected host cells with dry extract, which was shown to produce greater levels of TNF-α than murine macrophages treated with alcoholic and glycolic propolis extract. This is suggestive of host cell activation, which may have led to parasite killing via the TNF-α pathway. Furthermore, host cells treated with the alcoholic and glycolic extracts both were shown to effectively reduce the number of amastigotes, similarly to the dry extract, but with significant increases in SOD, which is indicative of the downmodulation of the oxidative stress response. Further investigations are warranted to elucidate the underlying mechanisms by which the alcoholic and glycolic extracts act upon specific pathways, potentially offering both control of parasite burden and an anti-inflammatory effect.
5. Conclusions
Our findings demonstrate that propolis induced-inflammatory imbalance involving cytokines and oxidative response hallmarks the outcome of Leishmania infection. This would be extremely beneficial in the context of CL treatment, due to the high inflammatory profile of this disease, which leads to severe tissue damage.
Acknowledgments
The authors thank Andrezza Souza for technical and logistics support. They thank Dr. Fábio Formiga for their helpful discussions and Dr. Adriana Rangel and Dr. Claudio Figueira for their technical assistance with the electron microscopy. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (Process no. 309988/2014-4 to Valéria M. Borges), Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (Process no. 2013/50496-2 to Andresa A. Berretta), and Financiadora de Estudos e Projetos, FINEP (Process no. 03.12.0056.00 to Andresa A. Berretta). Valéria M. Borges, Andresa A. Berretta, and Camila I. de Oliveira are senior investigators from CNPq. Jéssica Rebouças-Silva and Fabiana S. Celes have fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Hernane S. Barud has fellowship from CNPq.
Disclosure
The funders had no role in study design, data collection and analysis, decision of publishing, or preparation of the manuscript.
Conflicts of Interest
The authors declare that they do not have a commercial association that might pose conflicts of interest.
References
- 1.Chávez-Fumagalli M. A., Ribeiro T. G., Castilho R. O., Odília S., Fernandes A., Cardoso V. N., et al. Review article new delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Revista da Sociedade Brasileira de Medicina Tropical. 2015;48(3):235–242. doi: 10.1590/0037-8682-0138-2015. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R., Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunol Nature Publishing Group. 2014;3(3, article e13) doi: 10.1038/cti.2014.4. http://www.readcube.com/articles/10.1038/cti.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pace D. Leishmaniasis. The Journal of Infection. 2014;69(supplement 1):S10–S18. doi: 10.1016/j.jinf.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Leishmaniasis: worldwide epidemiology and drug access update. http://www.who.int/leishmaniasis/resources/Leishmaniasis_worldwide_epidemiological_and_drug_access_update.pdf, 2012.
- 5.Alvar J., Vélez I. D., Bern C., Herrero M., Desjeux P., Cano J., et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5, article e35671) doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministério da Saúde. Manual de vigilância da leishmaniose tegumentar americana. serie A. Normas e Manuais Técnicos. 2007:p. 180. [Google Scholar]
- 7.Salgado V. R., de Queiroz A. T. L., Sanabani S. S., de Oliveira C. I., Carvalho E. M., Costa J. M. L., et al. The microbiological signature of human cutaneous leishmaniasis lesions exhibits restricted bacterial diversity compared to healthy skin. Memorias do Instituto Oswaldo Cruz. 2016;111(4):241–251. doi: 10.1590/0074-02760150436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layegh P., Ghazvini K., Moghiman T., Hadian F., Zabolinejad N., Pezeshkpour F. Bacterial contamination in cutaneous leishmaniasis: its effect on the lesions' healing course. Indian Journal of Dermatology. 2015;60(2):p. 211. doi: 10.4103/0019-5154.152560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouhan G., Islamuddin M., Sahal D., Afrin F. Exploring the role of medicinal plant-based immunomodulators for effective therapy of leishmaniasis. Frontiers in Immunology. 5(5, 1993) doi: 10.3389/fimmu.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llanos-Cuentas A., Tulliano G., Araujo-Castillo R., Miranda-Verastegui C., Santamaria-Castrellon G., Ramirez L., et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clinical Infectious Diseases. 2008;46(2):223–231. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 11.Machado P., Araújo C., da Silva A. T., Almeida R. P., D’Oliveira A., Jr., Bittencourt A., et al. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clinical Infectious Diseases. 2002;34(12):e69–e73. doi: 10.1086/340526. http://www.ncbi.nlm.nih.gov/pubmed/12032913. [DOI] [PubMed] [Google Scholar]
- 12.de Smet P. A. The role of plant-derived drugs and herbal medicines in healthcare. Drugs. 1998;54(6):801–840. doi: 10.2165/00003495-199754060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Santos D. M., Petersen A. L. O. A., Celes F. S., Borges V. M., Veras P. S. T., de Oliveira C. I. Chemotherapeutic potential of 17-AAG against cutaneous leishmaniasis caused by leishmania (Viannia) braziliensis. PLoS Neglected Tropical Diseases. 2014;8(10):p. e3275. doi: 10.1371/journal.pntd.0003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda J. M., Fujita T. C., Faveri Pitz A. D., Amarante M. K., Felipe I., Saridakis H. O., et al. Ação do extrato de própolis nas leishmaniose. Semina: Ciências Biológicas e da Saúde. 2011;32(1):111–121. doi: 10.5433/1679-0367.2011v32n1p111. [DOI] [Google Scholar]
- 15.Bankova V. S., de Castro S. L., Marcucci M. C. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31(1):3–15. doi: 10.1051/apido:2000102. [DOI] [Google Scholar]
- 16.Machado J. L., Assunção A. K. M., Da Silva M. C. P., Reis A. S. D., Costa G. C., Arruda D. D. S., et al. Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evidence-Based Complementary and Alternative Medicine. 2012;2012:p. 10. doi: 10.1155/2012/157652.157652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitão D. P. S., Filho A. A. D. S., Polizello A. C. M., Bastos J. K., Spadaro A. C. C. Comparative evaluation of in-vitro effects of Brazilian green propolis and Baccharis dracunculifolia extracts on cariogenic factors of Streptococcus mutans. Biological and Pharmaceutical Bulletin. 2004;27(11):1834–1839. doi: 10.1248/bpb.27.1834. [DOI] [PubMed] [Google Scholar]
- 18.Amarante M. K., Watanabe M. A. E., Conchon-Costa I., et al. The effect of propolis on CCL5 and IFN-γ expression by peripheral blood mononuclear cells from leishmaniasis patients. Journal of Pharmacy and Pharmacology. 2012;64(1):154–160. doi: 10.1111/j.2042-7158.2011.01385.x. [DOI] [PubMed] [Google Scholar]
- 19.da Silva S. S., Thomé G. D. S., Cataneo A. H. D., et al. Brazilian propolis antileishmanial and immunomodulatory effects. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/673058.673058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado B. A. S., Silva R. P. D., Barreto G. D. A., et al. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0145954.e0145954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barud H. D. S., de Araújo Júnior A. M., Saska S., et al. Antimicrobial Brazilian propolis (EPP-AF) containing biocellulose membranes as promising biomaterial for skin wound healing. Evidence-Based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/703024.703024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Castro P. A., Savoldi M., Bonatto D., et al. Molecular characterization of propolis-induced cell death in Saccharomyces cerevisiae. Eukaryotic Cell. 2011;10(3):398–411. doi: 10.1128/ec.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Castro P. A., Bom V. L. P., Brown N. A., et al. Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genetics and Biology. 2013;60:74–86. doi: 10.1016/j.fgb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Berretta A. A., Nascimento A. P., Bueno P. C. P., Leite Vaz M. M. D. O. L., Marchetti J. M. Propolis standardized extract (EPP-AF ®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. International Journal of Biological Sciences. 2012;8(4):512–521. doi: 10.7150/ijbs.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hori J. I., Zamboni D. S., Carrão D. B., Goldman G. H., Berretta A. A. The inhibition of inflammasome by Brazilian propolis (EPP-AF) Evidence-Based Complementary and Alternative Medicine. 2013;2013:11. doi: 10.1155/2013/418508.418508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda, Suelen Santos Silva M. M., Costa I. N., et al. Leishmanicidal activity of brazilian propolis hydroalcoholic extract in Leishmania. 2015;36(2):25–34. [Google Scholar]
- 27.Ferreira F. M., Castro R. A. O., Batista M. A., et al. Association of water extract of green propolis and liposomal meglumine antimoniate in the treatment of experimental visceral leishmaniasis. Parasitology Research. 2014;113(2):533–543. doi: 10.1007/s00436-013-3685-8. [DOI] [PubMed] [Google Scholar]
- 28.Marcucci M. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83–99. doi: 10.1051/apido:19950202. [DOI] [Google Scholar]
- 29.Walker P., Crane E. Constituents of propolis. Apidologie. 1987;18(4):327–334. doi: 10.1051/apido:19870404. [DOI] [Google Scholar]
- 30.Salomão K., Pereira P. R. S., Campos L. C., et al. Brazilian propolis: correlation between chemical composition and antimicrobial activity. Evidence-Based Complementary and Alternative Medicine. 2008;5(3):317–324. doi: 10.1093/ecam/nem058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Sousa J. P. B., Bueno P. C. P., Gregório L. E., et al. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. Journal of Separation Science. 2007;30(16):2656–2665. doi: 10.1002/jssc.200700228. [DOI] [PubMed] [Google Scholar]
- 32.Andrade C. A. S., Oliveira M. D. L., Santos-Magalhães N. S., Correia M. T. S., de Melo C. P. Comparison of the interfacial properties of Eugenia uniflora and Triticum vulgaris lectins. Colloids and Surfaces B: Biointerfaces. 2009;68(1):7–12. doi: 10.1016/j.colsurfb.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Woiskya R. G., Salatinoa A. Analysis of propolis: some parameters and procedures for chemical quality control. Journal of Apicultural Research. 1998;37(2):99–105. doi: 10.1080/00218839.1998.11100961. [DOI] [Google Scholar]
- 34.Liu K., Liu P. C., Liu R., Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Medical Science Monitor Basic Research. 2015;(21):15–20. doi: 10.12659/MSMBR.893327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribble D., Goldstein N. B., Norris D. A., Shellman Y. G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnology. 2005;5, article 12 doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen A. L. D. O. A., Guedes C. E. S., Versoza C. L., et al. 17-AAG kills intracellular Leishmania amazonensis while reducing inflammatory responses in infected macrophages. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049496.e49496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elstner E. F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Analytical Biochemistry. 1976;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- 38.Washio K. W., Himamoto Y. S., Itamura H. K. Brazilian propolis extract increases leptin expression in mouse adipocytes. Biomedical Research. 2015;36(5):343–346. doi: 10.2220/biomedres.36.343. [DOI] [PubMed] [Google Scholar]
- 39.Siheri W., Zhang T., Ebiloma G. U., et al. Chemical and antimicrobial profiling of propolis from different regions within Libya. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155355.e0155355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayres D. C., Marcucci M. C., Giorgio S. Effects of Brazilian propolis on Leishmania amazonensis. Memórias do Instituto Oswaldo Cruz. 2007;102(2):215–220. doi: 10.1590/S0074-02762007005000020. [DOI] [PubMed] [Google Scholar]
- 41.Pontin K., da Silva Filho A. A., Santos F. F., et al. In vitro and in vivo antileishmanial activities of a Brazilian green propolis extract. Parasitology Research. 2008;103(3):487–492. doi: 10.1007/s00436-008-0970-z. [DOI] [PubMed] [Google Scholar]
- 42.Silva N. S., Muniz V. D. Epidemiology of American tegumentary leishmaniasis in the State of Acre, Brazilian Amazon. Cad saude publica/Minist da Saude, Fund Oswaldo Cruz, Esc Nac Saude Publica. 2009;25(6):1325–1336. doi: 10.1590/S0102-311X2009000600015. [DOI] [PubMed] [Google Scholar]
- 43.Miranda M. M., Panis C., Cataneo A. H. D., et al. Nitric oxide and Brazilian propolis combined accelerates tissue repair by modulating cell migration, cytokine production and collagen deposition in experimental leishmaniasis. PLoS ONE. 2015;10(5):1–19. doi: 10.1371/journal.pone.0125101.e0125101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J., Omene C., Karkoszka J., et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Letters. 2011;308(1):43–53. doi: 10.1016/j.canlet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hata T., Tazawa S., Ohta S., Rhyu M.-R., Misaka T., Ichihara K. Artepillin C, a major ingredient of brazilian propolis, induces a pungent taste by activating TRPA1 channels. PLoS ONE. 2012;7(11):1–9. doi: 10.1371/journal.pone.0048072.e48072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sousa J. P. B., Furtado N. A. J. C., Jorge R., Soares A. E. E., Bastos J. K. Perfis físico-químico e cromatográfico de amostras de própolis produzidas nas microrregiões de Franca (SP) e Passos (MG), Brasil. Brazilian J Pharmacogn. 2007;17(1):85–93. doi: 10.1590/S0102-695X2007000100017. [DOI] [Google Scholar]
- 47.Marcucci M. C., Ferreres F., García-Viguera C., et al. Phenolic compounds from Brazilian propolis with pharmacological activities. Journal of Ethnopharmacology. 2001;74(2):105–112. doi: 10.1016/S0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 48.Szliszka E., Mertas A., Czuba Z. P., Król W. Inhibition of inflammatory response by artepillin C in activated RAW264.7 macrophages. Evidence-Based Complementary and Alternative Medicine. 2013;2013:11. doi: 10.1155/2013/735176.735176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paulino N., Abreu S. R. L., Uto Y., et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. European Journal of Pharmacology. 2008;587(1–3):296–301. doi: 10.1016/j.ejphar.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 50.Larki-Harchegani A., Hemmati A. A., Arzi A., Ghafurian-Boroojerdnia M., Shabib S., Zadkarami M. R., et al. Evaluation of the effects of caffeic acid phenethyl ester on prostaglandin E2 and two key cytokines involved in bleomycin-induced pulmonary fibrosis. Iranian Journal of Basic Medical Sciences. 2013;16(7):850–857. [PMC free article] [PubMed] [Google Scholar]
- 51.Orsolic N., Terzic S., Mihaljevic Z., Sver L., Basic I. Effects of local administration of propolis and its polyphenolic compounds on tumor formation and growth. Biological and Pharmaceutical Bulletin. 2005;28(10):1928–1933. doi: 10.1248/bpb.28.1928. [DOI] [PubMed] [Google Scholar]
- 52.Filho A. A. D. S., Resende D. O., Fukui M. J., et al. In vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D. C. (Asteraceae) Fitoterapia. 2009;80(8):478–482. doi: 10.1016/j.fitote.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Dezaki E. S., Mahmoudvand H., Sharififar F., Fallahi S., Monzote L., Ezatkhah F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharmaceutical Biology. 2015;54(5):752–758. doi: 10.3109/13880209.2015.1079223. [DOI] [PubMed] [Google Scholar]
- 54.Sen G., Mukhopadhyay S., Ray M., Biswas T. Quercetin interferes with iron metabolism in Leishmania donovani and targets ribonucleotide reductase to exert leishmanicidal activity. Journal of Antimicrobial Chemotherapy. 2008;61(5):1066–1075. doi: 10.1093/jac/dkn053. [DOI] [PubMed] [Google Scholar]
- 55.Krol W., Scheller S., Shani J., Pietsz G. C. Z. Synergistic effect of ethanolic extract of propolis and antibiotics on the growth of Staphylococcus aureus. Arzneimittelforschung. 1993;43(5):607–609. [PubMed] [Google Scholar]
- 56.Vannier-Santos M. A., de castro S. L. Electron microscopy in antiparasitic chemotherapy: a (close) view to a kill. Current Drug Targets. 2009;10(3):246–260. doi: 10.2174/138945009787581168. [DOI] [PubMed] [Google Scholar]
- 57.Marinho F. A., Gonçalves K. C. S., Oliveira S. S. C., et al. The calpain inhibitor MDL28170 induces the expression of apoptotic markers in leishmania amazonensis promastigotes. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0087659.e87659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayres D. C., Fedele T. A., Marcucci M. C., Giorgio S. Potential utility of hyperbaric oxygen therapy and propolis in enhancing the leishmanicidal activity of glucantime. Revista do Instituto de Medicina Tropical de Sao Paulo. 2011;53(6):329–334. doi: 10.1590/S0036-46652011000600006. [DOI] [PubMed] [Google Scholar]
- 59.Barral A., Teixeira M., Reis P., Vinhas V., Costa J., Lessa H., et al. Transforming growth factor-beta in human cutaneous leishmaniasis. The American Journal of Pathology. 1995;147(4):947–954. [PMC free article] [PubMed] [Google Scholar]
- 60.de Oliveira C. I., Brodskyn C. I. The immunobiology of Leishmania braziliensis infection. Frontiers in Immunology. 2012;3, article 145:1–9. doi: 10.3389/fimmu.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barral-Netto M., Barral A., Brownell C. E., et al. Transforming growth factor-β in leishmanial infection: A parasite escape mechanism. Science. 1992;257(5069):545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 62.Barral A., Barral-Netto M., Yong E. C., Brownell C. E., Twardzik D. R., Reed S. G. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proceedings of the National Academy of Sciences of the United States. 1993;90(8):3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.dos Santos Thomazelli A. P. F., Tomiotto-Pellissier F., da Silva S. S., Panis C., Orsini T. M., Cataneo A. H. D., et al. Brazilian propolis promotes immunomodulation on human cells from American Tegumentar leishmaniasis patients and healthy donors infected with l. braziliensis. Cellular Immunology. 2016:10–15. doi: 10.1016/j.cellimm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Novais F. O., Santiago R. C., Báfica A., et al. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. Journal of Immunology. 2009;183(12):8088–8098. doi: 10.4049/jimmunol.0803720. [DOI] [PubMed] [Google Scholar]
- 65.de Sá R. A., de Castro F. A. V., Eleutherio E. C. A., de Souza R. M., da Silva J. F. M., Pereira M. D. Brazilian propolis protects Saccharomyces cerevisiae cells against oxidative stress. Brazilian journal of microbiology: publication of the Brazilian Society for Microbiology. 2013;44(3):993–1000. doi: 10.1590/S1517-83822013005000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira D., Rocha H. C., Kreutz L. C., et al. Bee products prevent agrichemical-induced oxidative damage in fish. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0074499.e74499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwon T. D., Lee M. W., Kim K. H. The effect of exercise training and water extract from propolis intake on the antioxidant enzymes activity of skeletal muscle and liver in rat. Journal of Exercise Nutrition and Biochemistry. 2014;18(1):9–17. doi: 10.5717/jenb.2014.18.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wali A. F., Avula B., Ali Z., et al. Antioxidant, hepatoprotective potential and chemical profiling of propolis ethanolic extract from kashmir himalaya region using UHPLC-DAD-QToF-MS. BioMed Research International. 2015;2015:10. doi: 10.1155/2015/393462.393462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.do Nascimento T. G., da Silva P. F., Azevedo L. F., et al. Polymeric Nanoparticles of Brazilian red propolis extract: preparation, characterization, antioxidant and leishmanicidal activity. Nanoscale Research Letters. 2016;11(1, article 301) doi: 10.1186/s11671-016-1517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khouri R., Bafica A., Silva M. D. P., et al. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. Journal of Immunology. 2009;182(4):2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]