Abstract
The ricinosome (precursor protease vesicle) is an organelle found exclusively in plant cells. Ricinosomes contain a 45-kDa pro-cysteine endopeptidase (CysEP) with a C-terminal KDEL endoplasmic reticulum retention signal. CysEP is a member of a unique group of papain-type cysteine peptidases found specifically in senescing and ricinosome-containing tissues. During seed development in the castor oil plant (Ricinus communis L.), the cells of the nucellus are killed as the major seed storage organ, the cellular endosperm, expands and begins to accumulate reserves. The destruction of the maternal seed tissues is a developmentally programmed cell death. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling revealed that nuclear DNA fragmentation occurs in the nucellar cells adjacent to the expanding endosperm. These cells exhibit ultrastructural features consistent with programmed cell death, including vesiculation of the cytosol, development of irregularly shaped nuclei, vacuolar collapse, and shrinkage of the cytoplasm. Ricinosomes containing the CysEP were identified in the nucellar cells by light and electron microscopy and immunocytochemistry. Both proCysEP and mature CysEP are present in protein extracts of the nucellar tissues during seed development. Upon collapse of the nucellar cells, the content of the ricinosomes is released into the cytoplasm, where the activated CysEP digests the remaining proteinaceous cellular debris. Digestion products of the nucellar cells are presumed taken up by the outermost cells of the endosperm, which have labyrinthine ingrowths of the outer walls typical of transfer cells.
Keywords: castor bean, papain-type KDEL peptidase, seed maturation
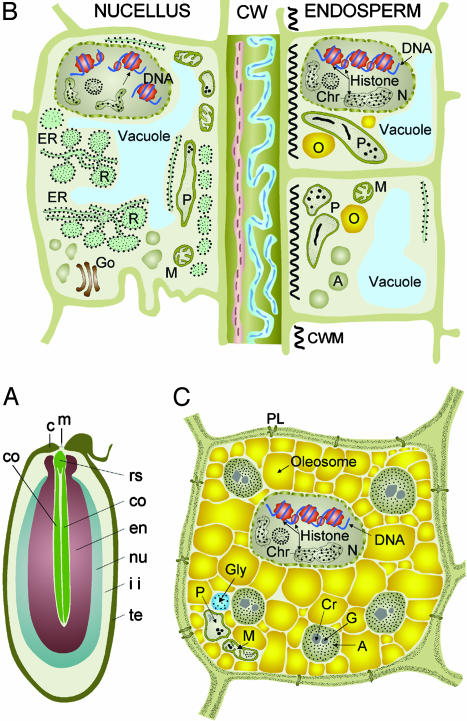
Developing angiosperm seeds provide unique opportunities to study the opposing roles of cell division and differentiation, and those of cell death, that occur in plants (1). The double fertilization of the egg and central cell leads to the formation of the embryo and endosperm (2). After fertilization, the ovule rapidly expands because of the proliferation and growth of cells of maternal tissues, the nucellus, and integuments (3). In Ricinus communis seed, the rapid growth of the maternal tissues accommodates early increases in the length of the (initially) free nuclear endosperm. At 10–25 days after pollination (DAP), the cotyledons grow through the central space of the successively expanding cellular endosperm (Fig. 1A). Filling of the endosperm cells with protein and oil starts 25–30 DAP accompanied by a 6-fold increase in amount of acetyl-CoA-carboxylase (4) and is completed at 60 DAP (Fig. 1C). By maturity, the living endosperm forms the major storage tissue surrounding the thin cotyledons of the centrally located embryo (5, 6). The expansion of the cellular endosperm takes place at the expense of the nucellus and inner integument tissues (Fig. 1 A). Degeneration of nucellar cells before fertilization (7, 8) and of both the nucellar (9, 10) and inner integument cells (11) after fertilization are considered programmed cell death (PCD) events.
Fig. 1.
Senescence and PCD of nucellus during Ricinus seed development. (A) Approximately 20 DAP. co, expanding cotyledons; rs, root–shoot axis; en, expanding endosperm; nu, nucellus undergoing PCD and elimination; ii, inner integument diminishing as endosperm expands to fill the seed; te, testa (seed coat); c, caruncle; m, micropyle. (B) Juncture between nucellus undergoing PCD and expanding endosperm. Shown in the nucellus are DNA fragmentation and a large number of ricinosomes; CW, wall remnants of eliminated nucellus cells. Shown in the endosperm are cells with developing oleosomes and aleuron storage vacuoles. CWM, cell wall material. (C) Mature endosperm cell after elimination of inner integument. A, aleuron grains; Chr, chromatin; Cr, crystalloid; G, globoid; Go, Golgi; M, mitochondria; N, nucleus; O, oleosome; P, plastid; R, ricinosome. C is reproduced from Gietl and Schmid (15) with the permission of Springer.
PCD also occurs in Ricinus seeds after germination. PCD of senescent endosperm cells is a well studied process (12–15) that occurs only after the complete mobilization of reserve proteins and lipids (16). Ricinosomes, organelles found only in plant cells, develop in the senescing endosperm cells concomitant with nuclear DNA fragmentation, a hallmark of PCD (14). Initially reported by Mollenhauer and Totten (17) and Vigil (18), ricinosomes are derived from the endoplasmic reticulum (ER). The bounding membrane of the organelle is studded with ribosomes, and the organelles contain ER resident proteins BiP and protein disulfide isomerase (14). Important to their role in PCD, ricinosomes harbor substantial amounts of a pro-cysteine endopeptidase (CysEP) with an N-terminal propeptide and C-terminal KDEL ER retention signal (13). The organelles act as suicide bombs (15) in the final stages of cellular disintegration, with CysEP being released from the ricinosomes upon vacuolar collapse and acidification of the cytosol triggering the maturation of the enzyme through removal of the N- and C-terminal propeptides (14).
The enzyme accepts a variety of amino acid residues at the catalytic site; determination of crystallized structure at 2-Å resolution revealed a more open catalytic pocket, as expected for an enzyme attacking a variety of proteins during PCD (19). Ricinus CysEP is a member of a unique group of the papain-type cysteine endopeptidases (20, 21) found specifically in senescing tissues (15).
The CysEP is also expressed during Ricinus seed development (13). In the present paper, we show that PCD of the nucellar cells is accompanied by fragmentation of nuclear DNA, and the cells have ultrastructural and morphological features consistent with PCD. The nuclear DNA fragmentation is concomitant with the expression of CysEP, proliferation of ricinosomes, and collapse of the nucellar cells. The expanding endosperm cells next to the crushed cell wall layers contain a few developing oleosomes and cytosolic vacuoles to be subsequently filled with the components of the aleurone grains. Their cytoplasm bordering the crushed cell wall layers is intruded by regularly spaced ingrowth of wall material as it is characteristic for transfer cells (3, 5, 22, 23). This ingrowth increases the area over which the plasma membrane is in contact with the extracytoplasmic environment facilitating the uptake of catabolites produced upon nucellar cell death (Fig. 1B).
Materials and Methods
Plant Material. Castor oil plants were grown in the greenhouse under ambient conditions. Maturing seeds were harvested according to a morphological timetable (3). Capsules were bisected longitudinally. Seed coat, caruncle, nucellus, and endosperm were separated by using scalpel and razor blade. The respective tissues of one seed from 10, 15, and 20 DAP, with the exception of the 20-DAP seed coat, were homogenized in buffer (50 mM Hepes, pH 7.5/1 mM DTT/100 μM PMSF/100 μM N-p-tosyl-l-Lys chloromethyl ketone) and analyzed by SDS/PAGE and immunoblotting.
Biochemical Procedures. SDS/PAGE (12% gels) and immunoblotting using the primary antibody rabbit anti-CysEP were performed as described (14); immunoreactive proteins were detected with horseradish peroxidase-conjugated anti-rabbit IgG, visualized by using chemiluminescent substrate (Perbio Science).
Light Microscopy, TUNEL Assay, and Immunolocalization. For the TUNEL assay, maturing seeds were fixed, dehydrated, embedded in Paraplast Plus (Sigma), sectioned, permeabilized with proteinase K, and nick end labeled as previously described (12). Terminal transferase was omitted for the negative control. Nuclear DNA was pretreated with DNase (3 units/ml in 10 mM Tris·HCl, pH 7.5, for 10 min at 37°C) and nick end labeled as a positive control. For light microscopy immunocytochemistry, maturing seeds were fixed and embedded in Technovit 8100 (Kulzer) as described (12); CysEP was localized by using rabbit anti-CysEP antibodies directly labeled with Alexa Fluor 546 (Molecular Probes) (14).
Electron Microscopy and ImmunoGold Localization. Central slices, 1 mm thick, of maturing seeds were prepared for electron microscopy, and ImmunoGold localization was performed as described (24) using anti-CysEP antibodies and indirect localization with 10-nm colloidal gold-conjugated goat anti-rabbit IgG (Sigma). Sections were examined in a JEOL 100 CX transmission electron microscope, operated at 80 keV.
Results
Morphology of Ricinus Seed Development. At 10 DAP, the Ricinus seed is 10–12 mm long. Nucellus and integument tissues form the major portion of the seed and surround the 7- to 8-mm-long central endosperm. A layer of expanding cellular endosperm, <1 mm thick, surrounds free nuclear endosperm. The embryo is globular. By 25 DAP, the seed has reached its final size of 13–14 mm, and the majority of the nucellus and inner integument have been reduced to a layer of crushed cells by the expanding cellular endosperm, which now is 9 mm long and 7 mm wide. The outer integument has sclerified, forming the testa. The embryo is 6 mm long, consisting of the root–shoot axis and a pair of flat cotyledons elongating through the center of the cellular endosperm and has reached two-thirds of its 10-mm final length (Fig. 1A).
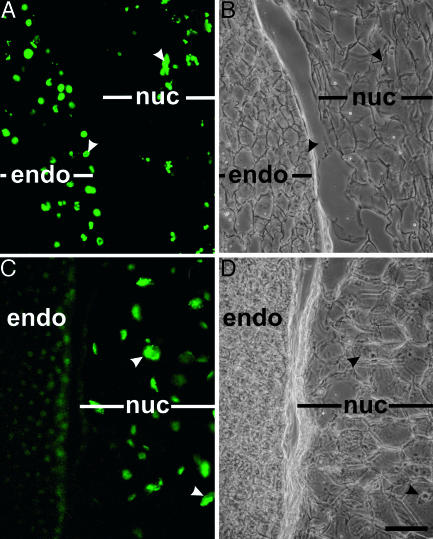
Nuclear DNA Fragmentation Occurs in Nucellar Cells Bordering the Expanding Endosperm. Nuclear DNA fragmentation in the endosperm and nucellus of developing Ricinus seeds was followed by using the TUNEL assay at 15 DAP (data not shown) and 20 DAP. In the positive control (Fig. 2A), all nuclei in both the degenerating nucellus and expanding endosperm were TUNEL-positive after DNase treatment. When the assay was performed without DNase pretreatment, only nuclei in the nucellar cells lying two to three cell layers proximal to the expanding endosperm were TUNEL-positive (Fig. 2C), suggesting that these nucellar cells are undergoing PCD. Nuclei of the endosperm cells, which act as an internal negative control, were not TUNEL-positive (Fig. 2C).
Fig. 2.
Nuclear DNA cleavage in nucellar cells adjacent to expanding endosperm. (A and B) Positive control treated with DNase before TUNEL assay. (C and D) TUNEL assay. Nuclei in nucellar cells adjacent to expanding endosperm are TUNEL-positive, endosperm cell nuclei are not. (A and C) Fluorescence. (B and D) Differential interference contrast (DIC) of identical areas. endo, endosperm; nuc, nucellus; arrowheads identify identical nuclei in fluorescence and DIC images. (Bar, 50 μm.)
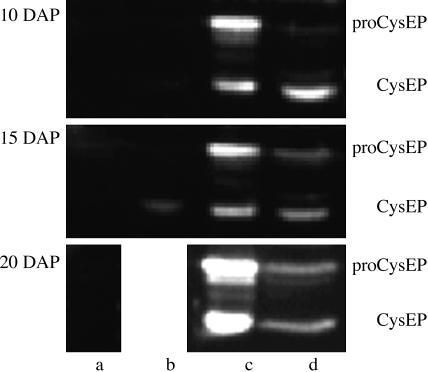
Ricinosomal CysEP Is Expressed in the Nucellar Cells Undergoing PCD. Antibodies raised against CysEP purified from ricinosomes of germinating Ricinus endosperm recognized the proform and mature form of the enzyme in nucellus dissected from the developing seed at 10, 15, and 20 DAP (Fig. 3). Lesser amounts of both forms of the enzyme were present in isolated endosperm (Fig. 3) because of enzyme activity in PCD of central endosperm cells at sites into which the cotyledons expand (not shown). CysEP was present in the seed coat during the final stages of sclerification (15 DAP, Fig. 3) but was absent from the caruncle (Fig. 3; see also label c in Fig. 1 A).
Fig. 3.
Western blot analysis of proCysEP and mature CysEP during seed development at 10, 15, and 20 DAP in caruncle (lane a), seed coat (lane b), nucellus (lane c), and endosperm (lane d) (seed coat at 20 DAP was already too sclerified for analysis).
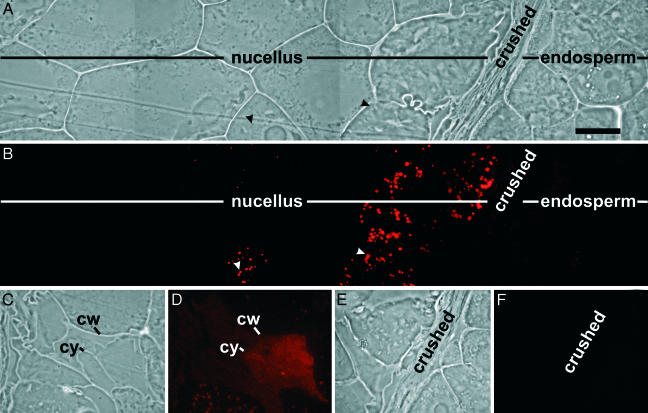
CysEP was localized to numerous organelles in the first two nucellar cell layers adjacent to the crushed cells lying between the degenerating nucellus and the expanding endosperm (Fig. 4A, red fluorescence Fig. 4B). Where the cell membrane was no longer appressed to the cell wall, CysEP spread diffusely over the nucellar cell cytoplasm because of enzyme release after vacuolar and cytoplasmic collapse (Fig. 4 C and D). Control sections did not exhibit fluorescence (Fig. 4 E and F).
Fig. 4.
Immunofluorescence microscopy with directly labeled antibodies to CysEP. (A and B) CysEP is localized to ricinosomes within nucellar cells fated to die. (C and D) CysEP is diffused throughout cytosol upon cell death and collapse. cw, cell wall; cy, cytoplasma. (E and F) Nonimmune control. (A, C, and E) Differential interference contrast. (B, D, and F) Fluorescence. (Bar in A, 50 μm.)
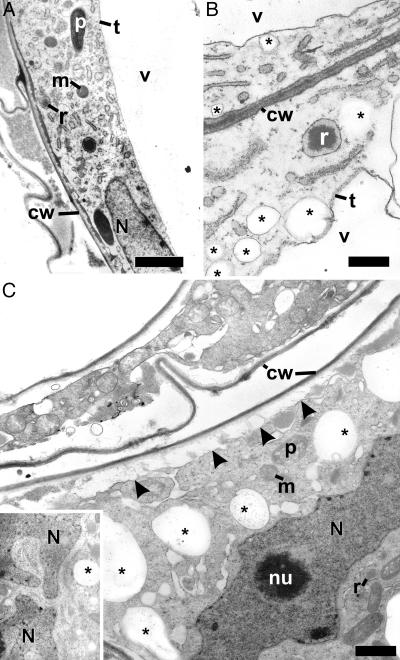
Ultrastructural Changes and Ricinosomes in the Nucellar Cells During PCD. Before the initiation of PCD, nucellar cells have a large single central vacuole, large nucleus, and a typical complement of organelles such as proplastids, mitochondria, ER, and Golgi complexes (Fig. 5A). With progression through PCD, the tonoplast becomes more electron dense, there is a marked vesiculation of the cells (Fig. 5 B and C), and nuclei become convoluted (Fig. 5C). In the final stages of PCD, in concert with the lysis of the central vacuole (Fig. 4D), the cytoplasm pulls away from the cell wall and mitochondria and proplastids become swollen (Fig. 6 J and K). Cytoplasmic remnants continue to be mobilized after cellular collapse (upper cell, Fig. 5C). The expanding endosperm compresses the remaining primary walls of the nucellar cells (Fig. 7).
Fig. 5.
Nucellar cells proximal to the expanding endosperm exhibit features consistent with progression through PCD. (A) Early in the process of PCD, nucellar cells have a large single central vacuole, regular nuclei, and the typical complement of organelles. (B and C) With progression through PCD, the tonoplast becomes electron dense (B), and there is a marked vesiculation of the cells (indicated by asterisks). Nuclei become convoluted (C and Inset), the cytoplasm pulls away from the cell wall (arrowheads in C), and ER and organelles swell (lower and upper cell in C). cw, cell wall; m, mitochondrion; N, nucleus; nu, nucleolus; p, proplastid; r, ricinosome; t, tonoplast; v, vacuole; *, vesicles. (Bar in A,5 μm; bars in B and C,1 μm.)
Fig. 6.
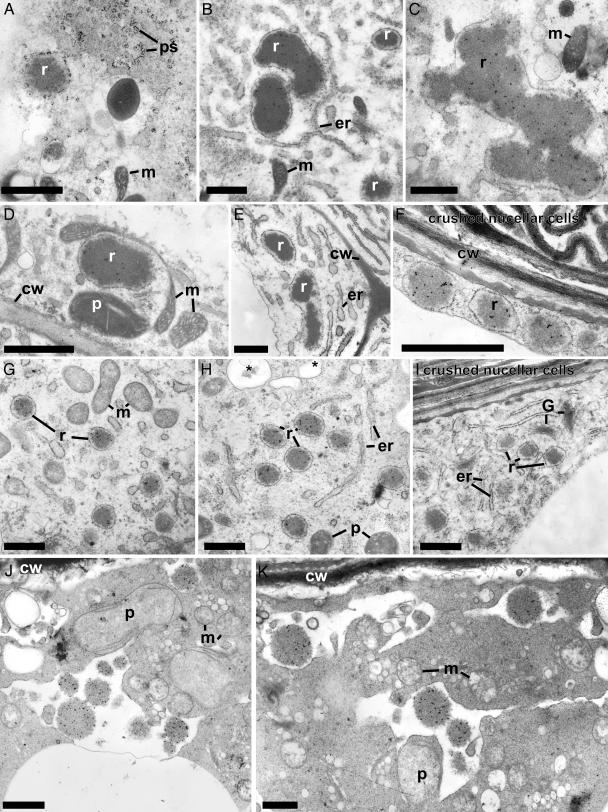
CysEP is localized to ricinosomes in nucellar cells undergoing PCD. (A–E) Ten DAP. (F–I) Twenty DAP. (J and K) During cell collapse. (A) Ricinosomes in the nucellar cells are derived from the ER. (B–D, F–H, J, and K) CysEP is localized to ricinosomes in nucellar cells fated to die. (A, E, and I) Preimmune control. er, endoplasmic reticulum; G, Golgi complex; ps, polysomes; all others as in Fig. 5. (Bars, 1 μm.)
Fig. 7.
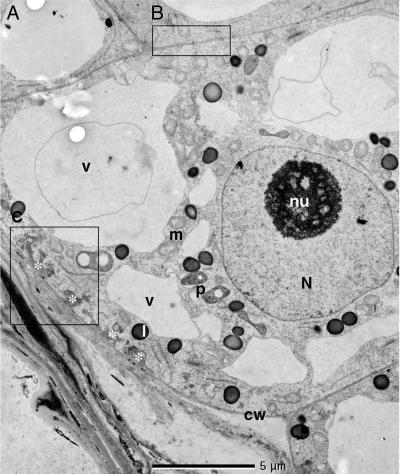
Electron micrograph of an endosperm cell adjacent to the crushed walls of nucellar cells. B, cell wall portions with plasmodesmata. C, cell wall portions with wall ingrowths. N, nucleus; nu, nuclealus; m, mitochondria; p, plastid; v, vacuole; L, lipid body, cw, cell wall; *, cell wall ingrowth. Enlargements of the regions B and C are depicted in Fig. 9.
The CysEP-containing organelles in the nucellar cells are ricinosomes. They are derived from the ER (Fig. 6A), having an electron-dense core and a more electron-translucent region immediately adjacent to the surrounding membrane. In planar section, attached ribosomes are arranged in helical arrays typical of polysomes (Fig. 6A). ImmunoGold labeling localizes CysEP to the dense interior of the ricinosomes (Fig. 6 B–E). At 10 DAP, nucellar cell ricinosomes are very large (>1 μm in diameter) and often have irregular proteinaceous deposits. A biochemical analysis of the protein composition of these ricinosomes (10 DAP) is presented in Fig. 8, which is published as supporting information on the PNAS web site. These ricinosomes contain less proCysEP and more additional proteins compared with ricinosomes from germinating seeds. By 20 DAP, the ricinosomes are more uniform, with a diameter of ≈0.5 μm (Fig. 6 F–I). During cellular collapse, CysEP is present as deposits within profiles possibly derived from the coalescence of the ricinosomes with numerous vesicles formed before collapse (Fig. 6H, *, J and K). Plastids, now gerontoplasts, and mitochondria are swollen, and the cytoplasm pulls away from the cell wall (Fig. 6 J and K).
The Thin Primary Cell Walls of the Expanding Endosperm Cells Form Irregular Fingers of Secondary Wall Material Penetrating into the Cytosol as Is Characteristic for Transfer Cells. The differential interference contrast micrograph of the endosperm cells in Fig. 4A shows a distinct bright layer of wall ingrowths emanating from the primary cell wall next to the crushed cell walls from the nucellus. These ingrowths are absent from the other wall portions of the cell and from cell walls of endosperm cells removed from the cell layer subtending the crushed walls. Instead, these cell wall portions and the cell walls connecting the endosperm cells removed from the cell layer subtending the crushed walls are traversed by plasmodesmata (Fig. 7, region B). Electron micrographs of the endosperm cells (Fig. 7, region C) reveal these structures to be regularly spaced intrusions into the cytosol of wall material ingrowths. They increase the area over which the plasma membrane is in contact with the extracytoplasmic environment. In the depicted portion of the endosperm cell wall, there is a direct contact of the primary wall with material that appears as an apoplastic remnant of a nucellar cell (Fig. 9, which is published as supporting information on the PNAS web site).
Discussion
The results of the present investigation of the elimination of the nucellus during the development of Ricinus seeds have been summarized in Fig. 1B. As the cellular endosperm expands, the nuclei in the cell layers of the nucellus proximal to the expanding face of the developing endosperm exhibit fragmentation of DNA, suggesting that the cells are undergoing PCD (25, 26). Although fragmentation of nuclear DNA is not a sufficient criterion for apoptotic PCD, morphological and ultrastructural features consistent with progression through PCD were seen in the nucellar cells (27). These include changes in the tonoplast density, vesiculation of the cytoplasm, nuclear and organelle deformation and condensation of nuclear chromatin, collapse and condensation of the cytoplasm, and cell collapse. Further, PCD has been implicated in the destruction of the nucellus and integument tissues during seed development in a number of other systems (7–11). Proliferation of the ricinosomes containing the ricinosomal CysEP ensues and is followed by the disintegration of the cells, leaving crushed and folded cell wall residues in the apoplastic space. In contrast to the ricinosomes operating in the PCD of the endosperm cells during germination of the castor bean seed after transfer of the mobilized stores to the developing cotyledons, the ricinosomes in the senescing nucellar cells are larger and structurally compound. Their protein composition indicates additional enzymes besides the CysEP.
As shown in Fig. 1B, the expanding endosperm cells are smaller than the mature endosperm cells and have not yet started on the major phase of protein and oil storage synthesis. The cells of the first endosperm cell layer adjacent to the crushed cell walls in the apoplast have the structure of transfer cells. These are characterized by wall invaginations, called ingrowths, that substantially increase the surface area of the plasma membrane. In the present case, the ingrowths are limited to the cell wall part of the endosperm cells bordering the crushed cell layer, whereas the other parts of their cell walls are connected to neighboring cells only by plasmodesmata, supporting a symplasmic transport of solutes and nutrients. Plasmodesmata seem to be the primary or only path for solute and nutrient transport among the developing endosperm cells in the Ricinus seed.
During expansion of the endosperm, the cells in the outermost layer develop labyrinthine ingrowths of the outer perclinal cell wall, a modification that increases the plasma membrane surface area and that is consistent with transfer cell morphology (5, 22, 23). Transfer cells are involved in the rapid uptake and delivery of amino compounds and photosynthate from the apoplast to subtending cells, the latter symplastic transport taking place through plasmodesmata. The Ricinus endosperm transfer cells are undoubtedly involved in the uptake of catabolites from nucellar and integument cell death during the early stages of endosperm expansion, but they most likely play a more important role later in development. The Ricinus ovule and developing seed have extensive vasculature entering from the chalazal end of the seed and branching in the maternal tissues to surround the embryo sac, developing endosperm. The vasculature remains active after the destruction of the nucellus and inner integument (3). It supplies the metabolites that allow for a 25-fold increase in the dry weight (storage proteins and oils) of the endosperm in the latter two-thirds of development after nucellar destruction (5). The endosperm transfer cells with their incipient ingrowth structures have at this stage developed into cells with highly elaborated ingrowths (J.S.G., unpublished results) and are likely to be involved in the uptake of these metabolites. It will be of interest to analyze if senescence and PCD of the nucellus as well as that of the inner integument provide low molecular weight metabolites through these incipient ingrowth structures for the endosperm cell growth and enlargement.
Supplementary Material
Author contributions: J.S.G., M.H., and C.G. designed research; J.S.G., M.H., and C.G. performed research; J.S.G., M.H., and C.G. analyzed data; and J.S.G. and C.G. wrote the paper.
Abbreviations: DAP, days after pollination; PCD, programmed cell death; ER, endoplasmic reticulum; CysEP, cysteine endopeptidase.
References
- 1.Lam, E. & Greenberg, J. (2000) Plant Mol. Biol. 44, vii-viii. [DOI] [PubMed] [Google Scholar]
- 2.Bewley, J. D. & Black, M. (1994) Seeds: Physiology of Development and Germination (Plenum, New York), 2nd Ed.
- 3.Greenwood, J. S. & Bewley, J. D. (1982) Can. J. Bot. 60, 1751-1760. [Google Scholar]
- 4.Simcox, P. D., Garland, W., De Luca, V., Canvin, D. T. & Dennis, D. T. (1979) Can. J. Bot. 57, 1008-1014. [Google Scholar]
- 5.Greenwood, J. S., Gifford, D. J. & Bewley, J. D. (1984) Can. J. Bot. 62, 255-261. [Google Scholar]
- 6.Greenwood, J. S. & Bewley, J. D. (1985) Can. J. Bot. 63, 2121-2128. [Google Scholar]
- 7.Chen, F. & Foolad, M. R. (1997) Plant Mol. Biol. 35, 821-831. [DOI] [PubMed] [Google Scholar]
- 8.Hiratsuka, R., Yamada, Y. & Terasaka, O. (2002) J. Plant Res. 115, 141-148. [DOI] [PubMed] [Google Scholar]
- 9.Xu, F.-X. & Chye, M.-L. (1999) Plant J. 17, 321-327. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez, F., Moreno, J. & Cejudo, F. J. (2001) Planta 213, 352-260. [DOI] [PubMed] [Google Scholar]
- 11.Wan, L., Xia, Q., Qui, X. & Selvaraj, G. (2002) Plant J. 30, 1-10. [DOI] [PubMed] [Google Scholar]
- 12.Schmid, M., Simpson, D. & Gietl, C. (1999) Proc. Natl. Acad. Sci. USA 96, 14159-14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid, M., Simpson, D., Kalousek, F. & Gietl, C. (1998) Planta 206, 466-475. [DOI] [PubMed] [Google Scholar]
- 14.Schmid, M., Simpson, D. J., Sarioglu, H., Lottspeich, F. & Gietl, C. (2001) Proc. Natl. Acad. Sci. USA 98, 5353-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gietl, C. & Schmid, M. (2001) Naturwissenschaften 88, 49-58. [DOI] [PubMed] [Google Scholar]
- 16.Gietl, C., Wimmer, B., Adamec, J. & Kalousek, F. (1997) Plant Physiol. 113, 863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollenhauer, H. H. & Totten, C. (1970) Plant Physiol. 46, 794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigil, E. L. (1970) J. Cell Biol. 46, 435-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Than, M. E., Helm, M., Simpson, D. J., Lottspeich, F., Huber, R. & Gietl, C. (2004) J. Mol. Biol. 336, 1103-1116. [DOI] [PubMed] [Google Scholar]
- 20.Gietl, C., Schmid, M. & Simpson, D. (2000) in Vacuolar Compartments, eds. Robinson, D. G. & Rogers, J. C. (Sheffield Academic, Sheffield, U.K.), Vol. 5, pp. 90-111. [Google Scholar]
- 21.Beers, E. P., Jones, A. M. & Dickermann, A. W. (2004) Phytochemistry 65, 43-58. [DOI] [PubMed] [Google Scholar]
- 22.Gunning, B. E. S., Pate, J. S. & Green, L. W. (1970) Protoplasma 71, 147-171. [Google Scholar]
- 23.Gunning, B. E. S., Pate, J.S., Minchin, F. R. & Marks, I. (1974) Symp. Soc. Exp. Biol. 28, 87-124. [PubMed] [Google Scholar]
- 24.Brown, M. J. & Greenwood, J. S. (1990) Can. J. Bot. 68, 2353-2360. [Google Scholar]
- 25.Wang, H., Li, J. & Gilchrist, D. G. (1996) Plant Cell 8, 375-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, M., Oppedijk, B. J., Lu, X., Van Duijn, B. & Schilperoort, R. A. (1996) Plant Mol. Biol. 32, 1125-1134. [DOI] [PubMed] [Google Scholar]
- 27.Gunawardena, A. H., Pearce, D. M., Jackson, M. B., Hawes, C. R. & Evans, D. E. (2001) Planta 212, 205-214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.