Abstract
Aims
We aimed to compare the diagnostic efficacy of serum cystatin C (sCyC) for contrast induced nephropathy (CIN) in Western Indians undergoing cardiac catheterization. We also aimed to propose a clinically applicable cut-off of sCyC for early identification of CIN in this ethnic group.
Methods
In this prospective study, 253 patients undergoing coronary angiography and/or percutaneous coronary intervention were enrolled. The demographic and risk factor details, levels of sCr at baseline, 24 and 48 h after the procedure, whereas baseline and 24 h levels of sCyC were noted. Increase of 0.5 mg/dl or ≥25% from baseline sCr was used to define CIN. Optimum cut off of sCyC for CIN diagnosis was obtained using Receiver Operating Characteristic (ROC) curve analysis.
Results
After 48 h of contrast media (CM) exposure, the incidence of CIN was 12.25% (31 patients) according to sCr definition, where only 3.9% (10 patients) had sCr rise in 24 h. Overall significant (p < 0.0001) rise in mean levels of sCr (48 h) and sCyC (24 h) was observed in CIN patients. However, the mean sCr rise at 24 h was non-significant. The optimum cut off of sCyC for diagnosing CIN was found to be a rise of ≥10% from baseline (AUC – 0.901; sensitivity – 100%, specificity – 77.89%). According to sCyC, 94 (37.15%) patients had CIN.
Conclusion
We may conclude that a rise of ≥10% in sCyC at 24 h could be used as a reliable marker for identification of CIN in western Indians undergoing cardiac catheterization.
Keywords: Contrast induced nephropathy, Creatinine, Cystatin C, Western Indians
1. Introduction
Contrast-induced nephropathy (CIN) is widely recognized as one of the complication of coronary angiography (CAG) and percutaneous coronary intervention (PCI) [1]. These procedures are reported to induce mild renal dysfunction in more than 15% of the population [2]. Risk of dialysis is lower than 1% in general but may be as high as 5–50% in patients with significant underlying renal impairment, chronic diabetes and hypertension [3], [4]. Though this renal insult is often transient and clinically insignificant in nature, several studies have documented its role in prolonged in-hospital stay and mortality and have represented it as a powerful predictor of unfavourable early and late outcome [5].
Contrast-induced nephropathy is generally defined as acute renal failure occurring within 48–72 h of exposure to intravenous radiographic contrast material (CM) that is not attributed to other causes. Most precisely it is an increase of 25% or more, or an absolute increase of 0.5 mg/dl or more in sCr level from baseline value, at 48–72 h following the exposure to CM. A rise in sCr or decrease in urine output is current golden standard for recognizing CIN. However, identification of CIN by sCr suffers from major drawback of prolonging hospital stay in the vast majority of patients who will not develop CIN due to its delayed rise tendency. Furthermore, sCr is insensitive to rapid acute reductions in kidney function, which may deteriorate more than 50% before sCr exceeds the normal range and it is influenced by a variety of non-renal factors, including body weight, nutritional status, race, age and gender [6]. Thus, it is necessary to establish another marker for early detection of CIN that could help in timely treatment of CIN and enable an appropriate preventive measure.
Serum Cystatin C (sCyC) is considered to be a more reliable marker than sCr in evaluating the glomerular filtration rate (GFR) in patients with acute renal failure during the first 24–48 h [7]. Furthermore, growing evidence suggest that sCyC is a stronger predictor of clinical outcomes associated with CKD than sCr [8]. However, limited data exist on whether changes in sCyC are superior to sCr in detecting CIN and predicting long-term renal impairment and mortality after exposure to CM. In contrast to majority of the studies favouring sCyC as a marker of CIN over sCr, Ribichini et al. [9] in 2012, performed first “head-to-head” comparison of both the markers and found that sCr had better diagnostic power for CIN as compared to sCyC. There is also a lacuna of research in the field of representative range of sCyC for particular ethnic group. In 2008, Darcy et al., showed that sCyC is significantly associated with race/ethnicity of the population and hence standardization of its cut-off for screening of patients with altered renal functions is of utmost important [10].
Here, we performed a prospective study comparing changes in sCr and sCyC in high risk patients undergoing CM administration during coronary or peripheral procedures at our center. The purpose was to assess, whether the changes in sCyC at 24 h after CM exposure is a reliable index for early identification of CIN as compared to sCr levels. We also aimed to propose a suitable cut-off of sCyC for CIN in Western Indians. Due to reported low incidences of CIN in normal population, we had selected majority of patients having risk of CIN (CIN score >6) to increase the probability of the incidence occurring and its effective diagnosis by the markers.
2. Subjects and methods
2.1. Patients and study design
In this prospective study, total 253 adult patients undergoing CAG and/or PCI from January 2013–January 2015 were enrolled. Out of these 253 patients, 180 (71.15%) were falling under moderate to very high risk category (>6 CIN risk score). The study was reviewed and approved by the Institutional Ethics Committee (UNMICRC/CARDIO/2013/19) of UN Mehta Institute of Cardiology and Research Centre, Ahmedabad, Gujarat. All patients had provided written informed consent, and the study was carried out in accordance to the institutional ethical guidelines and Declaration of Helsinki. The inclusion criteria for the study were – age above 18 years, patients having indication for CAG and/or PCI and patients providing voluntary written consent for the participation in the study. Patients unable to complete follow-up, pre-existing dialysis, multiple myeloma, pulmonary edema, acute myocardial infarction (≤2), recent exposure to CM (≤2 days), showing inability to give consent, having cardiogenic and septic shock, pregnant females and patients with administrated theophylline, dopamine, mannitol, Fenoldopam, thymol and patient with thyroid disease were excluded from the study.
2.2. Definition
Contrast induced nephropathy was defined as an increase in sCr of ≥0.5 mg/dl or ≥25% above the baseline value within 48 h after the CM administration. A scoring system with eight variables was developed and validated by Mehran et al. [11] consisting of hypotension (5 points), IABP (5 points), congestive heart failure (5 points), chronic kidney disease (4 points), diabetes (3 points), age ≥75 years (4 points), anemia (3 points), and volume of contrast (1 point for each 100cc). Based on the attained score, patients were further divided into low (<6 CIN score), moderate (6–10 CIN score), high (11–16 CIN score), very high risk (>16 CIN score) groups.
2.3. Procedure
Only non-ionic low osmolar CM were used during cardiac catheterization for all patients in the study. In all the patients, intra-arterial contrast was given. Baseline clinical, biochemical, and procedural characteristics of the patients were collected before procedure. Serum creatinine was assessed at baseline and at 24 and 48 h after CM exposure. Serum cystatin C were measured at baseline and at 24 h after angioplasty. All patients with eGFR <60 ml/min/1.73 m2 received hydration therapy with isotonic sodium bicarbonate at a rate of 1 ml/kg/h for 6 h before and 6 h after procedure, according to the institutional guidelines. Diuretics and non-steroidal anti-inflammatory agents were withheld 24 h prior to the procedure and resumed only if renal function was stable after 48 h of the procedure.
Measurements of sCr and sCyC were performed enzymatically and GFR was estimated using CKD-EPI formula. Serum cystatin C was measured by nephelometry and combined eGFR was estimated according to Inker et al. [12].
2.4. Statistical analysis
The statistical calculations were performed using SPSS software v 20.0 (Chicago, IL, USA). Quantitative data was expressed as mean ± SD whereas qualitative data was expressed as percentage. Univariate analysis of the continuous data was performed using student’s t-test (paired or unpaired whichever is applicable), whereas chi-square test was used for the categorical data. The cut off value of p < 0.05 was considered for the statistical significance. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic accuracy of sCyC (24 h) and sCr (48 h).
3. Results
Overall 253 patients undergoing CAG/PCI were included in the study. The demographic and clinical characteristics of the population are shown in the Table 1. Mean age of the patients was 56.54 ± 10.04 years. Majority of the population were male (81.42%), where 47.04% were hypertensive, 34.39% were diabetic and 66.01% had acute coronary syndrome (ACS). Reduced LVEF (<40%) was observed in 32.81% of the patients. Out of 253 patients, 117 (46.25%) had baseline eGFR <60 ml/min per 1.73 m2. Mean CIN risk score was 7.8 ± 3.3 and majority of patients (around 70%) had risk score between 6 and 16 and 4.35% had risk score more than 16. At 48 h of CM exposure CIN defined as sCr increase ≥ 0.5 mg/dL or ≥25% occurred in 31 patients (12.25%). The rise in sCr after 24 h was observed in 10 (3.9%) patients only.
Table 1.
Demographic and Clinical Characteristics of the population.
| Sr.No | Variable | Mean ± SD(N = 253) |
|---|---|---|
| 1 | Age | 56.54 ± 10.04 |
| 2 | Male | 206(81.42) |
| 3 | Female | 47(18.58) |
| 4 | Height(cm) | 157.55 ±17.55 |
| 5 | Weight(kg) | 62.97 ±13.13 |
| 6 | LVEF < 40% | 83(32.81) |
| 7 | HTN | 119(47.04) |
| 8 | DM | 87(34.39) |
| 9 | ACS | 167(66.01) |
| 10 | Procedure performed | |
| CAG | 96 (37.94) | |
| PCI | 68(26.88) | |
| CAG+ Adhoc PCI | 76(30.04) | |
| Peripheral | 13(5.14) | |
| 11 | CIN risk score | 7.8 ±3.3 |
| <6 | 74 (29.2) | |
| 6–10 | 128(50.6%) | |
| 11–16 | 40(15.8%) | |
| >16 | 11(4.35%) | |
| 12 | eGFR < 60(CKD) | 117(46.25%) |
| 13 | CIN incidence (sCr ≥0.5 mg/dl/25% after 48 h) | 31(12.5%) |
| 14 | CIN incidence (sCr ≥0.5 mg/dl/25% after 24 h) | 10(3.9%) |
LVEF: Left ventricular Ejection Fraction; HTN: Hypertension; DM: Diabetes Mellitus; ACS: Acute Coronary Syndrome; CAG: Coronary Angiography; CM: Contrast Media
Table 2 compares the clinical characteristics of CIN vs non CIN patients. Except for greater incidences of low eGFR (<60) in non-CIN group all other parameters in both the groups were comparable (p > 0.05). The biochemical profile of patients with CIN is presented in Table 3. The mean sCr value was 2.26 mg/dL at baseline and 24 h after CM exposure was 2.29 mg/dL whereas the mean sCyC value at baseline and 24 h was 2.02 mg/dL and 2.55 mg/dL respectively. Thus the sCyC value at 24 h after CM exposure was significantly higher as compared to the baseline whereas the sCr did not rise significantly at 24 h after CM exposure. The sCr value at 48 h after CM exposure was 2.96 mg/dL which was significantly higher as compared to the baseline value. This implies that sCr value increased only after 48 h of CM exposure whereas sCyC value increased significantly even after 24 h of CM exposure. The GFR calculated by sCr based formula was at baseline 45.77 (mL/min per 1.73m2), at 24 h 46.10 (mL/min per 1.73 m2) and at 48 h 25.17 (mL/min per 1.73 m2). There was no significant difference between the baseline and 24 h but there is statistical difference between baseline and 48 h. The mean GFR calculated by sCyC based formula was significantly (p = 0.0026) lower at 24 h after CM exposure. Similarly, the GFR calculated by the combined equation of sCyC and sCr was 36.73 and 26.37 ml at baseline and 24 h respectively showing a statistically significant difference.
Table 2.
Comparison between patients developing CIN vs Patients who did not develop CIN (CIN vs Non CIN).
| Sr. | Variable | NON-CIN (N = 222) n (%) | CIN (N = 31) n (%) | Sig. (p) |
|---|---|---|---|---|
| 1 | Age | 56.05 ± 10.29 | 60.03 ± 7.28 | 0.959 |
| 2 | Male | 183(81.3) | 23(74.19) | 0.192 |
| 3 | Female | 39(18.7) | 8(25.8) | 0.192 |
| 4 | HTN | 102(43.9) | 17(54.83) | 0.230 |
| 5 | DM | 82(30.4) | 11(35.48) | 0.515 |
| 6 | ACS | 176(62.0) | 24(77.41) | 0.466 |
| 7 | LVEF < 40% | 71(32) | 12(38.7) | 0.5870 |
| 8 | CM Volume(ml) | 101.35 ± 53.87 | 83.77 ± 49.43 | 0.0870 |
| 9 | eGFR < 60(CKD) | 88(39.6) | 27(87.1) | <0.0001 |
HTN: Hypertension; DM: Diabetes Mellitus; ACS: Acute Coronary Syndrome; LVEF: Left Ventricular Ejection Fraction; CM: Contrast Media; eGFR: Estimated Glomerular Filtraion Rate; CKD: Chronic Kidney disease.
Table 3.
Comparison between Baseline and Post- CM exposure Cystatin C and Creatinine values and respective GFRs.
| Sr.No | Variable | CIN (N = 31) | Sig |
|---|---|---|---|
| 1 | sCr (mg/dl)-BS | 2.26 ± 1.43 | |
| 2 | sCr (mg/dl)-24 h | 2.29 ± 1.52 | 0.9365 |
| 3 | sCr (mg/dl)-48 h | 2.96 ± 1.30 | 0.0482 |
| 4 | sCyC (mg/dl)-BS | 2.02± 0.89 | |
| 5 | sCyC (mg/dl)-24 h | 2.55 ± 0.98 | 0.0296 |
| 6 | GFR – sCr (ml/min per 1.73m2)-BS | 45.77 ± 32.97 | |
| 7 | GFR – sCr (ml/min per 1.73m2)-24 h | 46.10 ± 29.98 | 0.9672 |
| 8 | GFR – sCr (ml/min per 1.73m2)-48 h | 25.17 ± 10.28 | 0.0015 |
| 9 | GFR – sCyC-Bs(ml/min per 1.73m2) | 36.78 ± 15.95 | |
| 10 | GFR – sCyC (ml/min per 1.73m2)-24 h | 26.05 ± 10.38 | 0.0026 |
| 11 | GFR – sCr + sCyC (ml/min per 1.73m2)-BS | 36.73 ± 16.52 | |
| 12 | GFR – sCr + sCyC (ml/min per 1.73m2)-24 h | 26.37 ± 11.86 | 0.0062 |
Cyst-C: Cystatin-C; S.Creat: Serum Creatinine; BS: Baseline; GFR: Glomerular Filtration Rate; Significance as compared to baseline values of the respective variable
Receiver operating characteristic curve analysis showed that sCyC had maximum sensitivity and specificity of 100% and 77.89% respectively indicating high level of diagnostic efficacy for CIN incidence. The area under curve (AUC) of sCyC was 0.901 which was slightly lower than AUC of 48 h sCr (0.962; 95% CI: 0.938–0.986; p < 0.0001). The optimum cut-off of sCyC based on sensitivity and specificity analysis was 10% (Table 4). The comparison of diagnostic power of sCyC (24 h) and sCr (48 h) are presented in Fig. 1. The positive and negative predictive values were 32.98% and 100% respectively. Overall incidence of CIN according to sCyC was 37.15% (n = 94). The relationships between changes of sCyC levels and CIN, defined by sCr increase of ≥0.5 mg/dL or ≥25%, are represented in Table 5. All the cases affected from CIN according to sCr definition were effectively diagnosed by sCyC. Even a low rise of sCyC (≥0.3%) was observed in 27 out of 31 patients of CIN. Table 5 also indicates that patients having higher CIN score have greater tendency of developing CIN after CM exposure according to both the definition.
Table 4.
Receiver operating characteristic curve analysis of sCyC for CIN diagnosis.
| Value | 95% CI | |
|---|---|---|
| Optimum cut-off of sCyC | 10% rise | |
| Overall incidence of CIN according to sCyC (≥10%) | 94 (37.15%) | |
| Sensitivity | 100.00% | 88.78%–100% |
| Specificity | 77.89% | 72.62%–82.58% |
| Area under curve | 0.901 | |
| Positive likelihood ratio | 4.52 | 3.64–5.63 |
| Negative likelihood ratio | 0 | |
| Positive predictive value | 32.98% | 23.62%–43.44% |
| Negative predictive value | 100% | 98.35%–100% |
| Out of 31 CIN cases Cys increased in | 31 (100%) |
Fig. 1.
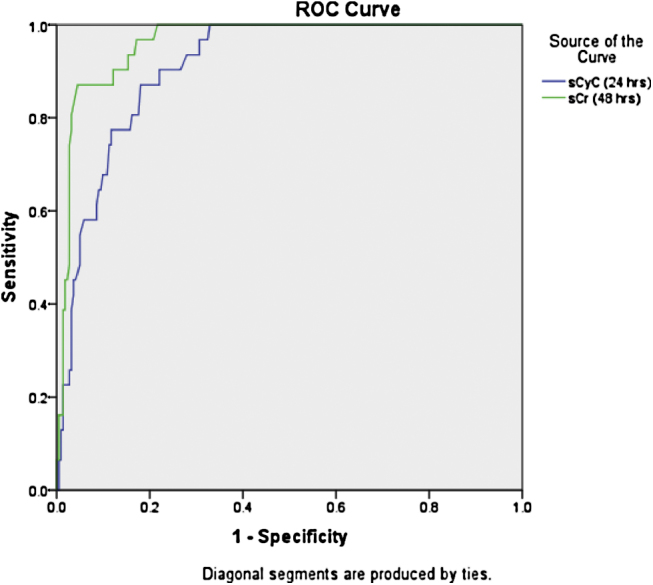
Comparison of diagnostic accuracy of sCyC (24 h) and sCr (48 h).
Table 5.
Changes in sCyC levels at 24 h after CM exposure and Incidence of CIN in various risk categories according to sCr and sCyC.
| Changes in sCyC at 24 h | sCr increase (≥0.5 mg/dL/25%) |
|---|---|
| Any Cys C increase (n = 150) | 31/31 |
| Any Cys C increase ≥ 10% (n = 102) | 31/31 |
| Any Cys C increase ≥ 15% (n = 82) | 26/31 |
| Any Cys C increase ≥ 25% (n = 43) | 20/31 |
| Any Cys C increase ≥ 0.3% (n = 43) | 27/31 |
| CIN incidence | <6 (n = 74) | 6–10 (n = 128) | 11–16 (n = 40) | >16 (n = 11) |
|---|---|---|---|---|
| CIN Risk Score | ||||
| According to sCr after 48 h | 0 (0%) | 12 (9.38%) | 13 (32.5%) | 6 (54.55%) |
| According to sCyC after 24 h | 12 (16.22%) | 45 (35.16%) | 29 (72.5%) | 8 (72.73%) |
4. Discussion
The exact mechanism of CIN is complex and poorly understood. Few reported mechanisms are (1) Renal ischemic injury, tubular epithelial cell toxicity or immunological reaction. (2) Osmolality and viscosity of CM increase hypoxia of renal medulla and free radicles production through post ischemic oxidative stress. (3) Direct effect of CM on kidney and toxic effect on tubular cell. (4) Contrast often induces natriuresis and diuretics which activate tubuloglomerular feedback response – a process involved in GFR regulation, ultimately causing glomerular afferent arterioles vasoconstriction and decline in GFR.
Koji Kato in 2008, reported that Cystatin C have highest discrimination power by ROC to diagnose CIN at cut-off value of 1.2 mg/L with sensitivity of 94.7% and 84.8% specificity [13]. In the same line Carlo Briguori et al., 2010 showed that <10% rise of sCyC at 24 h is reliable marker of CI-AKI whereas a rise of ≥10% at 24 h is an independent predictor of 1 year major adverse events [14]. To the best of our knowledge this the first study reporting sCyC cut-off for identification of CIN in Western Indians. Herewith we report that a rise of ≥10% in sCyC levels could be effectively used in clinical settings which is similar to other ethnic groups and internationally accepted diagnostic cut-off.
Acute renal insult is commonly encountered in the interventional cardiology setting. Contrast-induced nephropathy after cardiac catheterization and PCI is thought to be primarily caused by procedural exposure to contrast agent, which is nephrotoxic at high-doses [15]. The most important risk factor that has been linked to the development of CIN after cardiac catheterization and PCI is the presence of pre-existing CKD [16]. Other clinical factors like hemodynamic instability and diabetes mellitus, which are commonly prevalent in this population, may also contribute to its clinical course. In a small proportion of such patients, CIN may be due to renal atheroembolism from diffuse aortic atherosclerosis. Using a definition of contrast-induced nephropathy (a rise in sCr levels of ≥0.5 mg/dL or ≥25% increase from baseline), the reported incidence ranges from 8% to 15% in the general population and up to 28% in those with acute coronary syndromes (ACSs).[17]
A rise in sCr concentration is widely accepted method for detecting changes of renal function receiving CM. However, sCr possesses two important drawbacks: 1) The level of sCr is a result of both glomerular filtration rate and of renal tubular secretion and hence the changes in sCr will underestimate the actual alteration in GFR. 2) During acute deterioration of renal functions when GFR reduces drastically, less creatinine is excreted and the remaining creatinine gets distributed in total body water. Thus, the serum level can be expected to rise slowly and will continue to rise until new steady state has occurred. Therefore, although the injury induced by CM impairs GFR almost immediately, it requires 24 to 48 h for the fall in GFR to be reflected in an elevated level of sCr [18], [19], [20], [21].
Serum Cystatin C is a non-glycosylated protein produced in nucleated cells at a constant rate. Due to its low molecular weight it is filtered through the glomerular membrane without restriction and is fully reabsorbed in the proximal tubule [22]. In contrast to sCr, sCyC is secreted by all nucleated cells at a persistent rate. The serum concentration of cystatin C generally depends on filtration rather than tubular secretion, where a large distribution in body fluids, relatively low molecular weight and positive charge at physiological pH are the factors facilitating its glomerular filtration. The advantage of the sCyC–based equation over the sCr–based equation is that it is less subjected to the effects of age, sex and muscle mass [23]. Moreover the production rate of cystatin C is higher than the production rate of creatinine. Simultaneously, the renal clearance of cystatin C is faster than the creatinine clearance. In the net balance, the serum values are roughly the same namely 1.0 mg/dl for both. But the higher production rate of cystatin C makes it more rapidly increase than the creatinine if the clearance decreases hence make it a more suitable marker. The equation combining sCr and sCyC has been shown to provide the most precise and accurate estimate of GFR across the range of GFRs and in subgroups based on demographic and clinical characteristics [13]. However, limited data exist on whether changes in sCyc is superior to sCr in detecting CIN. This study was planned to investigate if sCyC and/or eGFR-CyC are superior to sCr and/or eGFR-Cr for early detection of peri-procedural CIN.
At 48 h after contrast media exposure, contrast-induced acute kidney injury (defined as a sCr increase of ≥0.5 mg/dL or ≥25% above baseline) occurred in 31 patients (12.25%). An increase in sCyC concentration ≥10% at 24 h after contrast media exposure was detected in 94 patients (37.15%). This was the best CyC cut-off for the early identification of patients at risk for contrast-induced acute kidney injury. In case of considering the higher cut-off such as 15%, the number of the false positive cases would have been reduced from 94 (10% cut-off) to 82 (15% cut-off), but then 5 true positive cases of CIN would have been missed. The patients subjected to CIN had to undergo dialysis only and have to face severe complications related to it and hence can’t be afford to be misdiagnosed. Therefore, in spite of achieving higher positive predictive value we would be compromising on sensitivity which would lead to more deteriorated outcome. Hence the cut-off of 10% was considered to be more suitable for this patient population. With an earlier rise of CyC at 24 h after CM exposure we were able to identify significant number of patients of CIN 24 to 48 h earlier as compared to creatinine.
When we compared baseline and post CM exposure sCyC and sCr values in the CIN group, it was found that sCyC value at 24 h was significantly higher as compared to baseline whereas sCr did not rise significantly at 24 h after CM exposure. However, the sCr value at 48 h after CM exposure was significantly higher. The CyC equation and the combined equation (sCr + sCyC) accurately identified the patients with CIN even at 24 h of CM exposure, in contrast to sCr which took 48–72 h post CM exposure to detect any significant changes from baseline. Hence CyC identifies the patients of CIN, 24–48 h earlier as compared to sCr. This observation may allow physicians an earlier discharge of the majority of patients, thus avoiding unnecessary prolonged hospitalization with associated practical and economic advantages. Early initiation of preventive measures may improve the outcome in CIN, a condition that substantially increases morbidity. So the main results of the present study is that sCyC seems to be a reliable marker for an early (24-h) diagnosis of CIN.
The few published longitudinal studies predominantly support our finding that sCyC reflects GFR changes more rapidly compared to sCr [7]. One explanation may be that cystatin C, unlike creatinine, resembles more closely an ideal endogenous marker of glomerular filtration. This is in contrast to the numerous non-renal factors that influence the generation of creatinine, its tubular secretion, and back leak, which may result in inaccurate reflection of GFR by creatinine. Cystatin C has been identified as a superior GFR marker to creatinine in chronic renal insufficiency with small variability and could be accurately measured using immunonephelometric assays [7]. Additionally, pre-analytic factors such as routine clinical storage conditions, freezing and thawing cycles, or interfering substances, such as bilirubin or triglycerides, do not affect Cystatin C measurement [24].
4.1. Limitations
Cystatin C levels have been assessed only at 24 h, and therefore its trajectory cannot be defined. We chose to record the CyC level at 24 h because elective patients are usually discharged the day after the procedure. We cannot exclude the possibility that an earlier rise (within h) may occur. Further studies are necessary to test whether an early sCyC rise (i.e., at 6 or 12 h) may be diagnostic. Moreover, sCyC possesses a positive predictive value of 32.98% indicating that all the patients showing a rise eventually do not develop CIN. Additionally, Cystatin C is a sensitive marker of reduction in GFR and not a marker of kidney injury. In case of sCr level, it has trend of rising within 48–72 h, however due to elective discharge and vacancy issues it could not be measured after 72 h and hence probability missing CIN cases due to late sCr rise could not be excluded.
5. Conclusion
In conclusion, a rise of ≥10% in sCyC levels at 24 h after CM exposure allows an early diagnosis of CIN as compared to sCr. Early initiation of preventive measures may improve the outcome in CIN, a condition that substantially increases mortality.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We thank our institute – U. N. Mehta Institute of Cardiology and Research Centre, Ahmedabad, Gujarat, India for funding the project. We also thank Dr. Hemal Nayak, M.D. and Dr. Payal Tripathi, M.D. from pathology department for providing their valuable support for the completion of this project.
References
- 1.Goldfarb S., McCullough P.A., McDermott J., Gay S.B. Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc. 2009;84(2):170–179. doi: 10.4065/84.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott F.A., Brahmajee K.N., Utpal D.P. Contrast induced acute kidney injury and the role of chronic kidney disease in percutaneous coronary intervention. In: Eric J.T., Paul S.T., editors. Textbook of Interventional Cardiology. 7th ed. 2012. P. 108. [Google Scholar]
- 3.McCullough P.A. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51(15):1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Gleeson T.G., Bulugahapitiya S. Contrast-induced nephropathy. Am J Roentgenol. 2004;183(6):1673–1689. doi: 10.2214/ajr.183.6.01831673. [DOI] [PubMed] [Google Scholar]
- 5.Smith G.L., Masoudi F.A., Shlipak M.G., Krumholz H.M., Parikh C.R. Renal impairment predicts long-term mortality risk after acute myocardial infarction. J Am Soc Nephrol. 2008;19(1):141–150. doi: 10.1681/ASN.2007050554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas M.E., Blaine C., Dawnay A. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87(1):62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 7.Herget-Rosenthal S., Marggraf G., Hüsing J. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 8.Murty M.S., Sharma U.K., Pandey V.B., Kankare S.B. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. 2013;23(3):180–183. doi: 10.4103/0971-4065.111840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribichini F., Gambaro G., Graziani M.S. Comparison of serum creatinine and cystatin C for early diagnosis of contrast-induced nephropathy after coronary angiography and interventions. Clin Chem. 2012;58(2):458–464. doi: 10.1373/clinchem.2011.170464. [DOI] [PubMed] [Google Scholar]
- 10.Groesbeck D., Köttgen A., Parekh R. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008;3(6):1777–1785. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehran R., Aymong E.D., Nikolsky E. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronaryintervention. J Am Coll Cardiol. 2004;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 12.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato K., Sato N., Yamamoto T., Iwasaki Y-k, Tanaka K., Mizuno K. Valuable markers for contrast-induced nephropathy in patients undergoing cardiac catheterization. Circulation. 2008;72(9):1499–1505. doi: 10.1253/circj.cj-07-1006. [DOI] [PubMed] [Google Scholar]
- 14.Briguori C., Visconti G., Rivera N.V. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121(19):2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 15.Becker C.R., Davidson C., Lameire N. High-risk situations and procedures. Am J Cardiol. 2006;98(6):37–41. doi: 10.1016/j.amjcard.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Hung Y.-M., Lin S.-L., Hung S.-Y., Huang W.-C., Wang P. Preventing radiocontrast-induced nephropathy in chronic kidney disease patients undergoing coronary angiography. World J Cardiol. 2012;4(5):157–172. doi: 10.4330/wjc.v4.i5.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senoo T., Motohiro M., Kamihata H. Contrast-induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2010;105(5):624–628. doi: 10.1016/j.amjcard.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 18.McCullough P.A. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51(15):1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Solomon R., Deray G. How to prevent contrast-induced nephropathy and manage risk patients: practical recommendations. Kidney Int. 2006;69:S51–S53. doi: 10.1038/sj.ki.5000375. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell A.M., Jones A.E., Tumlin J.A., Kline J.A. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010 Jan;5(1):4–9. doi: 10.2215/CJN.05200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harjai K.J., Raizada A., Shenoy C. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol. 2008;101(6):812–819. doi: 10.1016/j.amjcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 22.Gowda S., Desai P.B., Kulkarni S.S., Hull V.V., Math A.A., Vernekar S.N. Markers of renal function tests. N Am J Med Sci. 2010;2(4):170–173. [PMC free article] [PubMed] [Google Scholar]
- 23.Murty M., Sharma U., Pandey V., Kankare S. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. 2013;23(3):180–183. doi: 10.4103/0971-4065.111840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman D.J., Cystatin C. Ann Clin Biochem. 2002;39:89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]


