Abstract
The combined and relative contribution of glucose and fatty acid oxidation generates myocardial energy, which regulates the cardiac function and efficiency. Any dysregulation in this metabolic homeostasis can adversely affect the function of heart and contribute to cardiac conditions such as angina and heart failure. Metabolic agents ameliorate this internal metabolic anomaly, by shifting the energy production pathway from free fatty acids to glucose, resulting in a better performance of the heart. Metabolic therapy is relatively a new modality, which functions through optimization of cardiac substrate metabolism. Among the metabolic therapies, trimetazidine and ranolazine are the agents presently available in India. In the present review, we would like to present the metabolic perspective of pathophysiology of coronary artery disease and heart failure, and metabolic therapy by using trimetazidine and ranolazine.
Keywords: Metabolic therapy, Trimetazidine, Stable angina, Heart failure, Ischemia
1. Case study
A 72-year old woman presented with the complaints of chest pain and dyspnea occurring since the last 3–4 weeks, which is increased during her routine chores and resolved after rest, hampering her daily activities. Her medical history revealed that she has been a hypertensive for eight years. She had complained of chest discomfort 2 years ago and had undergone Percutaneous Coronary Intervention (PCI) with two drug eluting stents, following which her angina was relieved. Her left ventricular ejection fraction (LVEF) on 2D echo is 35%. Her blood pressure is under control at 138/82 mm Hg with amlodipine 5 mg and perindopril 8 mg. In addition, since the onset of angina, metoprolol 50 mg twice a day and nicorandil 5 mg twice a day has been added. In spite of these medications she presently needs to take nitroglycerin (0.4 mg sublingually) approximately 2–3 times a day for angina relief. This case scenario presents a challenge for the physician as the patient is already under treatment with most of the existing potential medications for stable angina. An alternate modality is the need in this case to effectively manage her troublesome angina and improve her quality of life. For personal reasons she is not willing to undergo further intervention.
As described by Opie L in 1999, “The heart is more than just a pump. It is also an organ that needs energy from metabolism. A metabolic disease, ischemia, should ideally be treated by metabolic therapy”.1 Hence, metabolic therapy has been evolved as a potential and effective treatment modality, which could be prescribed in this situation to overcome her ongoing complaints.
Usually stable coronary artery disease (CAD) patients would be prescribed two to three antianginal drugs such as beta-blockers, calcium channel blockers and nitrates.2 As these agents act by altering hemodynamic system, the addition of multiple drugs may be associated with side effects. Furthermore, usage of nitrates regularly can lead to tolerance. Therefore, in such situations, the need would be to treat the patients with an alternative and effective treatment modality.2
Metabolic therapy can be considered for cardiovascular ailments such as CAD and heart failure (HF). Indeed, recent several studies have shown that metabolic therapy can be a potential and promising approach in dealing with CAD and HF.3 Metabolic therapy functions by modulating metabolism, i.e. shift the energy production pathway from fatty acid to glucose in the presence of decreased oxygen supply. As these agents function without affecting the hemodynamic alterations, they would not add further to the side effects already produced by heavy multiple hemodynamically acting agents.2 Trimetazidine and ranolazine are the two main metabolic modulators, which are available in clinical practice today. Trimetazidine has been extensively studied for patients with angina and those with reduced left ventricular (LV) function, with or without HF. Recent guidelines published by European Society of Cardiology (ESC 2016), have recommended trimetazidine in patients with HF with ongoing angina.4 Moreover, trimetazidine has also been clinically tested for its cardioprotective role in combination with other interventions. The objective of this article is to present the role of metabolic management and the future perspectives of metabolic therapy in patients with angina and HF due to CAD.
2. Deranged myocardial metabolism in CAD and HF
During normal conditions, the heart generates most of its energy from free fatty acid (FFA) oxidation with the rest from glucose oxidation and lactate.5 The number of moles of adenosine triphosphate (ATP) produced per mole of carbon oxidized is approximately 29% higher for FFA compared to glucose.6 In hypoxia, glucose is preferred for energy production, since glycolysis requires less oxygen per mole of ATP generation compared to FFA oxidation.7 The number of moles of ATP produced per mole of oxygen spent is 12% higher for glucose than for FFA oxidation.8 Increasing the use of glucose and lactate can improve the oxygen utilization and efficiency of the myocardium by 16–26%.9, 10 Hence, generation of energy becomes more dependent on glucose metabolism.
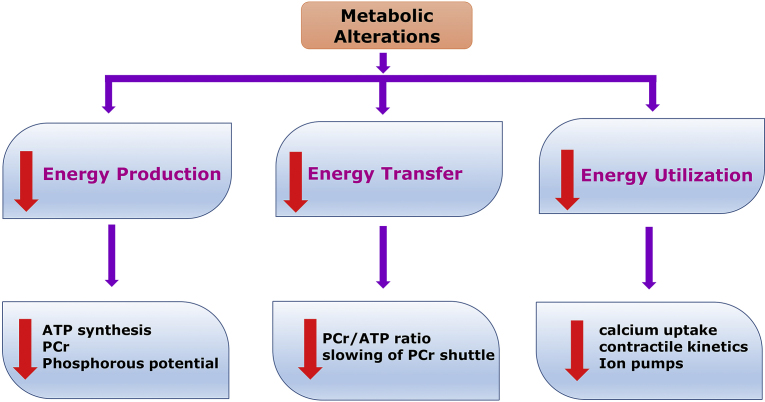
Meanwhile, continued availability of FFAs, inhibit pyruvate dehydrogenase (PDH), which in turn allows accumulation of pyruvate. Pyruvate converts into lactate resulting in intra cellular acidosis. This in turn causes metabolic, morphological and functional alteration of the myocardium leading to arrhythmias, contractile failure and electrophysiological abnormalities.7 In addition, FFAs reduce glucose uptake at cellular level, leading to further increased conversion to lactate, resulting in cell acidosis. Hence, FFA pathway slows down ATP production. These metabolic alterations ultimately lead to cell death. Heart failure is a late manifestation of CAD, because ischemia contributes to the advancement of LV systolic dysfunction. Therefore, altered metabolism is likely to be an indirect but important cause of HF (Fig. 1).5
Fig. 1.
Probable reasons of metabolic alterations in CAD and HF. ATP: adenosine triphosphate; PCr: phosphocreatine.
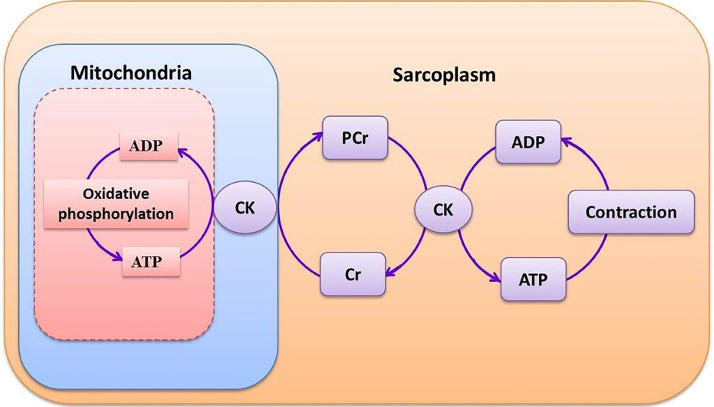
Abnormalities in cardiac functioning may also arise at the level of energy transfer from mitochondria to myocardial muscles. The generated ATP in mitochondria can be transferred to the myofibrils by the creatine kinase (CK) energy shuttle. In this process, the high-energy phosphate bond in ATP can be transferred to creatine by mitochondrial CK to form phosphocreatine (PCr). Phosphocreatine in turn diffuse through the mitochondrial membrane into myofibrils, where myofibrillar CK catalyses the reformation of ATP from PCr.11 The work of the heart is continuous, and this is reflected in the high rate of ATP hydrolysis (≈0.5 μmol/g wet weight per second).11 Consequently, the high-energy phosphate pool in the heart can be exhausted within a short time. Eventually, the PCr level decreases, but the free adenosine diphosphate (ADP) level rises. The amplified level of free ADP inhibits the function of many intracellular enzymes, resulting in failure of the heart's contractile mechanism. Thus, a metabolic imbalance in the cardiac cell can occur when PCr levels fall and free ADP levels rise.11 Hence, the PCr:ATP ratio is a measure of myocardial energetics and its reduction may cause reduced efficiency of myocardial contraction (Fig. 2, Fig. 3).
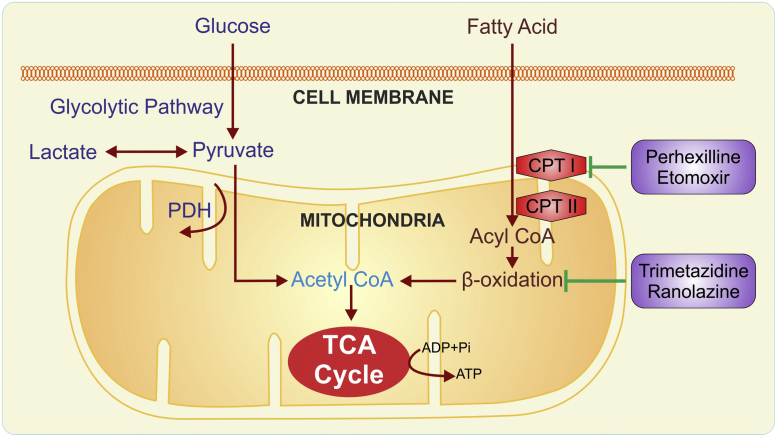
Fig. 2.
Metabolic alterations in CAD and HF. ADP: adenosine diphosphate; ATP: adenosine triphosphate; CPT: carnitine palmitoyl transferase; PDH: pyruvate dehydrogenase; TCA: tricarboxylic acid cycle.
Fig. 3.
Phosphocreatine shuttle system. ADP: adenosine diphosphate; ATP: adenosine triphosphate; CK: creatinine kinase; Cr: free creatinine; PCr: phosphocreatinine.
In principle, the approach of metabolic management is to switch the energy substrate preference from fatty acid oxidation to glucose oxidation.
3. Metabolic management of CAD and HF
Given the above described pathophysiological background and the difficulty of standard treatment to control the total symptomatic and prognostic burden in many patients with ischemia and HF, it seems logical to consider pharmacological manipulation of cardiac energy metabolism as an adjunctive therapeutic option. As mentioned earlier, optimization of cardiac energy metabolism is based on promoting cardiac glucose oxidation.
3.1. Trimetazidine
Trimetazidine is the most extensively studied drug among the metabolic therapies. It has multimodal action on cardiac energy metabolism. Major cardioprotective actions involve, (1) inhibitory effects on fatty acid oxidation, thus favoring glucose oxidation; (2) redirect fatty acids toward membrane phospholipids; (3) preserve PCr and ATP intracellular levels (PCr/ATP ratio).11
Trimetazidine inhibits the mitochondrial long chain 3-ketoacyl-CoA thiolase, the terminal enzyme, which participates in FFA β-oxidation. A decrease in FFA oxidation results in increase of glucose oxidation leading to ATP generation with less oxygen utilization. Trimetazidine also stimulates membrane phospholipid turnover during ischemia and reperfusion, thereby redirecting FFA toward phospholipids resulting in increased cell tolerance to ischemia-reperfusion damage.5 Moreover, it is clinically evident that trimetazidine may also preserve myocardial high-energy phosphate intracellular levels by improving PCr:ATP ratio by +33%.12
Additionally, trimetazidine delays the onset of anaerobic glycolysis and hence prevents free radical generation, maintains ATP production, and reduces intracellular acidosis. Trimetazidine potentially prevents membrane damage and cell death by decreasing sodium and calcium concentration.7 In contrast to classical antianginal therapies, trimetazidine does not show any effect on coronary flow, contractility, blood pressure, or heart rate. Moreover, it does not show inotropic or vasodilator properties at rest or during exercise; thus, it can be easily combined with conventional pharmacotherapy as an add-on therapy, as well as substitution therapy when traditional drugs are not tolerated.5
3.1.1. Trimetazidine in coronary artery disease
Trimetazidine has been examined in a number of studies as monotherapy for CAD. One of such studies, the Trimetazidine European Multicenter Study (TEMS) witnessed the comparable results of trimetazidine and propranolol with respect to time for ST segment depression on exercise testing and the time for onset of symptomatic angina.13 A similar study found significant reduction of anginal attack frequency and nitroglycerin consumption with trimetazidine versus placebo.14 Similarly, a comparative study suggested that trimetazidine is an effective and safe substitute for diltiazem in the treatment of patients with stable angina pectoris.15
Furthermore, trimetazidine was also examined as a combination therapy for CAD. The Trimetazidine in Poland (TRIMPOL-I) study suggested that 4 weeks treatment with trimetazidine in combination with conventional antianginal drugs, significantly decreased the number of angina episodes and improved myocardial ischemia and exercise capacity in patients with stable exercise-induced angina.16
Additionally, in the TRIMPOL-II study, 12 weeks treatment of trimetazidine in combination with metoprolol in patients with stable effort angina, exhibited significant improvements in exercise stress and the symptoms of angina relative to metoprolol alone.17 The Trimetazidine in Angina Combination Therapy (TACT) study demonstrated that trimetazidine with β-blockers or Long-Acting Nitrates (LAN) significantly improved exercise stress test parameters and decreases angina symptoms compared with placebo.18
The METRO (ManagEment of angina: a reTRospective cOhort) study suggested that inclusion of trimetazidine to other antianginal drugs (nitrates, beta-adrenoceptor antagonists, calcium channel antagonists, or nicorandil) early in the treatment of stable angina significantly reduced the risk of post MI mortality in patients surviving a myocardial infarction.19
Trimetazidine plus diltiazem demonstrated significant improvement in patients with stable angina pectoris compared to diltiazem plus placebo.20, 21 Similarly, trimetazidine in combination with standard therapies, improved LV function in diabetic patients with CAD and caused a significant reduction in mortality after surviving a myocardial infarction.22 In another study, trimetazidine plus standard therapy exhibited improvement of clinical complications and quality of life in elderly patients with ischemic heart disease (IHD) and reduced ventricular function compared to placebo.23
The VASCO-angina study examined the efficacy of trimetazidine on total exercise duration (TED) and time to 1-mm ST segment depression (T1), in symptomatic patients with chronic stable angina receiving background atenolol therapy. The study demonstrated that trimetazidine significantly increased TED. In addition, amongst patients with limiting angina during exercise test, trimetazidine markedly improved both T1 and TED.24 In a recent study, in patients with ischemic or non-ischemic cardiomyopathy microvolt T wave alternans has been found to be significantly improved in trimetazidine treated group compared to control group (63 ± 8 μV vs. 53 ± 7 μV). The study concluded that trimetazidine may be an effective drug to prevent arrhythmic complications in patients with stable CAD.70
The observations from monotherapy and combination therapy studies are reiterated by meta-analyses also. A large meta-analysis (n = 19,028) observed that trimetazidine substantially improved exercise tolerance, decreased weekly angina episodes, and use of short-acting nitrates compared to placebo.25 A later meta-analysis confirmed the efficacy of trimetazidine in the treatment of stable angina pectoris, in comparison with conventional antianginal agents, irrespective of treatment duration.26 Moreover, ESC-2013 guidelines recommended the use of trimetazidine as a second-line treatment for CAD (Recommendation: Class IIb, Level B).27 In addition, recently published management protocols of stable CAD, which are funded by Cardiological Society of India recommended use of trimetazidine besides to angina, microvascular and vasospastic angina.69
3.1.2. Trimetazidine in heart failure
Similar to the evidence in CAD, there is abundant evidence to support that the fact that trimetazidine not only improves symptoms of HF but also causes an improvement in cardiac ejection fraction (EF). A study with trimetazidine versus placebo in addition to standard therapy for patients with New York Heart Association (NYHA) class III/IV observed that at 6 months, LVEF increased in the trimetazidine group compared to the placebo group.28
In another study, trimetazidine improved the contractile response of chronically dysfunctional myocardium to dobutamine in patients with ischemic cardiomyopathy. Improvement in LVEF and peak VO2 (volume of oxygen) were also observed.29
In a study by Fragasso et al., trimetazidine improved functional class and LV function in patients with HF and increased the PCr/ATP ratio, which indicates the preservation of the myocardial high-energy phosphate levels.12 Another study by Fragasso et al. demonstrated that adding trimetazidine to conventional therapy in HF patients improved the NYHA functional class, decreased LV end-systolic volume and increased LVEF, while the same parameters deteriorated in placebo group.30
In a meta-analysis, trimetazidine treatment witnessed increased exercise tolerance, improved NYHA functional class, and increased LVEF in ischemic HF compared to placebo. Moreover, the review reported that trimetazidine reduced hospitalizations and overall mortality.31
Another meta-analysis involving chronic heart failure (CHF) patients showed that trimetazidine decreases hospitalization for cardiac causes and improves clinical symptoms, cardiac function, and LV remodeling.32 Moreover, trimetazidine had no harmful effect on heart rate or blood pressure.33
An International multicenter long term cohort study (retrospective) of 699 patients with NYHA class II to IV, and ejection fraction (EF) <45%, showed 11.3% improvement in survival over a five year period.34 A Single center prospective study, “Effects of Long-Term Treatment with Trimetazidine in 200 Ischemic Cardiomyopathy Patients” showed that at 24 months, a 30% improvement in survival rates when trimetazidine was added to the usual therapy. This was associated with an 8.3% increase in LV ejection fraction.33
Therapeutic efficacy of trimetazidine has been proved in Asian population too. A recent study from Bangladesh compared the treatment efficacy of trimetazidine (n = 55 patients; 35 mg sustained released tablet, bid) with placebo plus conventional medications (n = 53 patients). After 6 months, significantly more number of patients in trimetazidine group was in NYHA class I (22% vs. 8%) and class II (56% vs. 34%); higher number of patients in placebo group was in NYHA class III class IV. Anginal episodes and use of sublingual nitrate per week were significantly lower in the trimetazidine group. Left ventricular diastolic dimension (59.7 ± 5.2 vs. 65.1 ± 6.1) and the increase of LVEF (11% vs. 5.6%) were significantly improved. Hospitalizations were also very low in trimetazidine group (13 vs. 22).71 Furthermore, An India-based study demonstrated that trimetazidine plus conventional therapy vs conventional therapy alone in patients with dilated cardiomyopathy and HF, improved NYHA functional class (2.25 vs 1.85), 6 min walk test (349.7 vs 402 m), LV dysfunction-36 score (25.5 vs 21) and BNP (744.7 vs 248.3 pg/ml).72
European Society of Cardiology guidelines-2016 recommended use of trimetazidine when angina persists despite treatment with a β-blocker (or alternative) to relieve angina (effective anti-anginal treatment, safe in HF) (Recommendation: Class IIb, Level A).4
3.1.3. Additional actions of trimetazidine along with other cardiovascular interventions
Beyond the conventional use of trimetazidine in CVDs, trimetazidine has been studied in several other medical interventions such as PCI, coronary artery bypass grafting (CABG) and with thrombolytic agents.35 In several studies trimetazidine was found to demonstrate cardioprotective effects.
3.1.3.1. Trimetazidine with thrombolytic agents
In diabetic patients receiving thrombolytic treatment for anterior wall ST elevation myocardial infarction (STEMI), oral trimetazidine administration was associated with less myocardial damage, earlier successful reperfusion, improvement of LVEF and less cardiac adverse events such as reduced hospitalization for heart failure and re-infarction. Moreover, in this study, a complete resolution of ST-segment elevation in 90 min was found in 70% (n = 35) patients in trimetazidine (70 mg then 35 mg twice daily) group vs. 36% (n = 18) patients in placebo group.36
3.1.3.2. Trimetazidine in pre-PCI
Effect of pre-procedural acute oral administration of trimetazidine on PCI-induced myocardial injury was evaluated in a study (n = 266 patients); the findings witnessed that post-procedural cardiac troponin I levels were markedly decreased in the trimetazidine group compared to control group at 6 h (4.2 ± 0.8 vs 1.7 ± 0.2), 12 h (5.5 ± 1.5 vs 2.3 ± 0.4), 18 h (9 ± 2.3 vs 3 ± 0.5), and 24 h (3.2 ± 1.2 vs 1 ± 0.5) after PCI; this indicates a decreased PCI-evoked myocardial injury with trimetazidine administration.37 Further, in a study that randomized stable or unstable angina pectoris patients (n = 101) to receive or not trimetazidine, angina did not occur in trimetazidine group, compared with 25.5% in the control group. The changes of ST-segment and T-wave during balloon dilatation in PCI procedure were less noticeable in the trimetazidine group compared to control group (60.8% vs. 78.3%). Ejection fraction in the trimetazidine group was higher than that in the control group 66.6% versus 63.0%, 4 weeks after PCI.38 Moreover, a similar study evaluated the effect of pre-treatment with trimetazidine on the extent of ischemia for PTCA; the study findings observed significant lower mean and maximal ST segment elevations during balloon inflation and significant lower mean and maximal amplitude of the T-wave alteration.39
Furthermore, a recent meta-analysis examined the additional use of trimetazidine in patients undergoing PCI demonstrated that trimetazidine significantly improved the LVEF, decreased elevated cardiac troponin Ic level, angina attacks during PCI, and ischemic ST-T changes on the echocardiogram during PCI. Therefore trimetazidine can be used additionally in patients undergoing PCI for reducing myocardial injury during the procedure and improve cardiac function.40
3.1.3.3. Trimetazidine in post-PCI
A randomized controlled trial (RCT) examined the effect of trimetazidine in patients with acute STEMI without ST-segment resolution after primary PCI (n = 138); the study witnessed that trimetazidine improved the LV at days 30 and 180, as denoted by the LVEF, which was 51% versus 45% and 56% versus 49%, correspondingly.41 Another RCT examined the effects of trimetazidine on heart rate variability and LV systolic performance in patients with CAD receiving trimetazidine (n = 26 patients; 20 mg thrice a day) 24 h after PTCA. A statistically significant improvement of LV systolic performance and augmentation of the parasympathetic band of heart rate variability were observed in patients treated with trimetazidine, while no apparent changes were observed in controls.42
A single center study (n = 700 patients) conducted to evaluate the effects of trimetazidine in elderly coronary heart disease (CHD) patients with diabetes mellitus found that patients in the trimetazidine group (n = 255) compared with control group, exhibited marked improvements in the frequency and severity of angina pectoris (28.2% vs 37.6%), recurrent angina pectoris (40.4% vs 51%), as well as silent myocardial ischemia (34.5% vs 45.9%). In addition, LV function and structure in trimetazidine-treated patients were comparatively stable at 2-year follow-up, while they aggravated in the control group with a significant difference between groups.43 Similarly, the potential of trimetazidine in preventing in-stent restenosis was prospectively assessed in 635 patients after drug-eluting stent (DES) implantation. Trimetazidine administered for at least 30 days after the procedure significantly decreased the occurrence of stent restenosis from 11.1% to 4.2% on follow-up at 9–13 months. The frequency of major adverse cardiac and cerebrovascular events (MACCEs) were also reduced in the trimetazidine-treated group at 1-year follow-up (6.1% vs 10.8%).44
3.1.3.4. Trimetazidine in coronary artery bypass grafting (CABG)
The effects of trimetazidine on ischemic injury and myocardial reperfusion were also evaluated in patients undergoing CABG.45 Effect of trimetazidine on the cardiac biomarkers (troponin T and CpK-Mb) was compared with placebo in a randomized, double-blind, prospective study. Both troponin T and CpK-Mb reached highly significant values in the control group compared with the trimetazidine group at the 4 time points analyzed: 5 min after aortic declamping, and at subsequent 12, 24, and 48 h.46 In an RCT, patients (n = 40) undergoing elective CABG were treated with trimetazidine or placebo. Fractional area change increased by 12% in both groups. Lactate levels were markedly lowered in the trimetazidine group; only in the trimetazidine group less calcium channel blocking drugs were required in the early phase after aortic clamping.47 Furthermore, a systematic review investigated the effectiveness of myocardial preservation of preoperative trimetazidine therapy in CABG patients by assessing the postoperative levels of several blood-based biochemical markers of myocardial injury. The study witnessed a positive effect of trimetazidine on myocardial preservation.48
3.2. Ranolazine
Ranolazine functions by inhibiting the late inward sodium channel (late INa), which stays open in myocardial ischemia.49 Increased intracellular sodium burden results in major metabolic and electrophysiological disturbances.49 This is its main non metabolic mode of action; its metabolic effect is again through the conversion of fatty acids toward glucose metabolism. The activity of ranolazine is more significant in ischemic myocytes. Ranolazine is relatively safe with the main side effects of dizziness, nausea, asthenia, constipation, and headache. An anticipated worry about ranolazine is that it could cause dose-dependent prolongation of the QTc, the net effect of its inhibition of IKr, late INa, and late ICa. Metabolism of ranolazine occurs in the liver and eliminated in the urine and thus it is contraindicated in hepatic impairment. Ranolazine interferes with the metabolic pathways of simvastatin and digoxin, thus while concomitant use, dose adjustments of these drugs may be needed.50
3.2.1. Ranolazine in coronary artery disease
A number of studies examined the use of ranolazine in CAD. In one such study, ranolazine markedly reduced angina episodes and nitroglycerine usage and substantially increased exercise duration and time to exercise-induced myocardial ischemia in patients with stable CAD.51 Furthermore, Several clinical studies such as the Monotherapy Assessment of Ranolazine in Stable angina (MARISA),52 the Combination Assessment of Ranolazine in Stable Angina (CARISA)53 and Efficacy of Ranolazine in Chronic Angina (ERICA)54 have shown a significant benefit with ranolazine in patients with stable angina.
Furthermore, the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 study in patients with unstable coronary disease, did not show a significant decrease in the composite end point of cardiovascular death, myocardial infarction, or recurrent ischemia. However, ranolazine showed a marginal effect on disease-specific health status and quality of life (QOL) over around 12 months of follow-up.55 In addition, the MERLIN-TIMI 36 trial determined the effect of ranolazine on 1-year incidence of recurrent CV events in the patients with prior chronic angina. The results showed that ranolazine reduced recurrent ischemic events, regardless of whether patients did or did not receive PCI within 30 days of a non-ST-segment acute coronary syndrome (ACS).73 Ranolazine was also found to produce a favorable impact on diastolic function parameters in patients with chronic coronary disease and stable angina.74 A study examined the effect of ranolazine plus other standard therapies (β-blockers, LAN, calcium-channel blockers) in patients with type 2 diabetes, found that angina attacks occurred less frequently in patients received ranolazine than placebo.56 Similarly, another study assessed long-term safety of ranolazine witnessed that no patient was affected with torsade-de-pointe and QTc was increased on average by 2.4 ms.57 European Society of Cardiology-2013 guidelines recommended adding ranolazine as a second-line treatment in CAD patients according to heart rate, blood pressure and tolerance (Recommendation: Class IIa, Level B).27
3.2.2. Ranolazine in heart failure
Besides CAD, studies examined ranolazine in the management of HF also. A study in patients with HF with preserved ejection fraction (HFpEF), observed that inhibiting late Na+ current by ranolazine, improved hemodynamic measures. However, as this trial is only a proof of concept study, there is a requirement for further studies to corroborate the findings of the study.58 The latest meta-analysis reported that ranolazine was found to be effective in reducing the risk of atrial fibrillation (AF) when compared to control. Subgroup analysis showed a larger effect was seen in postoperative AF compared to no postoperative AF (Guerra, 2017). Some studies have demonstrated that ranolazine increases LVEF and when added to guideline-prescribed therapy in CHF.59 Therefore ESC guidelines recommended considering ranolazine in patients with stable angina and symptomatic (NYHA Class II-IV) HF with reduced ejection fraction and unable to tolerate a beta-blocker to relieve angina but safety in this patient group needs to be kept in mind (Recommendation: Class IIb, Level C).4
3.3. Other metabolic therapies
Besides trimetazidine and ranolazine there are few other metabolic modulators, which were examined for dealing with CAD and HF. They are: perhexiline, Glucose–Insulin–Potassium (GIK) infusion, oxfenicine, etomoxir, l-carnitine, dichloroacetate and β-adrenergic blockers. The following is a brief discussion of these agents.
3.3.1. Perhexiline
Perhexiline acts by shifting myocardial substrate consumption from fatty acids to carbohydrates through inhibition of Carnitine Palmitoyl Transferase-I (CPT-I) and, to a lesser extent, CPT-II, resulting in increased glucose and lactate utilization. Presently the drug is not available in India.3, 34
3.3.2. Glucose–insulin–potassium (GIK) infusion
The beneficial effect of GIK infusion could be due to increased glycolysis and decreased FFA uptake and metabolism by myocardial cells.1, 60 It can lead to lower myocardial oxygen requirement, decrease in proton and free radical accumulation, and improve myocardial energetics. Potential beneficial effects of GIK infusion can be anticipated when administration initiated within the first few hours of symptoms onset. The idea of early GIK therapy is backed by the recent findings from the IMMEDIATE trial, which are in line with the myocardial metabolic support that was hypothesized a long back ago for GIK. Previous futile experiences and relatively week beneficial effects of GIK in all the studies can be ascribed to late onset of infusion when the heart was already incurred significant damage.60 There are several studies, which have shown the beneficial effects of GIK infusion60 and presently it is used mainly in the acute setting of post myocardial infarction and LV dysfunction.
3.3.3. Etomoxir
Etomoxir inhibits the CPT1 enzyme, but to a lesser degree than perhexiline. It has been examined in several animal studies, for cardiovascular beneficial effects,61 and yet it has to be investigated in RCTs for cardiovascular effects. The long-term safety of etomoxir, has been questioned due to a recent clinical study involving 260 patients with moderate HF, which was stopped early due to four patients developed substantial hepatic dysfunction.62
3.3.4. l-Carnitine
l-Carnitine is a co-factor in fatty acid metabolism. It augments the FFA metabolism and increases glucose oxidation.63 Several clinical studies have shown a modest benefit in terms of LV energetics and function with l-carnitine use.63 However, a recent study by Hazen, Koeth et al. raised a debate over the use of l-Carnitine. The study claimed that ingestion of certain diets (ex: red meat) possessing containing l-carnitine, causes intestinal bacteria to produce trimethylamine-N-oxide (TMAO), which in turn correlates with an increased risk of arteriosclerosis and other CV complications. On the other hand, the critics argue that TMAO is formed not only from meat but from many foods including vegetables, the study conducted in mice cannot be correlated to humans and the data is incomplete to draw a contradictory conclusion over the well-established four decades research experience (Koeth, 2013). Presently there is a need for large clinical studies pertaining to l-carnitine in various cardiac diseases.
3.3.5. Beta-adrenergic blockers
Catecholamines induce lipolysis through β-adrenergic receptors. By blocking adrenergic receptors, activation of protein kinase A and hormone-sensitive lipase could be prevented, thereby lipolysis can be inhibited. Vasodilating β-blockers especially carvedilol can inhibit fatty acid oxidation by preventing lipolysis, and result in a decrease in plasma fatty acid levels.7 Beta blockers are strongly recommended for treatment of CHF and LV dysfunction and act through multiple mechanisms.64
4. Present and future of metabolic therapy in CAD and HF
Presently trimetazidine and ranoloazine are the predominantly metabolic agents used among the ones available (Table 1). Indeed, ESC guidelines-2013 recommended the use of trimetazidine (Recommendation: Class IIb, Level B) and ranolazine (Recommendation: Class IIa, Level B) as a second-line treatment for CAD according to heart rate, blood pressure, and tolerance.27 Moreover, ESC guidelines-2016 recommended considering use of trimetazidine (Recommendation: Class IIb, Level A) (effective antianginal treatment, safe in HF) and ranolazine (Recommendation: Class IIb, Level C) (effective antianginal treatment, but safety in HF uncertain) when angina persists despite treatment with a β-blocker (or alternative) to relieve angina.4 Further, the latest December 2016 recommendations – “Management protocols of stable coronary artery disease in India” recommend trimetazidine to be particularly beneficial in diabetic multivessel coronary artery disease patients who may have diffused vessel disease. In fact, the guidelines also emphasize on the addition of trimetazidine when 2 hemodynamic agents fail to provide adequate symptom relief.68 Moreover, as described in this review, presently, metabolic modulators especially trimetazidine has been demonstrated cardioprotective effects along with other interventions such as PCI, CABG and with thrombolytic agents. Presently these metabolic agents predominantly being used as add-on therapies. However, there is growing evidence to use them as first line agents along with hemodynamically active drugs. This results in better symptomatic relief with fewer side effects, than when two or three drugs all acting in a similar hemodynamic way are used. Metabolic management of CAD and HF holds great promise and additional large randomized trials would allow us to place these agents in their rightly deserved place.
Table 1.
Details of metabolic modulators.
| Features | Trimetazidine65, 66 | Ranolazine67, 68 | Perhexiline (PEXSIG 100 mg tablets Data Sheet) |
|---|---|---|---|
| Doses | 20 mg Film-coated tablets 35 mg modified-release film-coated tablet |
500 mg, 1000 mg; 375 mg; (Extended-release tablets) 500 mg; 750 mg (Prolonged-release tablets) | 100 mg tablets |
| Route of administration | Oral | Oral | Oral |
| Metabolism | Minimal biotransformation into several metabolites only detectable in the urine | O-demethylation and N-dealkylation | Principal metabolites of are monohydroxyperhexiline and dihydroxyperhexiline |
| Excretion | Half-life in young people: 7 h; people >65 years: 12 h | Half-life: 7 h | Half-life: Two to six days |
| Adverse effects | Dizziness, headache, Parkinsonian symptoms, palpitations, abdominal pain, diarrhea | Dizziness, headache, constipation, nausea | Peripheral neuropathy, hepatitis/cirrhosis, extrapyramidal dysfunction, muscle weakness and ataxia |
| Therapeutic indication | Add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled by first-line antianginal therapies | As add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled by first-line antianginal therapies | To reduce the frequency of moderate to severe attacks of angina pectoris due to coronary artery disease in patients who have not responded to other conventional therapy or in whom such therapy may be contraindicated |
| Availability | Several countries including India | World wide | Australia and New Zealand |
Vd: volume of distribution; VdSS: Vd at steady state.
Coming back to the patient we had discussed in the beginning, based on the concomitant diseases and the drugs she is currently taking, we consider trimetazidine 35 mg twice daily as an effective and safe drug to relieve her symptoms and improve her cardiac function and even possibly her survival.
5. Conclusion
With the presently available evidence, metabolic modulators such as trimetazidine deserve to be regarded as an early addition to classic hemodynamic agents. Being extensively studied in clinical setup, trimetazidine demonstrates promising therapeutic opportunities in patients with ischemic heart disease and those with LV dysfunction and HF. Patients with and without diabetes, diffuse vessel disease, recurrent angina post PCI, or post CABG and with or without LV dysfunction would get all benefit without the addition of any serious side effects.5
Conflicts of interest
The authors have none to declare.
References
- 1.Opie L.H. Proof that glucose-insulin-potassium provides metabolic protection of ischaemic myocardium? Lancet. 1999;353:768–769. doi: 10.1016/s0140-6736(98)00385-7. [DOI] [PubMed] [Google Scholar]
- 2.Daly C., Clemens F., Lopez-Sendon J.L. The impact of guideline compliant medical therapy on clinical outcome in patients with stable angina: findings from the Euro Heart Survey of stable angina. Eur Heart J. 2006;27:1298–1304. doi: 10.1093/eurheartj/ehl005. [DOI] [PubMed] [Google Scholar]
- 3.Chenniappan M., Udaysankar R. Metabolic management of Cad→ a way to have a healthy heart. Medicine (Baltimore) 2011;39 [Google Scholar]
- 4.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Narayanan S., Bhuvaneswaran J.S. Early metabolic intervention in the management of coronary artery disease. Medicine (Baltimore) 2008;18 [Google Scholar]
- 6.Marzilli M. Trimetazidine: a metabolic agent for the treatment of stable angina. Eur Heart J Suppl. 2001;3:O12–O15. [Google Scholar]
- 7.Wang W., Lopaschuk G.D. Metabolic therapy for the treatment of ischemic heart disease: reality and expectations. Expert Rev Cardiovasc Ther. 2007;5:1123–1134. doi: 10.1586/14779072.5.6.1123. [DOI] [PubMed] [Google Scholar]
- 8.Rosano G.M., Spoletini I., Vitale C. Evaluating the metabolic approach to treatment of diabetic coronary patients. IJC Metab Endocr. 2013;1:4–6. [Google Scholar]
- 9.Lopaschuk G.D., Stanley W.C. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 10.Fragasso G., Cuko A., Salerno A. The role of trimetazidine in heart failure. Arch Med Sci. 2007;3:S45–S51. [Google Scholar]
- 11.Doenst T., Nguyen T.D., Abel E.D. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragasso G., Perseghin G., De Cobelli F. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–948. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]
- 13.Detry J.M., Sellier P., Pennaforte S. Trimetazidine: a new concept in the treatment of angina. Comparison with propranolol in patients with stable angina. Trimetazidine European Multicenter Study Group. Br J Clin Pharmacol. 1994;37:279–288. doi: 10.1111/j.1365-2125.1994.tb04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passeron J. Effectiveness of trimetazidine in stable effort angina due to chronic coronary insufficiency. A double-blind versus placebo study. Presse Med. 1986;15:1775–1778. [PubMed] [Google Scholar]
- 15.Koylan N., Bilge A.K., Adalet K. Comparison of the effects of trimetazidine and diltiazem on exercise performance in patients with coronary heart disease. The Turkish trimetazidine study (TTS) Acta Cardiol. 2004;59:644–650. doi: 10.2143/AC.59.6.2005248. [DOI] [PubMed] [Google Scholar]
- 16.Szwed H., Pachocki R., Domzal-Bochenska M. Efficacy and tolerance of trimetazidine, a metabolic antianginal, in combination with a hemodynamic antianginal in stable exertion angina. TRIMPOL I, a multicenter study. Presse Med. 2000;29:533–538. [PubMed] [Google Scholar]
- 17.Szwed H., Sadowski Z., Elikowski W. Combination treatment in stable effort angina using trimetazidine and metoprolol: results of a randomized, double-blind, multicentre study (TRIMPOL II). TRIMetazidine in POLand. Eur Heart J. 2001;22:2267–2274. doi: 10.1053/euhj.2001.2896. [DOI] [PubMed] [Google Scholar]
- 18.Chazov E.I., Lepakchin V.K., Zharova E.A. Trimetazidine in Angina Combination Therapy – the TACT study: trimetazidine versus conventional treatment in patients with stable angina pectoris in a randomized, placebo-controlled, multicenter study. Am J Ther. 2005;12:35–42. doi: 10.1097/00045391-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Iyengar S.S., Rosano G.M. Effect of antianginal drugs in stable angina on predicted mortality risk after surviving a myocardial infarction: a preliminary study (METRO) Am J Cardiovasc Drugs. 2009;9:293–297. doi: 10.2165/11316840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Manchanda S., Krishnaswami S. Combination treatment with trimetazidine and diltiazem in stable angina pectoris. Heart. 1997;78:353–357. doi: 10.1136/hrt.78.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manchanda S.C. Treatment of stable angina with low dose diltiazem in combination with the metabolic agent trimetazidine. Int J Cardiol. 2003;88:83–89. doi: 10.1016/s0167-5273(02)00367-4. [DOI] [PubMed] [Google Scholar]
- 22.Rosano G.M., Vitale C., Sposato B. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol. 2003;2:16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marazzi G., Gebara O., Vitale C. Effect of trimetazidine on quality of life in elderly patients with ischemic dilated cardiomyopathy. Adv Ther. 2009;26:455–461. doi: 10.1007/s12325-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 24.Vitale C., Spoletini I., Malorni W. Efficacy of trimetazidine on functional capacity in symptomatic patients with stable exertional angina – the VASCO-angina study. Int J Cardiol. 2013;168:1078–1081. doi: 10.1016/j.ijcard.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Danchin N., Marzilli M., Parkhomenko A. Efficacy comparison of trimetazidine with therapeutic alternatives in stable angina pectoris: a network meta-analysis. Cardiology. 2011;120:59–72. doi: 10.1159/000332369. [DOI] [PubMed] [Google Scholar]
- 26.Peng S., Zhao M., Wan J. The efficacy of trimetazidine on stable angina pectoris: a meta-analysis of randomized clinical trials. Int J Cardiol. 2014;177:780–785. doi: 10.1016/j.ijcard.2014.10.149. [DOI] [PubMed] [Google Scholar]
- 27.Montalescot G., Sechtem U., Achenbach S. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 28.Brottier L., Barat J.L., Combe C. Therapeutic value of a cardioprotective agent in patients with severe ischaemic cardiomyopathy. Eur Heart J. 1990;11:207–212. doi: 10.1093/oxfordjournals.eurheartj.a059685. [DOI] [PubMed] [Google Scholar]
- 29.Belardinelli R., Purcaro A. Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischaemic cardiomyopathy. Eur Heart J. 2001;22:2164–2170. doi: 10.1053/euhj.2001.2653. [DOI] [PubMed] [Google Scholar]
- 30.Fragasso G., Palloshi A., Puccetti P. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006;48:992–998. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 31.Gao D., Ning N., Niu X. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–286. doi: 10.1136/hrt.2010.208751. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Lu Y., Jiang H. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol. 2012;59:913–922. doi: 10.1016/j.jacc.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 33.El-Kady T., El-Sabban K., Gabaly M. Effects of trimetazidine on myocardial perfusion and the contractile response of chronically dysfunctional myocardium in ischemic cardiomyopathy: a 24-month study. Am J Cardiovasc Drugs. 2005;5:271–278. doi: 10.2165/00129784-200505040-00006. [DOI] [PubMed] [Google Scholar]
- 34.Fragasso G., Rosano G., Baek S.H. Effect of partial fatty acid oxidation inhibition with trimetazidine on mortality and morbidity in heart failure: results from an international multicentre retrospective cohort study. Int J Cardiol. 2013;163:320–325. doi: 10.1016/j.ijcard.2012.09.123. [DOI] [PubMed] [Google Scholar]
- 35.Dalal J.J. Trimetazidine effects on cardiac biomarkers in acute coronary syndrome. Heart Metab. 2015;67:26–29. [Google Scholar]
- 36.Shehata M. Cardioprotective effects of oral trimetazidine in diabetic patients with anterior wall myocardial infarction treated with thrombolysis. Cardiol Res. 2014;5:58–67. doi: 10.14740/cr330w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonello L., Sbragia P., Amabile N. Protective effect of an acute oral loading dose of trimetazidine on myocardial injury following percutaneous coronary intervention. Heart. 2007;93:703–707. doi: 10.1136/hrt.2006.107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y.D., Zhao L.K., Tian F. Evaluation of the myocardial protection of trimetazidine during percutaneous coronary intervention: a multi-center randomized and controlled clinical study. Zhonghua Nei Ke Za Zhi. 2010;49:473–476. [PubMed] [Google Scholar]
- 39.Poloński L., Dec I., Wojnar R. Trimetazidine limits the effects of myocardial ischaemia during percutaneous coronary angioplasty. Curr Med Res Opin. 2002;18:389–396. doi: 10.1185/030079902125001146. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y., Ma X.J., Shi D.Z. Effect of trimetazidine in patients undergoing percutaneous coronary intervention: a meta-analysis. PLOS ONE. 2015;10:e0137775. doi: 10.1371/journal.pone.0137775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J.J., Ma Z.M., Ren W.L. Clinical outcomes of trimetazidine in patients with acute ST segment elevation myocardial infarction without ST segment resolution after primary percutaneous coronary intervention. Zhonghua Yi Xue Za Zhi. 2009;89:1399–1401. [PubMed] [Google Scholar]
- 42.Birand A., Kudaiberdieva G.Z., Batyraliev T.A. Effects of trimetazidine on heart rate variability and left ventricular systolic performance in patients with coronary artery disease after percutaneous transluminal angioplasty. Angiology. 1997;48:413–422. doi: 10.1177/000331979704800505. [DOI] [PubMed] [Google Scholar]
- 43.Xu X., Zhang W., Zhou Y. Effect of trimetazidine on recurrent angina pectoris and left ventricular structure in elderly multivessel coronary heart disease patients with diabetes mellitus after drug-eluting stent implantation: a single-centre, prospective, randomized, double-blind study at 2-year follow-up. Clin Drug Investig. 2014;34:251–258. doi: 10.1007/s40261-014-0170-9. [DOI] [PubMed] [Google Scholar]
- 44.Chen J., Zhou S., Jin J. Chronic treatment with trimetazidine after discharge reduces the incidence of restenosis in patients who received coronary stent implantation: a 1-year prospective follow-up study. Int J Cardiol. 2014;174:634–639. doi: 10.1016/j.ijcard.2014.04.168. [DOI] [PubMed] [Google Scholar]
- 45.Tünerir B., Colak O., Alataş O. Measurement of troponin T to detect cardioprotective effect of trimetazidine during coronary artery bypass grafting. Ann Thorac Surg. 1999;68:2173–2176. doi: 10.1016/s0003-4975(99)01126-1. [DOI] [PubMed] [Google Scholar]
- 46.Martins G.F., Siqueira Filho A.G., Santos J.B. Trimetazidine on ischemic injury and reperfusion in coronary artery bypass grafting. Arq Bras Cardiol. 2011;97:209–216. doi: 10.1590/s0066-782x2011005000079. [DOI] [PubMed] [Google Scholar]
- 47.Vedrinne J.M., Vedrinne C., Bompard D. Myocardial protection during coronary artery bypass graft surgery: a randomized, double-blind, placebo-controlled study with trimetazidine. Anesth Analg. 1996;82:712–718. doi: 10.1097/00000539-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang N., Lei J., Liu Q. The effectiveness of preoperative trimetazidine on myocardial preservation in coronary artery bypass graft patients: a systematic review and meta-analysis. Cardiology. 2015;131:86–96. doi: 10.1159/000375289. [DOI] [PubMed] [Google Scholar]
- 49.Undrovinas A.I., Belardinelli L., Undrovinas N.A. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson J.R., Nawarskas J.J. Ranolazine. A metabolic modulator for the treatment of chronic stable angina. Cardiol Rev. 2005;13:202–210. doi: 10.1097/01.crd.0000161979.62749.e7. [DOI] [PubMed] [Google Scholar]
- 51.Chaitman B.R. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 52.Chaitman B.R., Skettino S.L., Parker J.O. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375–1382. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 53.Chaitman B.R., Pepine C.J., Parker J.O. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 54.Stone P.H., Gratsiansky N.A., Blokhin A. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–575. doi: 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 55.Arnold S.V., Morrow D.A., Wang K. Effects of ranolazine on disease-specific health status and quality of life among patients with acute coronary syndromes: results from the MERLIN-TIMI 36 randomized trial. Circ Cardiovasc Qual Outcomes. 2008;1:107–115. doi: 10.1161/CIRCOUTCOMES.108.798009. [DOI] [PubMed] [Google Scholar]
- 56.Kosiborod M., Arnold S.V., Spertus J.A. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) J Am Coll Cardiol. 2013;61:2038–2045. doi: 10.1016/j.jacc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Koren M.J., Crager M.R., Sweeney M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE) J Am Coll Cardiol. 2007;49:1027–1034. doi: 10.1016/j.jacc.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 58.Maier L.S., Layug B., Karwatowska-Prokopczuk E. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Murray G.L., Colombo J. Ranolazine preserves and improves left ventricular ejection fraction and autonomic measures when added to guideline-driven therapy in chronic heart failure. Heart Int. 2014;9:66–73. doi: 10.5301/heartint.5000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grossman A.N., Opie L.H., Beshansky J.R. Glucose-insulin-potassium revived: current status in acute coronary syndromes and the energy-depleted heart. Circulation. 2013;127:1040–1048. doi: 10.1161/CIRCULATIONAHA.112.130625. [DOI] [PubMed] [Google Scholar]
- 61.Wolff A.A., Rotmensch H.H., Stanley W.C. Metabolic approaches to the treatment of ischemic heart disease: the clinicians’ perspective. Heart Fail Rev. 2002;7:187–203. doi: 10.1023/a:1015384710373. [DOI] [PubMed] [Google Scholar]
- 62.Holubarsch C.J., Rohrbach M., Karrasch M. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 63.Broderick T.L., Quinney H.A., Barker C.C. Beneficial effect of carnitine on mechanical recovery of rat hearts reperfused after a transient period of global ischemia is accompanied by a stimulation of glucose oxidation. Circulation. 1993;87:972–981. doi: 10.1161/01.cir.87.3.972. [DOI] [PubMed] [Google Scholar]
- 64.DiNicolantonio J.J., Lavie C.J., Fares H. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol) Am J Cardiol. 2013;111:765–769. doi: 10.1016/j.amjcard.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 65.SmPC VASTAREL 35 mg, modified-release film-coated tablet. SERVIER. Updated on Sep 2012. Available at http://www.servier.com.ve/sites/default/files/spc-pil/spc-vastarel.pdf (Visited on 13 Oct 2016).
- 66.SmPC VASTAREL 20 mg, film-coated tablet. SERVIER. Updated on Feb 2014. Available at http://www.servier.com/sites/default/files/RCP_Vastarel20.pdf (Visited on 13 Oct 2016).
- 67.USPI RANEXA® (ranolazine) extended-release tablets. Gilead Sciences. Updated on Jan 2016. Available at http://www.gilead.com/∼/media/files/pdfs/medicines/cardiovascular/ranexa/ranexa_pi.pdf (Visited on 13 Oct 2016).
- 68.SmPC Ranexa 375 mg, 500 mg and 750 mg (ranolazine) prolonged-release tablets. Menarini International Operations. Updated on Mar 2013. Available at https://www.medicines.org.uk/emc/print-document?documentId=21402 (Visited on 13 Oct 2016).
- 69.Mishra S., Ray S., Dalal J.J. Management protocols of stable coronary artery disease in India: executive summary. Indian Heart J. 2016;68:868–873. doi: 10.1016/j.ihj.2016.11.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yaman M., Arslan U., Gümrükçüoğlu H.A., Şahin M., Şimşek H., Akdağ S. Effects of trimetazidine on T wave alternans in stable coronary artery disease. Korean Circ J. 2016;46(May (3)):343–349. doi: 10.4070/kcj.2016.46.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Momen A., Ali M., Karmakar P.K. Effects of sustained-release trimetazidine on chronically dysfunctional myocardium of ischemic dilated cardiomyopathy – six months follow-up result. Indian Heart J. 2016;68:809–815. doi: 10.1016/j.ihj.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jatain S., Kapoor A., Sinha A. Metabolic manipulation in dilated cardiomyopathy: assessing the role of trimetazidine. Indian Heart J. 2016;68:803–808. doi: 10.1016/j.ihj.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gutierrez J.A., Karwatowska-Prokopczuk E., Murphy S.A. Effects of ranolazine in patients with chronic angina in patients with and without percutaneous coronary intervention for acute coronary syndrome: observations from the MERLIN-TIMI 36 trial. Clin Cardiol. 2015;38:469–475. doi: 10.1002/clc.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Babalis D., Tritakis V., Floros G. Effects of ranolazine on left ventricular diastolic and systolic function in patients with chronic coronary disease and stable angina. Hellenic J Cardiol. 2015;56:237–241. [PubMed] [Google Scholar]