Summary
Amino acid metabolism is a significant metabolic activity in humans, especially of sulphur‐containing amino acids, methionine and cysteine (Cys). Cys is cytotoxic and neurotoxic in nature; hence, mammalian cells maintain a constant intracellular level of Cys. Metabolism of Cys is mainly regulated by two thiol dioxygenases: cysteine dioxygenase (CDO) and 2‐aminoethanethiol dioxygenase (ADO). CDO and ADO are the only human thiol dioxygenases reported with a role in Cys metabolism and localized to mitochondria. This metabolic pathway is important in various human disorders, as it is responsible for the synthesis of antioxidant glutathione and is also for the synthesis of hypotaurine and taurine. CDO is the most extensively studied protein, whose high‐resolution crystallographic structures have been solved. As compared to CDO, ADO is less studied, even though it has a key role in cysteamine metabolism. To further understand ADO's structure and function, the three‐dimensional structures have been predicted from I‐TASSER and SWISS‐MODEL servers and validated with PROCHECK software. Structural superimposition approach using iPBA web server further confirmed near‐identical structures (including active sites) for the predicted protein models of ADO as compared to CDO. In addition, protein–protein interaction and their association in patho‐physiology are crucial in understanding protein functions. Both ADO and CDO interacting partner profiles have been presented using STRING database. In this study, we have predicted a 3D model structure for ADO and summarized the biological roles and the pathological consequences which are associated with the altered expression and functioning of ADO and CDO in case of cancer, neurodegenerative disorders and other human diseases.
Keywords: 2‐aminoethanethiol dioxygenase, cancer, cupin family, cysteine dioxygenase, neurodegenerative diseases
Introduction
Methionine (Met) and cysteine (Cys) are considered as principal sulphur‐containing primary amino acids. Metabolism of the sulphur‐containing amino acids is a crucial process inside the cell. In mammalian cells, many processes result in oxidation of thiol group, and thiol dioxygenation is an irreversible process. Mammalian thiol dioxygenase family comprises only two known proteins: cysteine dioxygenase (CDO, EC 1.13.11.20) and cysteamine (2‐aminoethanethiol) dioxygenase (ADO, EC 1.13.11.19) (Dominy et al. 2007; Stipanuk et al. 2011). These two mitochondrial proteins are members of the cupin superfamily and contain a β‐sandwich central domain with jelly‐roll topology and two consensus sequences (Dominy et al. 2007). Cysteine dioxygenase was first described in the 1960s by demonstrating that crude rat liver extracts contain an enzyme that produced L‐cysteine sulphinic acid from L‐cysteine (Lombardini et al. 1969; Yamaguchi et al. 1971). In 1966, Cavallini and co‐workers demonstrated the presence of another protein, ADO in the animal tissues, which converts cysteamine to hypotaurine (Cavallini et al. 1966). ADO has a much broader range of tissue expression than CDO. ADO has higher level of expression in the brain and muscle, whereas CDO expression is more specific and is present in organs, particularly liver, adipose tissue, and lungs, with little expression in the brain (Simmons et al. 2005; Ueki & Stipanuk 2009). ADO adds two atoms of oxygen to free cysteamine (2‐aminoethanethiol) to form hypotaurine. Hypotaurine further oxidizes to taurine (Figures 1 and 2) (Stipanuk et al. 2011). In response to dietary proteins or sulphur amino acid intake, CDO concentration may change up to 45 times and catalytic efficiency may change up to ten times. Overall, dynamic efficiency changes up to 450‐fold (Stipanuk et al. 2006, 2009). In mammals, Cys level is maintained by the liver; and in rat, intracellular Cys level is kept in the range of 20–100 nmol/g even though the sulphur amino acid intake can vary (Stipanuk et al. 2006). Cysteamine is an aminothiol compound and is cytotoxic at 10−4 to 10−3 M concentration. It has been experimentally proved that 10−4 M concentration of cysteamine kill cells by apoptosis. A study in 3T3‐L1 cells showed that CDO, CSDO and ADO protein levels increased during adipogenic differentiation and also significantly increased when these cells achieved a mature adipocyte phenotype. These changes were accompanied by a rise in hypotaurine and taurine production, particularly when cells were treated with Cys and cysteamine (Ueki & Stipanuk 2009). A study on rat and mouse model showed most probable pathway for ADO is coenzyme A to cysteamine and finally to hypotaurine.
Figure 1.
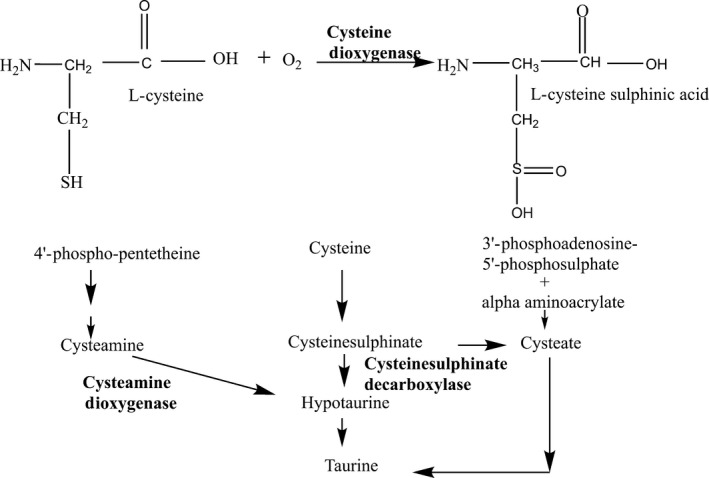
Cysteine metabolism catalysed by cysteine dioxygenase (CDO). L‐cysteine is converted into l‐cysteine sulfonic acid in the presence of oxygen, and taurine is the final product of pathway, three pathways exists for taurine biosynthesis however CDO and ADO mediated pathways are the main route for taurine biosynthesis.
Figure 2.
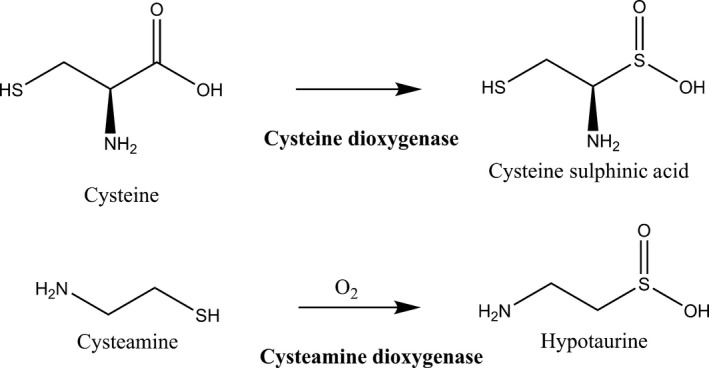
A comparison of the thiol dioxygenase reaction catalyzed by the two cysteine dioxygenase (CDO) and cysteamine dioxygenase (ADO) enzymes. These two enzymes are the rate‐limiting enzymes in cysteine metabolism.
Human thiol dioxygenases: the cupin superfamily members
Thiols are crucial in regulating oxidative stress, signal translation and transcriptional process in cells. Cysteamine, GSH, hypotaurine and taurine are widely known sulphur compounds that function as antioxidants. Cysteamine is known as a scavenger of OH and protects the cell against ionizing radiation (Guerin et al. 2001). There are only two thiol dioxygenases in mammals: CDO and ADO (Dominy et al. 2007; Stipanuk et al. 2011). These two proteins are placed in the cupin superfamily as they contain conserved cupin motifs. These family proteins are widely distributed from archaea, bacteria to eukaryotes. This superfamily has two very short signature sequence motifs, Gx5 HxHx3‐6 Ex6G (cupin motif‐1) and Gx5‐7PxGx2 Hx3N (cupin motif‐2), separated by a less conserved intermotif, that is separated by approximately 15–50 residues of amino acids. This two motif structure is not as conserved as thought earlier. The cupin superfamily is quite diverse and includes sugar‐binding metal‐independent, as well as metal‐dependent enzymes possessing dioxygenase, decarboxylase, hydrolase and isomerase activities. In this family, other non‐enzymatic functions are also present, such as binding to auxin, transcription factors and seed storage proteins (Woo et al. 2000; Adachi et al. 2001; Dominy et al. 2007). According to the structural classification of proteins (SCOP), cupin proteins are the members of the ‘RmlC‐like Cupins’ superfamily within the double‐stranded β‐helix (DSBH) multicatalytic fold (Giraud et al. 2000; Fusetti et al. 2002). The DSBH (also called as jelly‐roll) topology comprises of eight β‐strands that form a β‐sandwich structure comprised of two‐four‐stranded anti‐parallel β‐sheets. However, there are four types of jelly‐roll topology possible, but the right‐handed class‐I form is only present naturally (Uberto & Moomaw 2013).
The recent work on CDO identified that the functional enzyme is a monomer with a molecular weight of 23 KDa and 200 amino acids long (Joseph & Maroney 2007). Human CDO's crystal structure revealed high structural similarity to the mouse and rat origin. The conserved residues in CDO include Tyr58, Arg60, Trp77, His86, His88, His140, His155 and Tyr157, while Tyr58 is present at the entrance of the active site (McCoy et al. 2006). In the core of the active site, catalytically important ferrous ion is coordinated by N‐atom of His86, His88 and His140 (Ye et al. 2007). There is a thioether bond between the active site in between the residues Cys93 and Tyr157, a cross‐link factor, which was named as Cys‐Tyr cofactor (Arjune et al. 2015). This Cys‐Tyr cofactor has been found to be involved in post‐translational modification (McCoy et al. 2006). Cysteine dioxygenase catalyses the conversion of L‐cysteine to L‐cysteine sulphinic acid, CSA (Figure 1 ). CSA becomes a branch point, while one branch point leads to taurine via hypotaurine, and other branch leads to 3‐sulphinyl pyruvate, finally producing sulphate. In this process, taurine and hypotaurine synthesis is an important process, as taurine is the second most abundant amino acid (Vitvitsky et al. 2011). Taurine has a role in various metabolic activities like maintaining cardiac functions, protecting neural cells from excitotoxicity and damage induced by ischaemia. Taurine has been proposed as a neurotransmitter, as it is the second most abundant amino (sulphonic) acid in the central nervous system, CNS (Saransaari & Oja 2008). Taurine plays a key role in stabilizing mammalian skeletal muscles. In a study carried out by Vitvitsky et al., the exposure of cells to Cys or cysteamine resulted in an elevated intracellular hypotaurine without a corresponding increase in taurine levels. In addition, it was also suggested that oxidation of hypotaurine limits taurine synthesis in the cells. Consistent with its role as an organic osmolyte, taurine synthesis was stimulated by a hypertonic condition in neurons (Vitvitsky et al. 2011).
Another protein in mammals showing thiol dioxygenase activity is cysteamine dioxygenase (ADO), an ortholog of CDO (Dominy et al. 2007). Dominy Jr. and co‐workers reported that ADO has thiol dioxygenase activity, which shares some sequence similarity (14.2% identity) to CDO (Dominy et al. 2007). In ADO, the conserved glutamate (Glu) residue in cupin motif‐1 is replaced by either glycine (Gly) or valine (Val). These two proteins (CDO and ADO) represent unique clades within the cupin superfamily. These two proteins and many other proteins are also known as domain of unknown function 1637 (DUF1637) family. ADO binds to a transition metal (i.e. Fe2+) which is essential for its thiol dioxygenase activity (Dominy et al. 2007). It is also well characterized that there is no cross‐utilization of these two proteins, as ADO never uses Cys as its substrate, nor CDO uses cysteamine as its substrate (Dominy et al. 2006). In the brain, taurine gets synthesized, yet it has little CDO's expression (Dominy et al. 2004), to its contrast ADO's expression is very high (Dominy et al. 2007). One can say that ADO pathway is predominantly responsible for the hypotaurine and taurine biosynthesis in the brain (Dominy et al. 2007) for metabolic functions of the brain.
Disorder regions are responsible for protein function and interaction
Disordered regions in various proteins have been confirmed experimentally; they have often been found to be essential for protein function (Hegde et al. 2008, 2010). Intrinsically disordered regions are regions that lack stable secondary or tertiary conformation, and 30% of the human proteins are thought to contain large contiguous disordered regions (Ward et al. 2004). Interestingly, the intrinsic disorder appears to be significantly correlated with certain terms from functional ontologies and with specific functional motifs (Hegde et al. 2010). The disordered regions in proteins are involved in various functions like transcriptional regulation, signal transduction, cell cycle control, DNA damage sensing and repair, post‐translational modifications, such as phosphorylation, or sites of protein–protein interactions, often fall into the regions that are locally disordered or undergo order–disorder transition in different, biologically relevant situations (Hegde et al. 2008). Disordered regions in proteins show higher rates of mutations, presumably because changes in their protein sequences rarely affect protein stability and function as severely as that in ordered regions (Brown et al. 2002; Mittag et al. 2010). Recent studies have indicated that ‘hub’ protein complexes are widely present in higher eukaryotes, whose formation mostly involves interaction with disordered regions. Bioinformatics analyses of known protein interactions are suggestive that such interactions among disordered structures are significantly preferred among human proteins.
Commonly used disorder region prediction tools include PONDR, DisEMBL, MeDor, PrDOS, RONN, FoldIndex, GlobPlot, IUPred and FoldUnFold, and these tools are available in the public domain. Among these, PONDR is the most widely used and advanced version, which can predict the information upon single sequences. The PONDRs are typically feed‐forwarded neural networks that use sequence attributes taken over window of 9–21 amino acids. These attributes, such as the fractional composition of particular amino acids, hydropathy or sequence complexity, are averaged over these windows, and the values are used to train the neural network during predictor construction (Obradovic et al. 2003). The VSL predictors in PONDR take advantage of such differences to yield more accurate predictions. The PONDR score of 0.5 and higher indicates a disordered structure in any given protein.
To understand the role of ordered and disordered regions in the functioning of ADO and CDO, analyses with the multiple sequence alignments (MSA) were performed (Figure 3a,b). MSA were carried out to observe the evolutionary conservation of functionally important residues in ADO (His112, His114 and His194) and CDO (His86, His88 and His140) (McCoy et al. 2006). In this study, we used PONDR for prediction of CDO and ADO disordered regions (Vucetic et al. 2003). ADO comprises of 43.70% of disordered region and CDO contains 29% respectively. MSA of ADO and CDO indicate the conservation of length and location of disordered region in full‐length ADO and CDO. It also proves the intrinsic conservation amend functionally important residues in ADO (His112, His114 and His194) and CDO (His86, His88 and His140). The disordered regions are localized to the middle protein segment of both ADO and CDO proteins (Figure 4). ADO has a total of 270 amino acids and has disordered regions distributed in seven regions. Amino acids 137 to 182 in ADO form the longest disordered region of 46 amino acid residues, and this region has highest average strength of 0.6627. In CDO, disordered regions are distributed in five regions, and the longest disordered region is localized from 115 to 128 amino acids with an average strength of 0.7634.
Figure 3.
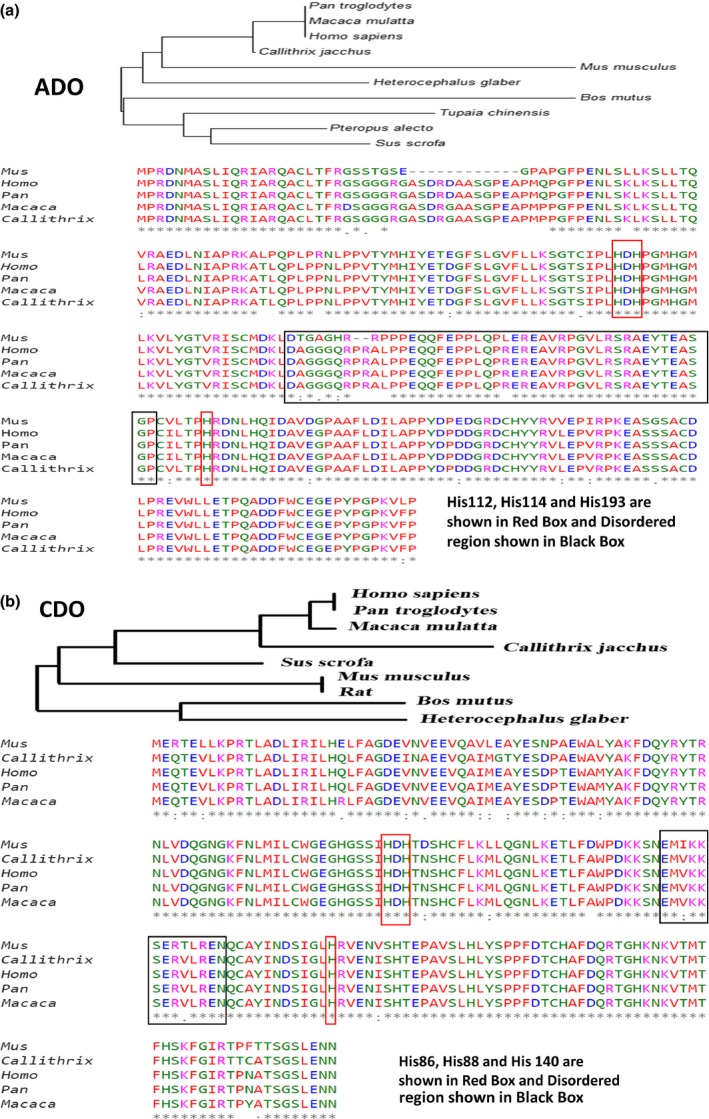
Multiple Sequence Alignment for ADO and CDO. (a) Multiple sequence alignment of ADO by Clustal Omega algorithm. Color indicated shows following color coding. RED: Small [small+ hydrophobic (incl. aromatic ‐Y)], BLUE: Acidic, MAGENTA: Basic‐H, GREEN: Hydroxyl + sulfhydryl + amine + G and GREY: Unusual amino/imino acids; and (b) Multiple sequence alignment of CDO by Clustal Omega algorithms.
Figure 4.
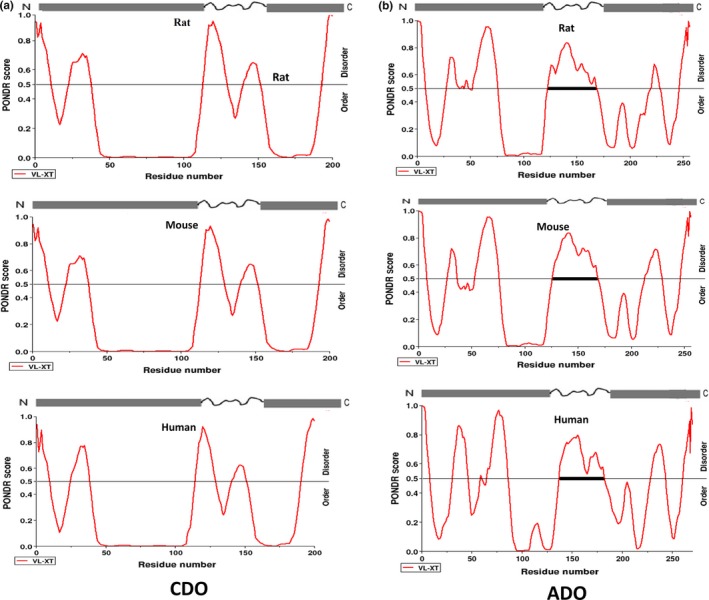
Comparison of disordered regions present in (CDO and ADO) thiol dioxygenase enzymes from the proteins of three different (rat, mouse and human) species being predicted by the PONDR software.
In silico structure prediction of ADO
Human ADO has no X‐ray crystal structure reported till now. The three‐dimensional (3D) structure is an essential requirement for understanding the structure and function of a protein. The 3D structure was predicted from I‐TASSER web server (Yang et al. 2015). I‐TASSER is a hierarchical protein structure modelling approach based on the secondary‐structure enhanced Profile‐Profile threading Alignment (PPA) and the iterative implementation of the Threading ASSEmbly Refinement (TASSER). The most probable predicted structure of ADO generated was validated with Ramachandran plot (Yang & Zhang 2015). PROCHECK analysis of protein revealed that approximately 3% amino acid residues have disallowed phi/psi angles in Ramachandran plot, and only approximately 55% amino acid residues are in the most favoured region and Verify_3D program showed good 3D_1D profile score of the residues, that is approximately 86% residues had an average 3D‐1D score of >0.2 (Bowie et al. 1991; Laskowski et al. 1993). According to I‐TASSER prediction, the predicted structure has C‐score of 2.14 and estimated RMSD of 10.9 ± 4.6 Å. This predicted structure has maximum coverage of 0.70 and normalized Z‐score of 1.06 with the template (PDB ID‐3ussA). According to Ramachandran plot, only approximately 55% amino acids of this model are in favoured region. The predicted active site has iron centre with coordination with four amino acid residues (His112, His114, His193 and Leu208). To understand the detailed picture and to determine more accurate 3D model, SWISS‐MODEL server was employed. The predicted 3D‐protein structure has been analysed by PROCHECK, and Ramachandran analysis of this protein revealed that approximately 2% of amino acid residues have phi/psi angles in disallowed region. The model has approximately 84% of amino acids in most favoured region, approximately 12% amino acids are in additional allowed region, and approximately 3% of amino acids are in generously allowed region (Figures 5 and 6 ). Ramachandran plot indicates that the protein is highly stable. Both the models have 8.4 Å RMSD and have approximately 91% similarity with the backbone atoms of complete template (CDO), but the active site of both models is similar with three His molecules at the centre. These iron coordination centres are organized in same geometry and same location in the model. The model from SWISS‐MODEL server has a loose active site with 6.45 Å distance between His114 and His193; 5.80 Å distance between His193 and His112; and 6.07 Å between His112 and His114. The model predicted by I‐TASSER has compact active centre with 5.59 Å distance between His114 and His 193; 6.07 Å between His193 and His112; and 5.68 Å between His112 and His114. The predicted ADO's 3D structures are not totally similar; however, the core structures of both the models are identical, and the difference lies in loop region and N‐ and C‐terminal regions. To understand more about ADO's thiol dioxygenase activity and correctness of SWISS‐MODEL server, predicted protein model's structural superimposition has been performed. Root mean square deviation (RMSD), between backbone chain atoms of the model and the respective template (PDB Id: 2IC1, Figure 7 ), was calculated by structural superimposition using iPBA web server (Tyagi et al. 2008). The comparative study of ADO model and its CDO template (PDB Id: 2IC1) by iPBA web server showed a RMSD of 0.16 Å. Both I‐TASSER and SWISS‐MODEL models are shown in Figure 8, and the topology model of CDO and ADO is shown in Figure 9.
Figure 5.
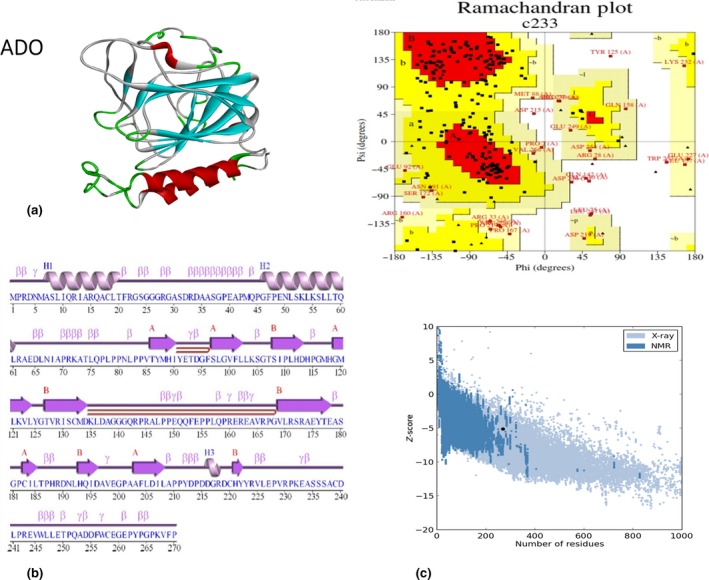
(a) Modelled structure of cysteamine dioxygenase (ADO) [SWISS‐MODEL server (Laskowski, 2009)]; (b) 2‐D structure of ADO from PDBsum is modelled from SWISS‐MODEL modeling software; and (c) PROCHECK analysis and Ramachandran plot of the 3‐D structure of ADO. The protein model has 83.6% of amino acids in the most favored region, 11.7% of amino acids as additional allowed region, 2.9% of amino acids generously allowed region, and 1.8% amino acids as disallowed region. Protein structure analysis (Pro SA‐web) predicted that ADO protein has Z‐score of −5.79.
Figure 6.
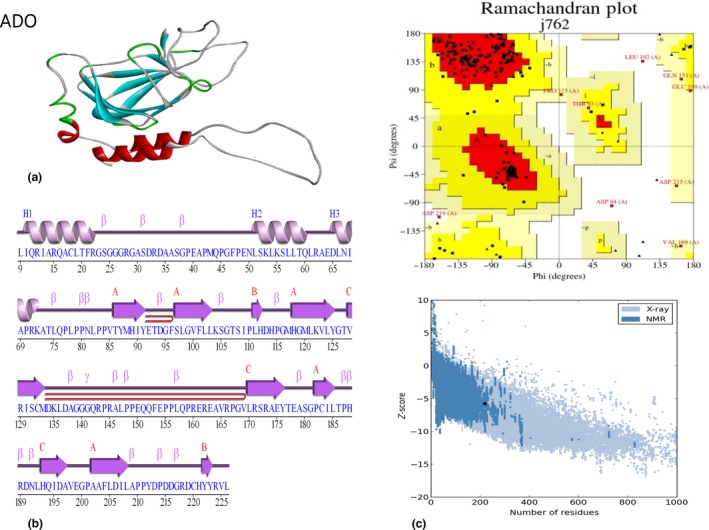
(a) Modelled structure of ADO predicted from the I‐TASSER server; (b) 2‐D structural details of ADO from PDBsum had been modeled from I‐TASSER modeling software; and (c) PROCHECK analysis and Ramachandran plot of the 3‐D structure of ADO. The Protein model has 54.5% amino acids in the most favored region, 33.3% amino acids in additional allowed region, 8.9% of amino acids in generously allowed region, and 3.3% amino acids in disallowed region. Protein structure analysis (Pro‐SA‐web) predicted that ADO protein has Z‐score of −5.15.
Figure 7.
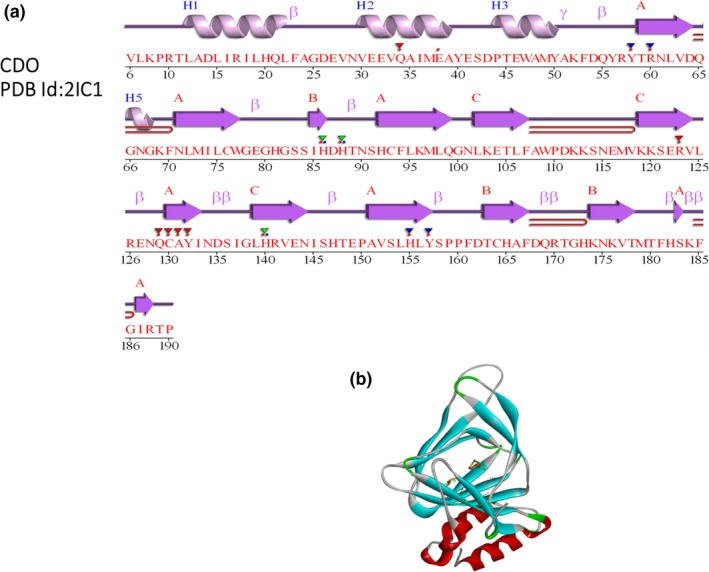
(a) 2‐D structural details (PDBsum) of Cysteine dioxygenase (CDO); and (b) 3‐D structure of CDO (PDB Id: 2IC1).
Figure 8.
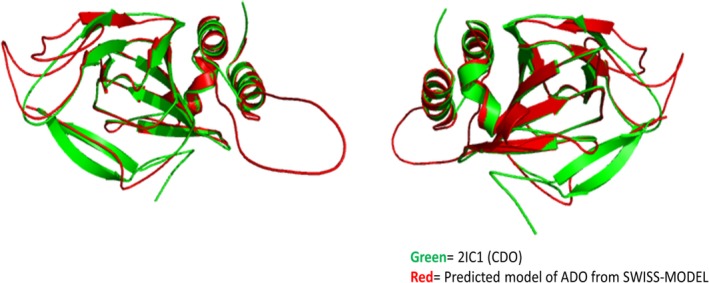
Superimposed structures of the predicted 3‐D structure of ADO in (green color) and CDO PDB Id: 2IC1 model of CDO in (red color) which have been predicted by the iPBA webserver (prediction based on structural alignment and a structural alphabet).
Figure 9.
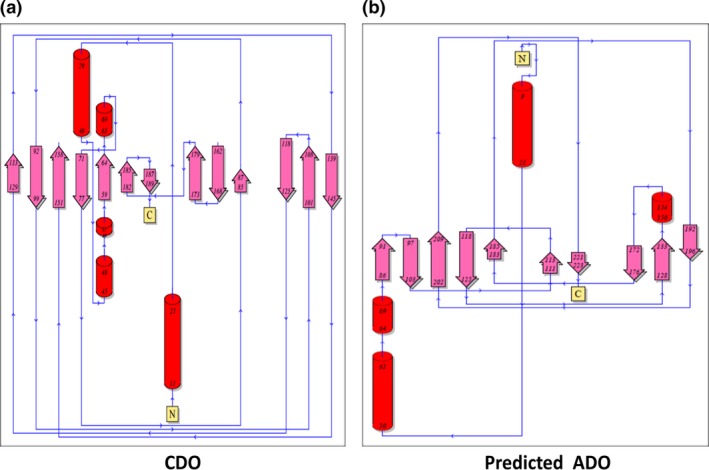
Comparison of topology model/diagram of CDO (PDB Id: 2IC1); (a) topology model/diagram of predicted model of ADO; and (b) topology diagrams derived from the PDBsum.
In the CDO, active site Fe (II) ion is coordinated by the N‐atoms of His86, His88 and His140 residues (McCoy et al. 2006). In ADO, the predicted Fe (II) ion is coordinated by His112, His114 and His193 residues (Figure 10). These amino acids are in ordered regions of both the proteins and are conserved. ADO is composed of four helices, including three alpha (α) helices (H1, H2 and H3) and one single turn 310 helix and twelve β‐strands, which forms two anti‐parallel β‐sheets. CDO is structurally conserved cupin β‐barrel protein constituted by all the three β‐sheets, by which the active centre at the chelated Fe2+ is surrounded (McCoy et al. 2006). There is a high structural similarity by 92% between human, mouse and rat CDO sequences (Ye et al. 2007). As compared to CDO, ADO also shows 90–95% sequence similarity in human, mouse and rat and it is also conserved through evolution, and this can be observed in MSA of ADO protein (Figure 3a). There are many similarities found between ADO and CDO structures and their active sites too. A recent study by Sallmann and group reported that a novel complex TpMe,PhFe(SCH2CH2NH2) which they have synthesized can act as a speculative model for ADO. They also reported that its reaction with O2 led to the dioxygenation of the S atom and thus to hypotaurine, therefore supporting the hypothesis that the active sites of CDO and ADO are quite similar (Sallmann et al. 2015).
Figure 10.
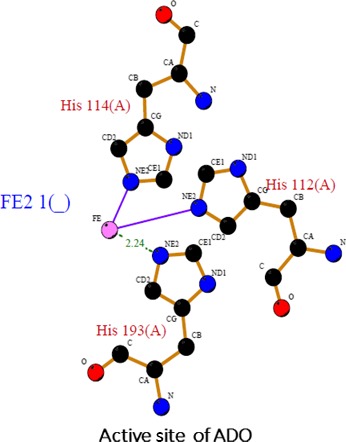
Active site prediction of ADO using PDBsum: Ball and Stick representation of the geometry of the active site of ADO (predicted structure SWISS‐MODEL server). The iron metallocenter in the (purple) is chelated by the three histidine (His 114, His 112, and His 193) residues.
Crosstalk of human thiol dioxygenases with other proteins
Proteins are the main workhorses of the cell; proteins control all biological functions and processes of the cell. The cellular responses are a dynamic process, and protein expression changes with the responses it encounters. Protein–protein interactions are governed by the interaction of domains that directs specific interactions with various target proteins, relative affinities of the partner protein and their modulation by covalent modifications as reviewed recently (Thakur et al. 2015). There are many pathways which are regulated by the protein–protein interactions. Human thiol dioxygenase enzymes, both CDO and ADO, interact with different proteins for regulation of different physiological processes. The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database‐based in silico interactions and known interactions of CDO and ADO proteins are listed in Tables 1 and 2 and also diagrammatically illustrated in Figures 11 and 12.
Table 1.
Predicted functional partners of CDO
| S. No | Proteins | Details of interacting proteins | String score | Prediction tools |
|---|---|---|---|---|
| 1. |
 CTH CTH |
Cystathionase (cystathionine gamma‐lyase) catalyses the last step in the trans‐sulphuration pathway from methionine to cysteine (405 aa) | 0.942 | STRING |
| 2. |
 GOT1 GOT1 |
Glutamic‐oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1); biosynthesis of L‐glutamate from L‐aspartate or L‐cysteine. (413 aa) | 0.922 | STRING |
| 3. |
 GOT2 GOT2 |
Glutamic‐oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) catalyses the irreversible transamination of the L‐tryptophan metabolite L‐kynurenine to form kynurenic acid (KA). (430 aa) | 0.919 | STRING |
| 4. |
 CSAD CSAD |
Cysteine sulphinic acid decarboxylase (520 aa) | 0.907 | STRING |
| 5. |
 GAD1 GAD1 |
Glutamate decarboxylase 1 (brain, 67 kDa) catalyses the production of GABA (594 aa) | 0.903 | STRING |
| 6. |
 GAD2 GAD2 |
Glutamate decarboxylase 2 (pancreatic islets and brain, 65 kDa) catalyses the production of GABA (585 aa) | 0.901 | STRING |
| 7. |
 CDON CDON |
Cdon homolog (mouse): component of a cell surface receptor complex that mediates cell–cell interactions between muscle precursor cells and promotes differentiation of myogenic cells (By similarity) (1264 aa) | 0.860 | STRING |
| 8. |
 GCLC GCLC |
Glutamate‐cysteine ligase, catalytic subunit (637 aa) | 0.829 | STRING |
| 9. |
 GCLM GCLM |
Glutamate‐cysteine ligase, modifier subunit (274 aa) | 0.825 | STRING |
| 10. |
 PPCS PPCS |
Phosphopantothenoylcysteine synthetase; catalyses the first step in the biosynthesis of coenzyme A from vitamin B5, where cysteine is conjugated to 4′‐phosphopantothenate to form 4‐phosphopantothenoylcysteine (311 aa) | 0.800 | STRING |
| 11. |
 CYP17A CYP17A |
Steroid 17‐alpha‐hydroxylase/17 | – | Biogrid |
| 12. |
 UBC UBC |
Polyubiquitin‐C; ubiquitin | – | I2D |
| 13. | VHL | Von Hippel–Lindau disease tumour suppressor | – | Biogrid |
| 14. | MAPK14 | Mitogen‐activated protein kinase 14 | – | Biogrid |
| 15. | JIP‐4 | C‐Jun‐amino‐terminal kinase‐interacting protein 4 | – | Biogrid |
Table 2.
Predicted functional partners of ADO
| S. No | Proteins | Details of interacting proteins | String score | Prediction tools |
|---|---|---|---|---|
| 1. |
 CSAD CSAD |
Cysteine sulphinic acid decarboxylase (520 aa) | 0.913 | STRING |
| 2. |
 GAD1 GAD1 |
Glutamate decarboxylase 1 (brain, 67 kDa); catalyses the production of GABA (594 aa) | 0.900 | STRING |
| 3. |
 GAD2 GAD2 |
Glutamate decarboxylase 2 (pancreatic islets and brain, 65 kDa); catalyses the production of GABA (585 aa) | 0.900 | STRING |
| 4. |
 CDON CDON |
CDON homolog (mouse); component of a cell surface receptor complex that mediates cell–cell interactions between muscle precursor cells. Promotes differentiation of myogenic cells (1264 aa) | 0.785 | STRING |
| 5. |
 ADK ADK |
Adenosine kinase; ATP‐dependent phosphorylation of adenosine and other related nucleoside analogs to monophosphate derivatives. Serves as a potential regulator of concentrations of extracellular adenosine and intracellular adenine nucleotides (362 aa) | 0.648 | STRING |
| 6. |
 RPIA RPIA |
Ribose 5‐phosphate isomerase A (311 aa) | 0.655 | STRING |
| 7. |
 C3 C3 |
Complement component 3; C3 plays a central role in the activation of the complement system. Its processing by C3 convertase is the central reaction in both classical and alternative complement pathways. After activation, C3b can bind covalently, via its reactive thioester, to cell surface carbohydrates or immune aggregates (1663 aa) | 0.610 | STRING |
| 8. |
 TRIM33 TRIM33 |
Tripartite motif containing 33: acts as an E3 ubiquitin‐protein ligase and promotes SMAD4 ubiquitination, nuclear exclusion and degradation via the ubiquitin‐proteasome pathway. (1127 aa) | 0.583 | STRING |
| 9. |
 ADORA2B ADORA2B |
Adenosine A2b receptor; receptor for adenosine. The activity of this receptor is mediated by G proteins which activate adenylyl cyclase (332 aa) | 0.581 | STRING |
| 10. |
 ADORA1 ADORA1 |
Adenosine A1 receptor; receptor for adenosine. The activity of this receptor is mediated by G proteins which inhibit adenylyl cyclase (326 aa) | 0.525 | STRING |
| 11. | UBC | Polyubiquitin‐C; ubiquitin | – | I2D |
| 12. | KCNJ10 | Potassium inward‐rectifying channel subfamily J member 10 | – | Biogrid |
| 13. | FAM67B | Family with sequence similarity 76, member B | – | Biogrid |
| 14. | C50RF24 | Chromosome 5 open reading frame 24 | – | Biogrid |
Figure 11.
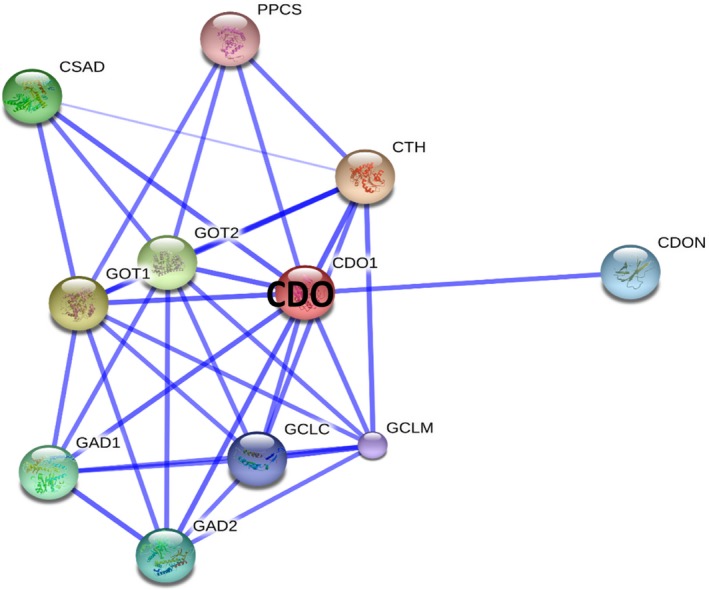
Protein‐Protein interaction network for human CDO. The figure shows a confidence view illustrating CDO‐associated protein interaction(s); those are displayed/predicted using STRING database. The thicker lines represent a stronger association between the proteins. Proteins which are interacting with CDO are listed in the Table 1.
Figure 12.
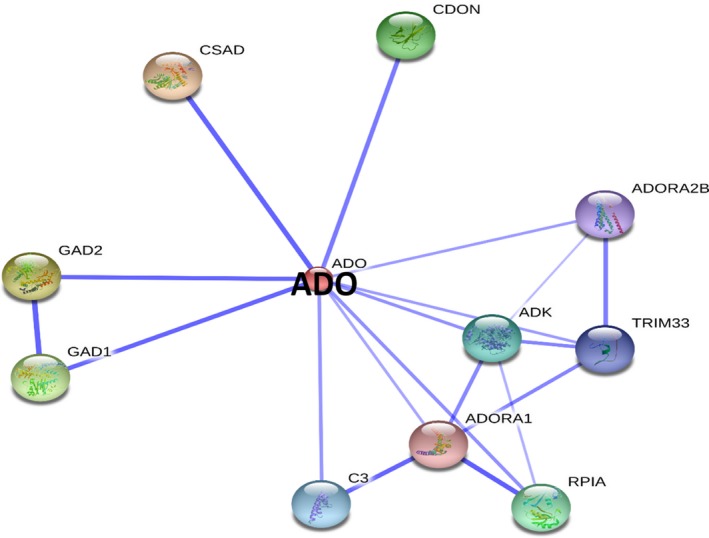
Protein‐Protein interaction network for human ADO. The figure shows a confidence view illustrating ADO‐associated protein interaction(s); those are displayed/predicted using STRING database. The thicker lines represent a stronger association between the proteins. Interactions of various proteins with ADO are listed in the Table 2.
Interactions of CDO with other proteins
STRING is a database of known and predicted protein interactions. The interactions include direct (physical) and indirect (functional) association (Szklarczyk et al. 2015). They are derived from the genomic study, high‐throughput experiments microarray analysis and previous resources. CDO interacts with various proteins for different activities are illustrated in Figure 11 and are listed in Table 1; cystathionase (cystathionine gamma‐lyase) [CTH] catalyses the last step in the transsulphuration pathway from methionine to cysteine (Sun et al. 2009), which generate endogenous signalling molecule hydrogen sulphide (H2S), and contributes to the regulation of blood pressure (Chiku et al. 2009). Glutamic‐oxaloacetic transaminase (GOT) is a pyridoxal phosphate‐dependent enzyme which exists in cytoplasmic and mitochondrial forms, GOT1 and GOT2 respectively. GOT plays a key role in amino acid metabolism and in urea and tricarboxylic acid cycles. Cysteine sulphinic acid decarboxylase (CSAD) acts as a rate‐limiting enzyme in taurine biosynthesis, catalysing the decarboxylation of cysteine sulphinate to hypotaurine (Skoldberg et al. 2004). Glutamate decarboxylase or glutamic acid decarboxylase (GAD) is an enzyme that catalyses the decarboxylation of glutamate to GABA and CO2. GAD is present in two forms, a 67‐kDa and 65‐kDa form (GAD1 and GAD2), one found in the brain, and the other one in pancreatic islets and brain, respectively (Burbaeva et al. 2014). Deficiency of this enzyme has been shown to cause the pyridoxine dependency with seizures. Alternative splicing of this GAD1 gene results in two products, the predominant 67‐kDa form and a less‐frequent 25‐kDa form (Curley et al. 2011). Cell adhesion associated, oncogene‐regulated (CDON) encodes a protein containing three fibronectin type III domains and five immunoglobulin‐like C2‐type domains. This protein is a member of a cell surface receptor complex that mediates cell–cell interactions between muscle precursor cells and positively regulates myogenesis (Gibert et al. 2014). Glutamate‐cysteine ligase also known as gamma‐glutamylcysteine synthetase is the first rate‐limiting enzyme of glutathione synthesis. The enzyme consists of two subunits, a heavy catalytic subunit and a light regulatory subunit (Lim et al. 2015). CDO also interacts with phosphopantothenoylcysteine synthetase (PPCS) and catalyses the first step in the biosynthesis of coenzyme A from vitamin B5, where Cys is conjugated to 4′‐phospho‐pantothenate to form 4‐phosphopantothenoylcysteine (Nakamura et al. 2012). The protein–protein interactions predicted thus advocates for further studies to understand CDO's association towards various biological functions.
Interactions of ADO with other proteins
STRING database‐based interactions of ADO with other proteins are illustrated in Figure 12 and are listed in Table 2. ADO also interacts with CSAD, CDON, GAD1 and GAD2 proteins, which interact with CDO (described in the section Interactions of CDO with other proteins). In addition, ADO interacts with adenosine kinase (ADK), ATP‐dependent phosphorylation of adenosine and other related nucleoside analogs to monophosphate derivatives (Mathews et al. 1998), which serves as a potential regulator of concentrations of extracellular adenosine and intracellular adenine nucleotides (Mathews et al. 1998). Adenosine has widespread effects on the cardiovascular, nervous, respiratory, and immune systems, and inhibitors of the enzyme could play an important pharmacological role in increasing intravascular adenosine concentrations and act as an anti‐inflammatory agent. ADO also interacts with ribose 5‐phosphate isomerase A (RPIA); RPIA interconverts ribose‐5‐phosphate and ribulose‐5‐phosphate. This enzyme also plays an essential role in both carbohydrate anabolism and catabolism; it is ubiquitous and is highly conserved evolutionarily (Zhang et al. 2003).
According to STRING prediction, ADO also interacts with complement component 3, generally called as C3, which is a protein of immune system and plays a significant role in the complement system and contribute towards innate immunity. This C3 protein is essential for activating the complement system. The presence of foreign invaders trigger the C3 protein to be cut (cleaved) into two smaller pieces. C3 convertase processes the central reaction in both classical and alternative complement pathways. After activation, C3b can bind covalently, via its reactive thioester, to cell surface carbohydrates or immune aggregates (Suresh et al. 2003). Tripartite motif containing 33 acts as an E3 ubiquitin‐protein ligase and promotes SMAD4 ubiquitination, nuclear exclusion and degradation via the ubiquitin‐proteasome pathway. The activity of adenosine A1, A2b receptors is mediated by the G proteins that activate adenylyl cyclase (Okada et al. 1996). Potassium inward‐rectifying channel subfamily J member 10 (KCNJ10) are a specific subset of potassium selective ion channels. Seven subfamilies have been identified in various mammalian cell types and are also found in plants. KCNJ10 are the targets of multiple toxins, and malfunction of the channels has been implicated in several human diseases. ADO also interacts with the family with sequence similarity 76, member B (FAM76B) and chromosome 5 open reading frame 24 (C50RF24) proteins. However, the biological consequences/functional implications associated with many of these proteins which are interacting either with ADO or CDO warrant for further studies in this regard.
Role of thiol dioxygenases in human health
Oxidative stress contributes significantly to many diseases such as atherosclerosis, cancer, Alzheimer's disease (AD) and age‐related macular degeneration. Sulphur exists stably in multiple oxidation states, which makes it a versatile component in biological systems. The most highly active and most reduced form of sulphur in biomolecules is the thiol, present in the amino acid cysteine (Moriarty‐Craige & Jones 2004). Cys is present in many proteins specifically in an active site, and this makes this amino acid a key target for many disease studies.
Cancer
In recent years, Cys metabolism in various human cancers has gained considerable interest, mostly focusing on its role in generating the antioxidant glutathione (Prabhu et al. 2014). Study on Cys metabolism pathway demonstrated the importance of this axis in glioma models (Chung et al. 2005; Ogunrinu & Sontheimer 2010). Yamaguchi and co‐workers reported the lack of CDO activity in malignant tumour cells such as rat hepatoma cells (AHZ440 and AH1009A) and mouse Ehrlich ascites tumour cells; it has been shown that CDO expression alters in cancer (Yamaguchi 1980). A study conducted by Dietrich et al. identified cysteine dioxygenase 1 (CDO1) as a strong DNA methylation biomarker in oestrogen receptor‐positive, lymph node receptor‐positive breast cancer treated with adjuvant anthracycline‐containing therapy (Dietrich et al. 2010). Human CDO1 gene is frequently deleted in advanced lung cancer (Ueno et al. 1998). A study conducted by Brait et al. identified promoter methylation of CDO, a specific marker of multiple types of human cancers. They also discovered a statistically significant difference in the frequency of CDO1 promoter methylation between normal and tumour tissue derived from colon, breast, oesophagus, lung, bladder and stomach. In addition, CDO1 also displayed tumour suppressive activities using in vitro cell culture and in vivo mouse model studies. These studies provided enough evidence for novel tumour suppressor activity of CDO1 as epigenetic regulator of human cancer (Brait et al. 2012; Jeschke et al. 2013). CDO1 also plays tumour suppressor role in human carcinogenesis; it was identified after use of algorithm utilizing pharmacological unmasking microarray (Minatani et al. 2016). In this study, promoter DNA methylation status of the CDO1 gene in 172 primary breast cancer tumour tissues, and strong association of CDO1 gene promoter DNA methylation with poor prognosis, was shown in primary breast cancer patients, especially for triple negative breast cancer (Minatani et al. 2016).
Prabhu et al. (2014) have identified a new metabolic pathway responsible for cancer development in glioblastomas. CDO1/cysteine sulphinic acid (CSA) pathway has been found to be a common pathway in the brain, and CSA is a by‐product of Cys metabolism. CSA inhibits pyruvate dehydrogenase (PDH) activity in mitochondria and subsequently attenuates oxidative phosphorylation, mitochondrial membrane potential and ATP production. Such a cellular status inhibits apoptosis and enhanced tumorigenesis in glioma cells (Prabhu et al. 2014).
Neurodegenerative disorders
Elevated level of Cys has been reported to be both neurotoxic as well as cytotoxic due to oxidative damage via formation of free radicals in the presence of iron (Stipanuk et al. 2009). It is observed that elevated Cys to sulphate ratio was found in patients with motor neuron disease (MND), Parkinson's disease (PD) and Alzheimer's disease (AD). The excess of Cys thiol group has been suggested to interfere with the neuronal protein function (Heafield et al. 1990). In Hallervorden–Spatz disease, a rare progressive extrapyramidal dysfunction and dementia, the activity of CDO was reduced in the globus pallidus. Results suggest that Cys accumulates locally in the globus pallidus in Hallervorden–Spatz disease. Accumulated Cys may further chelate iron, accounting for the local increase in iron content in Hallervorden–Spatz disease (Perry et al. 1985). The combined excess of Cys and ferrous iron may generate free radicals that damage neuronal membrane to cause the typical morphological changes which are observed in this disorder (Perry et al. 1985). Qusti et al. first developed the human medulloblastoma cell culture model for CDO expression, in a tumour‐derived cell line with a short doubling time and low media requirements (Qusti et al. 2000). Sulphur metabolism is observed by the use of probe drug S‐carboxymethyl Cys and measured the production of sulphoxides; this is an S‐oxidation reaction with high heritability that involves the enzyme CDO that converts Cys to sulphate. Neurodegenerative disorders like PD, AD and amyotrophic lateral sclerosis (ALS) in patients were all poor at this reaction and patients with AD being by far the worst (Williams et al. 1991). Research studies have indicated that there is an alteration in the ratio of oxidized to reduced glutathione (GSH) in the brains of patients with AD (Owen & Butterfield 2010). Cys levels are elevated in AD, and an increased Cys to the sulphur ratio in AD advocates for the defect in sulphur metabolism (Stipanuk et al. 2006). Thus making both CDO and ADO as susceptible targets associated with the vulnerability of neurological disorders. In addition, the metal‐induced toxicity is a serious concern in patients with AD; especially, aluminium (Al2+) and magnesium (Mg2+) levels in the brain are reported to be altered in patients with AD as compared to control groups (Maynard et al. 2005; Kawahara & Kato‐Negishi 2011).
Other human diseases
Mitochondria are the critical milieu for the synthesis of many essential biomolecules and are critical for cellular energy production (ATP synthesis) via oxidative phosphorylation (OXPHOS) (Scheibye‐Knudsen et al. 2015). CDO directly regulates mitochondrial membrane potential through inhibition of PDH activity. There are two pathways: one leads to taurine synthesis and other leads to glutathione synthesis. Lower levels of cytochrome c oxidase activity in liver mitochondria, higher levels of acid‐labile sulphide in tissues (liver, pancreas and lung) and higher levels of C4, C4‐hydroxy, C5, C5‐hydroxy and C3‐dicarboxylic acylcarnitines in blood were observed in CDO −/− mice compared with CDO +/+ mice (Ueki et al. 2011). The abnormalities exhibited by CDO −/− mice have some similarities to those reported for the rare human disease ethylmalonic encephalopathy, which is caused by the mutation of the Ethe1 gene as well as for the Ethe1 −/− mouse model. Ethe1 was recently shown to encode the mitochondrial sulphur dioxygenase, which catalyses a step in the mitochondrial pathway for oxidation of sulphide to thiosulphate (Depew 2008; Mancardi et al. 2009; Ueki et al. 2011).
Mesenchymal stem cells (MSCs) are multipotent cells, which can give rise to a variety of cell types, including adipocytes and osteoblasts. CDO inhibits osteogenesis in mouse bone marrow stromal cells. Zhao et al. identified that CDO regulates Wnt signalling pathway in primary mouse bone marrow stromal cells and regulates osteogenesis during cell differentiation (Zhao et al. 2016). In rheumatoid arthritis (RA), poor sulphoxidation and reduced formation of inorganic sulphate are responsible for this condition (Bradley et al. 1994). Cys regulates CDO1 turnover through ubiquitin‐26S proteasome‐mediated degradation, and high levels of Cys have been found to be cytotoxic, causing RA, PD and AD and increased risk of cardiovascular disease (CVD) and adverse pregnancy outcome (Heafield et al. 1990; Bradley et al. 1994; Brait et al. 2012). A study by Roman and co‐workers demonstrated that in CDO−/− mouse, production of the H2S/HS− in tissues can exceed the capacity of the animal to oxidize sulphide to sulphate. In addition, they showed that lung and pancreas are more susceptible to the toxicity from endogenous H2S/HS− production than the liver and kidney (Roman et al. 2013). Growing body of evidences advocate for conduction of further studies focusing on these two human thiol dioxygenases, especially ADO, which is merely studied as it is discovered recently.
Conclusion and future perspective
Cys metabolism became a critical pathway in the normal cellular activity as well as in the development and progression of various human diseases. Cys metabolic pathway leads to the second most abundant amino acid, taurine. ADO and CDO are human thiol dioxygenase enzymes which are responsible for Cys metabolism. These two are mitochondrial enzymes and are indirectly responsible for oxidative stress management. Taurine and hypotaurine are the most abundant amino acids in the human body, and they also play a role as antioxidants. In human body, availability of ADO and CDO is not similar in all the tissues. ADO is most abundant in the brain, heart, kidney skeletal muscles and spleen, while CDO is mostly abundant in adipocytes, lung and liver. These two thiol dioxygenase enzymes are mainly abundant in the mitochondria; however, it might be possible that they may localize to the nucleus also, which requires further studies. Our study reports the 3D structure of ADO based upon in silico studies with the available literature. However, crystal structure studies are required for its structure analysis and correlation towards its biological functions. In addition, the role of disordered regions in the function of these thiol dioxygenases should be carried out. Moreover, both CDO and ADO proteins may have crosstalk interactions with various proteins with key biological functions associated with Cys metabolism and thus may play a vital role in human health. Taurine has a role in different metabolic pathways, and the two thiol dioxygenases, CDO and ADO, have been correlated to be associated with the onset of various human diseases such as cancer, neurodegenerative disorders, rheumatoid arthritis and also various metabolic disorders.
Conflict of Interest
Authors declare that no conflict of interest exists.
Funding source
This work being supported to A.K.M. by the grants received from the Alzheimer's Association (NIRG‐11‐203527), USA, and the Department of Science and Technology [SR/CSI/288/2012(G)], Govt. of India. B.S. received financial support in the form of senior research fellowship (SRF) from the Indian Council for Medical Research (ICMR), New Delhi, India.
Acknowledgements
The CUPB institutional number provided for this article is P‐002/16. Because of the limited focus of the article, many relevant and appropriate references could not be included, for which the authors apologize.
Reference
- Adachi M., Takenaka Y., Gidamis A.B., Mikami B. & Utsumi S. (2001) Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol. 305, 291–305. [DOI] [PubMed] [Google Scholar]
- Arjune S., Schwarz G. & Belaidi A.A. (2015) Involvement of the Cys‐Tyr cofactor on iron binding in the active site of human cysteine dioxygenase. Amino Acids 47, 55–63. [DOI] [PubMed] [Google Scholar]
- Bowie J.U., Luthy R. & Eisenberg D. (1991) A method to identify protein sequences that fold into a known three‐dimensional structure. Science 253, 164–170. [DOI] [PubMed] [Google Scholar]
- Bradley H., Gough A., Sokhi R., Hassell A., Waring R. & Emery P. (1994) Sulfate metabolism is abnormal in patients with rheumatoid arthritis. Confirmation by in vivo biochemical findings. J. Rheumatol. 21, 1192–1196. [PubMed] [Google Scholar]
- Brait M., Ling S., Nagpal J.K. et al (2012) Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers. PLoS One 7, e44951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.J., Takayama S., Campen A.M. et al (2002) Evolutionary rate heterogeneity in proteins with long disordered regions. J. Mol. Evol. 55, 104–110. [DOI] [PubMed] [Google Scholar]
- Burbaeva G., Boksha I.S., Tereshkina E.B. et al (2014) A role of glutamate decarboxylase in Alzheimer's disease. Zh Nevrol Psikhiatr Im S S Korsakova 114, 68–72. [PubMed] [Google Scholar]
- Cavallini D., De Marco C., Scandurra R., Dupre S. & Graziani M.T. (1966) The enzymatic oxidation of cysteamine to hypotaurine. Purification and properties of the enzyme. J. Biol. Chem. 241, 3189–3196. [PubMed] [Google Scholar]
- Chiku T., Padvani D., Zhu W., Singh S., Vitvitsky V. & Banerjee R. (2009) H2S biogenesis by human cystathionine gamma‐lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 1601–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.J., Lyons S.A., Nelson G.M. et al (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 25, 7101–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley A.A., Arion D., Volk D.W. et al (2011) Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type‐specific features. Am. J. Psychiatry 168, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew M.J. (2008) Analysis of skeletal ontogenesis through differential staining of bone and cartilage. Methods Mol. Biol. 461, 37–45. [DOI] [PubMed] [Google Scholar]
- Dietrich D., Krispin M., Dietrich J. et al (2010) CDO1 promoter methylation is a biomarker for outcome prediction of anthracycline treated, estrogen receptor‐positive, lymph node‐positive breast cancer patients. BMC Cancer 10, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy J.E., Eller S. & Dawson R. Jr (2004) Building Biosynthetic Schools: reviewing Compartmentation of CNS Taurine Synthesis. Neurochem. Res. 29, 97–103. [DOI] [PubMed] [Google Scholar]
- Dominy J.E., Simmons C.R., Karplus P.A., Gehring A.M. & Stipanuk M.H. (2006) Identification and characterization of bacterial cysteine dioxygenases: a new route of cysteine degradation for eubacteria. J. Bacteriol. 188, 5561–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy J.E., Simmons C.R., Hirschberger L.L., Hwang J., Coloso R.M. & Stipanuk M.H. (2007) Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J. Biol. Chem. 282, 25189–25198. [DOI] [PubMed] [Google Scholar]
- Fusetti F., Schröter K.H., Steiner R.A. et al (2002) Crystal structure of the copper‐containing quercetin 2,3‐dioxygenase from Aspergillus japonicus. Structure 10, 259–268. [DOI] [PubMed] [Google Scholar]
- Gibert B., Delloye‐Bourgeois C., Gattolliat C.H. et al (2014) Regulation by miR181 family of the dependence receptor CDON tumor suppressive activity in neuroblastoma. J. Natl. Cancer Inst. 106, dju318. [DOI] [PubMed] [Google Scholar]
- Giraud M.F., Leonard G.A., Field R.A., Berlind C. & Naismith J.H. (2000) RmlC, the third enzyme of dTDP‐L‐rhamnose pathway, is a new class of epimerase. Nat. Struct. Biol. 7, 398–402. [DOI] [PubMed] [Google Scholar]
- Guerin P., El Mouatassim S. & Menezo Y. (2001) Oxidative stress and protection against reactive oxygen species in the pre‐implantation embryo and its surroundings. Hum. Reprod. Update 7, 175–189. [DOI] [PubMed] [Google Scholar]
- Heafield M.T., Fearn S., Steventon G.B., Waring R.H., Williams A.C. & Sturman S.G. (1990) Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci. Lett. 110, 216–220. [DOI] [PubMed] [Google Scholar]
- Hegde M.L., Hazra T.K. & Mitra S. (2008) Early steps in the DNA base excision/single‐strand interruption repair pathway in mammalian cells. Cell Res. 18, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M.L., Hazra T.K. & Mitra S. (2010) Functions of disordered regions in mammalian early base excision repair proteins. Cell. Mol. Life Sci. 67, 3573–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke J., O'Hagan H.M., Zhang W. et al (2013) Frequent Inactivation of Cysteine Dioxygenase Type 1 Contributes to Survival of Breast Cancer Cells and Resistance to Anthracyclines. Clin. Cancer Res. 19, 3201–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph C.A. & Maroney M.J. (2007) Cysteine dioxygenase: structure and mechanism. Chem. Commun. 32, 3338–3349. [DOI] [PubMed] [Google Scholar]
- Kawahara M. & Kato‐Negishi M. (2011). Link between Aluminum and the Pathogenesis of Alzheimer's disease: the Integration of the Aluminum and Amyloid Cascade Hypotheses. Int. J. Alzheimers Dis., 2011, 276393 http://doi.org/10.4061/2011/276393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski R.A. (2009) PDBsum new things. Nucleic Acids Res. 37, D355–D359. doi:10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur M.W., Moss D.S. & Thornton J.M. (1993) PROCHECK ‐ a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291. [Google Scholar]
- Lim J., Nakamura B.N., Mohar I., Kavanagh T.J. & Luderer U. (2015) Glutamate cysteine ligase modifier subunit (Gclm) null mice have increased ovarian oxidative stress and accelerated age‐related ovarian failure. Endocrinology 156, 3329–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardini J.B., Turini P., Biggs D.R. & Singer T.P. (1969) Cysteine oxygenase: 1. General properties. Physiol. Chem. & Physics 1, 1–23. [Google Scholar]
- Mancardi D., Penna C., Merlino A., Del Soldato P., Wink D.A. & Pagliaro P. (2009) Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim. Biophys. Acta 1787, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews I.I., Erion M.D. & Ealick S.E. (1998) Structure of human adenosine kinase at 1.5 A resolution. Biochemistry 37, 15607–15620. [DOI] [PubMed] [Google Scholar]
- Maynard C.J., Bush A.I., Masters C.L., Cappai R. & Li Q.X. (2005) Metals and amyloid‐β in Alzheimer's disease. Int. J. Exp. Pathol. 86, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy J.G., Bailey L.J., Bitto E. et al (2006) Structure and mechanism of mouse cysteine dioxygenase. Proc. Natl Acad. Sci. USA 103, 3084–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatani N., Waraya M., Yamashita K. et al (2016) Prognostic Significance of Promoter DNA Hypermethylation of cysteine dioxygenase 1 (CDO1) Gene in Primary Breast Cancer. PLoS One 11, e0144862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag T., Kay L.E. & Forman‐Kay J.D. (2010) Protein dynamics and conformational disorder in molecular recognition. J. Mol. Recognit. 23, 105–116. [DOI] [PubMed] [Google Scholar]
- Moriarty‐Craige S.E. & Jones D.P. (2004) Extracellular thiols and thiol/disulfide redox in metabolism. Annu. Rev. Nutr. 24, 481–509. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Pluskal T., Nakaseko Y. & Yanagida M. (2012) Impaired coenzyme A synthesis in fission yeast causes defective mitosis, quiescence‐exit failure, histone hypoacetylation and fragile DNA. Open Biol. 2, 120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic Z., Peng K., Vucetic S., Radivojac P., Brown C.J. & Dunker A.K. (2003) Predicting intrinsic disorder from amino acid sequence. Proteins 53, 566–572. [DOI] [PubMed] [Google Scholar]
- Ogunrinu T.A. & Sontheimer H. (2010) Hypoxia increases the dependence of glioma cells on glutathione. J. Biol. Chem. 285, 37716–37724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Mizuno K. & Kaneko S. (1996) Adenosine A1 and A2 receptors modulate extracellular dopamine levels in rat striatum. Neurosci. Lett. 212, 53–56. [DOI] [PubMed] [Google Scholar]
- Owen J.B. & Butterfield D.A. (2010) Measurement of oxidized/reduced glutathione ratio. Methods Mol. Biol. 648, 269–277. [DOI] [PubMed] [Google Scholar]
- Perry T.L., Norman M.G., Yong V.W. et al (1985) Hallervorden‐Spatz disease: cysteine accumulation and cysteine dioxygenase deficiency in the globus pallidus. Ann. Neurol. 18, 482–489. [DOI] [PubMed] [Google Scholar]
- Prabhu A., Sarcar B., Kahali S. et al (2014) Cysteine Catabolism: a Novel Metabolic Pathway Contributing to Glioblastoma Growth. Cancer Res. 74, 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qusti S., Parsons R.B., Abouglila K.D., Waring R.H., Williams A.C. & Ramsden D.B. (2000) Development of an in vitro model for cysteine dioxygenase expression in the brain. Cell Biol. Toxicol. 16, 243–255. [DOI] [PubMed] [Google Scholar]
- Roman H.B., Hirschberger L.L., Krijt J., Valli A., Kozich V. & Stipanuk M.H. (2013) The cysteine dioxgenase knockout mouse: altered cysteine metabolism in nonhepatic tissues leads to excess H2S/HS(‐) production and evidence of pancreatic and lung toxicity. Antioxid. Redox Signal. 19, 1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmann M., Braun B. & Limberg C. (2015) Dioxygenation of cysteamine to hypotaurine at a tris(pyrazolyl)borate iron(ii) unit‐cysteamine dioxygenase mimicking? Chem. Commun. 51, 6785–6787. [DOI] [PubMed] [Google Scholar]
- Saransaari P. & Oja S.S. (2008) Taurine in neurotransmission In: Handbook of Neurochemistry and Molecular Neurobiology, pp. 325–342 (eds Lajtha A. & Vizi E.S.), US: Springer. [Google Scholar]
- Scheibye‐Knudsen M., Fang E.F., Croteau D.L., Wilson Iii D.M. & Bohr V.A. (2015) Protecting the mitochondrial powerhouse. Trends Cell Biol. 25, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C.R., Hao Q. & Stipanuk M.H. (2005) Preparation, crystallization and X‐ray diffraction analysis to 1.5 A resolution of rat cysteine dioxygenase, a mononuclear iron enzyme responsible for cysteine thiol oxidation. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 61, 1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoldberg F., Rorsman F., Perheentupa J. et al (2004) Analysis of antibody reactivity against cysteine sulfinic acid decarboxylase, a pyridoxal phosphate‐dependent enzyme, in endocrine autoimmune disease. J. Clin. Endocrinol. Metab. 89, 1636–1640. [DOI] [PubMed] [Google Scholar]
- Stipanuk M.H., Dominy J.E. Jr, Lee J.I. & Coloso R.M. (2006) Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J. Nutr. 136, 1652S–1659S. [DOI] [PubMed] [Google Scholar]
- Stipanuk M.H., Ueki I., Dominy J.E. Jr, Simmons C.R. & Hirschberger L.L. (2009) Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids 37, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk M.H., Simmons C.R., Karplus P.A. & Dominy J.E. Jr. (2011) Thiol dioxygenases: unique families of cupin proteins. Amino Acids 41, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Collins R., Huang S. et al (2009) Structural basis for the inhibition mechanism of human cystathionine gamma‐lyase, an enzyme responsible for the production of H2S. J. Biol. Chem. 284, 3076–3085. [DOI] [PubMed] [Google Scholar]
- Suresh M., Molina H., Salvato M.S., Mastellos D., Lambris J.D. & Sandor M. (2003) Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 170, 788–794. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Franceschini A., Wyder S. et al (2015) STRING v10: protein‐protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S., Dhiman M., Tell G. & Mantha A.K. (2015) A review on protein‐protein interaction network of APE1/Ref‐1 and its associated biological functions. Cell Biochem. Funct. 33, 101–112. [DOI] [PubMed] [Google Scholar]
- Tyagi M., de Brevern A.G., Srinivasan N. & Offmann B. (2008) Protein structure mining using a structural alphabet. Proteins 71, 920–937. [DOI] [PubMed] [Google Scholar]
- Uberto R. & Moomaw E.W. (2013) Protein similarity networks reveal relationships among sequence, structure, and function within the Cupin superfamily. PLoS One 8, e74477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki I. & Stipanuk M.H. (2009) 3T3‐L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J. Nutr. 139, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki I., Roman H.B., Valli A. et al (2011) Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol. Endocrinol. Metab. 301, E668–E684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Kumagai T., Kijima T., Kishimoto T. & Hosoe S. (1998) Cloning and tissue expression of cDNAs from chromosome 5q21‐22 which is frequently deleted in advanced lung cancer. Hum. Genet. 102, 63–68. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V., Garg S.K. & Banerjee R. (2011) Taurine biosynthesis by neurons and astrocytes. J. Biol. Chem. 286, 32002–32010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic S., Brown C.J., Dunker A.K. & Obradovic Z. (2003) Flavors of protein disorder. Proteins 52, 573–584. [DOI] [PubMed] [Google Scholar]
- Ward J.J., Sodhi J.S., McGuffin L.J., Buxton B.F. & Jones D.T. (2004) Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J. Mol. Biol. 337(3), 635–645. [DOI] [PubMed] [Google Scholar]
- Williams A.C., Steventon G.B., Sturman S. & Waring R.H. (1991) Hereditary variation of liver enzymes involved with detoxification and neurodegenerative disease. J. Inherit. Metab. Dis. 14, 431–435. [DOI] [PubMed] [Google Scholar]
- Woo E.J., Dunwell J.M., Goodenough P.W., Marvier A.C. & Pickersgill R.W. (2000) Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat. Struct. Biol. 7, 1036–1040. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K. (1980) Natural Sulfur Compounds, pp. 175–186. New York: Plenum Press. [Google Scholar]
- Yamaguchi K., Sakakibara S., Koga K. & Ueda I. (1971) Induction and activation of cysteine oxidase of rat liver. I. The effects of cysteine, hydrocortisone and nicotinamide on hepatic cysteine oxidase and tyrosine transaminase activities of intact and adrenalectomized rats. Biochim. Biophys. Acta 237, 502–512. [Google Scholar]
- Yang J. & Zhang Y. (2015) I‐TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 43, W174–W181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yan R., Roy A., Xu D., Poisson J. & Zhang Y. (2015) The I‐TASSER Suite: protein structure and function prediction. Nat. Methods 12, 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Wu X., Wei L. et al (2007) An insight into the mechanism of human cysteine dioxygenase. Key roles of the thioether‐bonded tyrosine‐cysteine cofactor. J. Biol. Chem. 282, 3391–3402. [DOI] [PubMed] [Google Scholar]
- Zhang R.G., Andersson C.E., Savchenko A. et al (2003) Structure of Escherichia coli ribose‐5‐phosphate isomerase: a ubiquitous enzyme of the pentose phosphate pathway and the Calvin cycle. Structure 11, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Deng P., Feng J. et al (2016) Cysteine dioxygenase type 1 inhibits osteogenesis by regulating Wnt signaling in primary mouse bone marrow stromal cells. Sci. Rep. 6, 19296. [DOI] [PMC free article] [PubMed] [Google Scholar]


