Abstract
Background
Pioglitazone is a safe and effective option to manage patients with type 2 diabetes and nonalcoholic steatohepatitis (NASH). However, there is marked variability in treatment response.
Aim
To evaluate the relationship between concentrations of pioglitazone and its active metabolites and treatment outcomes in patients with NASH.
Methods
Pioglitazone concentrations were measured in patients with NASH treated with pioglitazone 45 mg/day for 18 months; liver biopsy samples were obtained at baseline and after treatment. The primary outcome was a ≥ 2-point reduction in NAFLD activity score (NAS) with at least one-point improvement in more than one liver histology category and without worsening of fibrosis. A novel marker, the pioglitazone exposure index, was calculated to consider the concentrations of pioglitazone as well as the two active metabolites.
Results
The response to pioglitazone was concentration-dependent as evidenced by the significant relationship between both pioglitazone concentration and pioglitazone exposure index with changes in NAS (r=0.48, p=0.0002 and r=0.51, p<0.0001, respectively), steatosis (r=0.41, p=0.002 and r=0.46, p=0.0005), and inflammation (r=0.44, p=0.0009 and r=0.40, p=0.0003). The pioglitazone exposure index was also associated with a change in ballooning (p=0.04). The pioglitazone exposure index was higher in patients with NASH resolution (2.85 ± 1.38 vs. 1.78 ± 1.48, p=0.018). A predictive model for the primary outcome was developed that incorporated baseline NAS and pioglitazone exposure index (AUC = 0.77).
Conclusion
This study demonstrates the importance of pioglitazone exposure to variable response in patients with NASH and identifies potential factors that may identify patients most likely to benefit from chronic pioglitazone treatment.
Keywords: NAFLD, NASH, pioglitazone, hydroxypioglitazone, ketopioglitazone
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, with a prevalence that is increasing in parallel with the obesity epidemic in both the United States and worldwide.1–3 In a study that used the sensitive and quantitative technique proton-magnetic resonance spectroscopy to measure hepatic triglyceride content, the prevalence of hepatic steatosis was 31%.4 The established risk factors for NAFLD are obesity, type 2 diabetes, and metabolic syndrome.5 Nonalcoholic fatty liver disease varies from relatively benign isolated steatosis, characterized by the accumulation of fat in the liver, to nonalcoholic steatohepatitis (NASH), where hepatic steatosis and inflammation with ballooning injury is present with or without fibrosis.5, 6 Nonalcoholic steatohepatitis can further progress to liver cirrhosis and hepatocellular carcinoma5 and is a rapidly growing indication for liver transplantation.7
Currently, the standard of care is lifestyle modification.5 The use of pioglitazone for NASH may be recommended based on several clinical studies showing improvement in liver histology scores and surrogate biomarkers.5, 8–12 However, limitations include that the response to pioglitazone is variable and improvement is not achieved in all treated patients. For example, in the PIVENS trial, 34% and 47% of the patients in the pioglitazone arm achieved the primary outcome and resolution of NASH, respectively.13 The reason why NASH improves in some but not all patients treated with pioglitazone is not understood. Thus, it is important to understand possible factors that may identify patients who are or are not likely to improve with pioglitazone treatment to optimize pharmacotherapy. These factors can help clinicians evaluate the benefit and risk of the treatment and assist in making therapeutic decisions. Currently, it is not known whether pioglitazone concentrations and other baseline characteristics are predictive of clinical outcome.
The relationship between pioglitazone plasma concentration and improvement in NAFLD has not been established. We hypothesized that the drug concentration achieved differs among patients with NAFLD and that the difference in the drug concentrations contributes to variability in clinical outcomes. Pioglitazone is metabolized in the liver mainly by CYP2C8 and to a lesser extent by CYP3A4 to two major active metabolites, hydroxypioglitazone (also known as M-IV) and ketopioglitazone (M-III).14, 15 The role of the active metabolites in the treatment of NAFLD is also unknown. The objectives of this study were to establish the pioglitazone concentration-response relationship in patients with NAFLD and to build a potential model that includes drug concentration and baseline characteristics to predict clinical outcomes for patients with NAFLD.
Methods
Study Population and Design
Pioglitazone, hydroxypioglitazone, and ketopioglitazone concentrations were measured in samples obtained from 54 participants in a clinical study (ClinicalTrials.gov NCT00994682).10 There were 36 patients from the initial randomized, placebo-controlled, blinded phase and 18 patients from the subsequent open-label phase where all patients received pioglitazone. A detailed description of the trial design and complete inclusion/exclusion criteria were previously reported.10 Briefly, all study participants received chronic pioglitazone treatment (ACTOS®, Takeda Pharmaceuticals, Deerfield, IL) for 18 months. The initial dose of 30 mg/day was titrated to 45 mg/day after 2 months, and the dose continued at 45 mg/day for the remaining 16 months of the study. The overall adherence to pioglitazone during the study was reported to be about 95%.10 The blood sample for drug concentration measurement was obtained in month 18 of pioglitazone treatment in the morning prior to the next dose. A baseline liver biopsy sample was required from all participants. Patients with type 1 diabetes, patients who had taken a thiazolidinedione, and patients with other etiologies of liver disease were excluded. A follow-up liver biopsy was conducted after 18 months of pioglitazone therapy. In addition to pioglitazone, all study participants were prescribed a hypocaloric diet (−500 kcal/day) by a research dietician, and counseling on physical activity was provided as a part of the standard of care. Reinforcement of the lifestyle modification was made at follow-up visits every 1–2 months. The research protocol was approved by the local institutional review board, and all participants granted written informed consent prior to their participation.
The primary outcome was defined as a ≥ 2-point reduction in NAFLD activity score (NAS)16 with at least a one-point improvement in more than one liver histology category and without worsening of fibrosis. Resolution of NASH, individual liver biopsy score (steatosis, inflammation, ballooning, and fibrosis), and NAS were also examined as secondary outcomes.
Analytic Procedures and Measurements
Plasma glucose was measured in the Clinical Research Center by the glucose oxidase method (Analox Glucose Analyzer, Analox Instruments, Lunenburg, MA). For other analyses, blood samples were placed on ice at the bedside, processed within 15–20 minutes, and frozen at −80°C until the samples were analyzed. Blood samples for the analysis of drug concentration were collected prior to the morning dose, and it was assured that patients were on chronic pioglitazone and that the drug concentration was at steady-state. A novel liquid chromatography tandem mass spectrometry assay described previously was used to measure plasma concentrations of pioglitazone, hydroxypioglitazone, and ketopioglitazone.17 The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from a single measurement of fasting insulin and glucose. Plasma insulin was determined by radioimmunoassay (Siemens, Los Angeles, CA). Adiponectin concentrations were measured by magnetic bead Milliplex technology (Luminex xMAP, Millipore, St. Charles, MO), and CK-18 levels were quantified by ELISA (M30-Apoptosense, DiaPharma, Columbus, OH).
Statistical Analyses
The Mann-Whitney test was used to compare drug concentrations between patients who achieved and did not achieve the primary outcome and NASH resolution. The paired t-test was used to compare the baseline and end-of-treatment biopsy results. Linear regression was used to calculate r2 values and to evaluate the association of drug concentration with liver biopsy scores. Multiple regression with stepwise selection was used to build predictive models for the primary outcome. In the multiple regression, covariates included in the analysis were: pioglitazone concentration, pioglitazone exposure index, baseline HOMA-IR, baseline alanine aminotransferase (ALT), baseline CK-18, baseline NAS, baseline fibrosis score, and other patient characteristics, including age, gender, BMI, presence of diabetes, presence of metabolic syndrome, and ethnicity. The ethnicity was self-identified and classified into either Caucasians, Hispanics, or Others. The pioglitazone exposure index was calculated as shown in Equation 1, where 356.4, 372.5, and 370.4 represent the molecular weights of pioglitazone, hydroxypioglitazone, and ketopioglitazone, respectively, and where 0.58 and 0.41 are factors to estimate relative potency of the metabolites based on a previous report.18 In addition, receiver operating characteristics (ROC) curves were used to assess the sensitivity and specificity of the final models. JMP version 10.0.0 (SAS Institute Inc, Cary, NC) was used to perform the statistical analyses.
| (Equation 1) |
Results
Baseline and follow-up biopsy samples were available in 54 participants. The baseline characteristics of the 54 participants are summarized in Table 1. The majority of participants were Hispanic males. The average steady-state pioglitazone, hydroxypioglitazone, and ketopioglitazone concentrations were 307.8 ± 357.8 ng/mL, 822.3 ± 437.7 ng/mL, and 263.8 ± 147.7 ng/mL respectively (mean ± standard deviation). The calculated pioglitazone exposure index was 2.43 ± 1.44. The measured drug concentrations were markedly variable among these patients with NAFLD.
Table 1.
Baseline characteristics of study participants (N=54).
| Characteristic | Percentage or Mean ± Standard Deviation |
|---|---|
| Age (years) | 54 ± 9 |
| Gender | Male 78%, Female 22% |
| Ethnicity | Caucasian 26%, Hispanic 61%, Other 13% |
| Body mass index (kg/m2) | 33.8 ± 4.4 |
| Type 2 diabetes | 56% |
| Metabolic syndrome | 89% |
| Fasting plasma glucose (mg/dl) | 122.7 ± 27.2 |
| Hemoglobin A1c (%) | 6.4 ± 0.9 |
| HOMA-IR | 4.6 ± 3.3 |
| Plasma ALT (IU/L) | 65 ± 40 |
| Plasma AST (IU/L) | 51 ± 35 |
| CK-18 (IU/L) | 353 ± 297 |
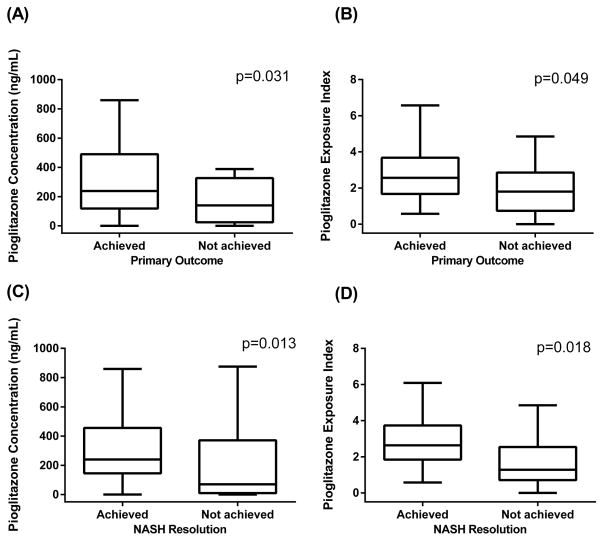
After 18 months of pioglitazone treatment, 33 (61%) patients achieved the primary outcome. Among 44 patients with confirmed definite NASH at baseline, 30 (68%) patients achieved NASH resolution. Figure 1 depicts significant differences in pioglitazone concentrations and pioglitazone exposure indices between patients who achieved the primary outcome and who did not [(A) and (B) respectively]. The mean (± SD) pioglitazone concentration was 380.7 ± 412.7 ng/mL in participants who achieved the primary outcome and 193.2 ± 209.9 ng/mL in participants who did not (p=0.031). Similarly, the average pioglitazone concentration was 387.7 ± 420.6 ng/mL in participants who achieved the resolution of NASH and 182.5 ± 247.1 ng/mL in participants who did not (p=0.013). Among participants who achieved and did not achieve the primary outcome, the pioglitazone exposure indices were 2.77 ± 1.39 and 1.91 ± 1.39 respectively (p=0.049). Among participants who achieved and did not achieve the resolution of NASH, the pioglitazone exposure indices were 2.85 ± 1.38 and 1.78 ± 1.48 respectively (p=0.018).
Figure 1.
Distribution of pioglitazone concentration and pioglitazone exposure index by achievement of the primary outcome [(A) and (B) respectively] and of NASH resolution [(C) and (D) respectively]. The box plots were drawn with Tukey method; the whiskers represent 75th percentile plus 1.5 times the interquartile range (IQR) and 25th percentile minus 1.5 times IQR.
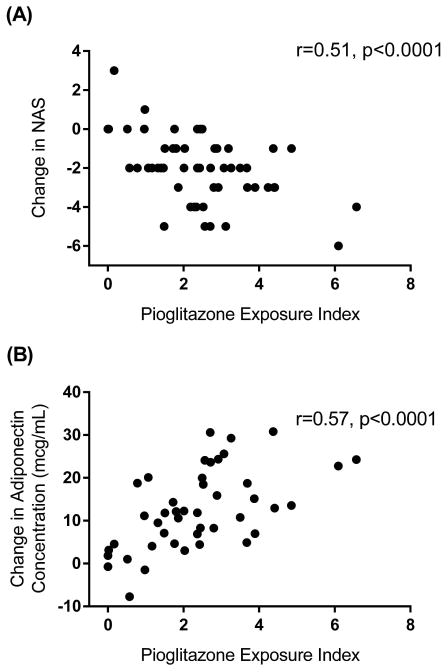
The liver biopsy results at baseline and after 18 months of pioglitazone treatment in these 54 patients are consistent with those in the full trial (Table S1).10 The relationship between changes in NAS and pioglitazone exposure indices and between changes in adiponectin concentrations and pioglitazone exposure indices are described in Figure 2(A) and (B) respectively. The pioglitazone concentration and the pioglitazone exposure index were significantly associated with changes in NAS (r=0.48, p=0.0002 and r=0.51, p<0.0001), steatosis (r=0.41, p=0.002 and r=0.46, p=0.0005), and inflammation (r=0.44, p=0.0009 and r=0.40, p=0.0003) after 18 months of therapy. Although pioglitazone concentration did not explain changes in ballooning, pioglitazone exposure index was significantly associated with changes in ballooning (r=0.28, p=0.04). Similarly, a concentration-dependent response was observed for change in adiponectin concentration with pioglitazone exposure index (r=0.40, p=0.038), but pioglitazone drug concentration alone was not significantly associated with the change in adiponectin concentration. Change in fibrosis was not associated with drug concentrations.
Figure 2.
The pharmacodynamic relationship between pioglitazone exposure index, which takes into account the concentrations of the parent drug and the two active metabolites, hydroxypioglitazone and ketopioglitazone (A). The pharmacodynamic response was also observed with change in adiponectin concentration (B).
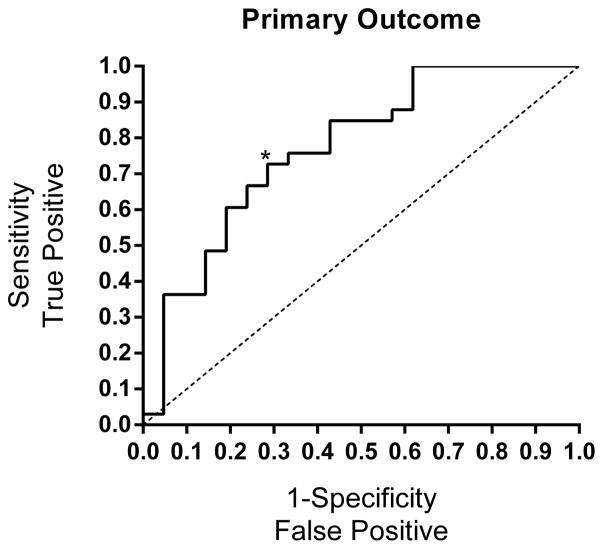
In the multiple regression analysis, the parameters that were significant and included in the final model are summarized in Table 2. For the primary outcome, the only significant factors predicting the response were baseline NAS and pioglitazone exposure index. Pioglitazone exposure index was a better determinant of response than pioglitazone concentration alone. The performance of the model to predict the primary outcome is described in Table 3. The area under the ROC curve was 0.77 for the primary outcome model (Figure 3).
Table 2.
Summary of parameters for the final model to predict the primary outcome (OR = odds ratio; 95%CI = 95% confidence interval of the OR).
| Outcome | Parameter | OR or Beta | Lower 95%CI | Upper 95%CI | Total R2 | p-value |
|---|---|---|---|---|---|---|
| Primary outcome | Baseline NAS | 2.01 | 1.24 | 3.54 | 0.117 | 0.008 |
| Pioglitazone exposure index | 1.69 | 1.06 | 2.96 | 0.186 | 0.042 |
Table 3.
ROC table for the primary outcome and NAS response. PPV = positive predictive value. NPV = negative predictive value.
| Outcome | Parameters | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Primary outcome | Baseline NAS | 72.7% | 71.4% | 80.0% | 57.7% |
| Pioglitazone exposure index |
Figure 3.
ROC curves for the primary outcome. Regressors included were baseline NAS and pioglitazone exposure index. The asterisk represents the optimal sensitivity and 1-specificity for the model.
Discussion
This is the first study to characterize the concentration-dependent response to pioglitazone in patients with NAFLD. Pioglitazone concentration and the novel pioglitazone exposure index, which incorporates concentrations of the two major active metabolites hydroxypioglitazone and ketopioglitazone, were shown to be associated with changes in NAS, steatosis, and inflammation from the liver biopsy results in patients with NAFLD. The pioglitazone exposure index also associated with change in ballooning. Similarly, higher pioglitazone concentration and higher pioglitazone exposure index were more likely to be observed in patients who achieved the primary outcome and in patients with NASH resolution. Baseline NAS and pioglitazone exposure index were significant factors to predict achievement of the primary outcome. The model including baseline NAS and pioglitazone exposure index to predict achievement of the primary outcome had 73% sensitivity and 71% specificity.
Both pioglitazone concentrations and the pioglitazone exposure index were shown in this study to differ significantly between patients who achieved and did not achieve the primary outcome and also between patients with and without resolution of NASH. However, the pioglitazone exposure index, which takes into account concentrations of both major active metabolite and the parent drug concentration, correlated better with changes in NAS, steatosis, and ballooning than pioglitazone concentration alone. This was also observed with adiponectin concentrations. Pioglitazone treatment has been shown to cause a more than 2-fold increase in adiponectin concentrations,19, 20 and in this study, the pioglitazone exposure index but not pioglitazone concentrations associated with the net change in adiponectin concentrations. The regression results similarly indicate that inclusion of the pioglitazone exposure index was preferred to that of pioglitazone concentration to build the predictive model of the primary outcome. The better association with the exposure index is likely because with chronic dosing, pioglitazone only accounts for approximately 25% of circulating active drug compounds; the active metabolites accumulate more in plasma than pioglitazone due to much longer elimination half-lives.21
Several factors may have contributed to the observed variability in pioglitazone and metabolite plasma concentrations. The variability (CV%) in pioglitazone clearance reported in the literature is typically about 40–50%, yielding a range in exposure (AUC) that is up to 10-fold.21, 22 Genetic polymorphisms in CYP2C8 contribute to variability in pioglitazone metabolism. For example, the CYP2C8*3 haplotype is associated with increased metabolism of pioglitazone, yielding faster clearance and lower systemic pioglitazone concentrations.23 This variant haplotype is found in Whites and Hispanics, but is not found in African-Americans. Additionally, NASH and other inflammatory conditions affect the expression of CYP2C8,24 while factors that do not appear to contribute to variability in CYP2C8-mediated metabolism include age and gender, which were shown to have no effect on CYP2C8 mRNA or protein expression in human liver.25
A limitation of this study is that the exact drug administration time was not available, which may contribute to the observed variability in drug concentrations. However, even though the exact time since the last dose administered in each patient was unknown, the blood sample was taken prior to the pre-morning dose, and fluctuations in drug concentrations around the trough were considered to be smaller. Additionally, adherence to drug treatment was confirmed to be high. The elimination half-life of pioglitazone is relatively short, ranging from 3–7 hours, but for the metabolites are longer, ranging from 16–24 hours.26 Thus, measuring the metabolite concentrations as a part of the pioglitazone exposure index provided a more stable measure of drug exposure. Another constraint is the limited number of factors considered to explain the outcomes. Although various baseline patient characteristics, as well as drug concentrations and biomarker levels, were considered, the best model from this study explained 18.6% of the total variability in the primary outcome. The possibility of other environmental and patient factors affecting the outcome cannot be ruled out, and further studies are warranted in a larger cohort.
In conclusion, the results of this study demonstrate that pioglitazone exposure is an important modulator of response in patients with NAFLD. Concentrations of pioglitazone and its major active metabolites were measured for the first time in this patient population, and marked variability was observed after chronic administration. The baseline NAS and pioglitazone exposure index explained inter-patient variability of drug response to a significant extent and were shown to be significant factors predicting the clinical outcomes. The pioglitazone exposure index may hold potential as a valuable marker to predict clinical response in patients with NAFLD. It may also have utility for individualizing pioglitazone therapy, which warrants further study.
Supplementary Material
Acknowledgments
Funding support: The work was supported in part by the Burroughs Wellcome Fund (KC) and the American Diabetes Association (KC). Additional support was provided by the National Center for Research Resources (MO1-RR-01346), the UTHSCSA Clinical Research Center and Research Imaging Center, and the Veterans Affairs Medical Research Fund.
Abbreviations
- CK-18
cytokeratin-18
- HOMA-IR
homeostasis model assessment of insulin resistance
- IQR
interquartile range
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- ROC
receiver operating characteristics
- 95%CI
95% confidence interval
Footnotes
Disclosures: The authors disclose no conflicts.
Writing assistance: None
Author Contributions: Guarantor of the article: Reginald F. Frye. MKS, FB, KC, and RF analyzed and interpreted the data and drafted the manuscript. All authors were involved in conceptualizing and designing the study, acquiring data, critically revising the manuscript for important intellectual content, and/or obtaining funding.
References
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28(4):339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107(6):811–26. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 6.Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic Fatty liver disease: current issues and novel treatment approaches. Drugs. 2013;73(1):1–14. doi: 10.1007/s40265-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 7.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–95. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 8.Malinowski SS, Byrd JS, Bell AM, Wofford MR, Riche DM. Pharmacologic therapy for nonalcoholic fatty liver disease in adults. Pharmacother. 2013;33(2):223–42. doi: 10.1002/phar.1190. [DOI] [PubMed] [Google Scholar]
- 9.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 10.Cusi K, Orsak B, Bril F, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165(5):305–15. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 11.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakoski MO, Singal AG, Rogers MAM, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32(10):1211–1221. doi: 10.1111/j.1365-2036.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. New Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckland DA, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocr Diab. 2000;108:S234–S242. [Google Scholar]
- 15.Lai XS, Yang LP, Li XT, Liu JP, Zhou ZW, Zhou SF. Human CYP2C8: structure, substrate specificity, inhibitor selectivity, inducers and polymorphisms. Curr Drug Metab. 2009;10(9):1009–47. doi: 10.2174/138920009790711832. [DOI] [PubMed] [Google Scholar]
- 16.Hjelkrem M, Stauch C, Shaw J, Harrison SA. Validation of the non-alcoholic fatty liver disease activity score. Aliment Pharmacol Ther. 2011;34(2):214–218. doi: 10.1111/j.1365-2036.2011.04695.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi-Suzuki M, Bril F, Sanchez PP, Cusi K, Frye RF. A validated liquid chromatography tandem mass spectrometry method for simultaneous determination of pioglitazone, hydroxypioglitazone, and ketopioglitazone in human plasma and its application to a clinical study. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:219–23. doi: 10.1016/j.jchromb.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Actos (Pioglitazone Hydrochloride) Tablets Pharmacology Review. U.S. Food and Drug Administration; [Accessed: March 13, 2017]. Published: March 30, 2001. [Google Scholar]
- 19.Gastaldelli A, Harrison S, Belfort-Aguiar R, et al. Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment Pharmacol Ther. 2010;32(6):769–775. doi: 10.1111/j.1365-2036.2010.04405.x. [DOI] [PubMed] [Google Scholar]
- 20.Otto C, Otto B, Goke B, et al. Increase in adiponectin levels during pioglitazone therapy in relation to glucose control, insulin resistance as well as ghrelin and resistin levels. J Endocrinol Invest. 2006;29(3):231–6. doi: 10.1007/BF03345545. [DOI] [PubMed] [Google Scholar]
- 21.Christensen ML, Meibohm B, Capparelli EV, Velasquez-Mieyer P, Burghen GA, Tamborlane WV. Single- and multiple-dose pharmacokinetics of pioglitazone in adolescents with type 2 diabetes. J Clin Pharmacol. 2005;45(10):1137–44. doi: 10.1177/0091270005279578. [DOI] [PubMed] [Google Scholar]
- 22.Eckland D, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocr Diab. 2000;108(Sup 2):234–242. [Google Scholar]
- 23.Kawaguchi-Suzuki M, Frye RF. Current clinical evidence on pioglitazone pharmacogenomics. Front Pharmacol. 2013;4:147. doi: 10.3389/fphar.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher CD, Lickteig AJ, Augustine LM, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087–94. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naraharisetti SB, Lin YS, Rieder MJ, et al. Human liver expression of CYP2C8: gender, age, and genotype effects. Drug Metab Dispos. 2010;38(6):889–93. doi: 10.1124/dmd.109.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACTOS (pioglitazone) Prescribing Information. Takeda Pharmaceuticals America Inc; [Accessed March 13, 2017]. http://general.takedapharm.com/content/file.aspx?filetypecode=actospi. Published 1999. Updated: December 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.