Abstract
In clinical setting, congestive heart failure (CHF) and chronic kidney disease (CKD) often co-exist in patients due to common underlying predisposing factors. An intricate equilibrium between the cardiovascular and renal system is maintained through rennin angiotensin–aldosterone axis and autonomic nervous system. Consequent to favorable hemodynamic modification, angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blocking (ARB) therapy have proven to be an indispensable aspect of heart failure management with morbidity and mortality benefit. Additionally, progression to end stage renal failure may be halted by renin angiotensin aldosterone system (RAAS) blockade in patients with preexisting renal dysfunction. However, concern over the safety of RAAS blockade in presence of renal impairment has led to profound underutilization of these drugs in CHF patients with renal insufficiency. This review aims to provide a simplified guide to pathophysiology and management options of this perplexing situation.
Keywords: Congestive heart failure, Chronic kidney disease, ACE inhibitors, Angiotensin receptor blockers, Creatinine
1. Introduction
Queries abound on the management strategy to follow when chronic kidney disease and heart failure co-exist in a patient. Among treatment options available, most perplexing question is whether to initiate or continue renin angiotensin aldosterone blocking agents in such patients. Despite of beneficial cardio-renal interactions of these medications, an apprehension over possible renal worsening often leads to its underuse in clinical practice. Through this review, we hope to outline guideline based simplified management strategy in such cases.
Renal insufficiency management is a significant aspect of heart failure (HF) treatment not only because of its high prevalence consequent to shared risk factors, but also its association with mortality.1 Major randomized trials have shown that ACEI and/or ARB in addition to standard cardiac medications have survival benefit in HF patients.2 However, under representation of coexistent renal insufficiency in HF trials has led to skewed data and failure to generalize these results among patients having concomitant kidney disease.3 Moreover chronic kidney disease (CKD) patients are frequently refractory to conventional treatment and are at increased risk of adverse effects with HF medications.4
Serum creatinine (sCr) level despite being an insensitive measure of glomerular filtration rate (GFR), it is often the favored parameter to assess the renal impairment in clinical practice. It has a nonlinear association with GFR, which varies with age, sex and body mass. To overcome these limitations, estimated glomerular filtration rate (eGFR) estimated by Cockcroft–Gault equation is now considered ideal for assessing renal function. Renal insufficiency is diagnosed with eGFR <90 ml/min/1.7 m2 and various stages categorized depending on eGFR values. Chronic kidney disease is defined as eGFR <60 ml/min/1.73 m2 for ≥3 months, with or without kidney damage.5
2. Pathophysiology
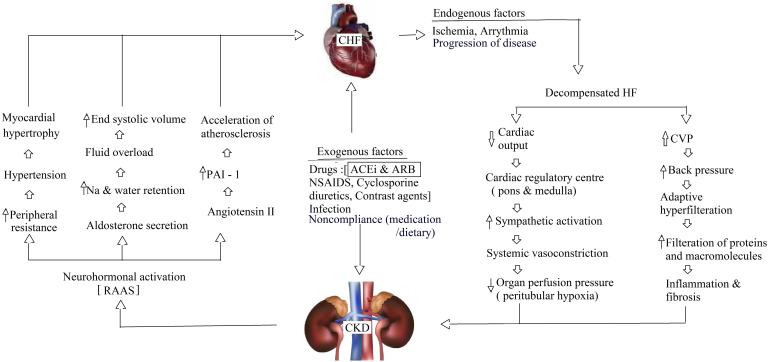
HF is a multifaceted syndrome that is consequent to any structural or functional impediment in ventricular filling or ejection of blood.6 It is diagnosed clinically based on manifestations secondary to congestion of systemic and/or pulmonary venous systems. HF classification based on left ventricular ejection fraction has prognostic and therapeutic importance. Even in presence of normal ejection fraction, excess volume overload consequent to renal dysfunction may result in clinical features of heart failure.7 Moreover assessment of cardiac failure is difficult in volume overloaded patients with renal dysfunction. Ventricular hypertrophy, diastolic dysfunction, pressure and volume overload in patients with CKD may contribute to the appearance or worsening of HF in patients with left ventricular dysfunction.8 Clinical manifestation of congestive heart failure (CHF) is present in approximately 20% of ESRD patients.9 Additionally, hemodynamic alteration produced by cardiac dysfunction may lead to worsening of renal function as adequate mean arterial pressure is necessary to maintain renal perfusion and glomerular filtration. Balance between the cardiovascular and renal system is maintained by an intricate link mediated through renin angiotensin–aldosterone axis and autonomic nervous system10 (Fig. 1).
Fig. 1.
Pathophysiologic pathways of RAAS interaction between heart failure and renal dysfunction. CHF – congestive heart failure, CKD – chronic kidney disease, RAAS – renin angiotensin aldosterone system, ACEI – angiotensin converting enzyme inhibitors, ARB – angiotensin receptor blocker, PAI-1 – plasminogen activator inhibitor-1, CVP – central venous pressure.
3. Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB)
ACEI and ARB are one of the most preferred therapeutic agents for management of hypertension. Their favorable hemodynamic alteration includes reduction of cardiac preload, afterload and systolic ventricular wall stress resulting in augmented cardiac output without proportionate increase in oxygen consumption.11 This hemodynamic alteration maintains glomerular filtration by improving the renal perfusion and promoting sodium excretion. Hence they are instrumental in long term management of patients with hypertension, CHF, diabetic and non-diabetic nephropathy providing mortality benefit.12 By preventing degradation of bradykinin, ACEI may improve organ perfusion enhancing kinin induced peripheral vasodilatation. But occasionally these drugs may result in mild renal insufficiency consequent to reduction in GFR. This functional renal insufficiency often occurs when renal perfusion is reduced secondary to decline in mean arterial pressure or when the GFR is highly angiotensin II dependent as in conditions like volume depletion, bilateral renal artery stenosis or renal artery stenosis in a single functional kidney as in transplant recipient.13 Although there is no creatinine value at which initiation of ACEI is contraindicated, it is advisable to be cautious while initiation of these medications when serum potassium is >5.5 mEq/L or systolic blood pressure is <90 mmHg. Usually ACEI and ARB associated renal insufficiency is transient and reversible with discontinuation of drugs.14
4. Renal hemodynamics in CHF
Renal blood flow and GFR are maintained over a wide range of mean arterial pressure by angiotensin II and sympathetic nervous system mediated renal auto-regulation.10 When renal perfusion pressure falls as in case of CHF, there is increased production of renin from juxtaglomerular cells and subsequent angiotensin II production. This leads to vasoconstriction of post-glomerular efferent arterioles and consequently glomerular capillary pressure increases. Angiotensin also increases distal convoluted tubule sodium reabsorption through aldosterone secretion. Thirst center activation along with free water absorption by ADH secretion helps to maintain intravascular volume. As a result, GFR is maintained despite reduction in mean arterial pressure.
RAAS blockade leads to reduction in systemic vascular resistance, sodium reabsorption, aldosterone and ADH secretion.11 These beneficial effects of ACEI and ARB in patients with CHF is also associated with fall in GFR due to preferential dilatation of post-glomerular efferent arterioles resulting in reduction in filtration pressure. In a normotensive patient, this leads to mild (usually <20%) increase in serum creatinine. But in patients with MAP is less than 60 mm of Hg, diuretic induced hypovolemia or those with significant renal arterial disease, use of ACEI and ARB can lead to marked reduction in glomerular filtration pressure and acute worsening in renal function leading to acute renal failure (ARF).13 In CONCENSUS II trial (Cooperative North Scandinavian Enalapril Survival Study II) there is 2.4% incidence of acute renal dysfunction15 and in SOLVD trial (Studies of Left Ventricular Dysfunction) there is 4% relative likelihood of developing ARF with use of ACEI and ARB.16 ACE inhibitors and ARBs can be used as alternatives of each other, if the preferred class cannot be used and should be gradually titrated to maximum tolerated dose. Concept of increased inhibition of the renin system to improve heart failure outcomes was marred with hurdles. There are conflicting reports about use of combination of ACEI and ARB in heart failure patients. While trials like ONTARGET and ValHeFT showed no clinical benefit of such combination, others trials like CHARM- Added showed decreased hospitalization.17, 18, 19 However, the important point to note in these trials are remarkably low proportion of patients having reduced GFR. Hence with all these inconsistencies, the generalizability of the findings to patients with chronic kidney disease is not justified.
5. Causes of ARF on ACEI and ARB therapy
ARF can be defined as increase in serum creatinine >0.5 mg/dl, if serum creatinine was <2 mg/dl or an increase of >1 mg/dl if initial serum creatinine was >2 mg/dl.13 ARF in the setting of chronic ACEI and/or ARB administration signify a change in systemic hemodynamics secondary to worsening heart failure causing extra cellular fluid accumulation and reduction in mean arterial pressure or aggressive diuresis causing intravascular volume depletion (Table 1). The probability of deterioration of renal function increases in CHF patients with CKD as the compensatory hyper filtration in remaining glomeruli is removed by ACEI and ARB. Renal filtration pressure is significantly reduced with ACEI and ARB especially in patients on with long acting formulations and in those requiring renal clearance when MAP falls below 60 mm of mercury. Higher incidence of elevated serum creatinine documented in patients already on diuretic therapy often return to pretreatment level when diuretics doses where reduced.20
Table 1.
Causes of renal dysfunction on RAAS blockade therapy.
| 1. Poor renal perfusion | Heart failure exacerbation, low cardiac output, dehydration, volume depletion |
| 2. Renovascular disease | Bilateral renal artery stenosis, stenosis of single or dominant kidney, diffuse atherosclerosis of pre-glomerular vessels |
| 3. Drugs | NSAIDS, cyclosporine, radiocontrast |
| 4. Infections | Bacterial, viral, fungal |
In patients with bilateral renal artery stenosis, diffuse atherosclerosis and functional single kidney, ACEI and ARB can significantly worsen renal function as they are dependent on RAAS to maintain glomerular filtration. Pre-glomerular afferent arteriolar constriction due to NSAID and hypertension can lead to ARF in patients on ACEI and ARB. A rise of creatinine up to 20% can be anticipated and this by itself is not an indication of discontinuing these medications. ACEI and ARB can also potentiate radiocontrast induced renal injury and this may be aggravated by concomitant use of NSAID.10
6. Management of ARF
Management of ARF centers on treatment of life threatening situations, attempts to halt or reverse renal deterioration, modifications of identifiable precipitating cause and if unsuccessful providing support by renal replacement anticipating functional recovery. The deterioration in renal function induced by inhibition of the RAAS does not have the same adverse prognostic implications as other causes of ARF in HF.21 Moreover diuretic induced hypokalemia is often offset by chronic ACEI and ARB therapy.
Anxiety ascribed to possible deterioration of renal functions and resultant complication is the key rationale implicated in underutilization of these drugs in CHF patients with renal insufficiency. ACEI and ARB are generally well tolerated by patients with mild to moderate renal insufficiency. Judicious monitoring of serum creatinine level in patients on RAAS blockade therapy may help in identification and prevention of progressive renal function deterioration. Degree of renal deterioration that mandates dose reduction or discontinuation of RAAS blockade is presently uncertain. Although the discontinuation of RAAS blockade often normalizes renal parameters, it is not the preferred initial therapeutic choice as mild elevation in serum creatinine is manifestation of hemodynamic effect of RAAS blockade.22 Higher creatinine level is evident in patient with more intensive blood pressure control as evidenced by recently published SRPINT trial.23 Raise in serum creatinine is usually transient as shown in CONSENSUS trial in which only 9% patients had persistent elevation creatinine as compared to 30% of study population in first follow-up. In SOLVD trial deterioration of renal function was comparable in patients on ACEI and placebo (16% vs 12%, respectively). These studies have proved beyond doubt that with proper monitoring of renal parameters, ACEI can be safely used in CKD patients. Though it is safe in majority, patients planned for institution of ACEI and ARB therapy should have baseline estimation of eGFR. Routine monitoring of serum creatinine and electrolytes must be done in all CHF patients in whom RAAS blockade is initiated. When RAAS blockade is initiated at GFR and potassium levels of <60 ml/min/1.73 m2 and ≥4.5 mEq/L, respectively, regular monitoring for renal parameters must be done preferably at ≤4 weeks interval. If the percentage of GFR decline after initiation is <15%, more liberal approach of monitoring creatinine and potassium levels at 4–12 weeks interval may be planned.
Studies suggest an increase in creatinine of up to 50% above baseline to an absolute value <3 mg/dl or reduction in eGFR of value less than 25 ml/min/1.73 m2 whichever is the smaller, is acceptable. In such cases serum creatinine should be checked after 2 weeks after stopping of offending nephrotoxic medications like NSAIDS, correction of hypovolemia and/or reduction in diuretic dosage. Azotemia in ACEI and ARB induced renal failure is usually completely reversible unless tubular necrosis sets in. Hence intensive follow-up in HF patients with CKD presenting with oliguria, azotemia and hypotension is vital. In cases with progressive renal failure despite above measures, the dose of the RAAS-inhibitor should be halved and serum creatinine strictly monitored. Guidelines advocate discontinuation of RAAS blockade with an increase in creatinine of up to 100% above baseline or to an absolute value >3.5 mg/dl (310 mmol/l) or an eGFR <20 ml/min/1.73 m2.21 In cases with severe renal dysfunction, fear of life threatening hyperkalemia hampers use of ACEI and ARB. It is advisable to initiate ACEI or ARB in incremental doses as tolerated in euvolemic patients. Majority of ACEI and ARB are eliminated during hemodialysis. Hence it is safe to continue these medications in CKD patients on renal replacement therapy, often needing post-dialysis substitution. Hypertensive patients having CHF with advanced renal dysfunction may be treated with non-dialyzable ACEI with hepatic clearance (e.g., Fosinopril).24
7. Conclusion
RAAS blockade is not only a vital aspect of HF management but also prevents progression of renal dysfunction providing morbidity and mortality benefit. CKD must not be considered as contraindication for institution of ACEI or ARB medications but caution must be exercised in elderly and patients with severe renal dysfunction as they are inadequately represented in trials. Their safety in this selected population needs to be substantiated by further studies. With Cardiovascular disease being the main cause of mortality in CKD patients, with proper monitoring of renal parameters, ACEI and ARB can be safely used in CHF patients having mild to moderate renal dysfunction.
Conflicts of interest
The authors have none to declare.
References
- 1.Sarnak M. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Davies M.K., Gibbs C.R., Lip G.Y.H. Management: diuretics, ACE inhibitors, and nitrates. BMJ. 2000;320(7232):428–431. doi: 10.1136/bmj.320.7232.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damman K., Tang W., Felker G. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency. J Am Coll Cardiol. 2014;63(9):853–871. doi: 10.1016/j.jacc.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Khalifeh N., Vychytil A., Hörl W. The role of peritoneal dialysis in the management of treatment-resistant congestive heart failure: a European perspective. Kidney Int. 2006;70:S72–S75. doi: 10.1038/sj.ki.5001919. [DOI] [PubMed] [Google Scholar]
- 5.KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Yancy C.W., Jessup M., Bozkurt B., ACCF/AHA Task Force Members 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;(June) doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Maeder M.T., Kaye D.M. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53(11):905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Di Lullo L., Gorini A., Russo D., Santoboni A., Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardioren Med. 2015;5(4):254–266. doi: 10.1159/000435838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosterd A., Hoes A. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh J., Kandala J., John Camm A. Non-pharmacological modulation of the autonomic tone to treat heart failure. Eur Heart J. 2013;35(2):77–85. doi: 10.1093/eurheartj/eht436. [DOI] [PubMed] [Google Scholar]
- 11.Brown N., Vaughan D. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97(14):1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 12.Molnar M., Kalantar-Zadeh K., Lott E. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63(7):650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoolwerth A., Sica D., Ballermann B., Wilcox C. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the council on the kidney in cardiovascular disease and the council for high blood pressure research of the American Heart Association. Circulation. 2001;104(16):1985–1991. doi: 10.1161/hc4101.096153. [DOI] [PubMed] [Google Scholar]
- 14.Main J. Atherosclerotic renal artery stenosis, ACE inhibitors, and avoiding cardiovascular death. Heart. 2005;91(4):548–552. doi: 10.1136/hrt.2003.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 16.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 17.ONTARGET Investigators, Yusuf S., Teo K.K. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 18.Cohn J.N., Tognoni G., For the Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin-receptor blocker Valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 19.McMurray J.J., Östergren J., Swedberg K., For the CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-added trial. Lancet. 2003;362(9386):767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 20.Heywood J., Burnett J. Cardiotext Pub.; Minneapolis: 2012. The Cardiorenal Syndrome. [Google Scholar]
- 21.Damman K., Tang W., Felker G. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. 2014;63(9):853–871. doi: 10.1016/j.jacc.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Bakris G.L., Weir M.R. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 23.A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inrig J. Antihypertensive agents in hemodialysis patients: a current perspective. Semin Dial. 2010;23(3):290–297. doi: 10.1111/j.1525-139X.2009.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]