Abstract
Inflammatory bowel disease reflects an aberrant mucosal CD4+ T cell response to commensal enteric bacteria. In addition to regulatory T cell subsets, recent studies have revealed a protective role of B cells in murine CD4+ T cell colitis, but the relationship of their action to T cell immunoregulation is unknown. Here we report that mesenteric lymph node (MLN) B cells protect mice from colitis induced by Gαi2–/– CD4+ T cells. Protection required the transfer of both B cells and CD8α+ T cells; neither cell type alone was sufficient to inhibit CD4+ T cell-mediated colitis. Similar results were also observed in colitis induced by CD4+CD45RBhi T cells. Immunoregulation was associated with localization of B cells and expansion of CD4–CD8– CD3+NK1.1+ T cells in the secondary lymphoid compartment, as well as expansion of CD4+CD8α+ T cells in the intestinal intraepithelial compartment. MLN B cells from Gαi2–/– mice were deficient in a phenotypic subset and failed to provide cotransfer colitis protection. These findings indicate that protective action of B cells is a selective trait of MLN B cells acquired through a Gαi2-dependent developmental process and link B cells with the formation of regulatory T cells associated with mucosal immune homeostasis.
Keywords: inflammatory bowel disease, G proteins, immune regulation, NKT cells, CD8αα+ T cells
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, is a group of immune-mediated disorders of the intestine characterized by dysregulated mucosal T cell activity, aberrant cytokine production, cellular inflammation, and resultant intestinal tissue damage. Although the phenotype and pathogenic pathways leading to IBD are heterogeneous, they share in common a disordered CD4+ T cell response to commensal enteric bacteria, due in part to host genetic susceptibility traits (1).
Much attention has focused on regulatory CD4+ T cell subsets that protect against IBD and other autoimmune diseases. Regulatory CD4+ T lymphocytes are a heterogeneous population analytically distinguishable by expression of CD25 and FoxP3, among other markers, and may differ in activation requirements and targets of functional activity (2–4). Recently, additional mucosal T cell subsets with regulatory function have been reported. Intraepithelial T cells (5) and certain subsets of CD8+ T cell [intraintestinal T cell receptor (TCR)αβ+CD4–CD8α+ T cells and CD4+CD8αα+ T cells] (6, 7) suppress CD4+CD45RBhi T cell colitis. Intraepithelial lymphocytes (IEL) and small intestinal CD4+CD8αα+ IEL were associated with resistance to acute colitis induced by Toxoplasma gondii or dextran sodium sulfate, respectively (8, 9). In most of these studies, genetic manipulations implicated intrinsic IL-10 expression for the protective action of both CD8+ T cell subsets and invariant class I MHC recognition for the pertinent CD4+CD8αα+ cells.
Accumulating evidence also implicates B cells in immunoregulation. The work of Mizoguchi et al. (10, 11) has revealed a protective role of B cells in TCRα–/– colitis and implicated intrinsic B cell expression of CD1d and IL-10 in their immunoregulatory function. A recent study further suggested IL-10-independent protective function of B cells in an intestinal inflammation caused by nonlymphocytes, implicating the regulatory role of B cells in innate immunity (12). In mouse models of oral tolerance, B cells are important for maintenance of a protective cytokine microenvironment in gut-associated lymphoid tissue (13). Similarly, B cells were also required for efficient induction of antigen-specific T cell unresponsiveness in the ocular anterior chamber (14, 15) and in the lung in the context of respiratory allergen (16). B cells also attenuate immune responses in certain autoimmune models. In experimental autoimmune encephalomyelitis, depletion of B cells promoted severe nonremitting disease (17, 18). In collagen-induced chronic arthritis, B cells from arthritogenic splenocytes treated in vitro with anti-CD40 inhibit arthritis in an IL-10-dependent manner (19). B cell immunoregulation typically required their intrinsic expression of IL-10 (11, 17, 19, 20) and, in some cases, the expression of certain invariant class I MHC molecules (11, 14, 15, 21). These observations introduce important questions about the developmental origin of protective B cell subsets and the cellular interactions through which they contribute to immunoregulation by regulatory T cell populations.
Recently, we and others have reported the selective capacity of marginal zone (MZ) B cells to produce IL-10 (22, 23). This B cell subset is selectively deficient in mice bearing restricted enteric microflora and in Gαi2–/– mice, a colitis-prone strain characterized by decreased IL-10 production and polarized T helper (Th) 1 reactivity (24, 25). Gαi2 protein is one subunit of the trimeric G protein family of signaling molecules involved in the regulation of cellular physiological function. The developmental deficiency of IL-10-producing B cells in restricted flora mice and Gαi2–/– mice raises the possibility that these subsets of B cells may have immune regulatory function in mucosal homeostasis and IBD resistance.
In the present study, we report that mesenteric lymph node (MLN) B cells protect mice from CD4+ T cell colitis through interactions with regulatory T cell populations. This protective role is displayed by selected B cell subsets, whose formation requires the Gαi2 gene. Protection requires the combined transfer of B cells and CD8+ T cells and is associated with the expansion of a CD4+CD8α+ T cell population in the intraepithelial intestinal compartment. However, transferred B cells strictly home to secondary lymphoid sites, and protection is associated with expansion of CD3+NK1.1+ T lymphocytes (NKT cells) in this compartment. Accordingly, these findings indicate that B cells contribute to intestinal immunoregulation through recruitment of novel regulatory T cell subsets. Furthermore, they suggest a sequential process involving B cells and NKT cells in the secondary lymphoid compartment, leading to activation and end-organ expansion of a regulatory CD4+CD8α+ T cell population.
Methods
Mice. Gαi2–/– (129Sv background) mice were from a breeding colony originally generated at Baylor College of Medicine (Houston) (25) and housed in the University of California, Los Angeles (UCLA) animal facility. RAG2–/– mice (129Sv background) and C.B-17scid mice were obtained from the UCLA Department of Radiation Oncology or purchased from Taconic Farms (Germantown, NY). BALB/c, C57BL/6, and 129Sv mice were obtained from The Jackson Laboratory. All of the mice were housed under specific pathogen-free conditions in the animal facilities at UCLA. The mice used in all experiments were 5–12 weeks old and were handled according to the guidelines and approved protocols of the UCLA Animal Research Committee.
Reagents. FITC-, phycoerythrin (PE)-, peridinin chlorophyll protein-, allophycocyanin- or biotin-conjugated mAbs specific for mouse B220 (RA3-6B2), CD19, IgM, CD3, CD4, CD8α, CD1d, CD21, CD23, or NK1.1 were purchased from BD Pharmingen. FITC- or biotin-conjugated anti-mouse IgD Abs were the products of eBioscience (San Diego). A CD4+ T cell isolation kit and anti-CD43, CD19, CD8α, anti-PE, or anti-biotin MicroBeads and MACS Separation LS Columns were from Miltenyi Biotec (Auburn, CA).
Flow Cytometry. B cells were stained with Abs against B220, CD19, CD1d, IgM, IgD, CD21, CD23, and CD24 to characterize splenic the MZ B cell population (CD21hiCD23loIgMhiCD1dhi) and the MLN B cell population. T cells were stained with Abs against mouse CD3, CD4, CD8α, and NK1.1. Flow cytometry analysis was usually performed on CD19 or CD3 gates for B cells and T cells, respectively. Data of multicolor-stained lymphocytes were then collected on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed with cellquest software.
Isolation of Intestinal Lymphocytes. Small and large intestinal IEL and lamina propria lymphocytes (LPL) were prepared as described in ref. 26.
Isolation of Colitigenic T Cells. Gαi2–/–CD3+ T cells were isolated from the spleens of Gαi2–/– mice by staining single splenic lymphocytes with biotinylated anti-CD3 Ab followed by anti-biotin MicroBeads and positive selection on a MACS column (Miltenyi Biotec). Gαi2–/–CD4+ T cells were isolated by using anti-CD4 MicroBeads or a CD4+ T cell isolation kit and were >90% pure by flow cytometry. CD4+CD45RBhi BALB/c T cells were isolated by staining splenic cells with anti-CD45RB-FITC and anti-CD4-PE Ab and sorted by using a FACSVantage instrument.
Isolation of B Cell Subsets. To prepare enriched splenic MZ B cells, B cells (B220+CD43–) were isolated by negative selection with anti-mouse CD43 (Ly-48) MicroBeads. The cells from flowthrough fraction (CD43–) were stained with PE-conjugated anti-CD23 followed by negative selection with anti-PE MicroBeads to enrich CD43–CD23lo B cells. To improve the purity of MZ B cells, CD43–CD23lo B cells were stained with anti-mouse CD21-FITC Ab followed by anti-FITC MicroBeads. This treatment yielded a population of 55% MZ B cells (CD19+CD21hiCD23lo). To prepare MLN B cells, MLN cells were positively selected with anti-CD19 MicroBeads. CD19+ B cells were then further stained with anti-IgM-PE Ab and positively selected with anti-PE MicroBeads. These populations were >95% CD19+IgM+ cells.
Assessment of Mucosal Inflammation. To establish colitis, 106 Gαi2–/– T cells (CD3+ or CD4+) or BALB/c T cells (CD4+CD45RBhi) were injected into the peritoneal cavity of recipients (RAG2–/– or C.B-17scid mice, respectively). In cases where CD8 T cells or B cells were also transferred, they were administered i.v. in 0.1 ml of sterile saline on the same day. The recipients were observed daily and weighed twice a week for clinical signs of disease. When the mice became moribund or show significant clinical signs of disease, the mice were killed for tissue harvest. The histological scoring for colitis utilizes an established scale using inflammatory and epithelial parameters, as previously reported (27). The histological scores were analyzed by the Student t test or one-way ANOVA (Tukey's multiple comparison test).
Results
Gαi2–/– T Cell Transfer Model for CD4+ T Cell Colitis. Native Gαi2–/– mice on a 129 or a mixed B6/129 background spontaneously develop colitis at the age of 6–8 weeks, a process that depends on Th1 CD4+ T cell response and enteric microflora (22, 24, 28–30). The model involves functional disorders in epithelial cells (31), professional antigen-presenting cells (32), B cells (22, 33), and CD4+ T cells (24, 29, 30, 32), suggesting multiple requirements for Gαi2 in the formation of normal mucosal immunoregulation. To better define these roles, we have established a transfer model permitting separate evaluation of different Gαi2–/– cell types in the development of colitis. Isolated total CD3+ or CD4+ T cells from spleens or MLN of Gαi2–/– mice were transferred i.v. or i.p. into RAG2–/– mice. Mice receiving 106 total CD3 or CD4 splenic Gαi2–/– T cells through i.v. injection developed acute colonic inflammation, manifested by bloody diarrhea within the first week, proceeding to death in 2–3 weeks. In comparison, mice receiving 0.5–1 × 106 Gαi2–/– T cells through i.p. injection developed chronic colitis beginning at 2–3 weeks, manifested by initial constipation followed by mild diarrhea, progressive weight loss, and colonic fibrosis. Lethality was observed in a minority of mice in 1–2 months.
To further characterize this chronic colitis, histological examination demonstrated severe mucosal inflammation of the large intestine (Fig. 1A), with mild inflammation also observed in the small intestine (Fig. 1B). By flow cytometry, CD4+ T cells were the predominant T cell population in spleen and MLN, with a relative increase compared with other T cell subpopulations in the inoculated lymphocyte population. In the large intestinal IEL and LPL compartments, resident lymphocytes are mainly CD4+ T cells, correlating with the severe mucosal damage in the large intestine. In the small intestine, where mucosal inflammatory damage was minimal or absent, CD8+ T cells are the dominant cell population in both IEL and LPL compartments (Fig. 1D). In contrast, wild-type T cells from 129Sv mice failed to induce colitis, demonstrating that the immunologic disorder required T cells formed in the Gai2–/– genotype (data not shown).
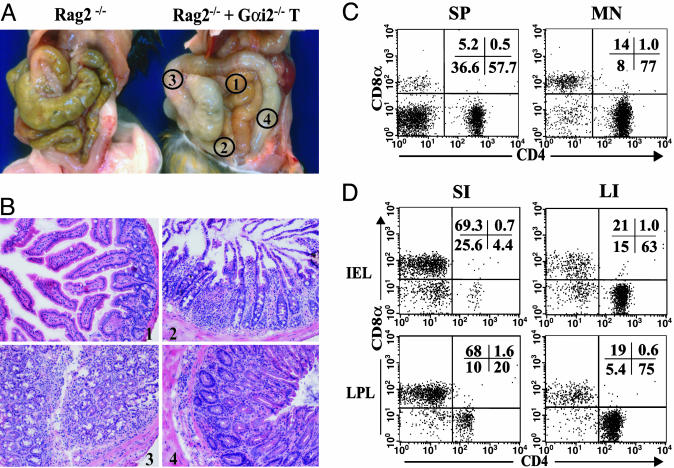
Fig. 1.
Features of colitis by Gαi2–/– T cell transfer to RAG2–/– mice. A total of 106 CD3+ T cells from spleens of Gαi2–/– mice were transferred i.p. into RAG2–/– mice. Two to 3 weeks after T cell transfer, the mice started to show the signs of clinic disease. Necroscopy was performed at ≈5 weeks after T cell transfer. (A) Organ examination showing RAG2–/– mice alone or after CD3+ T cell transfer. Regions selected for histologic examination were as follows: 1, small intestine; 2, ileum; 3, proximal colon; 4, distal colon. (B) Representative histology (stained with hematoxylin/eosin) of the four regions. (C and D) Flow cytometry of size-gated lymphocytes for CD4 and CD8 expression. (C) Splenic (SP) and MLN lymphocytes in 5-week-old recipients. (D) IEL and LPL in small intestine (SI) and large intestine (LI) in 5-week-old recipients.
Selective Protection by MLN B Cells in Gαi2–/– T Cell Colitis. MLN B cells are protective against colitis in the TCRα–/– mouse (10, 11), and we wondered whether an immunoregulatory role of B cells may be pertinent in Gαi2–/– colitis. To test this idea, total CD3+Gαi2–/– T cells were cotransferred with enriched splenic MZ B cells or MLN B cells in syngeneic RAG2–/– mice. Two to 3 weeks after Gαi2–/– T cell transfer, recipients of Gαi2–/– T cells alone showed signs of clinical disease, including weight loss and diarrhea (Fig. 2A). Histological examination demonstrated severe mucosal inflammation, especially in the large intestine (Fig. 2B). Cotransfer of MZ B cells delayed onset of weight loss and moderately reduced histologic inflammation. However, cotransfer of MLN B cells showed no evidence of clinical disease and no histological intestinal inflammation. As a negative control, transfer of B cell populations alone did not result in colitis or intestinal inflammation. These findings indicate a hierarchy of protective activity of MLN B cells in Gαi2 colitis. The scores of colitis severity indicated that the mucosal inflammation was slightly attenuated in the mice that received MZ B cells and Gαi2–/– T cells (Fig. 2B). MZ B cells delayed colitis onset but failed to provide sustained protection. In contrast, the CD19+IgMhi B cells from MLN protected mice from Gαi2–/– T cell-induced colitis.
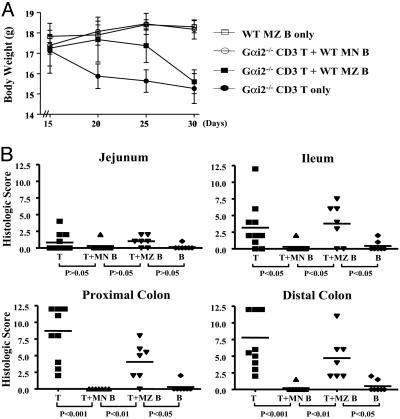
Fig. 2.
MLN B cells but not MZ B cells are protective in CD3+Gαi2–/– T cell colitis. CD3+Gαi2–/– T cells were transferred into RAG2–/– mice either alone or with cotransfer of wild-type splenic MZ or MLN CD19+IgMhi B cells. (A) Mice were monitored over time for clinical disease including body weight change (mean ± SD). (B) Approximately 5 weeks after cell transfer, mice were killed, replicate colon specimens from each mouse were prepared for histology, and inflammatory scores were prepared by an experienced pathologist blinded for specimen identity. Each dot represents the score of the specimen from an individual mouse. Lines indicate mean values of histologic scores in each group.
To understand whether the protective effect of MLN B cells on Gαi2–/– T cell colitis correlates with the localization of transferred B cells in the RAG2–/– recipients and how MLN B cells affect pathogenic Gαi2–/– T cells, we assessed the distribution of transferred B cells and Gαi2–/– T cells. CD19+IgMhi B cells in recipients of MZ and MLN B cells were present in the spleen (9.7% and 7.6%, respectively, for three or more experiments) and MLN (2.7% and 2.8%, respectively). Because the number of recovered CD19+IgMhi B cells in MZ B cell recipients and MLN B cell recipients was equivalent, it appeared that differential homing efficiency of MLN B cells did not account for their greater colitis protection. Notably, only rare CD19+IgMhi B cells were found in the intestinal epithelium-associated lymphoid follicles (0.7% and 0.8%) and LPL compartments (<0.5%), indicating that colitis protection is not likely to occur by the action of B cells in local intestinal sites.
Expansion of T Cell Subsets Correlates with MLN B Colitis Protection. We then assessed whether changes in T cell subsets were associated with colitis protection. The percentages of CD8α+ and CD4+ T cells were increased and reduced, respectively, in spleens and MLN of protected mice (MLN B cell cotransfer), as compared with unprotected mice (T cells alone or T cells plus MZ B cells) (Fig. 3A). The absolute numbers of splenic and MLN T cells was reduced ≈2-fold with protection. Accordingly, the major change appeared to be a 4-fold expansion of CD4+ T cells in mice with active disease. Notably, there was an expansion of NKT cells (CD3+NK1.1+) in the spleens/MLN of MLN B cell-protected healthy mice (Fig. 3B). No changes in percentage or absolute numbers of CD4+CD25+ T cells (that might include a regulatory CD4+ T lymphocyte population) were observed in central (spleens or MLN) or intestinal (LPL or IEL) compartments (data not shown). These findings indicated that central expansion of NKT cells might be involved in colitis resistance.
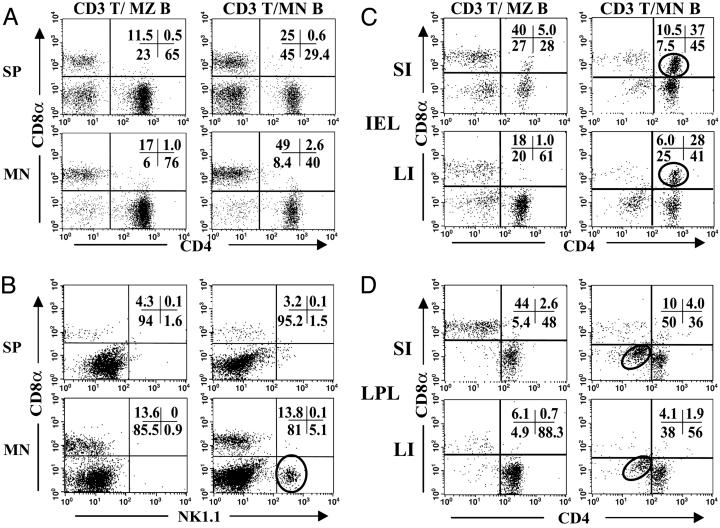
Fig. 3.
Protection is associated with expansion of central and intestinal T cell subsets. Mice were prepared as described in Fig. 2. Six to 8 weeks after transfer, “central” (spleen and MLN) (A and B) and large and small intestinal IEL and LPL (C and D) were isolated and stained for CD3 and subset markers. Flow cytometry was gated on CD3+ cells. (A) Central lymphocytes, CD4 and CD8α. (B) Central lymphocytes, NK1.1 and CD8α. (C) Intestinal IEL. (D) Intestinal LPL. Circles highlight the expanded cell subsets in MLN B cell-protected mice: NKT cells (B), DP T cells (C), and DN T cells (D).
In the intestine, MLN B cell cotransfer and colitis protection was associated with a large increase in CD4+CD8α+ double positive (DP) T cells in the small and large intestinal IEL compartments (Fig. 3C). In addition, CD3+CD4–CD8– double negative (DN) T cells were elevated in the large intestine LPL compartment (Fig. 3D). The DN T cells did not express NK1.1 (data not shown). The localization of these two expanded populations to the at-risk intestinal site (large intestine) and the selective correlation with MLN B cell transfer suggested that they might contribute locally to colitis protection.
CD8+ T Cells Are Required for MLN B Cell-Mediated Colitis Protection. Protection was associated with a relative and absolute expansion of CD8α+ T cells in central lymphoid and LPL compartments; therefore, we tested whether CD8α+ T cells might be required for this immunoregulatory phenotype. To test this idea, we first asked whether depletion of CD8α+ T cells from the CD3+Gαi2–/– T cell preparation abrogated the protective activity of cotransferred MLN B cells. Indeed, whereas colitis with total Gαi2–/– T cells was reversed by MLN B cells, colitis with CD4+Gαi2–/– T cells was resistant to MLN B cell protection (Fig. 4A). The resistance to MLN B cell protection was also reflected in the correlates of T cell expansion. Thus, neither central NKT cells nor intestinal CD4+CD8α+ DP T cells expanded in mice cotransferred with CD4+ T cells and MLN B cells (Fig. 4 B and C). It should be noted that detectable populations of NKT and CD4+CD8α+ DP T cells were observed in these mice. This finding probably reflects transfer and expansion of residual CD8α+ T cells in the donor populations and perhaps also the induction of CD8α expression in activated T cells (34).
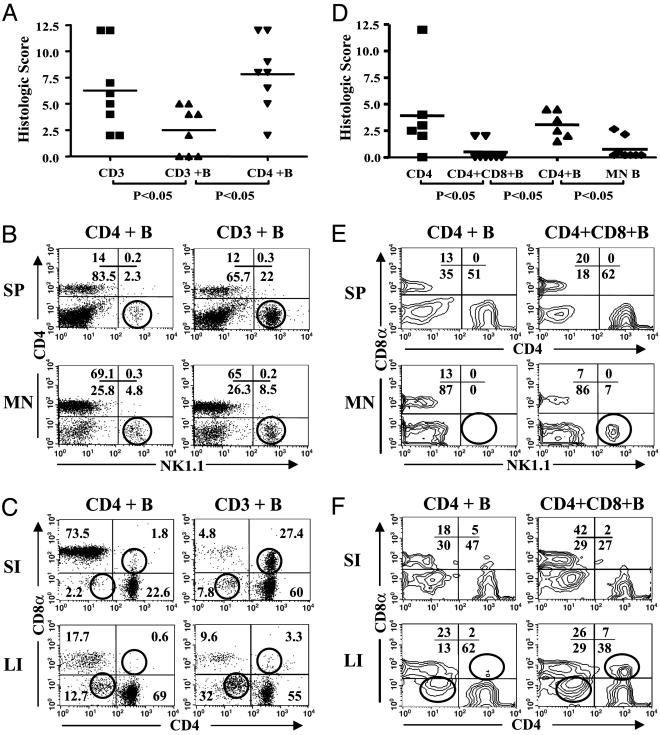
Fig. 4.
CD8α+ cells are required for the immunoregulatory effects of MLN B cells. (A–C) Total CD3+ (CD3) or CD8α-depleted CD4+ (CD4) Gαi2–/– T cells were cotransferred with wild-type MLN B cells from littermate control mice (on 129 and C57BL/6 mixed background) into RAG2–/– mice. (D–F) CD4+CD45RBhi BALB/c T cells (CD4) or T cells cotransferred with BALB/c MLN B cells and CD8α+ T cells into C.B-17scid mice. As a negative colitis control, MLN B cells alone were transferred in one group (B only). All mice were assessed at 6–8 weeks. (A and D) Histological scores for colitis severity. (B and E) Flow cytometry of splenic (SP) or MLN lymphocytes for NKT cells. (C and F) Flow cytometry of intestinal lymphocytes for DP and DN populations. The circles in FACS plots indicate the changes of cell subsets in MLN B cell-protected and unprotected mice.
Intestinal disease produced by transfer of CD4+CD45RBhi T cells is a widely used model for Th1-like immune colitis (1, 35). Therefore, we wondered whether this model also would be subject to protection by MLN B cells and CD8α+ T cells. As shown in Fig. 4D, CD4+CD45RBhi T cells induced colitis (significant compared with control mice receiving B cells alone) by 6–8 weeks, although it was moderate in severity compared with that produced by Gαi2–/– T cells. This moderate disease level, representative of four experiments, was also reported by another group at UCLA (27) and may be attributable in part to the ambient commensals at the UCLA animal facility. The recipients were then tested for the effect of cotransfer with MLN B cells and/or CD8α+ T cells (Fig. 4D). Indeed, mice receiving both of these cell types were significantly reduced in their histologic score for colitis, declining to a level insignificantly different from B cells alone. MLN B cells did not protect without CD8α+ T cells (Fig. 4D), and CD8α+ T cells were also ineffective without B cells (data not shown). In protected mice, flow cytometric analysis demonstrated an expansion of NK1.1+ T cells (Fig. 4E) and IEL DP and DN T cells (Fig. 4F). These expansions were consistent in protected versus unprotected mice. The relative amount of these changes in spleen and MLN or large and small intestine in different individuals correlates with colitis protection induced by MLN B cells.
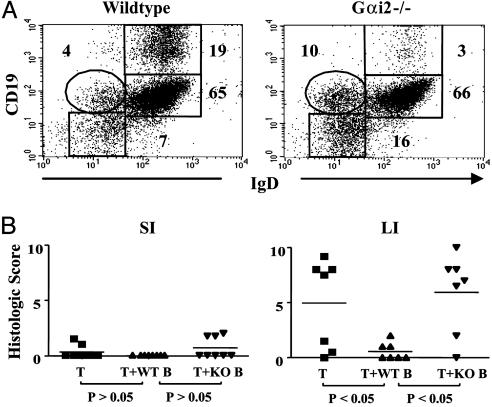
Deficiency of Protective MLN B Cells in Gαi2–/– Mice. Certain B cell subsets, including those efficient for IL-10 production, are deficient in the colitis-prone Gαi2–/– mouse (22); therefore, we wondered whether the B cell developmental impairment might also involve the protective MLN B cell population. We first compared the surface marker phenotype of MLN B cells in wild-type and Gαi2–/– mice. The phenotypes of B cell populations were indistinguishable between wild-type and mutant mice by several markers in unmanipulated MLN preparations. However, after our usual positive selection procedure for MLN B cells with anti-CD19 and anti-IgM, 20% of cells acquired a CD19hi phenotype in wild-type cells (Fig. 5A). In contrast, this subset of CD19hi did not form in Gαi2–/– cells. We then cotransferred standard numbers of wild-type and Gαi2–/– MLN B cells and evaluated their protective activity in Gαi2–/– T cell colitis. As shown in Fig. 5B, wild-type MLN B cells were protective, but Gαi2–/– MLN B cells failed to suppress colitis, implying that the protective subset might be included in the CD19hi MLN B cell subset. These findings indicate that Gαi2 protein is important to the development and function of regulatory B cells.
Fig. 5.
Gαi2–/– MLN B cells lack protective activity. (A) Flow cytometry analysis of MLN B cells (CD19 vs. IgD) after immunobead purification from wild-type (129Sv) or Gαi2–/– MLN lymphocytes. (B) Mice were prepared with total Gαi2–/– T cells and MLN B cells (from wild-type or Gαi2–/– cells) as described in Fig. 2, and intestinal tissues were collected at 6–8 weeks for determination of histological scores. Data are compiled from three independent experiments.
Discussion
Mechanisms of host resistance to mucosal inflammation are an important clue to pathogenesis and are potential targets for disease treatment and prevention. Investigation of protection and resistance in immune colitis has focused particularly on immunoregulatory CD4+ T cells. The present study provides new evidence for the regulatory participation of other T cell subsets, notably CD8α+ and NKT cells, in the regulation of Th1-mediated colitis. Moreover, it uncovers an unexpected, concerted role of MLN B cells in this protection and evidence that the formation of these cooperating B cells depends on Gαi2 sufficiency.
This study exploits the colitigenic activity of CD4+Gαi2–/– T cells after adoptive transfer into immunodeficient recipients. In this model, a small T cell inoculum produces rapid onset (2–3 weeks) and highly penetrant colitis, manifested by weight loss, gross and microscopic inflammation, and abundant CD4+ T cell mucosal infiltrate. These findings are consistent with the Th1 CD4+ T cell colitis in native Gαi2–/– mice (24, 25, 28). Unlike native disease, adoptive transfer included prominent early colonic fibrosis and subclinical ileitis (but not proximal small intestinal inflammation). Ileitis, a feature of human Crohn's disease, is observed in few of the many IBD animal models (e.g., HLA-B27 rats, SAMP1/Yit mice, and a genetic form of Th2 enteritis) (36–38). The reason for this additional localization is uncertain, but it may reflect impairments of host defense that normally prevent extension of cecal bacteria into the ileal region or a deficit of regional immune regulation that inhibits colitigenic T cell activity in the small intestine.
In the present study, cotransfer of MLN B cells protected mice from the formation of colitis in two adoptive transfer models (Gαi2–/– T cells and CD4+CD45RBhi T cells), analogous to the finding of such protection in TCRαβ–/– mice (10, 11). The latter studies reported that B cells with protective activity were anatomically localized to the MLN (but not to the spleen). In the present study, we also observed selective activity in the MLN B cell population. In our study, purified MZ B cells [a splenic subpopulation sharing with MLN B cells the CD21+IgMhiCD1dhi phenotype (39)] had reduced but detectable activity. The pertinent B cell protective traits associated with MLN compartmentalization might be attributed to the acquisition of enteric antigens, enabling presentation and regulatory cell–cell interactions with target T cell populations. Alternatively, differentiation or activation of B cells in this compartment might include up-expression of cell-interaction molecules important for protective activity, such as CD1d and IL-10 (11). The present study demonstrates that formation of protective B cells requires Gαi2 genetic sufficiency, which is also required for the formation of B cell subsets important for bacterial immune surveillance (22). Phenotypically, these subsets include splenic MZ B cells and a MLN B cell subset with elevated CD19 and membrane IgM expression. These findings provide clues to the phenotype of protective B cells and suggest that their impaired formation in Gαi2–/– mice might be a contributing factor in colitis susceptibility.
An important issue is the identity of the cell types targeted by protective B cells. A surprising observation was the requirement for CD8α+ T cell cotransfer, suggesting that a CD8α+ T cell subset either is a partner with or a target of protective B cells in the immunoregulatory circuitry. This finding is concordant with the reported role of CD8α+ T cells in mucosal immunoregulation (5–7, 9). One candidate regulatory subset is the CD8+ T cell population recognizing the invariant MHC class I molecule Qa-1 (HLA-E in human), which is expressed at high levels on certain B cell subpopulations. Qa-1 recognition may include mCD94/NKG2A (40) and induces negative regulatory activity in experimental autoimmune encephalomyelitis, oral tolerance, and B cell-dependent ocular tolerance (14, 21, 41). Another candidate is intestinal CD4+CD8α+ DP T cells, because they were expanded during B cell-mediated protection. Mucosal DP T cells represent a memory CD4+ T cell population enriched for agonist-selected, self-reactive clones (34, 42, 43). A regulatory role for DP T cells is suggested by recruitment of T cells with intraepithelial invariant MHC class I molecule (MICA) (9) and expansion of CD4+ T cells undergoing NF-κB- and GATA3-dependent Th2 polarization (7). Another invariant MHC class I molecule, MR1, selects for a distinct lamina propria and mucosal lymphoid TCRαβ+ T cell population. Although their regulatory role is not yet defined, it is notable that their formation depends on commensal bacteria and B cell interaction (44).
NKT cells in some settings suppress immune inflammation, including intraocular inflammation (15) and certain models of colitis (11, 45). The mechanism for this protection is uncertain. NKT cells are known to promote Th2 differentiation and, accordingly, exacerbate Th2-dependent mucosal inflammation in the lung and gut (46, 47). However, their protective role is unlikely to involve IL4 itself, because administration of this cytokine exacerbates common models of Th1 colitis (48), and CD1d-restricted immune function protects against a Th2-like colitis (11). NKT cells efficiently activate certain B cell populations (49), raising the possibility that they might efficiently activate B cell subsets contributing to colitis protection. The present study adds new evidence for this scenario by the demonstration of B cell-dependent expansion of NKT cells centrally and DN T cells intraepithelially during colitis protection. Yet to be resolved is the clonal relationship of the NKT and DN T cell populations and their functional interaction with CD8- and B cell-dependent colitis protection.
In many mouse models of mucosal inflammation, regulatory CD4+CD45RBlo T cells, in part through TGFβ and IL10 production, suppress formation of colitigenic T cells (1). A CD25+ subset of these cells appears to suppress activity of colitigenic cells already deployed in the lamina propria (3, 35, 50). As noted above, other T cell subsets [intraepithelial CD8+ (5, 6), CD4+CD8+ (7, 9), or NKT (11, 45) cells] also confer colitis resistance in some settings. At this juncture, it is not yet clear how B cell-dependent expansion of these central or mucosal T cell populations may reduce colitigenic CD4+ T cell activity. Protection may involve cognate mechanisms or nonspecific competitive processes such as homeostatic proliferation (51). An experimental starting point will be repletion of these activated T cell populations to test the specificity and numerical requirement for negative regulation. It is also possible that the B cells themselves may reduce inflammation by restoring mucosal IgA production, because secretory IgA reduces the scale and diversity of enteric microorganisms that can drive colitigenic T cell activity. Notably, formation of the Peyer's patch compartment (a major site of IgA B cell formation) is impaired in Gai2–/– mice (33). In conclusion, the present study confirms and expands the definition of a B cell contribution to mucosal immunoregulation. Further definition of this B cell role may be important in illuminating the manner in which different T cell populations are recruited and integrated to preserve mucosal homeostasis.
Acknowledgments
We thank Dr. William McBride (UCLA) for RAG2–/– mice and Dr. Tiffany Huang (UCLA) and Matthew Schrage (UCLA) for generation and maintenance of the Gαi2–/– mice. We are grateful to Dr. Hilde Cheroutre (La Jolla Institute for Allergy and Immunology, San Diego) and members of the J.B. laboratory for advice and critical reading of the manuscript. This study was performed at UCLA and supported by National Institutes of Health (NIH) Grants DK46763 (to J.B.), DK69434 (to J.B. and B.W.), DK19318 (to L.B.), and AI058919 (to P.V.) and a career development award from the Crohn's & Colitis Foundation of America (to B.W.). Flow Cytometry was performed in the UCLA Flow Cytometry Core Facility, supported by NIH Awards CA-16042 and AI-28697.
Author contributions: B.W., P.V., R.A., M.K., and J.B. designed research; B.W., P.V., O.T., and K.S. performed research; B.W., P.V., O.T., L.B., and J.B. analyzed data; B.W., P.V., O.T., R.A., M.K., L.B., and J.B. wrote the paper; and K.S., R.A., and L.B. contributed new reagents/analytic tools.
Abbreviations: DN, double negative; DP, double positive; IBD, inflammatory bowel disease; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes; MLN, mesenteric lymph node; MZ, marginal zone; NKT cells, CD3+NK1.1+ T lymphocytes; PE, phycoerythrin; TCR, T cell receptor; Th, T helper; UCLA, University of California, Los Angeles.
References
- 1.Strober, W., Fuss, I. J. & Blumberg, R. S. (2002) Annu. Rev. Immunol. 20, 495–549. [DOI] [PubMed] [Google Scholar]
- 2.McHugh, R. S. & Shevach, E. M. (2002) J. Immunol. 168, 5979–5983. [DOI] [PubMed] [Google Scholar]
- 3.Mottet, C., Uhlig, H. H. & Powrie, F. (2003) J. Immunol. 170, 3939–3943. [DOI] [PubMed] [Google Scholar]
- 4.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057–1061.12522256 [Google Scholar]
- 5.Laroux, F. S., Norris, H. H., Houghton, J., Pavlick, K. P., Bharwani, S., Merrill, D. M., Fuseler, J., Chervenak, R. & Grisham, M. B. (2004) Int. Immunol. 16, 77–89. [DOI] [PubMed] [Google Scholar]
- 6.Poussier, P., Ning, T., Banerjee, D. & Julius, M. (2002) J. Exp. Med. 195, 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das, G., Augustine, M. M., Das, J., Bottomly, K., Ray, P. & Ray, A. (2003) Proc. Natl. Acad. Sci. USA 100, 5324–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mennechet, F. J., Kasper, L. H., Rachinel, N., Minns, L. A., Luangsay, S., Vandewalle, A. & Buzoni-Gatel, D. (2004) Eur. J. Immunol. 34, 1059–1067. [DOI] [PubMed] [Google Scholar]
- 9.Park, E. J., Takahashi, I., Ikeda, J., Kawahara, K., Okamoto, T., Kweon, M. N., Fukuyama, S., Groh, V., Spies, T., Obata, Y., et al. (2003) J. Immunol. 171, 4131–4139. [DOI] [PubMed] [Google Scholar]
- 10.Mizoguchi, A., Mizoguchi, E., Smith, R. N., Preffer, F. I. & Bhan, A. K. (1997) J. Exp. Med. 186, 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizoguchi, A., Mizoguchi, E., Takedatsu, H., Blumberg, R. S. & Bhan, A. K. (2002) Immunity 16, 219–230. [DOI] [PubMed] [Google Scholar]
- 12.Gerth, A. J., Lin, L., Neurath, M. F., Glimcher, L. H. & Peng, S. L. (2004) Gastroenterology 126, 1115–1121. [DOI] [PubMed] [Google Scholar]
- 13.Gonnella, P. A., Waldner, H. P. & Weiner, H. L. (2001) J. Immunol. 166, 4456–4464. [DOI] [PubMed] [Google Scholar]
- 14.D'Orazio, T. J., Mayhew, E. & Niederkorn, J. Y. (2001) J. Immunol. 166, 26–32. [DOI] [PubMed] [Google Scholar]
- 15.Sonoda, K. H. & Stein-Streilein, J. (2002) Eur. J. Immunol. 32, 848–857. [DOI] [PubMed] [Google Scholar]
- 16.Tsitoura, D. C., Yeung, V. P., DeKruyff, R. H. & Umetsu, D. T. (2002) Int. Immunol. 14, 659–667. [DOI] [PubMed] [Google Scholar]
- 17.Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. (2002) Nat. Immunol. 3, 944–950. [DOI] [PubMed] [Google Scholar]
- 18.Wolf, S. D., Dittel, B. N., Hardardottir, F. & Janeway, C. A., Jr. (1996) J. Exp. Med. 184, 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauri, C., Gray, D., Mushtaq, N. & Londei, M. (2003) J. Exp. Med. 197, 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akbari, O., Freeman, G. J., Meyer, E. H., Greenfield, E. A., Chang, T. T., Sharpe, A. H., Berry, G., DeKruyff, R. H. & Umetsu, D. T. (2002) Nat. Med. 8, 1024–1032. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, H. & Chess, L. (2000) Annu. Rev. Immunol. 18, 185–216. [DOI] [PubMed] [Google Scholar]
- 22.Dalwadi, H., Wei, B., Schrage, M., Spicher, K., Birnbaumer, L., Su, T. T., Rawlings, D. J. & Braun, J. (2003) J. Immunol. 170, 1707–1715. [DOI] [PubMed] [Google Scholar]
- 23.Burke, F., Stagg, A. J., Bedford, P. A., English, N. & Knight, S. C. (2004) J. Immunol. 173, 2362–2372. [DOI] [PubMed] [Google Scholar]
- 24.Hornquist, C. E., Lu, X., Rogers-Fani, P. M., Rudolph, U., Shappell, S., Birnbaumer, L. & Harriman, G. R. (1997) J. Immunol. 158, 1068–1077. [PubMed] [Google Scholar]
- 25.Rudolph, U., Finegold, M. J., Rich, S. S., Harriman, G. R., Srinivasan, Y., Brabet, P., Boulay, G., Bradley, A. & Birnbaumer, L. (1995) Nat. Genet. 10, 143–150. [DOI] [PubMed] [Google Scholar]
- 26.Van der Heijden, P. J. & Stok, W. (1987) J. Immunol. Methods 103, 161–168. [DOI] [PubMed] [Google Scholar]
- 27.Aranda, R., Sydora, B. C., McAllister, P. L., Binder, S. W., Yang, H.-Y., Targan, S. R. & Kronenberg, M. (1997) J. Immunol. 158, 3464–3473. [PubMed] [Google Scholar]
- 28.Ohman, L., Franzen, L., Rudolph, U., Harriman, G. R. & Hultgren Hornquist, E. (2000) Scand. J. Immunol. 52, 80–90. [DOI] [PubMed] [Google Scholar]
- 29.Huang, T. T., Zong, Y., Dalwadi, H., Chung, C., Miceli, M. C., Spicher, K., Birnbaumer, L., Braun, J. & Aranda, R. (2003) Int. Immunol. 15, 1359–1367. [DOI] [PubMed] [Google Scholar]
- 30.Dalwadi, H., Wei, B., Kronenberg, M., Sutton, C. L. & Braun, J. (2001) Immunity 15, 149–158. [DOI] [PubMed] [Google Scholar]
- 31.Saha, C., Nigam, S. K. & Denker, B. M. (1998) J. Biol. Chem. 273, 21629–21633. [DOI] [PubMed] [Google Scholar]
- 32.He, J., Gurunathan, S., Iwasaki, A., Ash-Shaheed, B. & Kelsall, B. L. (2000) J. Exp. Med. 191, 1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohman, L., Franzen, L., Rudolph, U., Birnbaumer, L. & Hornquist, E. H. (2002) Gut 51, 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madakamutil, L. T., Christen, U., Lena, C. J., Wang-Zhu, Y., Attinger, A., Sundarrajan, M., Ellmeier, W., von Herrath, M. G., Jensen, P., Littman, D. R., et al. (2004) Science 304, 590–593. [DOI] [PubMed] [Google Scholar]
- 35.Powrie, F., Carlino, J., Leach, M. W., Mauze, S. & Coffman, R. L. (1996) J. Exp. Med. 183, 2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aiko, S. & Grisham, M. B. (1995) Gastroenterology 109, 142–150. [DOI] [PubMed] [Google Scholar]
- 37.Dohi, T., Fujihashi, K., Koga, T., Shirai, Y., Kawamura, Y. I., Ejima, C., Kato, R., Saitoh, K. & McGhee, J. R. (2003) Gastroenterology 124, 672–682. [DOI] [PubMed] [Google Scholar]
- 38.Kosiewicz, M. M., Nast, C. C., Krishnan, A., Rivera-Nieves, J., Moskaluk, C. A., Matsumoto, S., Kozaiwa, K. & Cominelli, F. (2001) J. Clin. Invest. 107, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, F. & Kearney, J. F. (2000) Immunol. Rev. 175, 70–79. [PubMed] [Google Scholar]
- 40.Vance, R. E., Kraft, J. R., Altman, J. D., Jensen, P. E. & Raulet, D. H. (1998) J. Exp. Med. 188, 1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panoutsakopoulou, V., Huster, K. M., McCarty, N., Feinberg, E., Wang, R., Wucherpfennig, K. W. & Cantor, H. (2004) J. Clin. Invest. 113, 1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheroutre, H. & Madakamutil, L. (2004) Nat. Rev. Immunol. 4, 290–300. [DOI] [PubMed] [Google Scholar]
- 43.Leishman, A. J., Gapin, L., Capone, M., Palmer, E., MacDonald, H. R., Kronenberg, M. & Cheroutre, H. (2002) Immunity 16, 355–364. [DOI] [PubMed] [Google Scholar]
- 44.Treiner, E., Duban, L., Bahram, S., Radosavljevic, M., Wanner, V., Tilloy, F., Affaticati, P., Gilfillan, S. & Lantz, O. (2003) Nature 422, 164–169. [DOI] [PubMed] [Google Scholar]
- 45.Saubermann, L. J., Beck, P., De Jong, Y. P., Pitman, R. S., Ryan, M. S., Kim, H. S., Exley, M., Snapper, S., Balk, S. P., Hagen, S. J., et al. (2000) Gastroenterology 119, 119–128. [DOI] [PubMed] [Google Scholar]
- 46.Akbari, O., Stock, P., Meyer, E., Kronenberg, M., Sidobre, S., Nakayama, T., Taniguchi, M., Grusby, M. J., DeKruyff, R. H. & Umetsu, D. T. (2003) Nat. Med. 9, 582–588. [DOI] [PubMed] [Google Scholar]
- 47.Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. (2002) Immunity 17, 629–638. [DOI] [PubMed] [Google Scholar]
- 48.Fort, M., Lesley, R., Davidson, N., Menon, S., Brombacher, F., Leach, M. & Rennick, D. (2001) J. Immunol. 166, 2793–2800. [DOI] [PubMed] [Google Scholar]
- 49.Galli, G., Nuti, S., Tavarini, S., Galli-Stampino, L., de Lalla, C., Casorati, G., Dellabona, P. & Abrignani, S. (2003) J. Exp. Med. 197, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maloy, K. J., Salaun, L., Cahill, R., Dougan, G., Saunders, N. J. & Powrie, F. (2003) J. Exp. Med. 197, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barthlott, T., Kassiotis, G. & Stockinger, B. (2003) J. Exp. Med. 197, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]