Abstract
In the Joint Center for Structural Genomics, one-dimensional (1D) 1H NMR spectroscopy is routinely used to characterize the folded state of protein targets and, thus, serves to guide subsequent crystallization efforts and to identify proteins for NMR structure determination. Here, we describe 1D 1H NMR screening of a group of 79 mouse homologue proteins, which correlates the NMR data with the outcome of subsequent crystallization experiments and crystallographic structure determination. Based on the 1D 1H NMR spectra, the proteins are classified into four groups, “A” to “D.” A-type proteins are candidates for structure determination by NMR or crystallography; “B”-type are earmarked for crystallography; “C” indicates folded globular proteins with broadened line shapes; and “D” are nonglobular, “unfolded” polypeptides. The results obtained from coarse- and fine-screen crystallization trials imply that only A- and B-type proteins should be used for extensive crystallization trials in the future, with C and D proteins subjected only to coarse-screen crystallization trials. Of the presently studied 79 soluble protein targets, 63% yielded A- or B-quality 1D 1H NMR spectra. Although similar yields of crystallization hits were obtained for all four groups, A to D, crystals from A- and B-type proteins diffracted on average to significantly higher resolution than crystals produced from C- or D-type proteins. Furthermore, the output of refined crystal structures from this test set of proteins was 4-fold higher for A- and B-type than for C- and D-type proteins.
Keywords: crystallographic resolution, NMR structure, x-ray structure, unfolded proteins
Efficient identification of protein targets (constructs) that ultimately leads to rapid determination of their three-dimensional (3D) structure is a key issue in high-throughput structural genomics. The most extensively used method for protein structure determination, x-ray crystallography, does not provide information on the biophysical state of a protein until crystals of diffraction quality are produced; this process can take from days to months, depending on the protein target. Alternative techniques for characterization of the biophysical state of the protein before crystallization trials are, therefore, of keen interest to make structure determination overall more efficient. These methods include circular dichroism (CD) spectroscopy, dynamic light scattering, NMR spectroscopy, and deuterium exchange by MS. Although CD spectroscopy and dynamic light scattering can be used, respectively, to characterize the regular secondary structure content and multimeric or aggregated states of proteins, NMR spectroscopy can provide information on the global fold of proteins (1), including secondary structure content and higher-order organization. With presently available NMR techniques, one-dimensional (1D) 1H NMR spectra can be recorded in a time frame of a few minutes, even with protein concentrations as low as 50–100 μM (2–10).
The Joint Center for Structural Genomics (JCSG; www.jcsg.org), which is one of nine centers funded by the National Institutes of Health Protein Structure Initiative (www.nigms.nih.gov/psi), has developed and implemented high-throughput structure determination pipelines (11–13) to obtain extensive structural coverage of a bacterial (Thermotoga maritima) and a eukaryotic (Mus musculus) proteome. The first pipeline used a multitier approach to process the ORFs in the T. maritima proteome (12). All targets were investigated by using novel high-throughput expression, purification, and nanocrystallization technologies, which so far yielded 3D structures of >100 proteins by using x-ray crystallography or NMR spectroscopy in solution.
A second pipeline is dedicated to processing bacterial and yeast homologues of mouse proteins (hereafter referred to as “mouse homologue proteins”; expressed in Escherichia coli) from their genes to x-ray diffraction-quality crystals or protein solutions for NMR structure determination. When compared with the T. maritima proteome, these targets may at times require additional care to optimize the production of diffraction-quality crystals and NMR-amenable protein solutions. The pipeline includes the following steps: target selection, cloning, microexpression (to verify target expression and solubility before scale-up), macroexpression, and purification by using immobilized metal chromatography, ion-exchange chromatography, and sometimes size-exclusion chromatography. The purified proteins then are screened with 1D 1H NMR spectroscopy to verify the folded state. Folded proteins are expected to have a higher likelihood of yielding diffraction-quality crystals, and, based on the results of this study, only well behaved proteins in the NMR screen are now forwarded for extensive coarse- and fine-screen crystallization trials (14).
This paper describes our experience with the incorporation of 1D 1H NMR screening into the JCSG structure determination pipeline to characterize the biophysical state of mouse homologue proteins before crystallization trials. Overall, NMR screening turned out to be a helpful tool for identifying targets with a high probability of producing x-ray diffraction-quality crystals or NMR-amenable protein solutions. Its incorporation into the structural genomics pipeline was readily achieved, including rapid dissemination of the results to the appropriate cores. Thus, 1D 1H NMR screening carries promise for improved focus of structural genomics projects on the most promising targets and, as a consequence, for significant cost reduction in the production of novel 3D protein structures. Moreover, because the sample requirements for NMR screening are further minimized from milligrams to micrograms of protein for a single spectrum (15), the use of NMR to identify the most promising targets for NMR structure determination and crystallization will further reduce this cost.
Materials and Methods
Protein Expression and Purification. Targets were selected by the Bioinformatics Core of the JCSG, using the criteria for target selection established by the Protein Structure Initiative plus additional filters, which were designed to increase the likelihood of success with structure determination. Once selected, targets were cloned into a standard JCSG expression vector pMH1, which contains an expression/purification tag, MGSDKIHHHHHH, immediately N-terminal to the gene-of-interest and is under the control of the pBAD promoter. Microexpression was performed by using 96-well blocks (2 ml deep) in either standard shaker incubators or using the Vertiga microfermentation device (750 μl of culture medium per well, Thomson Instruments, Carlsbad, CA). Macroexpression was performed by using the Genomics Institute of the Novartis Research Foundation 96-well fermentor (65 ml of culture medium per well). After purification by using immobilized metal chromatography, all samples underwent secondary purification by using ion-exchange chromatography and, when necessary, tertiary purification by using size-exclusion chromatography. Samples were concentrated to either 500 or 300 μl, depending on the amount of protein available. After concentration, the samples were flash frozen and stored at –80°C.
NMR Spectroscopy. All proteins were screened with sample volumes of 500 or 300 μl at concentrations of ≈0.5 mM, with a range from 0.05 to 1.0 mM. Disposable NMR tubes (NE-RG5-7, New Era, Vineland, NJ) were used for 500-μl samples, whereas reusable Shigemi tubes (Allison Park, PA) were used for 300-μl samples. An aqueous buffer containing 10 mM Tris·HCl (pH 7.8), 100 mM NaCl, and 10% D2O was used for all spectra. Then, 1D 1H NMR solvent presaturation experiments were recorded (presaturation of 2.0 sec during the relaxation delay) with a 9,000-Hz sweep width. The measurement time for the different proteins was between 20 sec and 20 min, depending on the sample concentration. The NMR measurements were performed at 285 K on an Avance600 spectrometer (Bruker, Billerica, MA) equipped with four radio-frequency channels and a triple resonance probe (TXI-HCN with z-gradient). The spectra were processed and analyzed by using the program xwinnmr3.5 (Bruker).
Results and Discussion
NMR Screening. For structural genomics projects, it is important to minimize sample processing time to maximize throughput. Thus, the number of additional sample processing steps in connection with the introduction of NMR screening into the JCSG high-throughput structure determination pipeline had to be minimal. An aqueous buffer with 10 mM Tris·HCl (pH 7.8), 100 mM NaCl, and 10% D2O was found to be compatible with both 1D 1H NMR screening and coarse-screen crystallization trials. Therefore, for the initial tests, the proteins needed to be expressed and purified only once (in either native or selenomethionine media, depending on the crystallographic requirements), and the same samples were used first for NMR screening and then for coarse-screen crystallization trials.
We established a rigorous protocol to ensure that each sample was processed in the same way. After purification and sample concentration, the protein solutions were flash frozen and stored at –80°C. Immediately before NMR screening, the proteins were thawed and transferred either into low-quality NMR tubes (New Era) or, in some cases when the amount of protein was limited, into microcell Shigemi tubes. All measurements were performed at 285 K. After the measurements, the samples were transferred back into Eppendorf tubes and forwarded to the crystallomics core for crystallization trials.
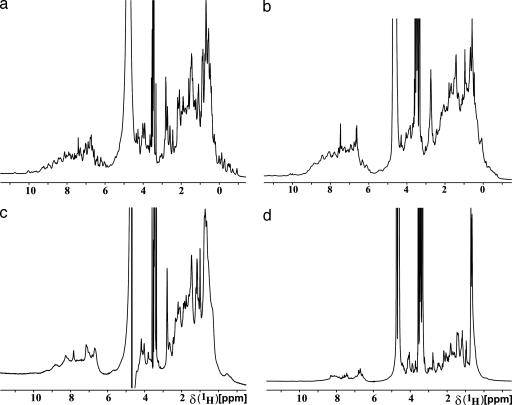
Although 2D 1H, 15N-correlation NMR spectroscopy (COSY) is quite commonly used in structural genomics initiatives (5, 6, 16), in the JCSG we used 1D 1H NMR as the primary experiment for NMR screening. We concluded from earlier experience (13) that for the purpose of the presently described screening, little or no additional information results from 15N-labeling of the proteins and recording of 2D NMR spectra. Therefore, we can achieve significant savings with the use of 1D 1H NMR experiments in combination with the high-throughput techniques developed for protein expression by the JCSG. In the 1D 1H NMR spectra, the dispersion of the NMR signals in the regions of the methyl protons (–0.5 to 1.5 ppm), α-protons (3.5–6 ppm), and amide protons (6–10 ppm) provides the main indicators of folded globular proteins (Fig. 1) (1, 16). A qualitative assessment of the line widths in the 1D 1H NMR spectra recorded with water presaturation was used as a further criterion to characterize the state of a protein. The results of the 1D 1H NMR screening were forwarded to the crystallographics core in the form of a classification of the proteins into four groups, “A” to “D,” with predicted differences in the probability of successful crystallization for high-resolution diffraction studies.
Fig. 1.
Representative 1D 1H NMR spectra from screening of 79 mouse homologue proteins. (a) A-grade protein, S. cerevisiae chromosome XII COSMID 8003 (GenBank accession no. YLR285W; molecular mass, 29.63 kDa). (b) B-grade protein, S. cerevisiae probable nicotinate phosphoribosyltransferase (Systematic name YOR209C; molecular mass, 49 kDa). (c) C-grade, S. cerevisiae small nuclear ribonucleoprotein E (accession no. YOR159C; molecular mass, 10.37 kDa). (d) D-grade, Nostoc sp. hypothetical protein Alr4516 (accession no. 17133652; molecular mass, 26.92 kDa). The sharp intense peak near 3.5 ppm comes from 10 mM Tris buffer.
Proteins in the groups “A” and “B” yielded 1D 1H NMR spectra with significant dispersion of resonance lines in the three spectral regions where ring current-shifted methyl resonances, downfield-shifted α-proton resonances, and downfield-shifted amide proton resonances were observed (1, 17). In A-type proteins, these resonances were well separated, sharp lines, and their intensities represented the entire population of protein molecules (Fig. 1a), whereas in B-type proteins the shapes and intensities of the dispersed resonance lines did not quite meet the stringent criteria for A-type classification (Fig. 1b). Proteins classified in group “C” showed clear evidence of chemical shift dispersion, particularly in the region between 1.0 and –2.0 ppm, but they also displayed line broadening over the entire spectrum (Fig. 1c), which may be caused by intrinsic large size, aggregation, or oligomerization (17). Finally, “D”-grade proteins showed no resonance lines in the spectral regions from 0.5 to –2.0 and from 5.0 to 6.5 ppm, and they had narrow chemical shift dispersion throughout the spectrum and particularly in the amide proton region (Fig. 1d).
The recommendations forwarded to crystallography, based on the results of this analysis, were that A- and B-grade proteins are primary targets for extensive crystallization trials. A-grade proteins are further potential candidates for NMR structure determination, and a decision may be taken to shift some of these proteins from crystallography to NMR structure determination. This decision also will be based on additional considerations, such as the molecular mass, and also might take into account possible cost savings from NMR structure determination for well behaved small proteins. If a protein is A- or B-grade and not flagged for NMR structure determination, considerable effort is expended to obtain diffraction-quality crystals. This process includes new large-scale expression and, for example, testing of variable protein concentrations, initial coarse-screening against a large number (up to 480) of different solvent compositions, and testing crystallization with known cofactors or other ligands, in addition to fine-screening when promising conditions are identified. For proteins of C- or D-grade, the NMR sample is further used for coarse-screen crystallization trials, but, if diffraction-grade crystals fail to form, the construct is removed from the JCSG target list and will not be further expressed and purified for additional crystallization trials.
Given that large proteins have long rotational correlation times (related to the Brownian motion in solution), the reliability of 1D 1H NMR to verify the folded state is limited by protein size and possibly by the multimerization state. Monomeric proteins of sizes up to ≈50 kDa are good candidates for 1D 1H NMR screening, but oligomerization or aggregation can cause the data on such proteins to be more difficult to interpret. It is for this reason that C-grade targets are still subjected to preliminary crystallization trials. In a follow-up step to NMR screening, C-grade targets will be screened in the future for potential multimerization by using dynamic light scattering, and proteins forming monodisperse oligomeric structures then also would be selected for fine-screen crystallization trials. D-grade targets are subjected to coarse-screen crystallization in as far as there is protein available from the sample used for the NMR screening.
To ensure seamless interaction between protein expression, protein purification, NMR screening, and crystallization, the results of the NMR screening are uploaded into the JCSG database (see Fig. 4, which is published as supporting information on the PNAS web site) to allow easy tracking of the results by other members of the JCSG. Similarly, whenever a protein is ready for NMR screening, the NMR core is automatically informed about the relevant target data (protein size, sequence, sample concentration, etc.).
Analysis of the Data on 79 Mouse Homologue Protein Targets. At this time, 79 target proteins (Tables 2 and 3, which are published as supporting information on the PNAS web site) have been successfully expressed, preselected for solubility (14), purified, and screened by using 1D 1H NMR spectroscopy. The average molecular mass of these proteins is 29.7 kDa, ranging from 9.8 to 65.8 kDa. The analysis showed that the average molecular masses of A- and B-grade proteins are 25.7 and 33.4 kDa, respectively, and the C- and D-grade proteins have an average molecular mass of 29.4 kDa. Thus, within the range considered here, there is no significant influence of molecular size on the results of the NMR grading.
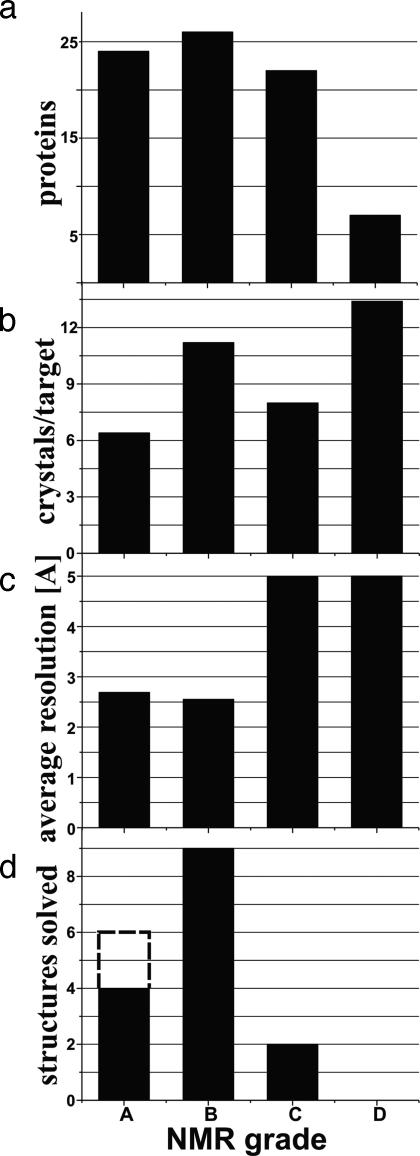
Of the total 79 mouse homologue proteins, 50 have been assigned A and B grades, whereas 29 have grades of C or D (Fig. 2a). This high yield of well behaved, folded proteins can be taken as a result of successful prescreening for soluble proteins. Surprisingly, the average number of crystal hits per protein target was found to be comparable for all four NMR grades (Fig. 2b). Furthermore, the percentage of proteins producing at least one crystal hit (between 1 and 92 hits per protein were obtained) is also similar, with 76% for A and B and 83% for C and D proteins (Table 1). In contrast, a clear-cut relation was observed between the quality of the crystals, as expressed by the resolution of the x-ray diffraction, and the NMR-based grade (Fig. 2c). Although C- and D-grade proteins tended to crystallize with similar success rates (Figs. 2b and 3; for example, one of the D-grade proteins yielded 22 crystallization hits), the resolution obtained from the resulting crystals was rather low, with average values for the diffracting crystals near 5.0 Å, as compared with average values close to 2.5 Å for A and B grade proteins. Furthermore, the crystal structures determined so far nearly all came from proteins in the A or B NMR grade category (Fig. 2d). The only two exceptions were two C-grade proteins that showed multimer formation in their crystal structures. In the NMR screening, both of these proteins showed characteristics of globular proteins, such as ring current-shifted methyl peaks between 0.5 and –2.0 ppm (Fig. 1c), but they had very broad line widths. The recommendation from the NMR screening to crystallography includes comments that the proteins might be either multimeric or nonspecifically aggregated.
Fig. 2.
NMR screening of 79 mouse homologue proteins and relations to the outcome of crystallization trials and crystal structure determination. (a) Distribution of the 79 targets among the NMR grades A–D based on 1D 1H NMR screening (see text). (b) Average number of crystal hits per protein in each of the NMR grades A–D. (c) Average resolution of the crystallographic diffraction obtained with the crystals from proteins of the NMR grades A–D. (d) Number of crystal structures determined so far for the NMR grades A–D. The broken line in grade A indicates the two proteins that had been removed from the crystallization pipeline for NMR structure determination. The two C-grade structures are multimeric proteins (see text).
Table 1. Results of crystallographic studies with 79 mouse homologue proteins that were graded A to D.
| Grade | Proteins* | Crystal hits† (%) | ≥5.0 Å diffraction, no structure‡ (%) | <5.0 Å diffraction, no structure§ (%) | Structures solved¶ (%) |
|---|---|---|---|---|---|
| A | 24 | 16 (67) | 0 (0) | 4 (17) | 4 (17) |
| B | 26 | 22 (85) | 0 (0) | 1 (4) | 9 (35) |
| C | 22 | 18 (82) | 4 (18) | 5 (23) | 2 (9) |
| D | 7 | 6 (86) | 2 (29) | 2 (29) | 0 (0) |
| Total | 79 | 62 | 6 | 12 | 15 |
The classification into four grades, A—D, by 1D 1H NMR screening is described in the text. A and B are proteins that are now routinely forwarded for extensive coarse- and fine-screen crystallization trials, whereas C and D proteins are only subjected to coarse-screen crystallization trials. For details on the 79 proteins, see Table 2.
Number of proteins in each category.
The number of proteins that crystallized in at least one coarse-screen crystallization condition. Two A proteins had been removed from the pipeline for structure determination by NMR.
The number of proteins for which the best crystals diffracted to no higher than 5.0 Å.
The number of proteins for which the best crystals diffracted to better than 5.0 Å, but no structure is as yet available.
The number of proteins for which high-resolution crystal structures have been determined.
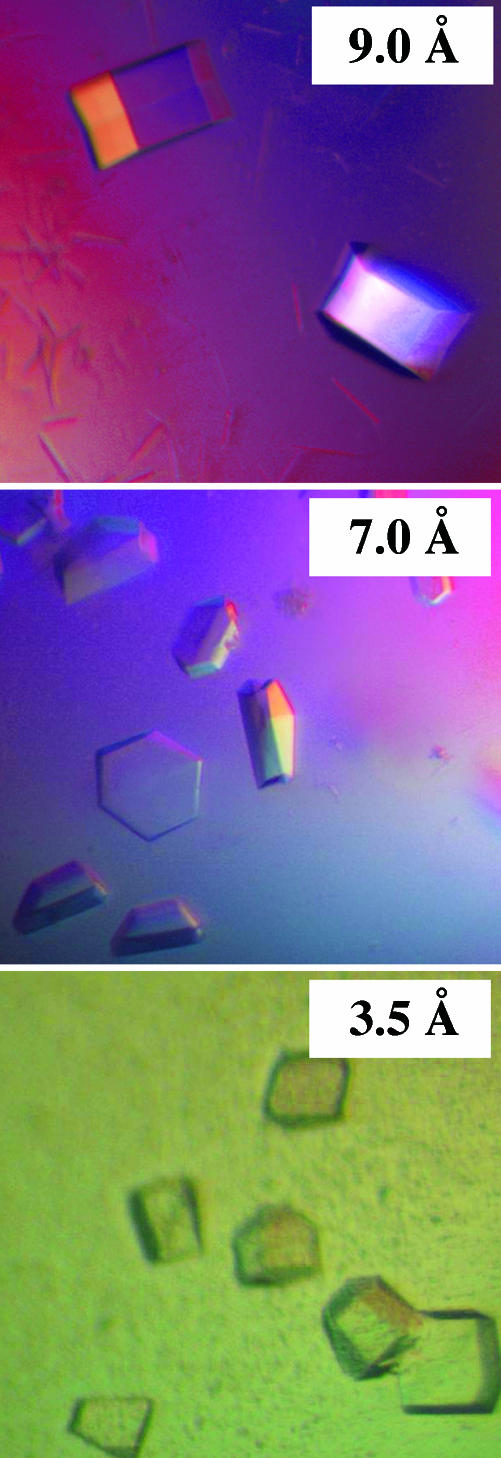
Fig. 3.
Examples of well formed, yet poorly diffracting, crystals obtained with three proteins of NMR grades C or D. The three proteins all were subjected to extensive coarse- and fine-screen crystallization trials (between 17 and 27 fine screens per target), which eventually resulted in the production of macroscopically well formed crystals. However, none of the between 28 and 117 crystals per target examined yielded sufficient resolution for high-resolution structure determination. (Top) A. tumefaciens hypothetical protein Atu1441 (15156516), 17 fine screens, 28 crystals screened; no structure; NMR grade, C. (Middle) S. cerevisiae α-α-trehalose phosphate synthase (YBR126C), 19 fine screens, 100 crystals screened; no structure; NMR grade, D. (Bottom) S. cerevisiae NGG1-interacting factor 3 (YGL221C), 27 fine screens, 117 crystals screened; no structure; NMR grade, C.
NMR Screening as a Guide for Optimizing Target Constructs. Among the group of 79 targets used in the present study, some proteins originated from constructs that previously failed to yield to high-resolution crystal structure determination. Based on sequence analysis with the programs pondr (18) and disembl (19), which are routinely used to predict “unstructured” polypeptide segments in proteins, variant constructs have been designed of such “parent targets,” provided that these had A- or B-grade 1D 1H NMR spectra. These variant constructs again were screened by using 1D 1H NMR spectroscopy, and the most highly graded constructs were forwarded for fine-screen crystallization efforts. So far, this approach allowed two otherwise intractable targets to proceed by means of variant constructs that subsequently yielded high-resolution crystal structures.
NMR screening also has been used to resurrect, without recloning, a number of targets that had been abandoned previously, because they initially failed to crystallize. Specifically, 12 such proteins that were found to have NMR grades A or B were reprocessed through the pipeline. To date, 3 of these 12 constructs, i.e., Saccharomyces cerevisiae probable nicotinate phosphoribosyltransferase (Systematic name YOR209C), Agrobacterium tumafaciens hypothetical protein Atu5508 (GenBank accession no. 15162326), and S. cerevisiae putative serine hydrolyase (Systematic name YDR428C), have produced diffraction-quality crystals after fine-screening, and their structures have been determined to high resolution. The remaining nine targets are currently in fine-screen crystallization trials.
Conclusion
NMR can be a highly beneficial part of a structural genomics pipeline because of its unique ability to provide detailed biophysical information about the protein targets under investigation at an early stage of the structure determination process. The NMR grading system described here enables efficient dissemination of the NMR results to the different cores of a large structural genomics effort. Two points stand out for their interest. First, experience has shown that 1D 1H NMR with proteins at natural isotope distribution is adequate for the screening in a structural genomics pipeline that uses primarily x-ray crystallography for structure determination. This finding makes the approach described in this paper also economically attractive. Second, although proteins that present poorly in the NMR screening tend to yield comparable numbers of crystal hits as proteins with high-quality solution 1H NMR spectra, proteins with high NMR grades nearly exclusively yield crystals that diffract to high resolution. Finally, the question arises about automation of the analysis of the NMR spectra recorded during extensive screening projects. Although this could become an attractive project in the future, it appears nonetheless that inspection of the spectra by an experienced NMR spectroscopist would be difficult to replace with a noninteractive procedure, particularly in situations where line shape effects from multimer formation or nonspecific aggregation has to be taken into account.
Supplementary Material
Acknowledgments
We thank all of the colleagues who contribute to the infrastructure of the JCSG. The JCSG consortium is funded by National Institutes of Health Grant GM062411. W.P. was a fellow of the Erwin Schrödinger Foundation (J2145) and the Max Kade Foundation. K.W. is the Cecil H. and Ida M. Green Professor of Structural Biology at The Scripps Research Institute.
Abbreviation: JCSG, Joint Center for Structural Genomics.
References
- 1.McDonald, C. C. & Phillips, W. D. (1967) J. Am. Chem. Soc. 89, 6332–6341. [DOI] [PubMed] [Google Scholar]
- 2.Edwards, A. M., Arrowsmith, C. H., Christendat, D., Dharamsi, A., Friesen, J. D., Greenblatt, J. F. & Vedadi, M. (2000) Nat. Struct. Biol. 7, Suppl., 970–972. [DOI] [PubMed] [Google Scholar]
- 3.Prestegard, J. H., Valafar, H., Glushka, J. & Tian, F. (2001) Biochemistry 40, 8677–8685. [DOI] [PubMed] [Google Scholar]
- 4.Rehm, T., Huber, R. & Holak, T. A. (2002) Structure (London) 10, 1613–1618. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy, M. A., Montelione, G. T., Arrowsmith, C. H. & Markley, J. L. (2002) J. Struct. Funct. Genomics 2, 155–169. [DOI] [PubMed] [Google Scholar]
- 6.Yee, A., Chang, X., Pineda-Lucena, A., Wu, B., Semesi, A., Le, B., Ramelot, T., Lee, G. M., Bhattacharyya, S., Gutierrez, P., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama, S. (2003) Curr. Opin. Chem. Biol. 7, 39–43. [DOI] [PubMed] [Google Scholar]
- 8.Styles, P., Soffe, N. F., Scott, C. A., Cragg, D. A., Row, F., White, D. J. & White, P. C. J. (1984) J. Magn. Reson. 60, 397–404. [DOI] [PubMed] [Google Scholar]
- 9.Styles, P., Soffe, N. F. & Scott, C. A. (1989) J. Magn. Reson. 84, 376–378. [Google Scholar]
- 10.Hajduk, P. J., Gerfin, T., Boehlen, J.-M., Häberli, M., Marek, D. & Fesik, S. W. (1999) J. Med. Chem. 42, 2315–2317. [DOI] [PubMed] [Google Scholar]
- 11.Lesley, S. A. (2001) Protein Expression Purif. 22, 159–164. [DOI] [PubMed] [Google Scholar]
- 12.Lesley, S. A., Kuhn, P., Godzik, A., Deacon, A. M., Mathews, I., Kreusch, A., Spraggon, G., Klock, H. E., McMullan, D., Shin, T., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 11664–11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peti, W., Etezady-Esfarjani, T., Herrmann, T., Klock, H. E., Lesley, S. A. & Wüthrich, K. (2004) J. Struct. Funct. Genomics 5, 205–215. [DOI] [PubMed] [Google Scholar]
- 14.Page, R., Grzechnik, S. K., Canaves, J. M., Spraggon, G., Kreusch, A., Kuhn, P., Stevens, R. C. & Lesley, S. A. (2003) Acta Crystallogr. D 59, 1028–1037. [DOI] [PubMed] [Google Scholar]
- 15.Peti, W., Norcross, J., Eldridge, G. & O'Neil-Johnson, M. (2004) J. Am. Chem. Soc. 126, 5873–5878. [DOI] [PubMed] [Google Scholar]
- 16.Christendat, D., Yee, A., Dharamsi, A., Kluger, Y., Savchenko, A., Cort, J. R., Booth, V., Mackereth, C. D., Saridakis, V., Ekiel, I., et al. (2000) Nat. Struct. Biol. 7, 903–909. [DOI] [PubMed] [Google Scholar]
- 17.Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids (Wiley, New York).
- 18.Dunker, A. K., Garner, E., Guilliot, S., Romero, P., Albrecht, K., Hart, J., Obradovic, Z., Kissinger, C. & Villafranca, J. E. (1998) Pacific Symp. Biocomput. 3, 473–484. [PubMed] [Google Scholar]
- 19.Linding, R., Jensen, L. J., Diella, F., Bork, P., Gibson, T. J. & Russell, R. B. (2003) Structure (London) 11, 1453–1459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.