Summary
The possibility of long‐term grape juice concentrate (GJC) consumption conferring a protective effect against cadmium (Cd)‐induced damage to the epididymis, completely preserving sperm profile, was evaluated here for the first time in the scientific literature. Male Wistar rats (n = 6/per group) received an intraperitoneal Cd injection (1.2 mg/Kg) at age 80 days and GJC (2 g/Kg) by gavage from 50 days until 136 days old. Groups receiving either Cd or GJC were added. An intraperitoneal injection of saline (0.9%) and water by gavage was administered in the absence of treatment with Cd or GJC. Animals were anaesthetized and exsanguinated at 136 days; the vas deferens, left testis and epididymis were removed; and perfusion continued with fixative. The right epididymis was collected for morphological analysis. Cd had a devastating effect demonstrated by reduced sperm count in testes and epididymis, sperm production and normal sperm count, besides increased epididymis sperm transit time and completely disorganized morphology. These alterations were attributed to higher Cd levels in the testes and a lipid peroxidation (LP) process. Consumption of GJC plus Cd intoxication was effective, reducing metal accumulation and LP. Consequently, we could identify a preserved sperm profile, with improvement in testis and epididymis sperm count, normal sperm structure and sperm transit time. Moreover, GJC extends its protective effect to the epididymis, allowing complete re‐establishment of its morphology, ensuring successful sperm maturation process. In conclusion, our study indicates long‐term GJC as a promising therapy against reproductive chemical intoxication injury damage, preserving sperm prior to ejaculation.
Keywords: heavy metal, male intoxication, male reproduction, morphometry, polyphenols
Recently, the therapeutic effect of natural products has been widely studied. It is known that high fruit and vegetable consumption contributes to the prevention of some metabolic disorders. The capacity of many natural compounds to reduce the risk of these alterations, which include obesity, intoxication and others, is, in part, associated with bioactive compounds present in vegetables and fruit (Dragano et al. 2013; Hu 2013; Lenquiste et al. 2015).
Among these bioactive molecules, phenolic compounds have been highlighted and are present in the grape and its derivates. They include the flavonoids anthocyanin, catechin, epicatechin, quercetin and an important molecule among non‐flavonoids, resveratrol (Ho et al. 2010; Domeneghini & Lemes 2011). Some properties of grape products have already been described in previous studies, such as the reduction in blood pressure, anti‐inflammatory action, reduction in cell proliferation and modulation of some enzymes (Andrade 2006; Gang et al. 2015; Pires et al. 2013; Mojica‐Villegas et al. 2014).
On the other hand, cadmium (Cd) is a toxic metal, occurring as a common environmental contamination (present in pesticides and cigarettes) that induces cellular damage, mainly due to the production of free radicals (El‐Shahat et al. 2009; Wang et al. 2011). Moreover, Cd has the ability to accumulate in organs, especially in testes, where its presence causes lipid peroxidation, destroys cell adhesion and promotes spermatic damages, among other alterations (Who, 1992; Gupta et al. 2004; Messaoudi et al. 2010). Recent studies have confirmed that this metal accumulates in the testis and can reduce sperm concentration and sperm motility and increase the percentage of abnormal sperm (Ola‐Mudathir et al. 2008; Wang et al. 2016). Cd can also increase peroxidation levels and alter antioxidant markers such as catalase, glutathione and superoxide dismutase, affecting the antioxidant status (Ola‐Mudathir et al. 2008; Wang et al. 2012). Moreover, Predes et al. (2016) demonstrated that Cd‐intoxicated rats showed a higher epithelium in the epididymis caput and cauda, reduced sperm and the presence of cell debris in the lumen of both epididymis compartments.
A previous study by our group revealed that when daily ingested, grape juice concentrate can mitigate Cd‐induced damages in testis morphology, possibly preventing the disruption of spermatogenic development caused by this metal (Lamas et al. 2015). Thus, the aim of this study was to verify whether grape juice concentrate protective effect is extended to other organs of the reproductive system, preserving sperm output. To accomplish this, the epididymis’ morphology, sperm parameters, oxidative profile and Cd accumulation analysis were developed.
Material and methods
Grape juice concentrate (GJC)
The grape juice concentrate used in the present research was G8000® provided by Golden Sucos (Farroupilha‐RS, Brazil). To calculate the grape juice concentrate dosage, the total phenols were measured by the Folin–Ciocalteu assay (Singleton & Rossi 1965), using gallic acid (Sigma‐Aldrich, St Louis, MO, USA) for the standard curve, and the results were expressed as mg EAG/kg. Readings (in triplicate) were taken at 740 nm, using a Genesis 2 (Mattson Instruments, East Lyme, CT, USA) spectrometer.
This analysis revealed the percentage of phenols in G8000®. It is known that an effective amount of polyphenols for an adult human is 2 g/day (Ada (American Dietetic Association) 2004). The proportion of this recommendation for a rat was 16 mg/day, considering that its metabolism is faster than human metabolism (Zorzano & Herrera 1990; Scollon et al. 2009). According to this higher metabolism and based on the American Dietetic Association's recommendation of 200–500 ml of grape juice (Ada (American Dietetic Association) 2004), the dosage of 2 mg/Kg of body weight (BW) was chosen. The G8000® components characterization and the nature of the bioactive compounds present in this concentrate can be verified in a previous study by Aguiar et al. (2011).
Ethical approval statement
All procedures were developed according to the Guide of Care and Use of Laboratory Animals and were approved by the Committee for Ethics in Animal Experimentation of the University of Campinas (#2900‐1).
Animals and experimental design
For this study, 24 Wistar rats (50 days old) were obtained from the Central Animal Raising Unit of Unicamp (State University of Campinas, Campinas, SP, Brazil). The animals were housed three per cage, with a 12‐h light–dark cycle. Food and water were provided ad libitum.
The rats were randomly divided into four groups (n = 6), that received the following treatments. The control group (CTg) received water by gavage. The cadmium group (Cdg) received water and a Cd injection (1.2 mg/Kg). The grape juice group (GJg) received 2 g G8000®/Kg/day. The fourth group received the same GJC dose and CdCl2 injection (CdGJg).
Cadmium chloride (CdCl2 P.A. Nuclear® cod. 318430) diluted in 0.5 ml of distilled water (Predes et al. 2010) was injected intraperitoneally in a single dose of 1.2 mg/Kg when the rats were considered adults (80 days old) (Zanato et al. 1994). This dose was chosen based in our previous work (Lamas et al. 2015) and in research that tested low doses of cadmium that can cause testicular injury (Predes et al. 2010). The GJC (2 g/Kg/day) was administered as a single daily dose by gavage. This procedure was always performed in the morning, in an anteroom of the vivarium of the Department of Cell Biology of Unicamp. The GJC treatment lasted 86 days, starting when animals were 50 days old and continued until the animals were 136 days old. This treatment period was chosen based on our previous research because it encompasses the sexual maturation of the rats, being administered from the beginning of sexual maturity (50 days old) until the end of one spermatogenic cycle in adult life (cycle lasts 56 days after the age of 80 days) (Zanato et al. 1994; Lamas et al. 2015). The groups CTg and GJg received an intraperitoneal injection of 0.9% saline solution (volume 0.5 ml), and groups CTg and Cdg received water by gavage, to maintain the same experimental conditions. At the end of the experiment, animals were anesthetized using a mixture of ketamine and xylazine (10 and 80 mg/kg respectively).
Sample collection and tissue preparation for microscopy analysis
After anaesthesia, the chest cavity was opened and animals were first perfused with saline solution (0.9%) to clear the testis vascular bed. The left testis and epididymis were removed. Half of this testis and the entire epididymis were frozen at −20°C for sperm counts, and the other half of the testis was frozen at −80°C for biochemical and Cd detection analyses. One centimetre of the vas deferens, proximal to the epididymis, was removed and then twisted and compressed in PBS buffer (pH = 7) where it remained for about 15 min to diffuse spermatozoa for morphological analysis. Then, the perfusion continued with Karnovsky fixative [5% glutaraldehyde, 2.5% paraformaldehyde 0.2 m in sodium phosphate buffer (pH 7.2)] for at least 20 min. The right epididymis was removed and weighed with an analytical scale (Ohaus®, Barueri, São Paulo, Brazil). The organs were postfixed overnight in the same solution. The following day, small blocks of the right epididymis were embedded in glycol methacrylate. They were sectioned at 3 μm thickness and stained with toluidine blue/1% sodium borate, for light microscopy analyses. For transmission electron microscopy analysis, samples were postfixed in osmium tetroxide (1%), dehydrated in acetone and embedded in epoxy resin. Ultrathin sections were obtained and stained with uranyl acetate (2%) and lead citrate (0.2%), before observation with a transmission electron microscope (Zeiss, Leo 906‐Oberkochen, Germany).
Biometry and morphometric analysis
The animals’ body weight was measured at the end of the experiment. The left epididymis was weighed to obtain the epididymis absolute weight (g), with an analytical scale (Ohaus®), immediately after being collected. The relative weight (%) of epididymis was calculated relating the epididymis absolute weight and the respective animal total body weight. The epithelium height evaluation was performed using 15 transverse sections of epididymis tubules with a circular shape (200× magnification), in the epididymis caput and cauda. The epithelium height was determined by the average of four diametrically opposed measurements.
Volumetric proportion of epididymis’ caput and cauda compartments
To determine the proportion of tubular (epithelium, lumen and smooth cell) and intertubular compartments in the epididymis caput and cauda, 10 randomly captured images (200× magnification) per animal were used. These photomicrographs were obtained using the light microscope, Olympus BX41 associated with the image‐pro plus 6.0 software (Media Cybernetics, Inc. MD, USA). A grid containing 100 intersections was superimposed on the imagens, and the points were classified as: epithelium, lumen, smooth muscle and interstitium. Thus, based on the total 1000 intersections per animal, it was possible to calculate the proportion of each component.
Sperm parameters
To analyse the daily sperm production (DSP), the half of the testicle tissue frozen at −20°C was used. The technique used was according to Robb et al. (1978), and sperm production value was corrected as proposed by Pires et al. (2013). The sperm transit time (STT) was performed using the epididymis frozen at −20°C. Sperm from the caput/corpus and cauda portions were counted in a Neubauer chamber according to Robb et al. (1978). The STT was calculated according to Kempinas et al. (1998).
To determine sperm abnormalities, a suspension of spermatozoa from the vas deferens was examined on a histological slide (Seed et al. 1996). Two hundred spermatozoa per animal were microscopically analysed at 400× magnification and separated into two categories: normal and abnormal (Seed et al. 1996; Oliveira et al. 2009; Favareto et al. 2011).
Determination of Cd concentration in testicular tissue
To determine Cd concentration in testicular tissue, 4 ml of deionized water was added to 0.15 g of the homogenized sample, allowed to stand for 5 min. Subsequently, 0.5 ml of tetramethylammonium hydroxide was added and again the mixture was homogenized for 30 min in an ultrasound cleaning tank. The total volume was completed to 10 ml, and analysis was performed by graphite furnace atomic absorption spectrometry (GF AAS model AAnalyst 600, Perkin‐Elmer, Norwalk, USA). The analytical calibration curve was obtained for concentrations of 1–9μg/l using aqueous metallic element standards and NH4H2PO4 as a chemical modifier. The pyrolysis and atomization temperatures were 500°C and 1500°C respectively. The same procedure was carried out with a certified reference material (NIST 1577b) to evaluate the method accuracy. All readings were performed in triplicate.
Oxidative stress markers
To perform these analyses, testicular tissue stored at −80°C was used. Part of the testis was homogenized and then centrifuged according to the recommendations of the respective commercial diagnostic kits used. The supernatant was removed and used for the assays. The amount of malondialdehyde (MDA) was measured to give the lipid peroxidation level, using TBARS Assay Kit from Cayman Chemical® (Ann Arbor, MI, USA) (cat no 10009055; lot no 0452078). The enzyme catalase (CAT) was determined spectrophotometrically following the decomposition of hydrogen peroxide (H2O2) with a Catalase Assay Kit from Sigma‐Aldrich® (cat no CAT100; lot no 110M4055). Superoxide dismutase (SOD) assay was performed using Superoxide Dismutase Assay Kit from Cayman Chemical® (cat noo 706002; lot no 0447900), which applies a tetrazolium salt for superoxide radical detection, generated by xanthine oxidase and hypoxanthine. To evaluate reduced glutathione (GSH), the Glutathione Assay Kit from Sigma‐Aldrich® was employed (cat no CS0260; lot no 082M4089).
Statistical analysis
The analysis of variance (anova) followed by Tukey's test (statistica®, StatSoft South America, São Caetano do Sul ‐ São Paulo, Brazil) was used to compare the control values and other groups. The results were considered significant for P < 0.05. Moreover, for all values, the means ± standard deviation was calculated.
Results
Total phenol content of G8000®
The results of the total phenol content of GJC showed a concentration of 54.3 g EAG/kg, with a standard deviation of 0.7. This value is equivalent to a percentage of 5.4 of polyphenols in the GJC used.
Biometric and morphometric evaluation
Considering the epididymis absolute (g) and relative (%) weights, no difference was found between the experimental groups. On the other hand, Cdg demonstrated higher epithelium height when compared to all other groups in epididymis’ caput and cauda. This parameter showed a positive effect for GJC, considering that the epithelium height found for CdGJg was the same as in CTg. These data are shown in Table 1.
Table 1.
Epididymis biometry and morphometric analysis (mean value ± standard deviation)
| CTg | Cdg | CdGJg | GJg | |
|---|---|---|---|---|
| Epididymis absolute weight (g) | 0.573 ± 0.04 | 0.572 ± 0.06 | 0.575 ± 0.04 | 0.583 ± 0.06 |
| Epididymis relative weight (%) | 0.108 ± 0.01 | 0.119 ± 0.01 | 0.127 ± 0.01 | 0.127 ± 0.01 |
| Epididymis caput | ||||
| Epithelium height | 23.93 ± 2.48 | 28.99 ± 2.20* | 24.12 ± 2.55† | 23.53 ± 2.53‡ |
| Epididymis cauda | ||||
| Epithelium height | 21.77 ± 1.89 | 25.25 ± 1.10** | 22.04 ± 1.60† | 22.10 ± 1.41‡ |
A significant difference was found for *P < 0.05 with CTg; **P < 0.01 with CTg; † P < 0.05 with Cdg and ‡ P < 0.01 with Cdg. CTg, control group; Cdg, cadmium group; CdGJg, cadmium and grape juice concentrate group; GJg, grape juice concentrate group (n = 6/group).
Volumetric proportion of epididymis’ caput and cauda compartments
The stereological evaluation revealed alteration of the epididymis caput and cauda components.
In the caput, a significant increase in the epithelium and smooth muscle proportions was observed in the Cdg, accompanied by a significant decrease in lumen proportion compared to the control group. Considering the tubular and intertubular compartment proportions, no difference was observed in the epididymis caput. In the groups treated with GJC, no difference was observed in relation to the control group. However, they had a lower epithelium and smooth muscle proportion when compared to Cdg group.
On the other hand, in the epididymis cauda, a significant increase in the tubular compartment, epithelium and smooth muscle proportions was observed in the Cdg associated with a significant reduction in the intertubular compartment. In the groups treated with GJC, no difference was observed in relation to the control group. However, they had a lower tubular compartment proportion when compared to the Cdg group.
These data are displayed in Table 2.
Table 2.
Epididymis stereological analysis (mean value ± standard deviation)
| CTg | Cdg | CdGJg | GJg | |
|---|---|---|---|---|
| Epididymis caput | ||||
| Tubular compartment (%) | 81.69 ± 4.78 | 70.84 ± 3.11 | 71.77 ± 6.98 | 69.77 ± 2.75 |
| Epithelium proportion (%) | 20.39 ± 0.68 | 29.53 ± 2.01*** | 21.90 ± 0,94§ | 17.80 ± 0,43§ |
| Lumen proportion (%) | 57.59 ± 4.54 | 35.11 ± 3.29** | 45.17 ± 8.44 | 47.37 ± 2.51 |
| Smooth muscle proportion (%) | 3.71 ± 0.25 | 6.19 ± 0.50** | 4.70 ± 0.24‡ | 4.59 ± 0.27‡ |
| Intertubular compartment (%) | 18.31 ± 4.80 | 29.16 ± 2.96 | 28.23 ± 8.33 | 30.23 ± 2.86 |
| Epididymis cauda | ||||
| Tubular compartment (%) | 77.12 ± 4.41 | 90.00 ± 1.57*** | 82.43 ± 3.52§ | 80.89 ± 1.23§ |
| Epithelium proportion (%) | 18.95 ± 1.28 | 25.87 ± 1.72*** | 23.02 ± 0.95 | 22.12 ± 1.24 |
| Lumen proportion (%) | 53.73 ± 3.54 | 54.16 ± 2.68 | 54.02 ± 4.43 | 53.52 ± 2.02 |
| Smooth muscle proportion (%) | 4.44 ± 0.39 | 9.97 ± 0.70*** | 5.40 ± 0.09 | 5.25 ± 0.37 |
| Intertubular compartment (%) | 22.89 ± 4.45 | 10.01 ± 1.42* | 17.57 ± 4.32 | 19.11 ± 1.33 |
A significant difference was found for *P < 0.05 with CTg; **P < 0.01 with CTg; ***P < 0.001 with CTg; † P < 0.05 with Cdg; ‡ P < 0.01 with Cdg and § P < 0.001 with Cdg. CTg, control group; Cdg, cadmium group; CdGJg, cadmium and grape juice concentrate group; GJg, grape juice concentrate group. (n = 6/group).
Epididymis light and transmission electron microscopy (TEM)
Typical tissue structure was found for the epididymis caput and cauda in groups CTg and GJg (Figure 1a,b,g,h and Figure 2). The principal cells had a columnar arrangement and basal nucleus (Figure 2a,b). TEM analysis confirmed the light microscopy observations and allowed us to identify regular apical stereocilia in principal cells (Figure 2a,b). Basal cells were observed intercalated with principal cells in the basal region (inset, Figure 2b). The eventual presence of a narrow cell with a cup shape was observed, containing vesicles produced by the secretory and endocytic function of this cell. All these cell types were distributed just inside the tunic. The sporadic presence of dense vesicles in the epithelial cell's cytoplasm was also noted (Figure 2a,b). Sperm was found in the lumen of the epididymis caput and cauda, as expected, considering their unaltered morphology (Figure 2c,d).
Figure 1.
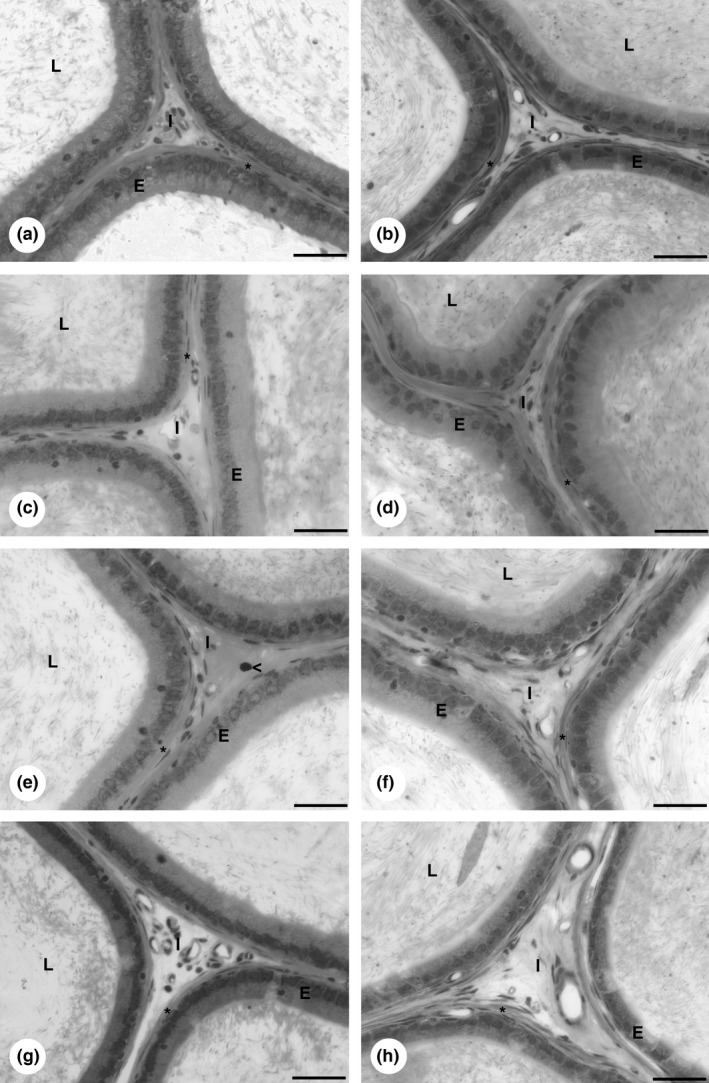
Epididymis morphology of rats exposed to cadmium and grape juice concentrate individually and in combination. Epididymis caput: (a) CTg group. (c) Cdg group. (e) CdGJg group. (g) GJg group. Epididymis cauda: (b) CTg group. (d) Cdg group. (f) CdGJg group. (h) GJg group. CTg, control group; Cdg, cadmium group; CdGJg, cadmium and grape juice concentrate group; GJg, grape juice concentrate group. (n = 6/group). Abbreviations: I, interstitium; L, tubular lumen; E, epithelium. Bar = 30 μm.
Figure 2.
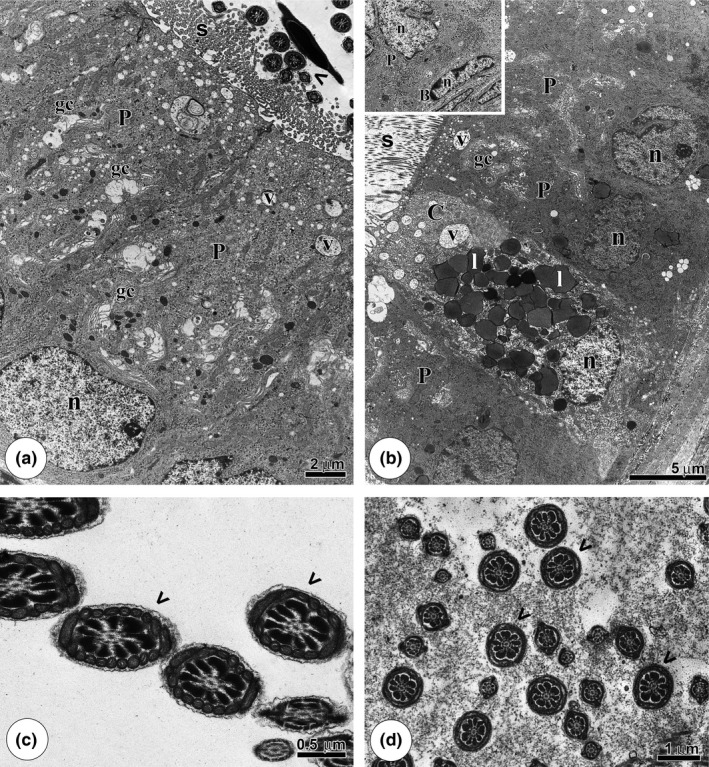
Epididymis ultrastructure of the CTg and GJg groups. (a): Epithelial overview of the caput region, showing the principal cells (P) with several vesicles (v) and Golgi complexes (gc). (b): Epithelium of the cauda portion with the clear cell (C) rich in lipid droplets (l) and other vesicles (v) and the principal cells (P) exposing the Golgi complex (gc) and many vesicles (v). Note, in the detail, the basal cells (B) position in relation to the principal cell (P). (c,d): Caput and cauda lumens, respectively, with healthy flagella (arrowheads). n: nucleus; s: stereocilia.
Cadmium aggressivity to this organ was demonstrated by the disorganized morphology of epididymis’ caput and cauda in Cdg (Figure 1c,dand Figure 3). The lack of adhesion between principal cells (Figure 3a) and between principal and basal cells (Figure 3b,c,e) was observed although these cells maintained their usual structure. A representative increase in dense vesicles in principal cell cytoplasm (Figure 3a) and an increased density of narrow cell vesicles were also noted (Figure 3b). Moreover, many lipid droplets were verified in principal and narrow cell cytoplasms (Figure 3b,d,f), occupying the entire cell cytoplasm in some cases (Figure 3d,f). In addition, regions of degenerated cytoplasm and a dilated tunic were recognized (Figure 3e). The sperm located in epididymis’ caput and cauda lumens had a considerable quantity of residual cytoplasm adhering to the flagella (Figure 3g). Furthermore, loose chromatin areas and partially adhering acrosomes occurred for some sperm heads (Figure 3h).
Figure 3.
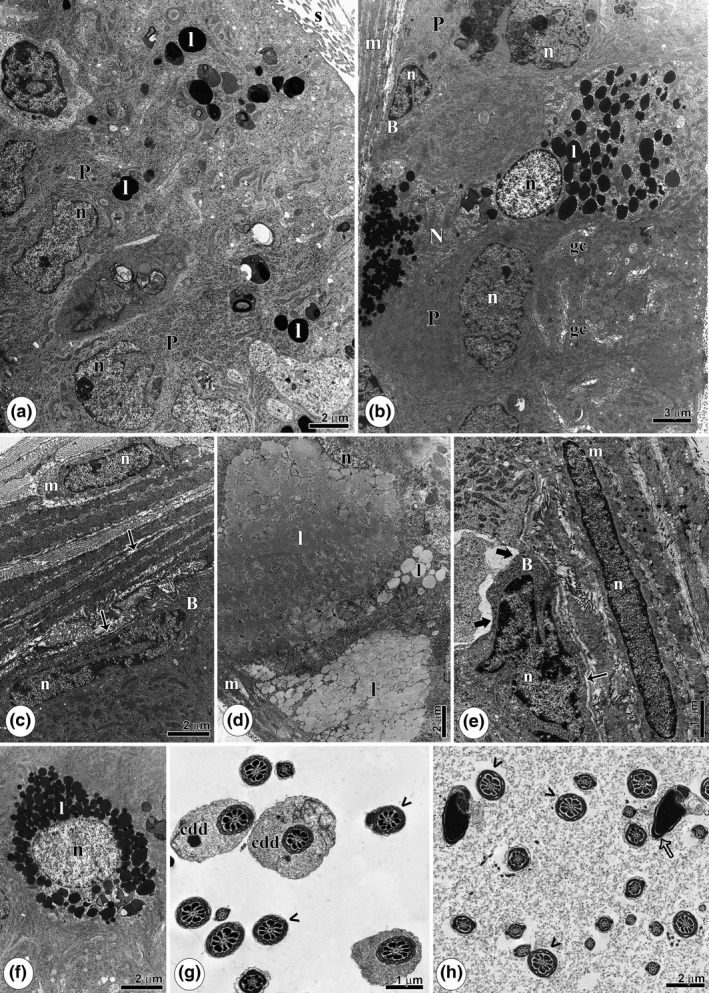
Epididymis ultrastructure of rats intoxicated with cadmium (Cdg). (a): Overview of the caput’ region epithelium, with the principal cells (P) with many lipids (l). (b): Epithelium of the cauda portion showing a basal cell (B), a narrow cell (N) with many lipid accumulation (l) and principal cells (P) with several Golgi complexes (gc). (c): Basal portion of the epithelium, highlighting the lack of adhesion between myoid (m) and basal cell (B) (thin arrow). (d): Lipid accumulation filling cell cytoplasm. (e): Caput portion with regions of degenerated cytoplasm (thick arrow) and lack of adhesion between basal (B) and myoid cell (m) (thin arrow). (f): Excess lipid deposits (l) in the cytoplasm. (g): Caput portion lumen showing sperm with distal cytoplasmic droplets (cdd) and normal flagella (arrowheads). (h): Cauda region showing sperm with areas of a poorly adhering acrosome (empty arrow) and the regular flagella (arrowheads). m, Myoid cells; n, nucleus; s: stereocilia.
The daily consumption of grape juice concentrate prior to and following a single cadmium injection demonstrated a positive action against cadmium‐induced damage in the epididymis caput and cauda (Figure 1e,f and Figure 4). In CdGJg, preserved tissue architecture was observed, exhibiting juxtaposed and morphologically normal cells, organized just inside the tunic (Figure 4a,b). Moreover, the dense vesicles of principal cell cytoplasm were less evident, and a significant reduction in the proportion of lipid droplets was verified (Figure 4a,b). The narrow cell morphology was the same as in CTg, with some dense vesicles characteristic of this cell (Figure 4c). The sperm observed in epididymis’ caput and cauda lumens also demonstrated the same structure as in CTg (Figure 4d,e).
Figure 4.
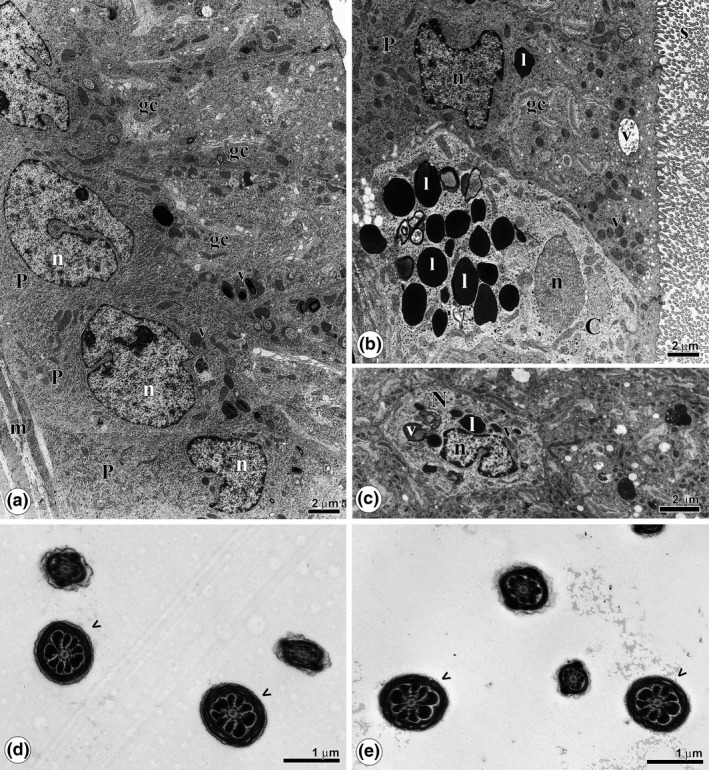
Ultrastructure of the epididymis of rats treated with cadmium and grape juice concentrate (CdGJg). (a): Overview of the epithelium of the caput portion, showing the principal cells (P), rich in vesicles (v) and Golgi complexes (gc). (b): Overview of the epithelium of the cauda region, with a clear cell (c), vesicles (v) and Golgi complexes (gc). (c): Narrow cell (N) of the cauda region, in cross section, showing a reduced amount of vesicles (v) and lipid drops (l). (d,e): Caput and cauda lumens, respectively, with sperm without abnormalities (arrowheads). l, lipids; n, nucleus; s: stereocilia.
Sperm parameters
Considering the DSP and the normal sperm percentage, the Cdg group showed significantly lower values than other groups (Figure 5a,b), while similar results were observed for the CTg and CdGJg groups. The normal sperm percentage in CdGJg was intermediate between groups CTg and Cdg. Thus, this is another positive aspect of GJC consumption after Cd intoxication. The same pattern occurred in relation to sperm number per testes and per gram of testes, where lower values were observed in the Cdg, relative to all other groups, while a re‐establishment of these parameters occurred with GJC ingestion in Cd‐intoxicated rats (Figure 5c). A qualitative analysis of the sperm profile revealed the most prevalent alterations after Cd intoxication, which is illustrated in Figure 6.
Figure 5.
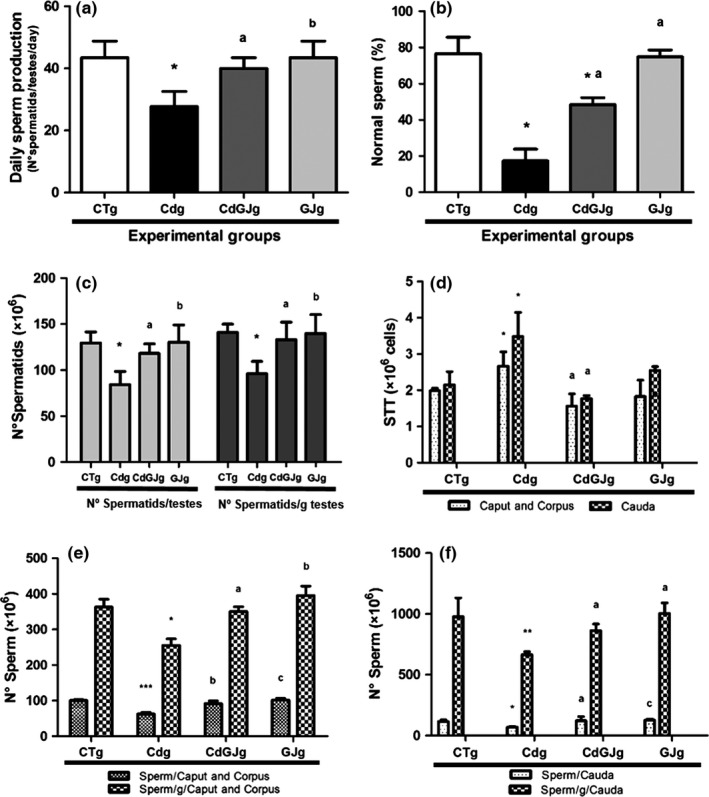
Sperm Parameters. (a) Daily sperm production. (b) Normal sperm percentage. (c) Sperm count in testis. (d) Epididymis sperm transit time. (e) Sperm count in epididymis’ caput. (f) Sperm count in epididymis’ cauda. A significant difference was found for *P < 0.05 with CTg; **P < 0.01 with CTg; ***P < 0.001 with CTg; a P < 0.05 with Cdg; b P < 0.01 with Cdg; c P < 0.001 with Cdg. CTg, control group; Cdg, cadmium group; CdGJg, cadmium and grape juice concentrate group; GJg, grape juice concentrate group. (n = 6/group).
Figure 6.
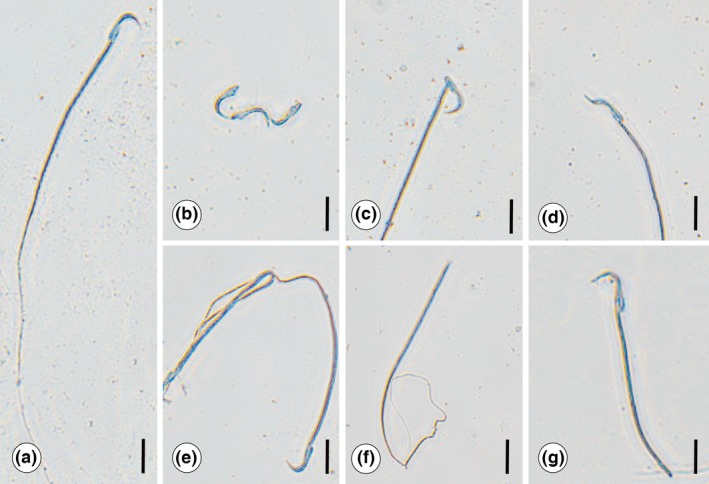
Illustration of sperm standard found for the experimental groups. (a) Normal sperm morphology, mostly observed in CTg, GJg and CdGJg. (b,g) Sperm head alterations found in group Cdg: (b) isolated head. (c) Broken head. (d) Amorphous head. Sperm flagellum alterations found in Cdg: (e) winding flagellum. (f) Isolated flagellum. (g) Broken flagellum. All these sperm alterations were also found in CDGJg, but they were less frequent. CTg, control group; Cdg, cadmium group; CdGJg, cadmium and grape juice concentrate group; GJg, grape juice concentrate group. (n = 6/group). Bar=10 μm.
GJC‐positive action was also observed in the STT and epididymis sperm count. Cdg group showed higher STT and reduced epididymis sperm count when compared to CTg group, in epididymis caput and cauda. Nevertheless, similar values between CTg and CdGJg were verified considering STT and epididymis sperm count, in both epididymis compartments, confirming GJC efficacy in maintaining these parameters (Figure 5d–f).
Determination of tissue Cd concentration
The instrumental conditions for Cd quantification were optimized by evaluating the recoveries of certified reference materials, and the results showed that suitable recoveries were obtained (103 ± 2%), as well as adequate relative deviations, below 10%. Limits of determination (LOD) and quantification (LOQ) were calculated, and the values are 0.04 and 0.14 μg/l respectively.
Cd ability to accumulate in the testes was observed in our research considering that Cd concentration in this organ was significantly higher in Cdg compared to CTg. Moreover, we could demonstrate the GJC protective effect with a reduction in cadmium accumulation in CdGJg relative to Cdg. These data can be seen in Table 3.
Table 3.
Cadmium accumulation in testis and antioxidant markers (mean value ± standard deviation)
| CTg | Cdg | CdGJg | GJg | ||
|---|---|---|---|---|---|
| Cd accumulation in testes | (μg/Kg) | 0.99 ± 0.94 | 47.75 ± 7.16*** | 33.01 ± 6.67*** , ‡ | 0 ± 0§ , †† |
| MDA | (μMol/ml) | 17.8 ± 2.16 | 27.8 ± 6.51* | 16.9 ± 2.78† | 19.5 ± 1.99 |
| GSH | (nMol/ml) | 86.7 ± 10.94 | 107.2 ± 9.00 | 104.6 ± 8.27 | 97.9 ± 4.65 |
| CAT | (U/ml) | 432 ± 36.85 | 349 ± 50,96 | 414 ± 68.39 | 417 ± 81.28 |
| SOD | (U/ml) | 0.13 ± 0.01 | 0.14 ± 0.03 | 0.17 ± 0.01 | 0.12 ± .0.01 |
A significant difference was found for *P < 0.05 with CTg; ***P < 0.001 with CTg; † P < 0.05 with Cdg and ‡ P < 0.01 with Cdg; § P < 0.001 with Cdg; †† P < 0.001 with CdGJg. CTg, control group; Cdg, cadmium group; CdGJg, cadmium and grape juice concentrate group; GJg, grape juice concentrate group. (n = 6/group).
Oxidative stress markers
The group that received only the metal demonstrated raised levels of lipid peroxidation illustrated by higher levels of MDA, compared to all experimental groups. After GJC ingestion in Cd‐intoxicated rats, it was possible to verify a reduction in lipid peroxidation level. Analyses of CAT, SOD and GSH levels were not altered among the groups. Table 3 displays the oxidative stress markers.
Discussion
Recently, many natural products have been demonstrated to make positive contributions to the prevention or mitigation of damage due to contamination (Ola‐Mudathir et al. 2008; Pires et al. 2013; Lamas et al. 2015). An evaluation of the total polyphenol content in the GJC used revealed the presence of a large amount of these molecules. Aguiar et al. (2011) showed that the major bioactive compounds in this concentrate are resveratrol, anthocyanins and quercetin. Thus, from a general perspective, our study demonstrated for the first time in the scientific literature that, when consumed over a long period, GJC promoted the maintenance of epididymis morphology and was able to regulate Cd action mechanism, allowing a complete re‐establishment of sperm production, possibly contributing to sperm maturation.
Grape polyphenols are considered potent metal‐chelating agents, mainly because of their high affinity for metal ions (Fraga et al. 2010). Thus, a significant reduction in metal accumulation was observed when GJC was administered to Cd‐contaminated rats. The fact that Cd can diffuse rapidly throughout the body after contamination (Monsefi et al. 2010), having a long biological half‐life (Oliveira et al. 2009), is crucial to its toxicity and highlights the importance of therapies that reduce its levels in the organism, as we demonstrated with continuous GJC ingestion.
Cd accumulation allows the direct action mechanism of this metal. Its ability to alter testicular architecture leads to reduced sperm output and development, as observed in the present research. A recent study by our group revealed that as a consequence of Cd contamination, disruption of cell adhesion, germ cell loss and altered sperm formation, as well as their liberation often after incomplete spermiogenesis, can occur (Lamas et al. 2015). Moreover, Cd accumulation has a direct effect inducing an inflammatory process, contributing to epithelium disruption (Al‐Azemi et al. 2010) and triggering permanent spermatogenesis loss (Abd‐Allah et al. 2009).
In our research, daily ingestion of GJC maintained the sperm number and production in the testes of intoxicated rats. Grape derivatives have been reported to interact with transcription factors involved in an inflammatory process and to improve testicular morphology, reducing the areas of disorganized and atrophic seminiferous epithelium caused by Cd (Makenzie et al. 2009; Fraga et al. 2010; Lamas et al. 2015). This activity together with the reduced metal accumulation due to the GJC can preserve testicular architecture, promoting proper sperm development, as observed in our research. Previous observations of Pires et al. (2013) found no improvement of daily sperm production in Cd‐intoxicated rats that received GJC only after the metal injury. This demonstrated the importance of GJC daily ingestion even before Cd intoxication, supporting the advantage of better eating habits.
On the other hand, Cd capacity to promote the formation of reactive oxygen species (ROS), developing a pro‐oxidative status, is a well‐known mechanism that is directly related to testicular disruption and altered sperm formation (Shaikh et al. 1999; Stohs et al. 2000; Ola‐Mudathir et al. 2008). Elevated ROS are able to stimulate lipid peroxidation (LP) (Sharma & Argawal 1996; Agarwal & Saleh 2002; Vernet et al. 2004; Ola‐Mudathir et al. 2008), promoting an altered signalling transduction, inflammatory process, DNA damages and also epithelium disruption (Siu et al. 2009; Wang et al. 2012). Although the LP process is generally accompanied by an alteration in enzymes involved in oxidative stress, this relation was not observed by us. This might be explained by the ability of organisms to re‐establish the levels of oxidative stress related enzymes, known to be time‐dependent (Predes et al. 2014). Thus, we believe there was a restoration in enzyme levels due to the time elapse between application of the metal and the biochemical analysis. Nevertheless, considering LP a more permanent event, it could be identified at the end of the experiment, indicating the probable occurrence of an oxidative stress process during this period.
Moreover, LP has a direct effect on sperm impairment; as this cell is rich in polyunsaturated fatty acids, it became an important ROS target, which is able to promote DNA damages, motility alterations, as well as sperm loss and abnormalities (El‐Demerdash et al. 2004; Vernet et al. 2004; Ola‐Mudathir et al. 2008; Zini et al. 2009; Aikten & Curry 2011). On the other hand, Cd accumulation can promote modifications in chromatin and DNA condensation considering that some molecules can bind to specific sites of sperm DNA (Aikten & Curry 2011). Alterations in sperm head morphology were very common in the present study and are, possibly, related to DNA modifications (Tanaka et al. 2005; Cho et al. 2011). Moreover, Cd accumulation has a direct effect on microtubules, inhibiting their formation or sliding, which could be associated with the defects observed in cell division and in sperm flagella (Brunner et al. 1991; Kanous et al. 1993). Thus, these aspects described above are directly related to reduced sperm production and to higher numbers of sperm with amorphous shapes that are observed after Cd intoxication.
According to Vernet et al. (2004), sperm damage caused by Cd can persist even in the epididymis tract. After an epididymis ultrastructure screening, we could note the presence of sperm with a considerable quantity of cytoplasm adhering to the flagella, as well as partially condensed areas and areas of poorly adhering acrosomes. Considering a previous investigation of Cd effects that showed residual cytoplasm adhering to the flagella after sperm release in the testicular lumen (Lamas et al. 2015), this study was able to confirm the continuing damage. Moreover, the damage is seen to be generalized, resulting in sperm alterations that could be verified throughout the epididymis tract.
Polyphenols in GJC, claimed to reduce oxidative stress and also LP, have been described (Ola‐Mudathir et al. 2008; Mojica‐Villegas et al. 2014). In a review, Fraga et al. (2010) stated that these molecules can neutralize and stabilize free radicals, reducing LP levels. Thus, together with its metal‐chelating properties, the polyphenols present in GJC have the ability to impede the LP cascade (Ola‐Mudathir et al. 2008; Fraga et al. 2010). The reduced levels of LP and Cd accumulation in Cd‐intoxicated animals that ingested GJC could result in preserved architecture and consequently improvement in sperm number, production and morphology, besides better chromatin condensation.
Reduced sperm production and increased sperm with altered morphology allowed fewer sperm to reach the epididymis, so that a lesser sperm concentration was observed in this organ caput/corpus and cauda after Cd intoxication. Thus, reduced lumen proportions are associated with the lower sperm concentration verified for this organ. In addition to a lower sperm count, Cd accelerated the time this cell takes to move through the epididymis compartments. When sperm reach the epididymis, this cell undergoes a maturation process, which confers protection, motility and other essential characteristics to the sperm, contributing to the fertilization process (Sullivan et al. 2007; Cornwall 2009; Fosseto da Silva et al. 2011). Moreover, the dilated tunic is in agreement with higher smooth muscle proportion observed in Cdg group, and both are related to the increased sperm transit time observed. Thus, morphological alteration of the epididymis tunic could contribute to the acceleration of sperm transit in this organ, reducing the exposition of this cell to the epididymis microenvironment, representing an impairment in sperm maturation and in gamete viability (Bedford 1966, 1967; Fernandez et al. 2007).
The relation between lower sperm count and acceleration in sperm transit time in epididymis caput/corpus and cauda has already been observed in previous studies using other toxic elements (Oliva et al. 2006; Fernandez et al. 2007; Fosseto da Silva et al. 2011; Fernandes et al. 2012). In this research, the lower sperm count and SST are associated with alterations in hormonal profile, because testosterone is essential to spermatogenesis and modulates epididymis contractility and luminal fluid viscosity (Sujarit & Pholpramool 1985; Fosseto da Silva et al. 2011). Moreover, Leydig cell atrophy and reduced serum testosterone have been described after the administration of 1.2 mg/Kg Cd dose (Pires et al. 2013; Lamas et al. 2015). Thus, hormone alteration triggered by the metal is possibly another cause of sperm damage in the testes and epididymis and culminates in the impairment of sperm maturation, diminishing sperm quality.
The modifications in epididymis morphology promoted by Cd are also associated with the sperm damage observed. Increased epithelium thickness is in agreement with the increased epithelium and reduced lumen proportion observed. This fact is directly related to the ultrastructural findings that revealed an increase in dense vesicles and lipid droplets, occupying the entire cell cytoplasm in some cases, promoting cell swelling. Moreover, Cd‐promoted epididymis injury also demonstrated a lack of cell adhesion and regions of degenerated cytoplasm.
The secretory activity of epididymis epithelium is attributed to its function, so it is common that these cells contain vesicles, which store substances related to sperm maturation mechanisms (Nazian 1988; Fosseto da Silva et al. 2011). Thus, the excessive accumulation of dense vesicles and lipid droplets may reflect changes in cellular metabolism which is accelerated in an effort to try to restore altered parameters and promote sperm viability. Moreover, LP and oxidative stress induction, as well as the direct action of this metal on adhesion proteins, are primarily responsible for general structural destabilization observed (Siu et al. 2009; Wang et al. 2012). Therefore, an epithelium increase and destabilization as well as vesicle accumulation may trigger alterations in the secretion pattern of the epididymis, possibly culminating in reduced sperm maturation and motility.
GJC daily ingestion extends its protective action against Cd intoxication to the epididymis parameters, possibly contributing to higher gamete quality. After GJC ingestion, a restoration of epididymis morphology and physiology was verified, which allows a proper sperm transit time and a regular maturation process. GJC metal‐chelating properties and its capacity to neutralize and stabilized free radicals are the main actions responsible for these events, reducing general Cd toxicity.
In addition, resveratrol, a polyphenol present in grapes, is also known for its ability to interact with oestrogen receptors, stimulating testosterone and gonadotrophin secretion (Juan et al. 2005). Moreover, GJC ingestion demonstrated the ability to improve Leydig cell percentage and serum testosterone in Cd‐intoxicated rats (Pires et al. 2013; Lamas et al. 2015). Thus, a possible hormonal modulation promoted by GJC exerts a positive action, also contributing to regulate epididymis contractility, secretion process and maturation mechanisms.
Considering epididymis relative and absolute weights, no alterations were observed after any treatments. When these parameters are reduced after intoxication, they have been associated with fewer sperm in the lumen of this organ (Oliva et al. 2006). Although a smaller quantity of sperm in the epididymis caput and cauda was found, in the present research it was accompanied by epithelial cell enlargement, which may have prevented the weight loss. Moreover, the maintenance of these parameters after GJC ingestion demonstrated that this concentrate does not contribute to epididymis toxicity.
The GJC by itself did not modify sperm development and epididymis morphology. This was not verified by Pires et al. (2013), who also used a GJC that corroborated with ours. Considering the testes metal concentration, the administration of G8000® in the GJg group appears to have contributed to attaining zero cadmium levels, as opposed to the CTg group that received the same water and commercial rat feed and showed a very low contamination, probably received in the commercial rat chow.
In conclusion, the present study demonstrated that long‐term GJC ingestion extends its protective effect to the epididymis tract, allowing a complete re‐establishment of its morphology, ensuring a successful sperm maturation process after Cd intoxication. In addition, the completely preserved sperm profile confirms GJC‐positive action throughout the spermatogenic process, mainly by modulating lipid peroxidation and Cd accumulation, which are the major action mechanisms for this metal. A fertility evaluation considering the results of mating these animals would be interesting and complement our findings and could be a limitation of this study. Therefore, an additional fertility investigation could be performed in near future to confirm GJC male reproductive preservation. Thus, we proved that long‐term consumption of grape juice concentrate possibly contributes to a better maintenance of gamete quality in Wistar rats after cadmium intoxication, preserving sperm prior to ejaculation, and therefore, it may be considered a promissory therapy against Cd‐induced infertility.
Conflict of interest
The authors declared no conflict of interest.
Funding source
This work was supported by the Coordination for the Improvement of Higher Education Personnel (Capes).
Acknowledgements
We are grateful to the Coordination of Higher Level Staff Improvement (Capes) for providing a scholarship for C.A. Lamas, to Henrique Ceretta Oliveira for the statistical support and to Maira Eduarda Macedo for the sperm count assistance. We thank Dr. Edson Rosa Pimentel for providing his laboratory to measure antioxidant markers dosages and for always being available to answer our questions and Dr. Solange Cadore for providing her laboratory to measure the Cd accumulation level in testicular tissue.
References
- Abd‐Allah A.R.A., Helal G.K., Al‐Yahya A.A., Aleisa A.M., Al‐Rejaie S.S. & Al‐Bakheet S.A. (2009) Pro‐inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L‐carnitine. Oxid. Med. Cell. Longev. 2, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ada (American Dietetic Association) . (2004) Position of the American Dietetic Association: functional foods. J. Am. Diet. Assoc. 104, 814–826. [DOI] [PubMed] [Google Scholar]
- Agarwal A. & Saleh R.A. (2002) Role of oxidants in male infertility: rationale, significance, and treatment. Urol. Clin. North Am. 29, 817–827. [DOI] [PubMed] [Google Scholar]
- Aguiar O., Gollücke A.P., De Moraes B.B. et al (2011) Grape juice concentrate prevents oxidative DNA damage in peripheral blood cells of rats subjected to a high‐cholesterol diet. Br. J. Nutr. 105, 694–702. [DOI] [PubMed] [Google Scholar]
- Aikten R.J. & Curry B.J. (2011) Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 14, 367–381. [DOI] [PubMed] [Google Scholar]
- Al‐Azemi M., Omu F.E., Kehinde E.O., Anim J.T., Oriowo M.A. & Omu A.E. (2010) Lithium protects against toxic effects of cadmium in the rat testes. J. Assist. Reprod. Genet. 8, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade A.C.M. (2006) Ação do vinho tinto sobre o sistema nervoso simpático e a função endotelial em pacientes hipertensos e hipercolesterolêmicos. PhD Tesis: University of São Paulo. [Google Scholar]
- Bedford J.M. (1966) Development of the fertilizing ability of spermatozoa in the epididymis of the rabbit. J. Exp. Zool. 163, 319–330. [Google Scholar]
- Bedford J.M. (1967) Effect duct ligation on the fertilizing ability of spermatozoa in the epididymis of rabbit. J. Exp. Zool. 166, 271–282. [DOI] [PubMed] [Google Scholar]
- Brunner M., Albertini S. & Würgler F.E. (1991) Effects of 10 known or suspected spindle poisons in the in vitro porcine brain tubulin assembly assay. Mutagenesis 6, 65–70. [DOI] [PubMed] [Google Scholar]
- Cho C., Willis W.D., Goulding E.H. et al (2011) Haploinsufficiency of protamine‐1 or ‐2 causes infertility in mice. Nat. Genet. 28, 82–86. [DOI] [PubMed] [Google Scholar]
- Cornwall G.A. (2009) New insights into epididymal biology and function. Hum. Reprod. Update 15, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeneghini D.C.S.J. & Lemes S.A.F. (2011) Effects of wine components on cardiovascular function. Nutrire 36, 163–176. [Google Scholar]
- Dragano N.R.V., Marques A.C., Cintra D.E.C. et al (2013) Freeze‐dried jaboticaba peel powder improves insulin sensitivity in high‐fat‐fed mice. Br. J. Nutr. 110, 447–455. [DOI] [PubMed] [Google Scholar]
- El‐Demerdash F.M., Yousef M.I., Kedwany F.S. & Baghdadi H.H. (2004) Cadmium‐induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta‐carotene. Food Chem. Toxicol. 42, 1563–1571. [DOI] [PubMed] [Google Scholar]
- El‐Shahat A.E., Gabr A., Meki A.R. & Mehana E.S. (2009) Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. Int. J. Morphol. 27, 757–764. [Google Scholar]
- Favareto A.P.A., Fernandez C.D.B., Da Silva D.A.F., Anselmofranco J.A. & Kempinas W.G. (2011) Persistent impairment of testicular histology and sperm motility in adult rats treated with cisplatin at peri‐puberty. Basic Clin. Pharmacol. Toxicol. 109, 85–96. [DOI] [PubMed] [Google Scholar]
- Fernandes G.S., Arena A.C., Campos K.E. et al (2012) Glutamate‐induced obesity leads to decreased sperm reserves and acceleration of transit time in the epididymis of adult male rats. Reprod. Biol. Endocrinol. 5, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C.D.B., Porto E.M., Arena A.C. & Kempinas W.G. (2007) Effects of altered epididymal sperm transit time on sperm quality. Int. J. Androl. 31, 427–437. [DOI] [PubMed] [Google Scholar]
- Fosseto da Silva D.A., Teixeira C.T., Scarano W.R. et al (2011) Effects of methylmercury on male reproductive functions in Wistar rats. Reprod. Tocxicol. 31, 431–439. [DOI] [PubMed] [Google Scholar]
- Fraga C.G., Galleano M., Verstraeten S.V. & Oteiza P.I. (2010) Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 31, 435–445. [DOI] [PubMed] [Google Scholar]
- Gang L.S., Song D.Y., Qiang N. et al (2015) Grape seed proanthocyanidin extract alleviates arsenic‐induced oxidative reproductive toxicity in male mice. Biomed. Environ. Sci. 28(272), 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.S., Gupta E.S., Dhakal B.K., Thakur A.R. & Ahnn J. (2004) Vitamin C and vitamin E protect the rat testes from cadmium – induced reactive oxygen species. Mol. Cells 17, 132–139. [PubMed] [Google Scholar]
- Ho C.T., Rafi M.M. & Ghai G. (2010) Substâncias bioativas: nutracêuticas e tóxicas In: Química de alimentos de Fennema, pp. 585–608 (eds Damodaran S., Parkin K.L. & Fennema O.R.), Porto Alegre: Artmed. [Google Scholar]
- Hu F.B. (2013) Plant‐based foods and prevention of cardiovascular disease: an overview. Am. J. Clin. Nutr. 78, 544S–551S. [DOI] [PubMed] [Google Scholar]
- Juan M.E., González‐Pons E., Munuera T., Ballester J., Rodríguez‐Gil J.E. & Planas J.M. (2005) Trans‐resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 135, 757–760. [DOI] [PubMed] [Google Scholar]
- Kanous K.S., Casey C. & Lindemann C.B. (1993) Inhibition of microtubule sliding by Ni2+ and Cd2+: evidence for a differential response of certain microtubule pairs within the bovine sperm axoneme. Cell Motil. Cytoskeleton 26, 66–76. [DOI] [PubMed] [Google Scholar]
- Kempinas W., Suarez J., Roberts N. et al (1998) Rat epididymal sperm quantity, quality, and transit time after guanethidine‐induced sympathectomy 1. Biol. Reprod. 59, 890–896. [DOI] [PubMed] [Google Scholar]
- Lamas C.A., Gollucke A.P.B. & Dolder H. (2015) Grape juice concentrate (G8000®) intake mitigates testicular morphological and ultrastructural damage following cadmium intoxication. Int. J. Exp. Pathol. 95, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenquiste S.L., Marineli R.L., Moraes E.A., Dionísio A.P., Brito E.S., Maróstica M.R. (2015) Jaboticaba peel and jaboticaba peel aqueous extract shows in vitro and in vivo antioxidant properties in obesity model. Food Res. Int. 77, 162–170. [Google Scholar]
- Makenzie G.G., Delfino J.M., Keen C.L., Fraga C.G. & Oteiza P.I. (2009) Dimeric procyanidins are inhibitors of NF‐қβ‐DNA binding. Biochem. Phermacol. 78, 1252–1262. [DOI] [PubMed] [Google Scholar]
- Messaoudi I., Hammouda F., El Heni J., Baati T. & Sai D.K. (2010) Reversal of cadmium‐induced oxidative stress in rat erythrocytes by selenium, zinc or their combination. Exp. Toxicol. Pathol. 62, 281–288. [DOI] [PubMed] [Google Scholar]
- Mojica‐Villegas M.A., Izquierdo‐Vega J.A., Chamorro‐Cevallos G. & Sanchez‐Gutierrez M. (2014) Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 6, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsefi M., Alaee S., Moradshahi A. & Rohani L. (2010) Cadmium‐induced infertility in male mice. Environ. Toxicol. 25, 94–102. [DOI] [PubMed] [Google Scholar]
- Nazian S.J. (1988) Serum concentrations of reproductive hormones after administration of various anesthetics to immature and young adult male rats. Proc. Soc. Exp. Biol. Med. 187, 482–487. [DOI] [PubMed] [Google Scholar]
- Ola‐Mudathir K.F., Suru S.M., Fafunso M.A., Obioha U.E. & Faremi T.Y. (2008) Protective roles of onion and garlic extracts on cadmium‐induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 46, 3604–3611. [DOI] [PubMed] [Google Scholar]
- Oliva S.U., Messias A.G., Silva D.A.F., Pereira C.O.M., Gerardin D.C.C. & Kempinas W.G. (2006) Impairment of adult male reproductive function in rats exposed to ethanol since puberty. Reprod. Toxicol. 22, 599–605. [DOI] [PubMed] [Google Scholar]
- Oliveira H., Spanò M., Santos C. & Pereira M.L. (2009) Adverse effects of cadmium exposure on mouse sperm. Reprod. Toxicol. 28, 550–555. [DOI] [PubMed] [Google Scholar]
- Pires V.C., Gollücke A.P., Ribeiro D.A., Lungato L., D'Almeida V. & Aguiar O. (2013) Grape juice concentrate protects reproductive parameters of male rats against cadmium‐induced damage: a chronic assay. Br. J. Nut. 9, 1–10. [DOI] [PubMed] [Google Scholar]
- Predes F.S., Diamante M.A.S. & Dolder H. (2010) Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 91, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predes F.S., Diamante M.A.S., Foglio M.A. et al (2014) Hepatoprotective effect of Arctium lappa root extract on cadmium toxicity in adult rats. Biol. Trace Elem. Res. 160, 250–257. [DOI] [PubMed] [Google Scholar]
- Predes F.S., Diamante M.A.S., Foglio M.A., Dolder H. (2016) Effects of Arctium lappa on cadmium‐induced damage to the testis and epididymis of adult wistar rats. Biol. Trace Elem. Res. 173, 362–371. [DOI] [PubMed] [Google Scholar]
- Robb G.W., Amann R.P. & Killian G.J. (1978) Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fert. 54, 103–107. [DOI] [PubMed] [Google Scholar]
- Scollon E.J., Starr J.M., Godin S.J., DeVito M.J. & Hughes M.F. (2009) In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome P450 isoforms. Drug Metab. Dispos. 37, 221–228. [DOI] [PubMed] [Google Scholar]
- Seed J., Chapin R.E., Clegg E.D. et al (1996) Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. Reprod. Toxicol. 10, 237–244. [DOI] [PubMed] [Google Scholar]
- Shaikh Z.A., Vu T.T. & Zaman K. (1999) Oxidative stress as a mechanism of chronic cadmium‐induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 154, 256–263. [DOI] [PubMed] [Google Scholar]
- Sharma R.K. & Argawal A. (1996) Role of reactive oxygen species in male infertility. Urology 48, 835–850. [DOI] [PubMed] [Google Scholar]
- Singleton V.L. & Rossi J.A. (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 16, 144–158. [Google Scholar]
- Siu E.R., Mruk D.D., Porto C.S. & Cheng C.Y. (2009) Cadmium‐induced testicular injury. Toxicol. Appl. Pharmacol. 238, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D., Hassoun E. & Bagchi M. (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 19, 201–213. [PubMed] [Google Scholar]
- Sujarit S. & Pholpramool C. (1985) Enhancement of sperm transport through the rat epididymis after castration. J. Reprod. Fertil. 74, 497–502. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Frenette G. & Girouard J. (2007) Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 9, 483–491. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Iguchi N., Isotani A. et al (2005) HANP1/H1T2, a novel histone H1‐like protein involved in nuclear formation and sperm fertility. Mol. Cell. Biol. 25, 7107–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet P., Aitken R.J. & Drevet J.R. (2004) Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 216, 31–39. [DOI] [PubMed] [Google Scholar]
- Wang L., Xu T., Lei W.W., Liu D.M. & Li Y.J. (2011) Cadmium‐Induced Oxidative Stress and Apoptotic Changes in the Testis of Freshwater Crab, Sinopotamon henanense. PLoS ONE 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sun Y., Liu J. et al (2012) Protective effect of the flavins on cadmium‐induced testicular toxicity in male rats. Food Chem. Toxicol. 50, 3243–3250. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Fu J et al (2016) Cadmium inhibits mouse sperm motility through inducing tyrosine phosphorylation in a specific subset of proteins. Reprod. Toxicol. 63, 96–106. [DOI] [PubMed] [Google Scholar]
- Who . Cadmium (Environmental Health Criteria No. 134). Geneva: WHO;1992. [Google Scholar]
- Zanato V.F., Martins M.P., Anselmo‐Franci J.A., Petenusci S.O. & Lamano‐Carvalho T.L. (1994) Sexual Development of male Wistar rats. Braz. J. Med. Bio. Res. 27, 1273–1280. [PubMed] [Google Scholar]
- Zini A., Phillips S., Courchesne A. et al (2009) Sperm head morphology is related to high deoxyribonucleic acid stainability assessed by sperm chromatin structure assay. Fertil. Steril. 91, 2495–2500. [DOI] [PubMed] [Google Scholar]
- Zorzano A. & Herrera H. (1990) In vivo ethanol elimination in man, monkey and rat: A lack of relationship between the ethanol metabolism and the hepatic activities of alcohol and aldehyde dyhydrogenases. Life Sci. 46, 223–230. [DOI] [PubMed] [Google Scholar]


