Abstract
To further study the role of GPR54 signaling in the onset of primate puberty, we used the monkey to examine the ability of kisspeptin-10 to elicit the release of gonadotropin-releasing hormone (GnRH) precociously, and we describe the expression of GPR54 and KiSS-1 in the hypothalamus during the peripubertal period. Agonadal juvenile male monkeys were implanted with a lateral cerebroventricular cannula and a jugular vein catheter. The responsiveness of the juvenile pituitary to endogenous GnRH release was heightened with a chronic pulsatile i.v. infusion of synthetic GnRH before kisspeptin-10 (112-121) injection. Intracerebroventricular (30 μg or 100 μg) or i.v. (100 μg) bolus injections of kisspeptin-10 elicited a robust GnRH discharge, as reflected by luteinizing hormone secretion, which was abolished by pretreatment with a GnRH-receptor antagonist. RNA was isolated from the hypothalamus of agonadal males before (juvenile) and after (pubertal) the pubertal resurgence of pulsatile GnRH release and from juvenile, early pubertal, and midpubertal ovary-intact females. KiSS-1 mRNA levels detected by real-time PCR increased with puberty in both male and female monkeys. In intact females, but not in agonadal males, GPR54 mRNA levels in the hypothalamus increased ≈3-fold from the juvenile to midpubertal stage. Hybridization histochemistry indicated robust KiSS-1 and GPR54 mRNA expression in the region of the arcuate nucleus. These findings are consistent with the hypothesis that GPR54 signaling by its cognate ligand in the primate hypothalamus may be activated at the end of the juvenile phase of development and may contribute to the pubertal resurgence of pulsatile GnRH release, the central drive for puberty.
Keywords: gonadotropin-releasing hormone, GPR54, kisspeptins, monkey, development, hypothalamus
Puberty represents a critical stage of human development, but the causation of this milestone remains an intriguing mystery. In particular, the neurobiological mechanisms underlying the resurgence of pulsatile hypothalamic gonadotropin-releasing hormone (GnRH) release, which represents the neuroendocrine initiator of the onset of primate puberty, are poorly understood. In this regard, inactivating mutations in the gene that encodes for GPR54, a G protein-coupled receptor (1), have been recently shown to be associated with hypogonadotropic hypogonadism and pubertal delay in human (2, 3), and a similar phenotype has been described for mice lacking GPR54 (3, 4). Although ligands to this receptor remain to be fully defined, kisspeptins encoded by the metastasis suppressor gene, KiSS-1, exhibit agonistic properties (5). Among the peptides derived from the KiSS-1 product, the decapeptide kisspeptin-10 (112-121) has been shown to be most potent in activating GPR54 (6). To further examine the hypothesis that GPR54 signaling is a critical event in the pubertal resurgence of GnRH release in primates, we used the rhesus monkey to determine whether central or peripheral administration of kisspeptin-10 to juvenile animals elicits precocious GnRH release and whether hypothalamic expression of GPR54 and KiSS-1 is developmentally regulated at the time of puberty. GnRH responses to kisspeptin-10 administration were measured indirectly by monitoring plasma luteinizing hormone (LH) secretion after pituitary responsiveness to GnRH had been increased by a chronic intermittent i.v. infusion of synthetic GnRH (7), whereas developmental changes in GPR54 and KiSS-1 were estimated by real-time PCR.
Materials and Methods
Exp. 1: Effect of Central or Peripheral Administration of Kisspeptin-10 on GnRH Release in Agonadal Juvenile Male Monkeys. Four juvenile (17-23 mo of age and 2.9-3.9 kg of body weight) male rhesus monkeys (Macaca mulatta) were used for this experiment. The monkeys were bilaterally castrated between 16 and 21 mo of age and implanted with an indwelling jugular vein catheter by using standard aseptic surgical procedures under anesthesia with isoflurane inhalation (1.5-2.5% with oxygen). After implantation of the catheters, the animals were fitted with a jacket and tether and housed in remote sampling cages for i.v. infusion of GnRH, i.v. injection of kisspeptin-10, and sequential blood samples. To administer kisspeptin-10 centrally, the monkeys were implanted with a 22-gauge stainless steel cannula in the lateral cerebroventricle as previously described (7). The remote sampling system allowed drug administration and blood sampling with minimal restraint and without sedation.
The sensitivity of the pituitary to GnRH in these animals was first enhanced with a chronic pulsatile i.v. infusion of GnRH (0.3 μg every hour for ≈3 weeks) as previously described (8). After cessation of the GnRH priming infusion, circulating LH levels fall abruptly to undetectable concentrations, but the response of the pituitary to GnRH is maintained for several days. In the present study, the GnRH priming infusion was interrupted at least 3 days before administration of kisspeptin-10 and subsequently reinitiated between trials to maintain pituitary responsiveness to GnRH. For central administration, vehicle [7.5% DMSO in artificial cerebrospinal fluid (aCSF)] or 30 or 100 μg of kisspeptin-10 in 200-μl volumes was introduced slowly into the intracerebroventricular (i.c.v.) line via a three-way connector above the animal's cage and immediately chased into the cerebroventricular system with 450-570 μl of aCSF. The doses of kisspeptin-10 were based on previous experience with i.c.v. injections of 1229U91, a potent peptide secretagogue of GnRH effective in the monkey at the 100- to 300-μg range (7, 9). 1229U91 is a recognized mixed neuropeptide Y (NPY) Y1 receptor antagonist/Y4 receptor agonist (10). The doses of kisspeptin-10 were injected on different days in a semirandom manner. Three hours after the central injection of kisspeptin-10, a bolus injection of 200 μg of 1229U91 was administered via the i.c.v. line as a positive control. Two of the animals also received 100 μg of kisspeptin-10 or vehicle (1.4% DMSO in saline) as an i.v. bolus (1 ml). On separate occasions, animals were treated with acyline, a GnRH-receptor (GnRH-R) antagonist (60-120 μg/kg at ≈0900 and 1600 hours, s.c.); the next day, animals were challenged with 100 μg of kisspeptin-10 administered by the i.c.v. or i.v. route. After the GnRH-R blockade had dissipated after i.c.v. injection of kisspeptin-10, the experiment was repeated to confirm the patency of the i.c.v. line.
All kisspeptin-10 injections were given between 0900 and 0915 hours. Blood samples were obtained at 30-min intervals for 30 min before injections (-30 and 0 min) and for 240 min thereafter. Plasma was harvested and stored at -20°C until assayed.
The animals were housed in individual cages maintained under a controlled environment (lights on between 0700 and 1900 hours at 20°C) and fed daily at ≈1100 hours a high-protein monkey diet, supplemented with fruit in the afternoon. Drinking water was available ad libitum. The animals were maintained according to the National Institutes of Health Guide for Care and Use of Laboratory Animals, and the protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Exp. 2: Developmental Changes in Expression of KiSS-1 and GPR54. Levels of KiSS-1 and GPR54 mRNA in medial basal hypothalamus (MBH) of agonadal juvenile (17-20 mo of age, n = 3) and pubertal (32-34 mo of age, n = 3) male monkeys and ovary-intact juvenile (12-23 mo of age, n = 8), early pubertal but premenarcheal (26-30 mo of age, n = 6) and midpubertal (36-43 mo of age, n = 7) female monkeys were determined by using real-time PCR. RNA derived from MBH and cortex (CTX) of agonadal male monkeys was obtained from an RNA bank established during a previous study (9). This RNA had been isolated with RNAzol (RNA STAT-60, Tel-Test, Friendswood, TX) and stored at -80°C. Tissue from ovary-intact female rhesus monkeys that had been euthanized for a variety of reasons was obtained through the Oregon National Primate Research Center necropsy program. MBH and CTX were dissected and preserved overnight at 4°C in RNAlater (Ambion, Austin, TX). Tissue was then removed from RNAlater and stored at -85°C until RNA extraction. For this purpose, tissues were homogenized (100 mg/ml) in TRI Reagent (MRC, Cincinnati), and the aqueous and organic phases were separated by the addition of 0.1 vol of bromochloropropane (BCP; Sigma) followed by centrifugation at 4°C. RNA was precipitated from the aqueous phase with 1 vol of isopropanol by centrifugation at 14,500 × g for 15 min at 4°C. The pellets were resuspended in diethyl pyrocarbonate (DEPC)-treated H2O, and the suspension incubated with Ambion's DNA-free DNase I (two units per reaction) for 30 min at 37°C. RNA concentrations were determined spectrophotometrically, and RNA integrity was verified on denaturing agarose gels.
In males, juvenile and pubertal stages were evaluated by monitoring circulating LH levels (9): LH concentrations were undetectable (<0.09 ng/ml) in juveniles but exceeded 3 ng/ml in pubertal monkeys. The pubertal stages of female monkeys were assigned according to criteria previously reported by Watanabe and Terasawa (11).
Exp. 3: Localization of GPR54 and KiSS-1 mRNA in the Hypothalamus. Coronal 25-μm sections of brain containing MBH obtained from previously described ovariectomized/hysterectomized adult rhesus monkeys (n = 4) treated with estradiol, either alone or in combination with progesterone (12), were used to identify sites of expression of KiSS-1 and GPR54 mRNA in the hypothalamus. A total of 20 sections (five per animal) was processed for each mRNA examined.
Reagents. Human kisspeptin-10 (112-121) was synthesized by the Peptide/Protein Core Facility of the Massachusetts General Hospital Endocrine/Reproductive Endocrine Unit. Kisspeptin-10 was dissolved in monkey artificial cerebrospinal fluid (aCSF; GIBCO/BRL/Life Technologies, Grand Island, NY) or normal saline containing 15% or 5% DMSO (Sigma), respectively. Working kisspeptin-10 solutions contained 7.5% or 1.4% DMSO for i.c.v. and i.v. administration, respectively. GnRH (GMP-26 code no. 230-110-40) and the GnRH-receptor (GnRH-R) antagonist, acyline, were synthesized at The Salk Institute (contract N01-HD-0-2906) and BIOQUAL (Rockville, MD), respectively, and made available by the Contraception and Reproductive Health Branch of the Center for Population Research at the National Institute of Child Health and Human Development. GnRH was dissolved in saline, and acyline was dissolved in 5% aqueous mannitol.
LH RIA. Plasma LH concentrations were measured with a double-antibody RIA system that uses recombinant cynomolgus LH (AFP342994) as standard and radioiodinated tracer and a rabbit polyclonal antiserum to recombinant monkey LH (AFP342994) as first antibody. The RIA reagents were provided by the National Hormone and Peptide Program. The sensitivity of the assay varied from 0.06 to 0.19 ng/ml and the intra- and interassay coefficients of variation were <3% and <6%, respectively.
DNA Cloning. To PCR-clone a cDNA fragment from monkey GPR54 mRNA, primers derived from the human sequence (GenBank accession no. AF343725) were used. The sense primer (5′-GCTCGTGCCGCTCTTCTTC-3′) corresponded to nucleotides 129-147, and the antisense primer (5′-CGCGCAACGGGAACACC-3′) was complementary to nucleotides 432-448. Because this reaction did not yield a single PCR product, a seminested PCR was performed using the same forward primer described above and a new reverse primer (5′-CACCGAGACCTGCTGGATGTA-3′) complementary to nucleotides 358-378. This procedure resulted in a single PCR product that was cloned into the plasmid pGEM-T (Promega) and sequenced from both ends to verify its identity. This cDNA was used as a template to generate either sense or antisense RNA.
A cDNA derived from monkey KiSS-1 mRNA was similarly cloned by using primers derived from the human sequence (GenBank accession no. NM_002256). For the first PCR, a 5′ primer (5′-GCTACTGCTTTTCCTCTGTGCC-3′) corresponding to nucleotides 24-45 in human KiSS-1 mRNA, and a 3′ primer (5′-AGCGCTTCTGCCGTGGTT-3′) complementary to nucleotides 388-405 was used. The nested PCR was carried out by using a 5′ forward primer (5′-GGGGAGCCATTAGAAAAGGTG-3′) corresponding to nucleotides 55-75 and a 3′ reverse primer (5′-CCGAAGCGCAGGCCGAAGGAGT-3′) complementary to nucleotides 344-365. The 311-bp PCR product obtained was also cloned into pGEM-T and sequenced.
Real-Time PCR. GPR54 and KiSS-1 mRNA contents in brain were quantitated by real-time PCR, as previously described (13). After reverse transcription of 200 ng of total RNA, aliquots of each reaction (10 ng/μl cDNA) were diluted 1:10 (KiSS-1) and 1:25 (GPR54) before using 2 μl for real-time PCR. Each sample was run in triplicate along with a relative and an absolute standard curve. Relative standard curves were generated by diluting one sample 1:10 to 1:10,000 times and served to estimate the content of 18S ribosomal RNA of each sample. The primers used to detect 18S ribosomal RNA were purchased as a kit (TaqMan Ribosomal RNA Control Reagents Kit, PerkinElmer/Applied Biosystems). Absolute standard curves were constructed by using serial dilutions (1:10) of sense RNA (2 ag to 2 ng). The threshold cycle number (CT) from each sample was referred to this curve to estimate the corresponding RNA content, and each RNA value was then normalized for procedural losses by using the 18S ribosomal RNA values estimated from the relative standard curve. The CT was the fractional cycle number at which the fluorescence accumulated to a level 10 times 1 SD from basal values. The GPR54 and KiSS-1 primers and fluorescent probes used were designed to target a segment comprised within the cloned cDNA described above. All primers and fluorescent probes for real-time PCR were selected with the assistance of the program primerexpress (PerkinElmer/Applied Biosystems). The primer sequences (Invitrogen Life Technologies) for GPR54 were 5′ forward (5′-AACTCGCTGGTCATCTACGTCAT-3′) and 3′ reverse (5′-TCTGTGGCCGCCAGGTT-3′). The internal fluorescent oligodeoxynucleotide probe sequence was 5′-ACAAGCCGATGCGGACCGTGA-3′ (PerkinElmer/Applied Biosystems) and was covalently linked to the fluorescent dye FAM at the 5′ end and to the quencher dye TAMRA at the 3′ end. The KiSS-1 real-time PCR primers used were 5′ forward (5′-AGAAAAGGTGGCCTCTGTGGA-3′) and 3′ reverse (5′-AGGCTCTGCTCCCACGG-3′). The internal fluorescent oligodeoxynucleotide probe was 5′-CAGGCCAGCAGCTGGAATCCCTG-3′. Real-time PCRs were performed in a total vol of 10 μl, each reaction containing 2 μl of the diluted reverse transcribed sample or 2 μl of standard, 5 μl of TaqMan Universal PCR Master Mix (PerkinElmer/Applied Biosystems), 250 nM each gene-specific and ribosomal fluorescent probe, 300 nM each gene-specific primer, and 10 nM each ribosomal primer. The real-time PCR program consisted of an initial annealing period of 2 min at 50°C, followed by 10 min of denaturing at 95°C and 40 cycles of 15 sec at 95°C and 1 min at 60°C.
Hybridization Histochemistry. To localize KiSS-1 and GPR54 mRNA in the hypothalamic sections, homologous 35S-UTP-labeled cRNA probes were used. The hybridization procedure was that described by Simmons et al. (14) and used earlier by us (15). After an overnight hybridization at 56°C, the slides were washed and processed for cRNA detection. After dehydration, the slides were dipped in NTB-2 (Kodak) photographic emulsion and exposed for 3 weeks at 4°C. Sections were counterstained with thionin.
Statistical Analyses. Significance of difference between mean LH concentrations after kisspeptin-10 and vehicle administration was determined by two-way ANOVA with repeated measures. Those between mean mRNA contents were determined by one-way ANOVA, t test, or χ2 test. Statistical significance was set at P < 0.05.
Results
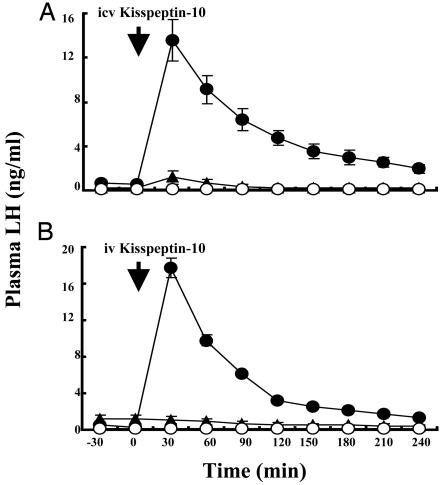
Exp. 1: Effect of Central or Peripheral Administration of Kisspeptin-10 on GnRH Release in Agonadal Juvenile Male Monkeys. Both i.c.v. and i.v. administration of 100 μg of kisspeptin-10 to agonadal juvenile monkeys induced a robust discharge of LH (Fig. 1). Within 30 min after either central or peripheral administration of kisspeptin-10, plasma LH levels increased by >25-fold and remained elevated for 2-3 h. i.c.v. administration of 30 μg of kisspeptin-10 induced a similar increase in plasma LH levels. Acyline pretreatment abolished the stimulatory action of both centrally and peripherally administered kisspeptin-10 on LH secretion (Fig. 1).
Fig. 1.
Effect of central (A; n = 3-4) or peripheral (B; n = 2) administration of 100 μg of kisspeptin-10 (•), vehicle (▴), or 100 μg of kisspeptin-10 with acyline pretreatment (○) on GnRH release, as reflected by plasma LH levels (mean ± SEM), in agonadal juvenile male monkeys. Kisspeptin was injected at time 0 (arrow). The LH-releasing action of i.c.v. or i.v. kisspeptin was abolished by acyline pretreatment. Statistical analysis revealed significant effects of treatment, time, and treatment × time interaction.
Exp. 2: Developmental Changes in Expression of KiSS-1 and GPR54. In agonadal male monkeys, hypothalamic content of KiSS-1 mRNA was significantly greater in the pubertal group than in the juvenile animals (Fig. 2). In contrast, GPR54 expression was similar in juvenile and pubertal animals. Levels of KiSS-1 and GPR54 mRNA in CTX were very low.
Fig. 2.
Changes in KiSS-1 and GPR54 mRNA content (mean ± SEM) determined by real-time PCR in MBH of agonadal male monkeys before (Juv) or after (Pub) the pubertal resurgence of GnRH release. KiSS-1 mRNA content in the MBH was significantly greater (*, P < 0.005) after the pubertal resurgence of GnRH, whereas changes in GPR54 mRNA content during these developmental stages were unremarkable. Three animals were examined per group.
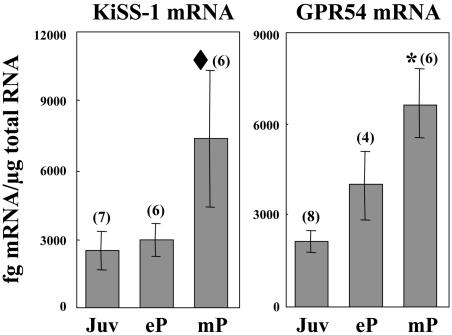
In intact females, KiSS-1 mRNA content in MBH of midpubertal monkeys was 3-fold greater than that in the juvenile or early pubertal group (Fig. 3). Although variability in the midpubertal group was marked, a χ2 test revealed significance (Fig. 3). GPR54 mRNA levels in MBH of female monkeys increased progressively from juvenile to midpubertal stages. As in males, levels of KiSS-1 and GPR54 mRNA in CTX from females were also low.
Fig. 3.
Changes in KiSS-1 and GPR54 mRNA content (mean ± SEM) determined by real-time PCR in MBH of intact female monkeys during pubertal development. KiSS-1 and GPR54 mRNA content increased in MBH with pubertal development. Numbers in parentheses indicate the number of animals per group. *, P < 0.005 (ANOVA), ♦, P < 0.05 (χ2 with threshold for bins set at 6,000 fg of KiSS-1 mRNA per μg of total RNA). Juv, juvenile; eP, early pubertal; mP, midpubertal.
Exp. 3: Localization of GPR54 and KiSS-1 mRNA in the Hypothalamus. Hybridization histochemistry with homologous cRNA probes demonstrated that KiSS-1 mRNA was discretely expressed in cells in the region of the arcuate nucleus (ARC) (Fig. 4). Expression of GPR54 was also observed in this region, although the pattern was less discrete (Fig. 5). KiSS-1 mRNA-containing cells were prominently circumscribed to the medial ARC, whereas GPR54 mRNA transcripts were observed in cells of both the medial and lateral portions of the ARC. GPR54 mRNA-expressing cells were also observed in the ventral aspect of the ventromedial hypothalamus. Because pale, large nuclei are usually considered to belong to neurons, most of the cells expressing either KiSS-1 mRNA or GPR54 mRNA are likely to be neuronal. Sections hybridized to a sense GPR54 RNA probe showed an artifactual “edge” hybridization effect but no specific hybridization to cells in either the medial or lateral ARC (Fig. 5C).
Fig. 4.
Selective expression of KiSS-1 mRNA in neurons of the medial ARC of the female monkey (ovariectomized and estrogen-treated) hypothalamus. (A) Low-magnification view. (Scale bar: 400 μm.) (B) Merged bright- and darkfield higher-magnification image showing the presence of KiSS-1 mRNA transcripts in neurons of the ARC (arrows). (Scale bar: 20 μm.)
Fig. 5.
GPR54 mRNA is expressed in cells of the ARC. (A-C) Medial ARC (A; MARC), lateral ARC (B; LARC), and section hybridized with a sense RNA probe (C). (Scale bar: 200 μm.) V, third ventricle; ME, median eminence. (D) Higher-magnification image showing expression of GPR54 mRNA in neurons of the ARC (arrows). (Scale bar: 20 μm.)
Discussion
The finding that central or peripheral administration of kisspeptin-10 to juvenile agonadal male monkeys elicited a robust release of LH that was abolished by prior treatment with a GnRH-receptor (GnRH-R) antagonist indicates that activation of hypothalamic GPR54 receptor signaling before puberty in higher primates induces precocious GnRH release. A GnRH-dependent stimulation of LH secretion after peripheral or central administration of kisspeptins has also been recently described in adult male mice (16) and in prepubertal and adult rats (17-19). In the monkey, the comparable LH-releasing action of centrally and peripherally administered kisspeptin-10 indicates that the 100-μg dose used may provide a supramaximal stimulus. Although the precise site of this hypothalamic action of kisspeptin in the monkey remains to be determined, it is probably exerted within the region of ARC and median eminence. This view is supported by our finding that peripheral injection of kisspeptin-10 elicited a marked discharge of LH and that high levels of expression of GPR54 were observed in the region of ARC. This nucleus is adjacent to the median eminence, a circumventricular organ that possesses fenestrated capillaries and that therefore is highly permeable to circulating factors (20). The finding that, in the monkey as in the mouse (16), KiSS-1 is expressed in the region of the ARC and median eminence is consistent with a site of action of exogenously administered kisspeptin in this region. It is interesting to note that expression of KiSS-1 and GPR54 in monkey MBH appears to be similar in the mouse (16), although the distribution of GnRH perikarya is very different in rodents (21).
Whether the GnRH-releasing action of kisspeptins is exerted directly on the GnRH neuron or indirectly through afferent neurons or ensheathing glial cells remains to be clarified. In support of a direct action of kisspeptin on the GnRH neuron, it may be noted that, in a cichlid fish, GnRH neurons were reported to express GPR54 transcripts (22) and that, in the rat, the immediate early gene c-fos was rapidly induced in GnRH neurons after s.c. metastin (kisspeptin-54) administration (17). Additionally, in the present study GPR54 expression was also observed in the ventral aspect of the ventromedial hypothalamus, an area in the monkey hypothalamus where neuroendocrine GnRH neurons are concentrated (21). On the other hand, the possibility that kisspeptin influences GnRH neurons indirectly cannot be excluded. In this regard, GPR54 expression was also evident in hypothalamic areas devoid of GnRH neurons. Based on relatively crude morphological criteria used in the present study, the majority of cells expressing GPR54 mRNA in MBH appeared to be neuronal. However, confirmation by dual labeling is required for a definitive conclusion on this issue.
The ability of the network of GnRH neurons in the hypothalamus of the juvenile primate to produce a robust discharge of this releasing factor in response to kisspeptin-10 stimulation is consistent with earlier findings that repetitive i.v. injections of an NMDA receptor agonist elicited a sustained train of hypothalamic GnRH discharges at this stage of development (23). In striking contrast to GnRH release, GnRH gene expression and GnRH peptide content in the hypothalamus are maintained during juvenile development in the monkey (24, 25). Together, these findings reinforce the view that the molecular machinery necessary for GnRH pulse generation is extant before puberty in higher primates and that the deficit underlying the arrest of spontaneous GnRH release at this stage of development lies upstream of the GnRH neuron.
Kisspeptin must now be considered as a candidate for this upstream signal because in the present study an increased hypothalamic expression of KiSS-1 was observed in association with the pubertal resurgence in pulsatile GnRH release in agonadal males and with the progression of pubertal development in intact females. Hypothalamic KiSS-1 expression has also been observed to increase in association with puberty in male and female rats (26). In the monkey, the pubertal elevation of KiSS-1 expression appears to be an intrinsic neurobiological event that occurs independently of gonadal steroids. In the case of the male, the pubertal increase in KiSS-1 expression was observed in the absence of the testes. In the case of the female, although the animals used in the present study were ovarian-intact, the increase in KiSS-1 expression observed at puberty was unlikely to be induced by the rise in circulating estradiol concentrations that would be occurring at this stage of development. This is because hypothalamic KiSS-1 expression has been reported to be inhibited by estradiol treatment in both adult ovariectomized rats (26) and monkeys (unpublished observations). Parenthetically, testosterone has also been shown to inhibit hypothalamic KiSS-1 expression both in adult male monkeys (M. Shibata, R. Friedman, M.S., and T.M.P., unpublished observations) and rats (26).
In contrast to the pubertal changes in KiSS-1 expression, which were similar in male and female monkeys, a pubertal increase in hypothalamic expression of GPR54 was only observed in the females. Changes in GPR54 expression have also been reported during the peripubertal period in rats (26). Whether the difference in the developmental expression of GPR54 in pubertal male and female monkeys is related to gender or hormonal status remains to be clarified. At the present time, however, we favor the former possibility because hypothalamic GPR54 expression appears to be estrogen-insensitive in the adult female monkey (M. Shibata and T.M.P., unpublished observations) and inhibited by estrogens in the female rat (26). GPR54 expression in the female was markedly greater than in the male. The reason for this sex difference, which has not been reported for the rat (26), is unclear, but it may be unrelated to differences in hormonal status because it was manifest in juveniles, a stage of development when testicular and ovarian hormone secretion is minimal (27).
The present results in the monkey complement the signal clinical observations that loss-of-function mutations in GPR54 are associated with isolated hypogonadotropic hypogonadism and pubertal delay (2, 3). Taking the findings together, it may be proposed that increased kisspeptin signaling at the GPR54 receptor occurs in the primate hypothalamus at the time of puberty and that reduced kisspeptin tone during childhood and juvenile development may contribute to the hypogonadotropic state characteristic of these phases of development. To substantiate this hypothesis it would be important to demonstrate that developmental changes in hypothalamic kisspeptin content parallel those in KiSS-1 expression and that continuous kisspeptin stimulation before puberty leads to sustained GnRH release and precocity.
Also, it should not be forgotten that compelling evidence implicates several other upstream signaling systems in the developmental control of GnRH neuronal function in primates and, thereby the timing of puberty in these species. Most notable in this regard are the systems using GABA (28), glutamate (29), neuropeptide Y (NPY) (9), and astroglial factors (15). For the future, it will be important, when defining the role of hypothalamic kisspeptin signaling at the GPR54 receptor in the onset of primate puberty, that new models accommodate and attempt to integrate all information relevant to the developmental regulation of the GnRH neuronal network.
Acknowledgments
We thank Dr. Cynthia Bethea (Oregon National Primate Research Center) for kindly providing hypothalamic sections for hybridization histochemistry; Dr. Clifford R. Pohl (Duquesne University, Pittsburgh)for statistical advice; the staff of the Primate and Assay Cores of the Specialized Cooperative Centers Program in Reproduction Research at the University of Pittsburgh School of Medicine for exceptional technical assistance; Dr. A. F. Parlow (National Hormone and Pituitary Program) for the reagents for the LH RIA; and the Contraceptive and Reproductive Health Branch of the National Institute of Child Health and Human Development for kindly providing GnRH and acyline. This work was supported by National Institutes of Health Grants HD08610, HD13254 (to T.M.P.), HD25123, HD18185, RR00163 (to S.R.O.), and HD28138 (to W.F.C.).
Author contributions: M.S., C.M., S.B.S., W.F.C., S.R.O., and T.M.P. designed research; M.S., C.M., S.R.O., and T.M.P. performed research; M.S., C.M., S.R.O., and T.M.P. analyzed data; M.S., C.M., S.B.S., W.F.C., S.R.O., and T.M.P. wrote the paper; and W.F.C. contributed new reagents/analytic tools.
A preliminary report of this work was presented at the 86th Annual Meeting of the Endocrine Society, New Orleans, June 16-19, 2004 (abstract pp. 3-269).
Abbreviations: GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; i.c.v., intracerebroventricular; MBH, medial basal hypothalamus; CTX, cortex; ARC, arcuate nucleus.
References
- 1.Lee, D. K., Nguyen, T., O'Neill, G. P., Cheng, R., Liu, Y., Howard, A. D., Coulombe, N., Tan, C. P., Tang-Nguyen, A.-T., George, S. R. & O'Dowd, B. F. (1999) FEBS Lett. 446, 103-107. [DOI] [PubMed] [Google Scholar]
- 2.de Roux, N., Genin, E., Carel, J.-C., Matsuda, F., Chaussain, J.-L. & Milgrom, E. (2003) Proc. Natl. Acad. Sci. USA 100, 10972-10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seminara, S. B., Messager, S., Chatzidaki, E. E., Thresher, R. R., Acierno, J. S., Shagoury, J. K., Bo-Abbas, Y., Kuohung, W., Schwinof, K. M., Hendrick, A. G., et al. (2003) N. Engl. J. Med. 349, 1614-1627. [DOI] [PubMed] [Google Scholar]
- 4.Funes, S., Hedrick, J. A., Vassileva, G., Markowitz, L., Abbondanzo, S., Golovko, A., Yang, S., Monsma, F. J. & Gustafson, E. L. (2003) Biochem. Biophys. Res. Commun. 312, 1357-1363. [DOI] [PubMed] [Google Scholar]
- 5.Kotani, M., Detheux, M., Vandenbogaerde, A., Communi, D., Vanderwinden, J.-M., Poul, E. L., Brezillon, S., Tyldesley, R., Suarez-Huerta, N., Vadenput, F., et al. (2001) J. Biol. Chem. 276, 34631-34636. [DOI] [PubMed] [Google Scholar]
- 6.Muir, A., Chamberlain, L., Elshourbagy, N. A., Michalovich, D., Moore, D. J., Calamari, A., Szekeres, P. G., Sarau, H. M., Chambers, J. K., Murdock, P., et al. (2001) J. Biol. Chem. 276, 28969-28975. [DOI] [PubMed] [Google Scholar]
- 7.Shahab, M., Balasubramaniam, A., Sahu, A. & Plant, T. M. (2003) J. Neuroendocrinol. 15, 965-970. [DOI] [PubMed] [Google Scholar]
- 8.Suter, K. J., Pohl, C. R. & Plant, T. M. (1998) Endocrinology 139, 2774-2783. [DOI] [PubMed] [Google Scholar]
- 9.El Majdoubi, M., Sahu, A., Ramaswamy, S. & Plant, T. M. (2000) Proc. Natl. Acad. Sci. USA 97, 6179-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker, E. M., Babij, C. K., Balasubramaniam, A., Burrier, R. E., Guzzi, M., Hamud, F., Mukhopadhyay, G., Rudinski, M. S., Tao, Z., Tice, M., et al. (1998) Eur. J. Pharmacol. 349, 97-105. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe, G. & Terasawa, E. (1989) Endocrinology 125, 92-99. [DOI] [PubMed] [Google Scholar]
- 12.Gundlah, C., Lu, N. Z. & Bethea, C. L. (2002) Psychopharmacology 160, 271-282. [DOI] [PubMed] [Google Scholar]
- 13.Romero, C., Paredes, A., Dissen, G. A. & Ojeda, S. R. (2002) Endocrinology 143, 1485-1494. [DOI] [PubMed] [Google Scholar]
- 14.Simmons, D. M., Arriza, J. L. & Swanson, L. W. (1989) J. Histotechnol. 12, 169-181. [Google Scholar]
- 15.Ma, Y. J., Costa, M. E. & Ojeda, S. R. (1994) Neuroendocrinology 60, 346-359. [DOI] [PubMed] [Google Scholar]
- 16.Gottsch, M. L., Cunningham, M. J., Smith, J. T., Popa, S. M., Acohido, B. V., Crowley, W. F., Seminara, S., Clifton, D. K. & Steiner, R. A. (2004) Endocrinology 145, 4073-4077. [DOI] [PubMed] [Google Scholar]
- 17.Matsui, H., Takatsu, Y., Kumano, S., Matsumoto, H. & Ohtaki, T. (2004) Biochem. Biophys. Res. Commun. 320, 383-388. [DOI] [PubMed] [Google Scholar]
- 18.Navarro, V. M., Castellano, J. M., Fernandez-Fernandez, R., Tovar, S., Roa, J., Mayen, A., Nogueiras, R., Vazquez, M. J., Barreiro, M. L., Magni, P., et al. (2005) Endocrinology 146, 156-163. [DOI] [PubMed] [Google Scholar]
- 19.Thompson, E. L., Patterson, M., Murphy, K. G., Smith, K. L., Dhillo, W. S., Todd, J. F., Ghatei, M. A. & Bloom, S. R. (2004) J. Neuroendocrinol. 16, 850-858. [DOI] [PubMed] [Google Scholar]
- 20.Weindl, A. (1973) in Frontiers in Neuroendocrinology, eds. Ganong, W. F. & Martini, L. (Oxford Univ. Press, New York), pp. 3-32.
- 21.Silverman, A.-J., Livne, I. & Witkin, J. W. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 1683-1709.
- 22.Parhar, I., Ogawa, S. & Sakuma, Y. (2004) Endocrinology 145, 3613-3618. [DOI] [PubMed] [Google Scholar]
- 23.Gay, V. L. & Plant, T. M. (1988) Neuroendocrinology 48, 147-152. [DOI] [PubMed] [Google Scholar]
- 24.Fraser, M. O., Pohl, C. R. & Plant, T. M. (1989) Biol. Reprod. 40, 972-980. [DOI] [PubMed] [Google Scholar]
- 25.El Majdoubi, M., Sahu, A. & Plant, T. M. (2000) Endocrinology 141, 3273-3277. [DOI] [PubMed] [Google Scholar]
- 26.Navarro, V. M., Castellano, J. M., Fernandez-Fernandez, R., Barreiro, M. L., Roa, J., Sanchez-Criado, J. E., Aguilar, E., Dieguez, C., Pinilla, L. & Tena-Sempere, M. (2004) Endocrinology 145, 4565-4574. [DOI] [PubMed] [Google Scholar]
- 27.Plant, T. M. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 453-485.
- 28.Terasawa, E. & Fernandez, D. L. (2001) Endocr. Rev. 22, 111-151. [DOI] [PubMed] [Google Scholar]
- 29.Plant, T. M., Gay, V. L., Marshall, G. R. & Arslan, M. (1989) Proc. Natl. Acad. Sci. USA 86, 2506-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]