Abstract
Intravascular imaging has improved our understanding of in vivo pathophysiology of coronary artery disease (CAD) and predicted decision-making in percutaneous coronary intervention (PCI). Intravascular ultrasound (IVUS) has emerged as the first clinical imaging method contributing significantly to modern PCI techniques. This modality has outlived many other intravascular techniques 26 years after its inception. It has assisted us in understanding dynamics of atherosclerosis and provides several unique insights into plaque burden, remodeling, and restenosis. It is useful as an imaging endpoint in large progression-regression trial and as workhorse in many catheterization laboratories. IVUS guidance appears to be most beneficial in complex lesion subsets that are being treated with drug-eluting stents. The recent introduction of optical coherence tomography (OCT), a light based imaging technique, has further expanded this field because of its higher resolution and faster image acquisition. The omnipresence of OCT raises the question: Does IVUS have a role in the era of OCT? Whether OCT is superior to IVUS in routine clinical practice? Even if OCT is currently gaining clinical significance in detailed planning of interventional strategies and stent optimization in complex lesion subsets, it is the much younger technique and has to prove its worth. Nevertheless, undoubtedly IVUS plays significant role in studies on coronary atherosclerosis and for guidance of PCI. In fact, both the methods are complementary rather than competitive.
Keywords: Percutaneous coronary intervention, Intravscular ultrasound, Optical coherence tomography, Vulnerable plaque, Biodegradable vascular scaffold
1. Introduction
More than 26 years after its inception, intravascular ultrasound (IVUS) is still alive and has outlived many intravascular techniques. IVUS has played a pivotal role in understanding the pathophysiology of coronary atherosclerosis and has facilitated the refinement of diagnostic and therapeutic strategies.1 It assists in understanding of the dynamics of atherosclerosis because of its capability to depict the arterial wall and lumen of the coronary arteries across the full 360° circumference of the vessel. It is not only an established imaging endpoint in progression-regression trials, but also an important workhorse in many catheterization laboratories across the globe. The advent of drug-eluting stents (DES) expands the horizon of complex percutaneous coronary intervention (PCI) where in application of IVUS could be useful. Recently, the introduction of optical coherence tomography (OCT) with better resolution allows for increased ability to visualize vessel wall, characterize plaque, and assist with opimization of coronary stenting with short-and long-term follow up. The omnipresence of OCT questions if IVUS has a future in OCT era.
2. Plaque characterization
Arterial morphology could be better delineated by OCT due to its superior resolution (Table 1). OCT is more accurate than IVUS in measuring intima media thickness, intimal hyperplasia, and external and internal elastic lamina.2, 3 But it lacks depth of penetration to visualize the external elastic lamina in the presence of heavy plaque burden.4 Plaque burden, an important predictor of clinical outcome, is more readily quantified with IVUS.
Table 1.
Comparative technical summary of Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT).
| OCT | IVUS | |
|---|---|---|
| Technology | Near-infrared | Ultrasound |
| Axial resolution (um) | 10–20 | 100–200 |
| Lateral resolution (um) | 20–90 | 200–300 |
| Frame rate (frame per second) | 100 | 30 |
| Pull-back speed (mm/s) | 1–20 | 0.5–1.0 |
| Rotation speed (Hz) | 16–160 | 30 |
| Scan diameter-field of view(mm) | 7–11 | 15 |
| Tissue penetration (mm) | 1–3 | 10 |
| Image through blood field | No | Yes |
| Blood removal with contrast | Yes | No |
| Catheter size | 3.2 Fr | 3.5 Fr |
| Wavelength | 10–40 MHz | 1.3 um |
Newer applications such as integrated backscatter, wavelet analysis, and virtual histology, currently allow IVUS to characterize plaques as lipid, fibrous tissue, calcification, or necrotic core with high accuracy.5, 6, 7, 8, 9 Because of its ability to visualize plaque microstructures and tissue adjacent to calcium, OCT is superior to both grayscale and radiofrequency IVUS in characterizing plaque.10 This superiority is apparent while identifying lipid-rich plaques. However, full visualization of large plaques is precluded because of its limited depth of penetration (Fig. 1). IVUS, however, can accurately quantify large lipid pool and see the entire vessel wall, even in presence of large plaque burden.4, 11
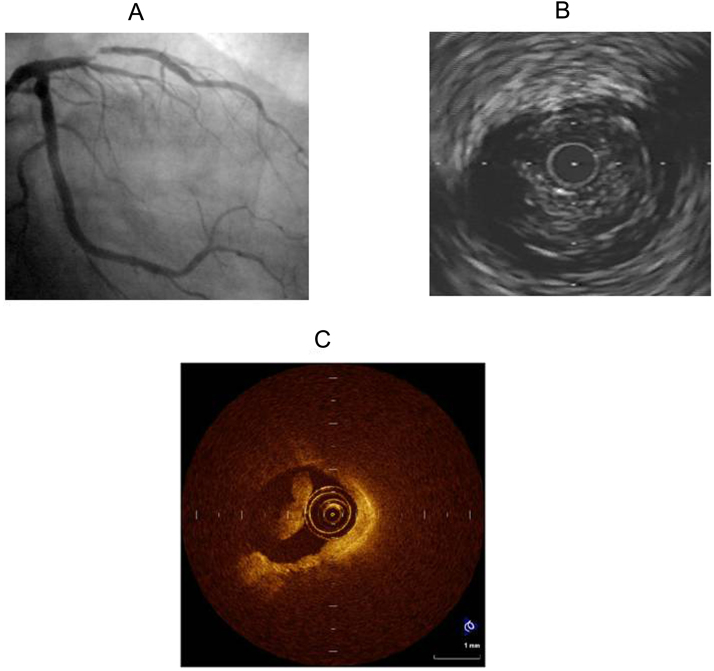
Fig. 1.
A) 43 year old gentleman with non ST-elevation myocardial infarction depicting significant lesion in mid segment of left anterior descending artery. B) Suspicion of thrombus in IVUS intrrogation. C) Clearly visible thrombus in OCT that precludes plaque characterization.
3. Plaque vulnerabilty
Identification of vulnerable plaque has emerged as a potential tool in preventing acute coronary syndromes. Pathologically vulnerable plaque has been characterized as hypocellular, lipid rich with necrotic core, and covered by a thin cap (<65 um). OCT is superior to IVUS in identifying features of vulnerability for plaque rupture including thin fibrous caps (Fig. 2), large lipid cores (Fig. 2), microchannels, macrophage infiltration, superficial spotty calcification, and cholesterol crystals.2, 12 It can visualize and qualify intracoronary thrombus as white or red.4 OCT may misinterpret signal-poor regions in the deeper vessel wall as necrotic core that may can label plaque falsely as vulnerable.13 It can interpret mural thrombi as lipid-rich fibroatheroma, due to smaller OCT single attenuation patterns produced by these two plaque components. Due to its depth of resolution, IVUS can assess plaque burden and vessel remodeling, whereas OCT cannot.13
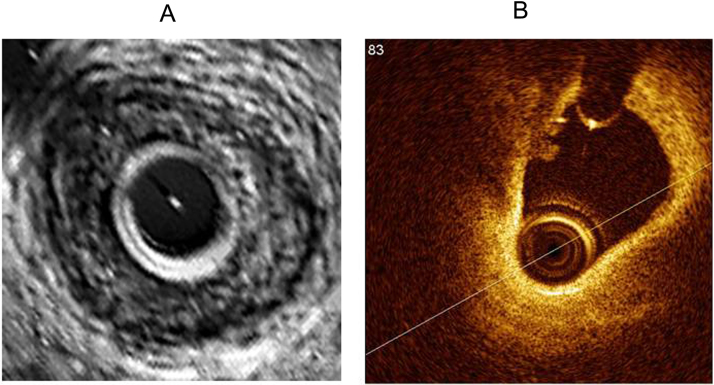
Fig. 2.
A. IVUS showing large lipid pool, but fails to delineate fibrous cap and thrombus clearly. B. OCT revealing large lipid pool, thin fibrous cap with a lobulated homogenous signal rich region known as thrombus which is obscured by IVUS imaging.
4. Vessel sizing
Due to shallow penetration of OCT, there may be a limit in the detail of the whole vessel structure visualized as compared to IVUS imaging.14 OCT measured reference lumen diameters are almost identical to those measured with IVUS.15 OCT derived minimal lumen area (MLA) is smaller than IVUS.
5. Optimisation of PCI
OCT allows detailed evaluation of stent apposition and expansion. It detects stent edge dissection, tissue protrusion and incomplete stent apposition that may not be visualized by IVUS (Table 2).15, 16 Although, long-term clinical implications of these findings are unclear, the ability to visualize clearly these phenomena will motivate the researchers further to conduct more large, long-term prospective trials to study their impact on clinical outcomes.
Table 2.
Comparative characterization of pathology using Intravascular Ultrasound and Optical Coherence Tomography.
| OCT | IVUS | |
|---|---|---|
| Necrotic core | +++ | + |
| TCFA | +++ | – |
| Thrombus | +++ | + |
| Calcium | ++ | +++ |
| Stent expansion/sizing | +++ | +++ |
| Stent apposition | +++ | ++ |
| Vascular injury | +++ | ++ |
| PCI guidance | + | ++ |
| Stent restenosis/NIH | ++ | +++ |
| Stent coverage Ostial lesion |
+++ + |
– ++ |
IVUS, intravascular ultrasound; NIH, neointimal hyperplasia; PCI, percutaneous coronary intervention; OCT, optical coherence tomography; TCFA, thin cap fibroatheroma. +++ = excellent capability; ++ =good capability; + = poor capability; − = impossible
6. Neointimal coverage
Strut coverage is an important surrogate risk factor for stent thrombosis. Most DES appeared uncovered by neointima in IVUS examination. The thickness and extent of neointimal coverage are difficult to be delineated by IVUS due to limited resolution. On the other hand, OCT clearly demonstrates both the coverage of individual struts and thickness of neointimal coverage (Table 2).17 Unlike IVUS, OCT can also be used for qualitative assessment of neointimal coverage (to determine if it is homogenous, heterogeneous, or layered)18 which could improve the understanding of the mechanism of in-stent restenosis. Histological validation of these measurements remains to be performed, and the long- term prognostic implications are unknown.
7. Biodegradable Vascular Scaffold (BVS)
Because of translucency and radiolucent, visualization of BVS is difficult with traditional imaging modalities. OCT has the potential to quantitatively assess strut thickness and biodegradation making it an ideal imaging modality for monitoring these stents. This modality precisely characterizes stent apposition and strut coverage and demonstrates structural changes in the bioresorbable DES over time.19
8. Artifacts
Both IVUS and OCT experience artifacts during image acquisition. Certain artifacts are shared by both, whereas others are unique to their respective imaging technique. OCT's smaller profile and simplified rotational mechanisms make it less prone to mechanical artifacts. A study using a phantom model found that OCT experienced less non-uniform rotational distortion than IVUS, particularly in more tortuous vessel.
9. Application of IVUS in OCT era
OCT-“the new kid on the block”- still has to prove its value. Because of shallower penetration, OCT may not be able to visualize the whole vessel structures, including external elastic lumina, especially in presence of heavy lipid-rich plaque burden. It is inferior to IVUS in assessment vascular remodeling,and progression-regression trials.1 However, OCT is able to depict and measure clearly thin cap fibroatheroma prone to rupture rather than IVUS. On the other hand, radiofrequency (RF) IVUS provides quantification of different plaque components which are displayed in simply color-coded images. Many experts agree to usefulness of IVUS guidance during stenting of bifurcations, left main, long lesions, small vessels, and in diabetes.20 Forward looking IVUS may improve the ease and success of PCI in coronary chronic total occlusion in the near future.21 IVUS guided DES stenting has been shown to reduce late stent thrombosis and other major adverse cardiac events as well as the need for repeat revascularization.22 High density IVUS (HDi) reinvents IVUS technology with improved image quality, a touch panel interface, a highly deliverable high-definition catheter, and superfast pullback speed. It also offers a high depth of penetration to allow assessment of full plaque burden.
The interpretation of pseudo-microscopic OCT images is more difficult. Differentiation of lipidic and calcified plaques may be quite challenging with OCT.1 Another drawback of this modality is the need to replace the coronary blood pool with contrast. The increased contrast volume during OCT may impair renal function. The clinical value of higher resolution images in guiding decision-making is still unclear.23 There are few data on the ability of OCT to measure stent lumen area and to identify stent underexpansion. It remains unknown if IVUS criteria could be translated to OCT-guided stent implantation. Several studies demonstrated that OCT-guided lumen dimensions are smaller compared to IVUS- guidance.14, 24 Okamura et al.25 found OCT derived MLA to be smaller as compared to IVUS derived. This measurement difference might lead to stent underexpansion resulting in restenosis and stent thrombosis when PCI is performed by OCT guidance using IVUS criteria. However, optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI), a randomised controlled trial demonstrates that OCT-guided stent sizing strategy results in similar minimal stent area compared to IVUS-guided PCI. [26]
The author feels that OCT is not yet ready to replace IVUS.27 OCT stent studies continue to focus on mechanisms of stent failure (restenosis and thrombosis), long-term comparisons of different stent platforms (extent of tissue coverage and malapposition) and qualitative analysis of the composition of neointimal tissue. There are few clinical studies attempted to assess the utility of the OCT, either diagnostically or during stent-implantation procedures. The paradigms for IVUS guidance and criteria for optimization do not translate directly to OCT. Much work needs to be done to define the best OCT-guided stent sizing strategy and the appropriate endpoints for OCT-guided stent optimization.
10. Conclusion
Both IVUS and OCT remain useful imaging guiding tools. It is unclear if additional information obtained by OCT translates into improved patient outcome. OCT is now on the stage and only will have only a niche role. There is obviously a need for more data particularly randomized studies versus IVUS and coronary angiography to better understand the clinical implications. OCT has limitations (penetration, true vessel sizing, assessment of plaque burden, etc) and really does not add important information. The lack of clinical data and standardized OCT criteria for optimizing stent implantation may further delay its penetration. In an era of more complex PCI, IVUS remains still an important armamentarium for the modern-day interventional cardiologist. A good IVUS study provides all the information needed to optimize stenting. In the era of OCT, IVUS is still necessary to characterize and measure plaque burden, assess vessel remodelling, and view deep vascular structures. At present, OCT is not ready for prime time and IVUS remains the most validated imaging modality and is author's first choice in catheterization laboratories. The future would certainly witness usage of both modalities in combination, at least for certain indications.
Conflicts of interest
Nil.
Funding
Nil.
References
- 1.Dash D., Daggubati R. An update on clinical applications of intravascular ultrasound. J Cardiovasc Dis Diagn. 2015;3:4. [Google Scholar]
- 2.Jang I., Bouma B.E., Kang D. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 3.Kume T., Akasaka T., Kawamoto T. Assessment of coronary intima-media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69:903–907. doi: 10.1253/circj.69.903. [DOI] [PubMed] [Google Scholar]
- 4.Kubo T., Imanishi T., Takarada S. Assessment of culprit lesion morphology in acute myocardial infarction. Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 5.Murashige A., Hiro T., Fujii T. Detection of lipid-laden atherosclerotic plaque by wavelet analysis of radiofrequency intravascular ultrasound signals: in vitro validation and preliminary in vivo application. J Am Coll Cardiol. 2005;45:1954–1960. doi: 10.1016/j.jacc.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 6.Nair A., Kuban B.D., Obuchowski N., Vince D.G. Assessing spectral algorithms to predict atherosclerotic plaque composition with normalized and raw intravascular ultrasound data. Ultrasound Med Biol. 2001;27:1319–1331. doi: 10.1016/s0301-5629(01)00436-7. [DOI] [PubMed] [Google Scholar]
- 7.Nair A., Kuban B.D., Tuzcu E.M. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki M., Takatsu H., Noda T. In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation. 2002;105:2487–2492. doi: 10.1161/01.cir.0000017200.47342.10. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki M., Sano K., Okubo M. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol. 2005;45:1946–1953. doi: 10.1016/j.jacc.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 10.Yabushita H., Bouma B.E., Houser S.L. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 11.Kume T., Akasaka T., Kawamoto T. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol. 2006;97:1172–1175. doi: 10.1016/j.amjcard.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki M., Hattori A., Ishihara Y. Tissue characterization of coronary plaques and assessment of thickness of fibrous cap using integrated backscatter intravascular ultrasound: comparison with histology and optical coherence tomography. Circ J. 2010;74:2641–2648. doi: 10.1253/circj.cj-10-0547. [DOI] [PubMed] [Google Scholar]
- 13.Kume T., Okura H., Yamada R. Frequency and spatial distribution of thin-cap fibroatheroma assessed by 3-vessel intravascular ultrasound and optical coherence tomography: an ex vivo validation and an initial in vivo feasibility study. Circ J. 2009;73:1086–1091. doi: 10.1253/circj.cj-08-0733. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T., Terashima M., Akasaka T. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol. 2008;101:562–567. doi: 10.1016/j.amjcard.2007.09.116. [DOI] [PubMed] [Google Scholar]
- 15.Kawamori H., Shite J., Shinke T. The ability of optical coherence tomography to monitor percutaneous coronary intervention: detailed comparison with intravascular ultrasound. J Invasive Cardiol. 2010;22:541–545. [PubMed] [Google Scholar]
- 16.Bouma B.E., Tearney G.J., Yabushita H. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89:317–320. doi: 10.1136/heart.89.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto D., Shite J., Shinke T. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–967. doi: 10.1093/eurheartj/ehl413. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalo N., Serruys P.W., Okamura T. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–293. doi: 10.1016/j.ahj.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Ormiston J.A., Serruys P.W., Regear E. A bioabsorbable everolimus-eluting coronary stent system for patients with single de novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 20.Minz G.S., Painter J.A., Pichard A.D. Atherosclerosis in angiographically normal coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995;25:1479–1485. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- 21.Dash D., Li Li. Intravascular ultrasound guided percutaneous coronary intervention for chronic total occlusion. Curr Cardiol Rev. 2015;11:323–327. doi: 10.2174/1573403X11666150909105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy P., Steinberg D.H., Sushinsky S.J. The potential clinical utility of intravascular ultrasiund guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008;29:1851–1857. doi: 10.1093/eurheartj/ehn249. [DOI] [PubMed] [Google Scholar]
- 23.Meneveau N., Ecarnot F., Souteyrand G. Does optical coherence tomography optimize results of stenting? Rationale and study design. Am Heart J. 2014;168:75–81. doi: 10.1016/j.ahj.2014.05.007. [e1-2] [DOI] [PubMed] [Google Scholar]
- 24.Gonzalo N., Serruys P.W., Garcia-Garcia H.M. Quantitative ex vivo and in vivo comparison lumen dimensions measured by optical coherence tomography and intravascular ultrasound in human coronary arteries. Rev Esp Cardiol. 2009;62:615–624. doi: 10.1016/s1885-5857(09)72225-x. [DOI] [PubMed] [Google Scholar]
- 25.Okamura T., Onuma Y., Garcia-Garcia H.M. First-in-man evaluation of intravascular optical frequency domain imaging (OFDI) of Terumo: a comparison with intravascular ultrasound and quantitative coronary angiography. EuroIntervention. 2011;6:1037–1045. doi: 10.4244/EIJV6I9A182. [DOI] [PubMed] [Google Scholar]
- 26.Ali Z.A., Maehara A., Généreux P. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI), a randomised controlled trial. Lancet. 2016 doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 27.Dash D. Application of intravascular ultrasound in the era of optical coherence tomography. EC Cardiol. 2015;2(1):90–93. [Google Scholar]