Abstract
Coronary chronic total occlusion (CTO) intervention remains one of the most challenging domains in interventional cardiology. Due to the technical challenges involved and potential procedural complications, CTO percutaneous coronary intervention (PCI) attempt and success rates remain less than standard PCI. However, the use of several adjunctive tools such as intravascular ultrasound, optical coherence tomography, rotational atherectomy, orbital atherectomy, excimer laser coronary atherectomy and percutaneous left ventricular assist device may contribute to improved CTO PCI success rates or provide better hemodynamic assessment of CTO lesion (i.e., using fractional flow reserve). In this review we present the current literature describing the utility and efficacy of these adjunctive modalities in CTO intervention.
Keywords: Chronic total occlusion, Percutaneous coronary intervention, Adjunctive modalities, Fractional flow reserve, Intravascular ultrasound
1. Introduction
Coronary CTO, commonly seen in patients with chronic stable angina, is characterized by the presence of atherosclerotic plaque resulting in complete or near complete occlusion of coronary artery for greater than 3 months duration.1, 2 Although over time the myocardial territory supplied by CTO tends to form well developed collaterals from a donor artery, the potential for myocardial ischemia still persists. CTO treatment is primarily aimed at improving angina symptoms, inducible myocardial ischemia, left ventricular function, quality of life and possibly overall survival.3, 4, 5 Often, a hard cap composed of dense fibrous tissue and calcium is present at both ends of CTO plaque with a soft portion in the middle, which makes guidewire crossing and subsequent balloon inflation more difficult.6 The difficulty and complexity of recanalizing renders lower success rates for CTO PCI than PCI of subtotal stenosis. CTO PCI results in greater procedural cost due to incremental equipment use, increased radiation exposure to both operator and patient due to prolonged fluoroscopic and procedural times, and increased utilization of catheterization laboratory staff compared to less complex interventions.1, 2, 3, 4, 5
For patients, undergoing complex CTO PCI increases the risk of coronary artery perforation, collateral vessel loss, higher radiation exposure and contrast induced nephropathy.7 In addition, CTO PCI attempts are higher among high volume operators (>200 PCIs per year) compared to low or intermediate volume (<200 PCIs per year) operators.4 Brilakis et al. using the data from National Cardiovascular Data Registry (NCDR) between July 2009 to March 2013 reported that CTO PCI represented only 3.8% of total 594,510 PCI cases for stable coronary artery disease.8 In addition, patients undergoing CTO PCI had lower procedural success rates (59% vs 96%) and higher in-hospital major adverse cardiac event rates (MACE, 1.6% vs 0.8%) compared with non-CTO PCI patients. Thus, procedural complexity, economic disincentives, operator inexperience, failure of medical management, and potential risks associated with CTO PCI result in most cases referred for coronary artery bypass surgery. However, some studies have shown improved long-term survival rates for successful CTO PCI compared with failed CTO or non-CTO PCI,9, 10 thereby emphasizing the need for more aggressive CTO revascularization in the appropriate patient subset. In the midst of emergence of novel guidewires and CTO crossing tools,11 advances in adjunctive imaging and debulking modalities in the era of drug eluting stents have contributed to increased CTO PCI success rates and have made percutaneous CTO intervention more amenable. In this review, we will describe such adjunctive modalities and the current literature evidence supporting their utility in CTO PCI.
1.1. Fractional flow reserve (FFR)
FFR, a measure of intracoronary physiology, is a lesion specific index used to determine the functional importance of coronary stenosis. FFR is the ratio of mean distal coronary pressure to mean aortic pressure in the setting of maximum hyperemia. FFR of ≤0.8 is currently recommended as a threshold for invasive determination of myocardial ischemia.12, 13 The information on myocardial ischemia obtained from FFR is more specific than myocardial perfusion studies, because every possible diseased artery could be analyzed individually using FFR. In FFR verses angiography for multivessel evaluation (FAME) study among patients with multivessel disease, FFR guided (Fig. 1) PCI resulted in reduced mortality and myocardial infarction rates compared with standard angiography guided PCI.14 In addition, compared to non-FFR guided PCI, FFR guided PCI resulted in fewer stents and procedural contrast volume use, similar health related quality of life and significant decrease in 2-year major adverse cardiac events.15 Although FFR use has been increasingly recommended, especially in multivessel PCI, its utility in CTO evaluation is not well-known. Often, the presence of collaterals from the donor artery in the CTO myocardial territory renders operators less inclined towards the need for CTO revascularization. Sachdeva et al., in a study of 100 patients (50 each in CTO and non-CTO groups), reported that in symptomatic patients with CTO, FFR use helps identify ischemic zones within the CTO territory even in the presence of well-developed coronary collaterals and regional ventricular dysfunction. The severity of ischemia was greater in CTO territory compared with non-CTO regions, and 78% of patients with CTO had ischemia at rest, i.e., FFR ≤ 0.8. Pre-PCI FFR was lower in the CTO group compared with non-CTO group (0.45 ± 0.15 vs 0.58 ± 0.17), but was similar post-PCI. Post-PCI, the FFR in the CTO ischemic zone reverted back to normal. Interestingly, the rate of all-cause mortality, target lesion revascularization and angina were similar among CTO-PCI and non-CTO PCI groups.16 Varghese et al., in a prospective study of 50 CTO lesions with well-developed collaterals, demonstrated significant improvement in FFR post-PCI. The pre-PCI FFR (median 0.54, range 0.27–0.83) normalized in most cases post-PCI (median 0.9, range 0.61–1.12).17 These findings suggest that in patients with complex coronary lesions, using FFR could help identify ischemia in the CTO territory which could be normalized post-PCI. Furthermore, the above findings also suggest that the mere presence of robust collaterals and absence of wall motion abnormality alone do not indicate absence of ischemia in the corresponding CTO territory.
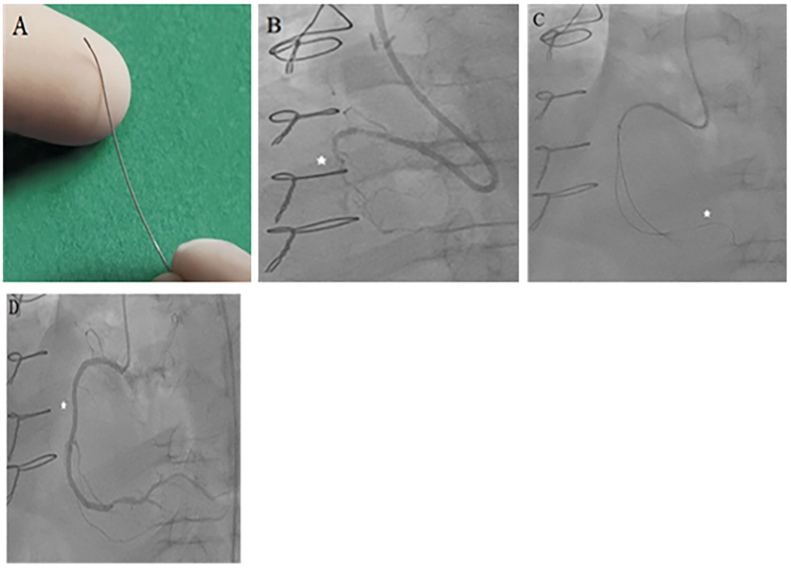
Fig. 1.
Device and case images of coronary fractional flow reserve measurement (FFR). A. FloWire Doppler Guide Wire for FFR measurement (Image provided courtesy of Volcano Corporation, San Diego, CA); B. Case image demonstrating a proximal right coronary artery (RCA) chronic total occlusion (CTO) (*); C. pre-intervention FFR wire positioning in the distal RCA (*) through a Corsair catheter after crossing the CTO with pre-FFR of 0.45; D. and final post-intervention image of revascularized RCA CTO (*) for post-FFR of 0.85.
The CTO myocardial territory is dependent on the donor artery blood flow for tissue perfusion. In multivessel disease involving the donor artery, using FFR may help determine the need for donor artery revascularization. In patients with CTO and intermediate donor artery stenosis, FFR in the donor artery is frequently abnormal and reverts to normal after successful CTO PCI.18 Therefore, recanalizing CTO and resultant normal FFR in the donor artery would obviate the need for donor artery PCI or multivessel bypass surgery.19 However, the change in donor vessel FFR after CTO PCI may be related to lesion severity in the donor vessel, and any improvement in very low pre-PCI FFR associated with severe stenotic lesions may not be enough to cross the ≤ 0.8 treatment threshold.20 FFR, therefore, remains a useful tool in the setting of multivessel disease with concomitant CTO to evaluate donor artery lesion physiology and determine the need for donor artery and even CTO lesion revascularization.
1.2. Intra-vascular ultrasound (IVUS)
IVUS has been a valuable adjunct to coronary angiography due to its ability to provide direct high resolution (100–150 μm) view of vessel wall and to precisely allow measurements of lumen area, plaque size and composition.12, 21, 22 In non-CTO interventions, IVUS use during stent placement has been associated with lower rates of restenosis, repeat revascularization and major adverse cardiac events.21, 23 IVUS is particularly useful in complex interventions involving ostial and bifurcation lesions and segments with multiple overlapping vessels and branch points21––23. Contrary to conventional angiography, IVUS provides a cross-sectional assessment of CTO plaque morphology, size and distribution, and provides real time images of the exact location of guidewire within the atherosclerotic plaque to help discriminate the true lumen from a false lumen.12, 21, 22 In addition, IVUS can identify intramural hematomas resulting from guidewire penetration in the medial space during recanalization.24
The use of IVUS (Fig. 2) to successfully recanalize technically challenging CTO lesions has been reported. The success rates in penetrating the proximal cap of the blunt CTO lesion in the presence of a side branch at the occlusion site are low due to lack of ability of conventional angiographic images to identify the proper CTO entry site and frequent slipping of guidewire into the side branch.25 In this setting, if a side branch is large enough to accommodate the IVUS catheter, IVUS can help guide the operator to optimally penetrate the guide wire into the proximal cap without disrupting the external elastic membrane.26 At times, the guidewire can penetrate the subintimal layer, but by using IVUS in the subintimal layer, the operator can often advance a second guidewire through the true CTO lumen.27 Similarly, in the presence of a side branch, IVUS images obtained while withdrawing the catheter from the bifurcated branch can help direct the wire into the true CTO occlusion cap.27, 28 The ‘tandem wire technique’ (i.e., advancing a second guidewire to create an alternate channel through the CTO lesion using the first guidewire as a landmark) could also be used under IVUS guidance to successfully recanalize the CTO.26
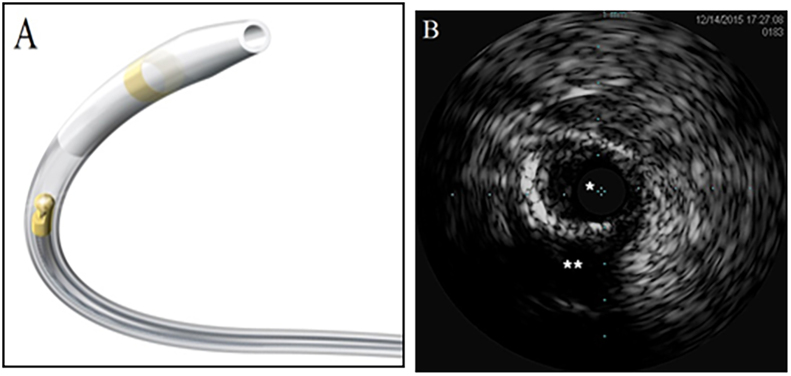
Fig. 2.
Device and case images of coronary intravascular ultrasound (IVUS). A. OptiCross IVUS coronary imaging catheter (Image provided courtesy of Boston Scientific Corporation, Natick, MA); B. IVUS case image demonstrating recanalized calcified true lumen (*) and subintimal lumen (**) of chronic total occlusion.
Park et al., assessed the feasibility of the above technique in recanalizing the stumpless CTO lesions. They reported that out of 32 lesions, 96.9% of them had CTO entry points successfully identified under IVUS guidance, in which the guidewire passage was impossible in 12.5% lesions due to complications and lesion tortuosity. In this subset, the rate of successful recanalization was 81%.29 Hong et al., using the Korean-CTO registry, investigated 2-year follow-up outcomes from IVUS-guided CTO PCI with drug eluting stents. The IVUS-guided PCI group showed significantly less stent thrombosis (0% vs 3%) and trend towards lesser myocardial infarction compared with non-IVUS-guided PCI. Additionally, target lesion revascularization occurred less frequently in IVUS-guided PCI group, especially for CTO lesions ≥3 cm.30 Kim et al., in a randomized study of 402 patients with CTO, compared IVUS-guided versus angiography-guided interventions. After 12-month follow-up, IVUS-guided intervention group had significantly lower MACE rates (2.6% vs 7.1%) and composite of cardiac death or myocardial infarction (0% vs 2%) compared with angiography-guided intervention group. Interestingly, high pressure post-stent ballooning was more frequently performed in the IVUS-guided group probably resulting in a larger post-procedural minimum lumen diameter.31 Additionally, IVUS imaging may help optimize stent placement pre- and post- intervention which may contribute to high long term success rates. Post CTO revascularization IVUS imaging may also help to determine restenosis and late-stent malapposition.21 Therefore, the above data supports IVUS use as an important imaging tool during angiography-guided PCI: to facilitate successful CTO crossing by visualization of the CTO cap; and to optimize stent expansion and apposition to improve outcomes.
1.3. Optical coherence tomography (OCT)
OCT uses light in the infrared range which reflects off tissues to provide high resolution (10–15 μm) cross-sectional images. At such resolution, OCT could help assess the degree of intima, media, and fibrous cap thickness of an atherosclerotic plaque and details of plaque morphology. OCT can further be used to determine lesion length, vessel diameter, length of subintimal track, and the constituents of arterial wall.21, 32 Therefore, OCT could be used to better determine stent length accurately and limiting inadequate stent expansion or incomplete stent strut apposition, thus minimizing the risk for stent thrombosis or restenosis.33 In addition, OCT may help determine plaque burden and composition, thereby allowing operators to decide if additional debulking tools are necessary prior to intervention. Furthermore, OCT could help visualize microchannels within the CTO, which may greatly assist in guidewire passage across the lesion.34
Use of OCT (Fig. 3) to assess long-term success of drug eluting stents in coronary lesions is well-described.35, 36 The risk for in-stent restenosis remains significant in morphologically complex CTO, and OCT imaging may be incrementally valuable in such cases. Naganuma et al. reported a case of 1-year follow-up after bioresorbable vascular scaffold placement for CTO lesion. They reported that OCT demonstrated acceptable scaffold and luminal area with homogenous neointimal hyperplasia and partial strut malapposition.37 Recently, Jia et al. reported OCT use to assess long-term vascular response post-CTO PCI with drug eluting stents. They reported that compared to non-CTO group, the CTO group had higher incidence of stent malapposition, uncovered struts, and protruding struts.38 Thus, OCT could be the imaging modality of choice to determine the extent of strut coverage and degree of in-stent restenosis in CTOs.32 Due to its ability to provide high definition visualization of the lesion and optimal stent sizing and placement, OCT often is a useful tool in CTO interventions. Although, there is lack of clinical trial data on OCT use in CTO PCI, it remains a useful adjunct tool in CTO revascularization.
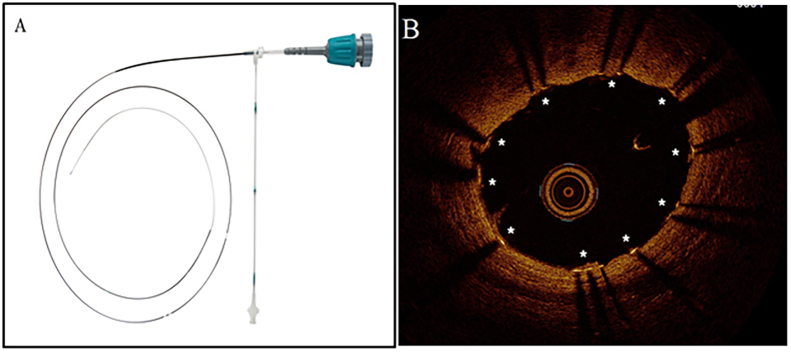
Fig. 3.
Device and case images of coronary optical coherence tomography (OCT). A. Dragonfly duo OCT imaging catheter (Image provided courtesy of St. Jude Medical Inc., Little Canada, MN); B. OCT case image demonstrating adequately expanded and apposed stent struts (*) following PCI of right coronary artery chronic total occlusion.
1.4. Rotational atherectomy (RA) and orbital atherectomy (OA)
RA is primarily used for plaque modification in calcified vessels, to smoothen the vessel lumen and disrupt the plaque continuity to facilitate balloon dilation and stent implantation. RA catheter consists of an elliptical burr that rotates concentrically at high speeds to provide ablation by gradually advancing across the lesion over a guidewire. RA is commonly used to treat complex lesions that are heavily calcified, ostial lesions, bifurcation lesions, or CTO.39, 40 In CTO, RA (Fig. 4) may be useful in the setting of successful guidewire passage through the lesion and subsequent failure of balloon to cross or dilate the stenosis. Pagnotta et al., reported that out of 648 CTO lesions with successful guidewire passage, 45 CTO lesions were resistant to subsequent balloon angioplasty and stent implantation, of which, 95.5% were successfully treated with rotational atherectomy without procedural complications.41 Similarly, in a study of 145 CTOs, Omoigui N reported 90.1% success rate without major complications when using RA after crossing the CTO with a guidewire; non-diabetics and those with relatively larger vessel diameter (2.8 ± 0.6 vs 2.0 ± 0.5 mm) benefited the most.42
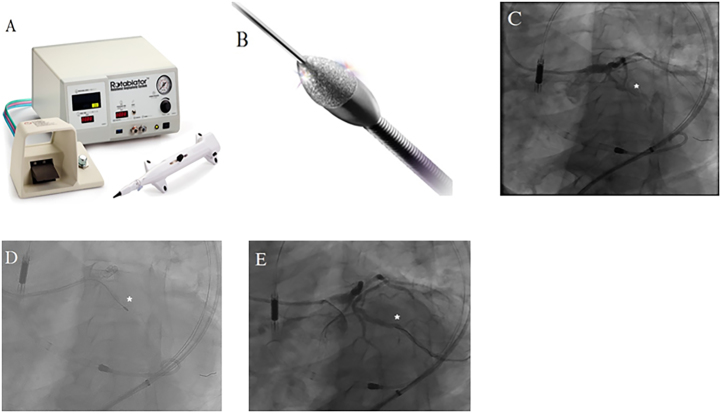
Fig. 4.
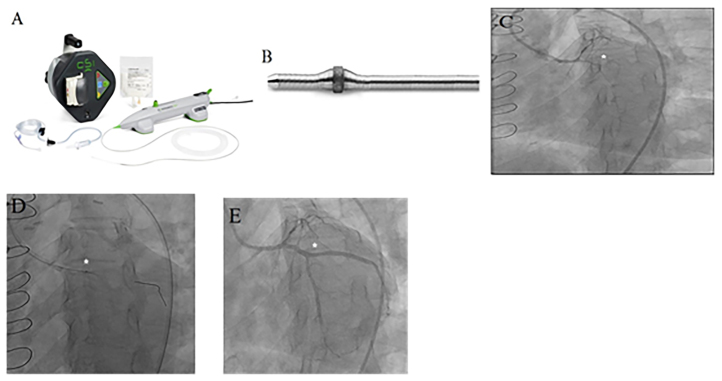
Device and case images of coronary rotational atherectomy (RA). A. RA system and B. Rotablater diamond tipped burr (Images provided courtesy of Boston Scientific Corporation, Natick, Massachusetts); C. Case image demonstrating left circumflex (LCX) chronic total occlusion (CTO) (*); D. Use of RA (*) to treat a heavily calcified segment of LCX CTO [4c]; E. Final angiography of revascularized LCX CTO (*) after RA and stenting.
Few studies have assessed the short and long-term outcomes post-debulking of CTOs.43, 44 Tsuchikane et al. in a randomized study of 266 patients with CTO (177 in RA group) reported that pre-stent debulking strategy led to higher 30-day MACE rate (15.9% vs 8.5%), lower restenosis rate (23.8% vs 34.6%) and lower 1-year MACE rate (27.5% vs 39.8%) compared with non-debulking strategy.43 However, Gruberg et al. showed that using debulking techniques (including RA) prior to stent placement in CTOs led to similar short (in-hospital mortality, myocardial infarction and repeat angioplasty rates) and long-term (deaths and target lesion revascularization) outcomes compared with stent placement alone.44 Interestingly, in this study, the protocol for debulking strategy was not standardized, and the study did not evaluate the impact of “optimal” debulking. Furthermore, mean CTO length was markedly longer in the debulking group compared to stent-alone group (23 mm vs 10.4 mm). Further clinical studies would help establish the efficacy of RA debulking in CTO intervention. Nevertheless, the above studies indicate that RA could be a useful adjunctive tool in treating challenging CTO lesions.
A novel plaque debulking tool, Diamondback 360° OA System (Fig. 5), has been shown to be very effective in treating heavily calcified coronary lesions.45, 46, 47 OA uses eccentrically mounted diamond coated crown, which can revolve at very high speeds. In contrast to the concentric motion of RA burr, the orbital motion of OA creates a sanding effect while removing plaque, and facilitates greater luminal gain at progressively higher speeds obviating the need for frequent catheter upsizing.48, 49, 50 Trayer et al. reported a case demonstrating the utility of OA in treating heavily calcified right coronary artery near-total occlusion with a drug eluting stent.51 The prospective, non-randomized, multicenter trial to Evaluate the Safety and Efficacy of OA in Treating Severely Calcified Coronary Lesions (ORBIT II) assessed the safety and efficacy of OA in debulking severely calcified lesions prior to stent placement.52 In ORBIT II, the OA device was able to cross the lesion in 98% of 440 trial participants. Of 440 total lesions, 29.3% were American College of Cardiology/American Heart Association (ACC/AHA) type C (which also includes CTOs). The study reported 89.6% rate of freedom from MACE at 30 days post-procedure, 97.7% rate of successful stent placement, 98.6% rate of achieving <50% stenosis post-procedure and very low rates of cardiac death, in-hospital Q wave myocardial infarction and target vessel revascularization; however, the trial did not report outcomes based on different ACC/AHA lesion types. Thus OA could potentially be useful in treating coronary CTOs, which are often heavily calcified. Prospective studies exclusively focused on assessing OA use in CTO intervention would be valuable.
Fig. 5.
Device and case images of orbital atherectomy (OA). A. Diamondback OA system; B. OA catheter with diamond coated crown (Image provided courtesy of Cardiovascular Systems Inc., St. Paul, MN); C. Case image demonstrating left circumflex (LCX) chronic total occlusion (CTO) (*); D. use of OA (*) in percutaneous coronary intervention of a calcified LCX CTO; E. Final angiography of revascularized LCX CTO (*) after OA and stenting.
1.5. Excimer laser Coronary atherectomy (ELCA)
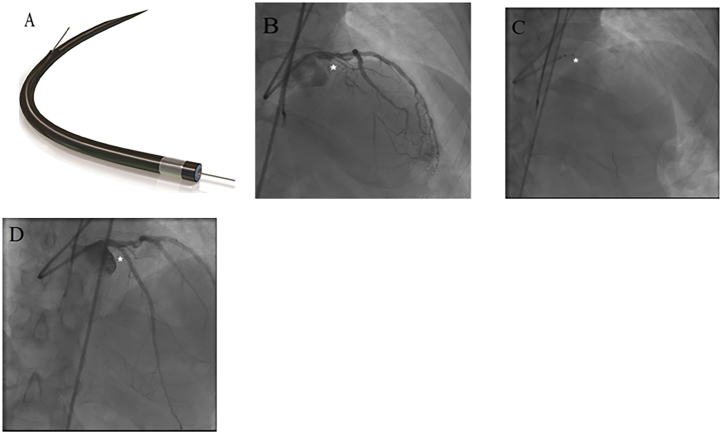
ELCA is an alternative technique for debulking complex lesions including CTOs crossable by guidewire. Absorption of pulsed excimer laser by tissue leads to formation of gas and acoustic shock waves within the target biologic tissue, which dessicates the atherosclerotic plaque and thrombus.53, 54 ELCA is an alternative, when the advancement of the proprietary guidewire for RA is not possible. In addition, ELCA is deliverable using 0.014″ standard guidewire (Fig. 6) and can be readily utilized when a CTO has been crossed.55 Fernandez et al., in a retrospectively studied 18 CTO lesions in which balloon either failed to cross or expand, showed 83.3% treatment success rate using ELCA alone with no ELCA-related complications. Recent studies have shown increasing utility and efficacy of ELCA as a tool for CTO intervention.56 Badr et al., in a study of 32 patients with CTO, demonstrated that ELCA catheter was able to cross the lesion in 93.8% of cases. In addition, in 90.6% of cases ELCA achieved <50% residual stenosis in the absence of in-hospital adverse events. Although, out of 32 CTOs, 8 required the use of adjunctive techniques, the technical success rate of ELCA was significant.57 Don et al. in a retrospective case series reported subintimal ELCA use for CTO revascularization by advancing the laser catheter through the hypertrophied medial layer of a chronically occluded vessel.58 Lesions that cannot be initially treated successfully by RA can be treated with ELCA. In such cases, ELCA may not be able to debulk the lesion completely, but may create a channel within the lesion, which can then be used to perform additional debulking or aggressive balloon dilatation. Although data on long-term efficacy and safety of ELCA use in CTO intervention is lacking, ELCA remains an effective tool to achieve successful CTO revascularization.
Fig. 6.
Device and case images of Excimer laser coronary atherectomy. A. ELCA catheter (Image provided courtesy of Spectranetics, Colorado Springs, CO); B. Case images demonstrating a proximal left anterior descending (LAD) chronic total occlusion (CTO) (*); C. Use of ELCA (*) to debulk the proximal LAD CTO after crossing; D. Final angiography of revascularized LAD CTO (*) after ELCA and stenting.
1.6. Percutaneous left ventricular assist device (pLVAD)
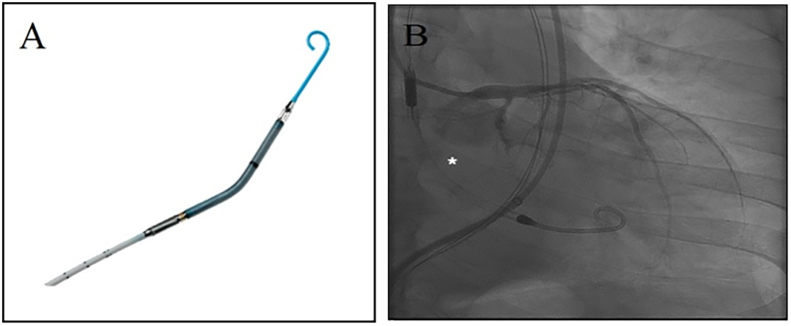
Complex interventions such as CTO-PCI commonly require extensive catheter manipulations and balloon dilations, which increase the risk of fatal arrhythmias and worsening hemodynamic instability in patients with depressed left ventricular (LV) function. In such settings, minimizing LV work load and myocardial oxygen demand is imperative. The use of pLVAD, such as Impella 2.5 LP and CP systems (Abiomed, Danvers, MA), delivered percutaneously and positioned across the aortic valve into the left ventricle (Fig. 7), incorporates an axial flow pump which ejects blood from the LV into the ascending aorta at the rate of up to 2.5–3.5 L/min.59, 60 The pLVAD is commonly used as a hemodynamic support in the setting of cardiogenic shock or high-risk PCI with depressed LV function, but the data on its use for CTO PCI is limited.61 Murarka et al. reported a case of pLVAD use to successfully treat LAD CTO in a patient with LV ejection fraction (EF) of 15%.62 Alasnag et al. studied 60 patients with depressed LVEF (23 ± 15) undergoing elective high-risk PCI in the presence of Impella 2.5, of which 14 patients had CTO. The study showed 96% angiographic success with mean Impella flow rate of 2.1 ± 0.2 L/min and duration of 38 ± 15 min, low procedural complication rate and low in-hospital stay. Although, only 23% of the patients had CTO, the above study suggests that pLVAD could be successfully used in high-risk CTO interventions.63 Larger clinical trials evaluating the short and long-term efficacy of pLVAD in complex CTO-PCI would be valuable.
Fig. 7.
Device and case images of Impella 2.5 LP system. A. Impella 2.5 LP system (Image provided courtesy of Abiomed Corporation, Danvers, MA); B. Case image of percutaneous left ventricular assist device use (*) during percutaneous coronary intervention of left circumflex chronic total occlusion.
2. Summary
Each of the above modalities, if used in the appropriate clinical settings, could significantly aid in successful CTO PCI. In addition, using adjunct modalities may potentially decrease procedural time, contrast volume, fluoroscopic time and patient related risks associated with CTO PCI. The field of CTO intervention remains dynamic and novel imaging and interventional modalities are rapidly emerging. With use of these adjunctive modalities, CTOs are becoming more amenable to intervention and successful revascularization; robust clinical trial data is needed to reinforce their widespread adoption in CTO PCI.
Disclosures
Hemal Bhatt – None
Sean Janzer – Consultant for Avinger, Bard, Boston Scientific, Cardinal Health, and Medtronic.
Jon George – Consultant for Avinger, Boston Scientific, Cardinal Health, and Medtronic.
Contributor Information
Hemal Bhatt, Email: cardiothorax@gmail.com.
Jon C. George, Email: jcgeorgemd@hotmail.com.
References
- 1.Shah P.B. Management of coronary chronic total occlusion. Circulation. 2011;123:1780–1784. doi: 10.1161/CIRCULATIONAHA.110.972802. [DOI] [PubMed] [Google Scholar]
- 2.Bardaji A., Rodriguez-Lopez J., Torres-Sanchez M. Chronic total occlusion: to treat or not to treat. World J Cardiol. 2014;6:621–629. doi: 10.4330/wjc.v6.i7.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz S., Ramsdale D.R. Chronic total occlusions–a stiff challenge requiring a major breakthrough: is there light at the end of the tunnel? Heart. 2005;91(Suppl. (3)):42–48. doi: 10.1136/hrt.2004.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grantham J.A., Marso S.P., Spertus J. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479–486. doi: 10.1016/j.jcin.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Levine G.N., Bates E.R., Blankenship J.C. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2011;2013(82):E266–355. doi: 10.1002/ccd.23390. [DOI] [PubMed] [Google Scholar]
- 6.Dash D. Guidewire crossing techniques in coronary chronic total occlusion intervention: A–Z. Indian Heart J. 2016;68(3):410–420. doi: 10.1016/j.ihj.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes D.R., Jr., Williams D.O. Catheter-based treatment of coronary artery disease: past, present, and future. Circ Cardiovasc Interv. 2008;1:60–73. doi: 10.1161/CIRCINTERVENTIONS.108.783134. [DOI] [PubMed] [Google Scholar]
- 8.Brilakis E.S., Banerjee S., Karmpaliotis D. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry) JACC Cardiovasc Interv. 2015;8:245–253. doi: 10.1016/j.jcin.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Suero J.A., Marso S.P., Jones P.G. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001;38:409–414. doi: 10.1016/s0735-1097(01)01349-3. [DOI] [PubMed] [Google Scholar]
- 10.Mehran R., Claessen B.E., Godino C. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc Interv. 2011;4:952–961. doi: 10.1016/j.jcin.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Mishra S. Language of CTO interventions – focus on hardware. Indian Heart J. 2016;68(4):450–463. doi: 10.1016/j.ihj.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotfi A., Jeremias A., Fearon W.F. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the Society of Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2014;83:509–518. doi: 10.1002/ccd.25222. [DOI] [PubMed] [Google Scholar]
- 13.Pijls N.H., Van Gelder B., Van der Voort P. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183–3193. doi: 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- 14.Tonino P.A., De Bruyne B., Pijls N.H. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 15.van Nunen L.X., Zimmermann F.M., Tonino P.A. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00057-4. pii: S0140-6736(15)00057-4. [DOI] [PubMed] [Google Scholar]
- 16.Sachdeva R., Agrawal M., Flynn S.E. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv. 2014;83:9–16. doi: 10.1002/ccd.25001. [DOI] [PubMed] [Google Scholar]
- 17.Varghese V., George J., Salamat J. Physiological assessment of ischemia using fractional flow reserve in coronary chronic total occlusions. J Am Coll Cardiol. 2016;67(13_S):149. [Google Scholar]
- 18.Matsuo H., Kawase Y. Physiological impact of CTO recanalization assessed by coronary pressure measurement: a case report. Catheter Cardiovasc Interv. 2013;82:E459–464. doi: 10.1002/ccd.24842. [DOI] [PubMed] [Google Scholar]
- 19.Sachdeva R., Agrawal M., Flynn S.E. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter Cardiovasc Interv. 2013;82:E453–458. doi: 10.1002/ccd.25031. [DOI] [PubMed] [Google Scholar]
- 20.Ladwiniec A., Cunnington M.S., Rossington J. Collateral donor artery physiology and the influence of a chronic total occlusion on fractional flow reserve. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002219. pii: e002219. [DOI] [PubMed] [Google Scholar]
- 21.Courtney B.K., Munce N.R., Anderson K.J. Innovations in imaging for chronic total occlusions: a glimpse into the future of angiography's blind-spot. Eur Heart J. 2008;29:583–593. doi: 10.1093/eurheartj/ehm634. [DOI] [PubMed] [Google Scholar]
- 22.Nissen S.E., Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–616. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 23.Parise H., Maehara A., Stone G.W. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107:374–382. doi: 10.1016/j.amjcard.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Fujii K., Ochiai M., Mintz G.S. Procedural implications of intravascular ultrasound morphologic features of chronic total coronary occlusions. Am J Cardiol. 2006;97:1455–1462. doi: 10.1016/j.amjcard.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 25.Dong S., Smorgick Y., Nahir M. Predictors for successful angioplasty of chronic totally occluded coronary arteries. J Interv Cardiol. 2005;18:1–7. doi: 10.1111/j.1540-8183.2005.00390.x. [DOI] [PubMed] [Google Scholar]
- 26.Ito S., Suzuki T., Ito T. Novel technique using intravascular ultrasound-guided guidewire cross in coronary intervention for uncrossable chronic total occlusions. Circ J. 2004;68:1088–1092. doi: 10.1253/circj.68.1088. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara T., Murata A., Kanyama H. IVUS-guided wiring technique: promising approach for the chronic total occlusion. Catheter Cardiovasc Interv. 2004;61:381–386. doi: 10.1002/ccd.10796. [DOI] [PubMed] [Google Scholar]
- 28.Furuichi S., Airoldi F., Colombo A. Intravascular ultrasound-guided wiring for chronic total occlusion. Catheter Cardiovasc Interv. 2007;70:856–859. doi: 10.1002/ccd.21219. [DOI] [PubMed] [Google Scholar]
- 29.Park Y., Park H.S., Jang G.L. Intravascular ultrasound guided recanalization of stumpless chronic total occlusion. Int J Cardiol. 2011;148:174–178. doi: 10.1016/j.ijcard.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 30.Hong S.J., Kim B.K., Shin D.H. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second-generation drug-eluting stents for chronic total occlusions (from the Multicenter Korean-Chronic Total Occlusion Registry) Am J Cardiol. 2014;114:534–540. doi: 10.1016/j.amjcard.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Kim B.K., Shin D.H., Hong M.K. Clinical impact of intravascular ultrasound-Guided chronic total occlusion intervention with zotarolimus-Eluting versus biolimus-Eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8:e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 32.Viceconte Nicola, Teijeiro-Mestre Rodrigo, Foin Nicolas. Optical coherence tomography to guide the treatment of chronic total occlusions. In: Waksman R., Saito S., editors. Chronic total occlusions: a guide to recanalization. 2nd ed. John Wiley & sons; Hoboken: 2013. pp. 60–66. [Print] [Google Scholar]
- 33.Vaquerizo B., Barros A., Pujadas S. Bioresorbable everolimus-eluting vascular scaffold for the treatment of chronic total occlusions: CTO-ABSORB pilot study. EuroIntervention. 2015;11:555–563. doi: 10.4244/EIJY14M12_07. [DOI] [PubMed] [Google Scholar]
- 34.Teijeiro Mestre R., Alegría-Barrero E., Di Mario C. Microchannels in recent chronic total occlusions assessed with frequency-domain optical coherencetomography. Rev Esp Cardiol (Engl Ed) 2013;66:907. doi: 10.1016/j.rec.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Barlis P., Regar E., Serruys P.W. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial substudy. Eur Heart J. 2010;31:165–176. doi: 10.1093/eurheartj/ehp480. [DOI] [PubMed] [Google Scholar]
- 36.Moore P., Barlis P., Spiro J. A randomized optical coherence tomography study of coronary stent strut coverage and luminal protrusion with rapamycin- eluting stents. JACC Cardiovasc Interv. 2009;2:437–444. doi: 10.1016/j.jcin.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Naganuma T., Latib A., Panoulas V.F. One year followup optical coherence tomography after implantation of bioresorbable vascular scaffolds for achronic coronary total occlusion. JACC Cardiovasc Interv. 2014;7:e157–159. doi: 10.1016/j.jcin.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Jia H., Li N., Wang X. Chronic total occlusion: optical coherence tomography findings at six months following treatment with sirolimus eluting stents. J Am Coll Cardiol. 2013;61(10_S) [Google Scholar]
- 39.Barbato E., Carrié D., Dardas P. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30–36. doi: 10.4244/EIJV11I1A6. [DOI] [PubMed] [Google Scholar]
- 40.Tomey M., Kini A., Sharma S. Current status of rotational atherectomy. J Am Coll Cardiol Interv. 2014;7:345–353. doi: 10.1016/j.jcin.2013.12.196. [DOI] [PubMed] [Google Scholar]
- 41.Pagnotta P., Briguori C., Mango R. Rotational atherectomy in resistant chronic total occlusions. Catheter Cardiovasc Interv. 2010;76:366–371. doi: 10.1002/ccd.22504. [DOI] [PubMed] [Google Scholar]
- 42.Omoigui N., Booth Joan, Reisman Mark. Rotational ahterectomy in chronic total occlusions. J Am Coll Cardiol. 1995;25 [97A–97A. [Google Scholar]
- 43.Tsuchikane E., Suzuki T., Asakura Y. Debulking of chronic coronary total occlusions with rotational or directional atherectomy before stenting: final results of DOCTORS study. Int J Cardiol. 2008;125:397–403. doi: 10.1016/j.ijcard.2007.07.117. [DOI] [PubMed] [Google Scholar]
- 44.Gruberg L., Mehran R., Dangas G. Effect of plaque debulking and stenting on short- and long-term outcomes after revascularization of chronic total occlusions. J Am Coll Cardiol. 2000;35:151–156. doi: 10.1016/s0735-1097(99)00491-x. [DOI] [PubMed] [Google Scholar]
- 45.Parikh K., Chandra P., Choksi N. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81(7):1134–1139. doi: 10.1002/ccd.24700. [DOI] [PubMed] [Google Scholar]
- 46.Lee M., Shlofmitz E., Kaplan Barry. Percutaneous Coronary intervention in severely calcified unprotected left main Coronary artery disease: initial experience with orbital atherectomy. J Am Coll Cardiol Interv. 2016;9(4_S) [S8–S8. [PubMed] [Google Scholar]
- 47.Shlofmitz E., Jauhar R., Meraj P. Initial commercial experience with orbital atherectomy in calcified coronary artery disease. J Am Coll Cardiol Interv. 2016;9(4_S):S24–S25. [Google Scholar]
- 48.Akkus N.I., Abdulbaki A., Jimenez E. Atherectomy devices: technology update. Med Devices (Auckland, NZ) 2015;8:1–10. doi: 10.2147/MDER.S50594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heuser R.R. Treatment of lower extremity vascular disease: the diamondback 360° orbital atherectomy system. Expert Rev Med Devices. 2008;5(3):279–286. doi: 10.1586/17434440.5.3.279. [DOI] [PubMed] [Google Scholar]
- 50.Chambers J.W., Diage T. Evaluation of the Diamondback 360 Coronary Orbital Atherectomy System for treating de novo, severely calcified lesions. Expert Rev Med Devices. 2014;11(5):457–466. doi: 10.1586/17434440.2014.929493. [DOI] [PubMed] [Google Scholar]
- 51.Trayer Troy. Kintur sanghvi complex Coronary intervention using Coronary orbital atherectomy (Diamondback 360°) via a transradial approach. Cath Lab Digest. 2014;22(2) [Google Scholar]
- 52.Chambers J.W., Feldman R.L., Himmelstein S.I. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II) JACC Cardiovasc Interv. 2014;7(5):510–518. doi: 10.1016/j.jcin.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 53.Ben-Dor I., Maluenda G., Pichard A.D. The use of excimer laser for complex coronary artery lesions. Cardiovasc Revasc Med. 2011;12(69):e1–8. doi: 10.1016/j.carrev.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Akkus Nuri I., Abdulbaki Abdulrahman, Jimenez Enrique. Atherectomy devices: technology update. Med Devices (Auckl) 2015;8:1–10. doi: 10.2147/MDER.S50594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez J.P., Hobson A.R., McKenzie D. Beyond the balloon: excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9:243–250. doi: 10.4244/EIJV9I2A40. [DOI] [PubMed] [Google Scholar]
- 56.Badr S., Ben-Dor I., Dvir D. The state of the excimer laser for coronary intervention in the drug-eluting stent era. Cardiovasc Revasc Med. 2013;14:93–98. doi: 10.1016/j.carrev.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Dor I., Maluenda G., Pichard A.D. The use of excimer laser for complex coronary artery lesions. Cardiovasc Revasc Med. 2011;12:e1–8. doi: 10.1016/j.carrev.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Don C., Kalyanasundaram A., Lombardi W. Subintimal laser atherectomy for chronic total occlusion revascularization. J Am Coll Cardiol. 2014;64(11_S) [Google Scholar]
- 59.Morine K.J., Kapur N.K. Percutaneous mechanical circulatory support for cardiogenic shock. Curr Treat Options Cardiovasc Med. 2016;18:6. doi: 10.1007/s11936-015-0426-6. [DOI] [PubMed] [Google Scholar]
- 60.Mohamad Tamam, Alani Anas, Munir Ahmad. Use of the impella 2.5 device with multi-Vessel stenting in severe peripheral artery and left main disease. Cath Lab Digest. 2011;19(4) [Google Scholar]
- 61.Henriques J.P., Remmelink M., Baan J., Jr. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol. 2006;97:990–992. doi: 10.1016/j.amjcard.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 62.Murarka S., Lassetter J., Waters K. Chronic total occlusions in the coronary vasculature. Cath Lab Digest. 2010;18(10) [Google Scholar]
- 63.Alasnag M.A., Gardi D.O., Elder M. Use of the Impella 2.5 for prophylactic circulatory support during elective high-risk percutaneous coronary intervention. Cardiovasc Revasc Med. 2011;12:299–303. doi: 10.1016/j.carrev.2011.02.002. [DOI] [PubMed] [Google Scholar]