Abstract
Background
The ability of anti-cytokine antibodies to play a disease-causing role in the pathogenesis of immunodeficiencies is widely accepted. The aim of this study was to investigate whether autoantibodies against BAFF (important B cell survival signal), APRIL (important plasma cell survival signal), or Interleukin-21 (important cytokine for immunoglobulin class switch) present an alternative mechanism for the development of the following primary antibody deficiencies (PADs): common variable immune deficiency (CVID) or selective IgA deficiency (sIgAD).
Results
Two hundred thirty-two sera from patients with PADs were screened for autoantibodies against cytokines by ELISA. Statistical data analysis yielded a significant difference (p < 0.01) between the healthy donor sera and both PAD cohorts. The analysis was deepened by subdividing the patient collective into groups with distinct B cell phenotypes but no significant differences were found. For selected sera with notable high ELISA-read outs functional analysis ensued. Anti-BAFF and anti-APRIL antibodies were further examined by a B cell survival assay, whilst the functional relevance of putative anti-IL-21 autoantibodies was investigated by means of a STAT3 phosphorylation assay. However, the results of these experiments revealed no discernible functional effect.
Conclusion
Whilst statistical analysis of ELISA results showed significant differences between patients and healthy controls, in our set of patients functional tests yielded no evidence for an involvement of autoantibodies against BAFF, APRIL, or IL-21 in the pathogenesis of CVID or sIgAD.
Keywords: Autoimmunity, Autoantibodies, Cytokines, Primary antibody deficiency, Common variable immunodeficiency, Selective IgA deficiency, BAFF, APRIL, IL-21
Background
In recent years the role of autoantibodies against cytokines in disease pathogenesis has received an increased amount of attention. The existence of cytokine autoantibodies has been known for more than 30 years, but their significance in disease pathogenesis remains uncertain for many patients. In the context of viral infection [1], malignancy, extensive trauma, and therapy with recombinant proteins [2, 3], autoantibodies are mostly regarded as an epiphenomenon rather than disease-causing. Yet lately several studies were able to link autoantibodies against cytokines directly to disease pathogenesis: Kitamura et al. identified antibodies against GM-CSF in pulmonary alveolar proteinosis (PAP), which, by impairing alveolar macrophages, affected the immune-response [4]. Similarly, anti-erythropoetin antibodies have been found in patients with pure red-cell aplasia (PRCA) [5, 6] and many more have been described in literature [7–9]. Furthermore, anti-IL6 antibodies were discovered in a patient with recurrent staphylococcal cellulitis [10] and Kampmann et al. was able to show, that antiIFN-γ antibodies in patients resulted in a predisposition to mycobacterial diseases [11]. In view of these findings, autoantibodies against cytokines have sparked a special interest in the field of immunodeficiencies [7, 12].
Within the group of primary immunodeficiencies (PID), primary antibody deficiencies (PAD) constitute the lion’s share with approximately 50–70% [13]. While most PIDs are caused by single gene mutations, the underlying pathogenesis of PAD still remains poorly understood [14]. For common variable immunodeficiency (CVID), the most prevalent symptomatic PAD, several mutations have been described [15–19], although they only account for approximately 25% of CVID [17, 20]. In other PADs such as selective IgA Deficiency (sIgAD) no causal mutations have been found to this day [21]. However, in sIgAD several linkage studies were able to demonstrate an association to HLA-DQ/DR loci [22] as well as IFIH1, a locus associated with type 1 diabetes and systemic lupus erythematodes [23], strongly suggesting an autoimmune origin in CVID/sIgAD. Adding to this, autoimmune manifestations are frequent in PAD patients [24, 25].
Combining the growing relevance of autoantibodies against cytokines with the putative autoimmune etiology of primary antibody deficiencies, in this study we aimed to investigate whether autoantibodies directed against key-immunocytokines, were able to impair the maturation of an antibody response and ultimately culminating in a hypogammaglobulinemic phenotype.
The cytokines, which were investigated in this study, were chosen based on their role in the immune system. The presence of the antibody will prevent the cytokine to bind to its receptor and will thereby block further signaling.
BAFF and its homologous counterpart APRIL belong to the TNF-family of cytokines [26] and play a key role in the differentiation as well as the homoeostasis of the B cell- and plasma cell pool [27–29]. Both have been extensively studied in the context of CVID: BAFF receptor mutations have been described to cause CVID [15, 16] and BAFF and APRIL dysregulation have been reported in CVID patients [29, 30]. Considering that anti-cytokine autoantibodies have been reported to mimic the phenotype caused by a mutation of the respective cytokine, we picked BAFF and APRIL as appropriate candidate cytokines to investigate. IL-21 was chosen due to its significance in the regulation of B cell development as well as its role in the process of class-switching. Parrish-Novak et al. were able to show that IL-21 was key in activating the proliferation and differentiation of B cells into memory B cells and plasma cells [31, 32], whilst Okazi et al. reported that IL-21-deficient transgenic mice had markedly reduced serum levels of IgG1-3. Moreover, IL21-/IL4-deficient mice presented with a CVID phenotype [33–35], making it a particularly interesting cytokine.
Following up on the aforementioned studies, we screened for inhibitory autoantibodies against BAFF, APRIL and IL-21 in over 200 PAD patients. Overall, several significant differences were detected between PAD patients and healthy controls, but the absolute OD values of detected autoantibodies were low and, most significantly, sera positive for auto-antibodies failed to show neutralizing activity. Thus, no conclusive evidence for an involvement of autoantibodies against BAFF, APRIL or IL-21 in the pathogenesis of PAD could be established in our study.
Results
Screening for autoantibodies by ELISA
In order to search for autoantibodies, the ELISA-method was chosen, due to its high sensitivity as well as its capability to screen large numbers of samples in a time- and cost-efficient manner. Since all reported autoantibodies against cytokines have been of the IgG isotype [12, 36], we focused our search on this isotype only.
After having established a screening ELISA able to detect anti-cytokine IgG immunoglobulin, healthy donor sera and patient sera were analysed. Each sample was analysed three times. Samples were considered positive if the read-out was above the cut-off value, defined as the healthy donor mean + 2 standard deviation.
For the anti-BAFF ELISA 68 healthy donor sera and 174 PAD-patients were investigated. The PAD-patients consisted of 52 CVID-patients and 118 patients with sIgAD. After screening, 18 samples of the CVID group, and 52 of the sIgAD group produced a read-out higher than the cut-off.
For the APRIL-ELISA, a total of 41 healthy donor sera and 232 patient sera (113 CVID, 119 sIgAD) were screened. The results identified 27 CVID sera and 27 sIgAD sera with optical densities (OD) above the cut-off value. The anti-IL-21 ELISA included a total of 67 HD sera and 206 patient sera (83 CVID, and 118 sIgAD). Ten CVID patients and 52 sIgAD patients had values above the defined threshold.
The screening results can be viewed in Fig. 1. Data analysis with the t-test showed a statistically significant difference (p < 0.01) between the healthy donor groups and CVID- and sIgAD patients for all three cytokines.
Fig. 1.
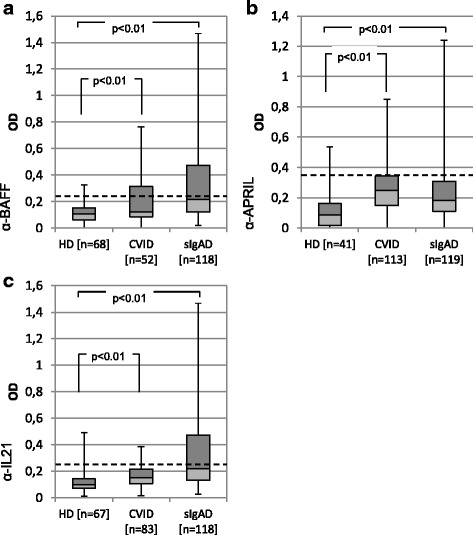
Results of Screening-ELISA. In this Box-and-Whiskers-Plot the bottom and top of the box represent the first and third quartiles of the dataset, the band inside the median, and the whiskers show the minimum and maximum values. In Panel (a), anti-BAFF ELISA read-outs show a significant difference between healthy donors and the CVID and sIgAD cohort. Similar results were obtained with anti-APRIL-ELISA (Panel b) and anti-IL-21 ELISA (Panel c). The dashed line marks a cut-off value for each ELISA, defined as the healthy donor mean + 2 standard deviation, above which samples were considered positive
Subgroup analysis
Investigating whether the patient groups with high read-outs in the ELISA shared common characteristics, we sorted the patient pool depending on their cellular B cell phenotype and clinical manifestations, in particular autoimmune manifestations, cytopenia, and lymphadenopathy [26, 29, 37, 38]. The results can be viewed in Fig. 2 Panel a, c, and e. Following the EURO Classification Trial [39], B cells were subdivided into decreased numbers of total B cells (<1% CD19+), decreased switched memory B cells (<2% IgM- IgD- CD27+) and expanded transitional B cells (>9% CD38hi IgM hi CD27-). Further subgroups were defined as decreased marginal zone B cells (<2.5% IgM + IgD+ CD27+) and expanded naive B cells (>85% CD19+ IgM + IgD+ CD27-) (see Fig. 2 Panel b, d and f).
Fig. 2.
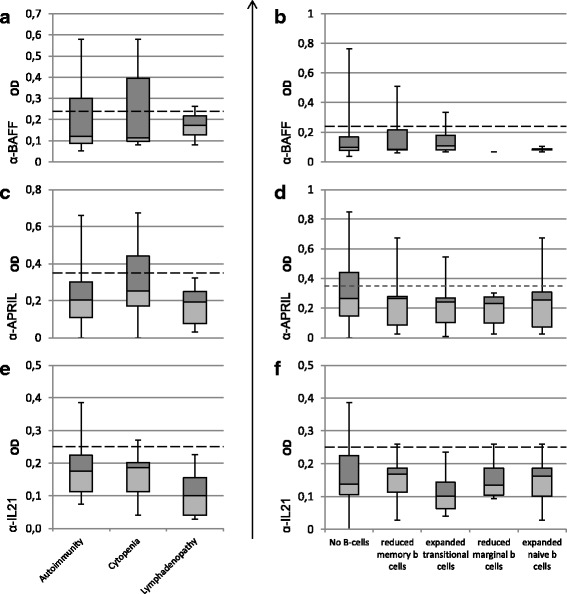
Subgroup analysis. Panel (a) shows the results of the BAFF-ELISA assorted by clinical characteristics. Comparing the distribution of these data subsets with the results of the healthy donor cohort was unable to show a significant difference. In Panel (b) the BAFF-ELISA results are sorted according to their B cell phenotype. In Panel (c) and (d) the results of the APRIL-ELISA are grouped by clinical manifestations and (b) cell phenotypes respectively. Panel (e) and (f) show the subgroups analysis for IL-21. The statistical analysis for none of the above yielded a significant result
Considering that blocking the BAFF- and APRIL receptor BCMA led to a marked reduction of peripheral B cells [40] and that BAFF knock-out mice have markedly reduced mature B cells [41] as well as marginal zone B cells [37, 42], patients exhibiting these cellular characteristics were of particular interest. Furthermore, patients with expanded T1-transitional B cells were investigated, since a developmental block at the transitional B cell stage had been reported in BAFF receptor-mutated humans [15, 16] and BAFF knock-out mice [37, 43, 44]. However, the statistical analysis between these subgroups yielded no significant difference at either anti-cytokine autoantibody.
Functional analysis
In order to address the ambiguity surrounding the functional significance of elevated autoantibody titres, we set up an array of functional tests to assess the effects that autoantibodies might have on the immune system. A specific assay was established for each cytokine. Based on the property of BAFF and APRIL to promote the survival of B cells [37, 40, 45–47] a survival assay was devised. To detect dead cells, FACS analysis with DAPI-staining was chosen. Pre-testing yielded an ideal incubation period of 9 days.
The samples selected for functional analysis had to comply with several criteria: Firstly, an ELISA read-out above the cut-off was required. Polyreactive sera, i.e. sera with high read-outs in every ELISA, were omitted. Secondly, the cellular phenotype should match with the involved cytokine. In case of BAFF, we would expect low B cell numbers, expanded transitional B cells, and low BAFF levels. Lastly, a known mutation in BAFF should be excluded.
After the incubation period, viable cells were counted. Each functional experiment was repeated twice. The results can be seen in Fig. 3a and b. Since it was known that the selected patient sera had low BAFF-levels, we presumed that the cell culture with HD serum would contain the highest concentration of BAFF and therefore retain the largest amount of viable cells after 9 days. This prediction was confirmed by our experiment. If the patient with ID#1147 had anti-BAFF antibodies able to block BAFF signalling, we would expect a decreased amount of living cells. The results however, showed a similar cell count to the HD control, rendering functional antibodies in this sample unlikely. In the case of patient ID#1185, a marked reduction of living B cells was observed, making this result compatible with the presence of BAFF autoantibodies. However, after examining the patient’s medical history, an anti-B cell directed therapy with the monoclonal anti-CD20 antibody rituximab was reported. This detail sheds a different light on our results and makes the presence of anti-BAFF autoantibodies an unlikely reason for the observed B cell deficiency.
Fig. 3.

Functional Analysis. In Panel (a) and (b) the results of the B cell survival assay are displayed. In Panel (a) viable (b) cells (CD19+,DAPI-) are shown after 9 days-incubation with patient sera, which were selected for their high anti-BAFF-ELISA results. Pat.1185 had received Rituximab-treatment prior to serum withdrawal; hence the results were not conclusive. Panel (b) shows the amount of viable (b) cells after the addition of sera with putative anti-APRIL antibodies. The results show no significant difference. In Panel (c) the results of the STAT3 phosphorylation assay are shown. PBMCs were incubated with IL-21 as well as selected healthy donor and patient sera. pSTAT3 was measured by FACS analysis. No marked disparity was observed between patients and healthy donors
Contrary to the BAFF survival assay, APRIL levels in patients were unknown, hindering prediction on the outcome of the experiment. Although APRIL levels are reported to be high in patients with CVID [30], patients with normal or reduced APRIL levels are not uncommon [30].
High levels of APRIL promote B cell survival and in case of autoantibodies against APRIL a notable decrease in live cells would be expected. The experimental results (Fig. 3b) however, do not match our expectations and thus the existence of functional autoantibodies seems improbable.
To investigate the functionality of supposed anti-IL21 antibodies, a STAT3-phosphorylisation assay was used. To detect the phosphorylated STAT3 (pSTAT3) by FACS-analysis we followed the STAT3-phosphlow protocol (BD Pharmigen) according to the manufacturer’s instructions. When IL-21 binds to its receptor, a signaling cascade is started, which includes the phosphorylation of STAT3. Autoantibodies against IL-21 should therefore lead to a decrease in pSTAT3.
In Fig. 3c we show that the difference between the healthy donor pSTAT3 and the ones of our patients is only marginal, rendering a functional involvement of putative autoantibodies negligable.
Discussion
Although the screening-ELISAs returned a considerable amount of positive output with a statistical analysis hinting a significant difference (p < 0.01), we were not able to demonstrate any functional effect of those autoantibodies, which seemed to be elevated just above the baseline without any functional impact. This hypothesis is supported by the observation that often a single sample had several anti-cytokine antibodies elevated (polyreactivity), which may be an intrinsic feature to patients with PADs.
Compared to commercially available ELISA kits, the sensitivity and specificity of our in-house established ELISA tests was unknown. Even though positive and negative controls were implemented, several altering factors have to be taken into account: The most crucial aspect were non-specific bindings of antibodies to a natural occurring antigen (e.g. plastic), potentially masking correct binding with the coating cytokine. Whilst our ELISA protocol accounted for some of these disturbances by blocking the wells with BSA and duplicating each step on a non-coated well plate, inadequacies were difficult to quantify. Furthermore, it has to be considered that some autoantibodies may have been bound to its respective cytokine, thus not detectable by ELISA.
Conclusion
Addressing the initial hypothesis, our experimental data suggests that antibodies against the cytokines BAFF, ARIL, and IL-21 do not play a role in the pathogenesis of primary antibody deficiencies. Although our ELISA screening results pointed towards elevated anti-cytokine antibody titers, our functional analysis demonstrated no relevant involvement of these putative anti-cytokine antibodies in the etiopathophysiology of antibody deficiencies. Thus, the existence of autoantibodies cannot be revoked; their role in the formation of disease however, remains elusive. It is in particular the clinical heterogeneity of PADs such as CVID that should encourage scientists to consider alternative pathomechanisms, and an autoimmune origin of antibody deficiencies remains a promising area of research.
Methods
Patient cohorts and cell culture
The study followed the Declaration of Helsinki and all subjects provided informed consent for participation. Patient serum samples (n = 232) were collected from the Institute of Immunity and Transplantation at the Royal Free Hospital London and from the Karolinska University Hospital Stockholm. 115 patients had CVID, and 116 had sIgAD. Patients were diagnosed according to the criteria established by the European Society for Immunodeficiencies [48]. Upon receipt, samples were aliquoted and frozen until analysis. Informed consent was obtained from all patients and approval was granted by the local ethics committee.
Enzyme-linked immunosorbent assay (ELISA)
Maxisorp 96-well ELISA plates (Maxisorp; Nunc) were coated with the recombinant cytokines by incubation overnight at 4 °C at their respective concentration. They were then washed (PBS-Tween 0.05%) and incubated for 1 h with the same buffer supplemented with 5% nonfat milk powder, in order to block free binding sites. After being washed twice, the patient sera were added and incubated 2 h at room temperature. Thereafter plates were thoroughly washed and Fc-specific HRP-conjugated IgG-fractions of polyclonal goat antiserum against human IgG (Peprotech) were added. After an incubation time of 1 h at room temperature, the plates were washed, substrate TMB was added and absorption was determined in an ELISA reader at 450 nm. For the positive control we used polyclonal antibodies against the cytokine (Peprotech), which were produced from sera of goats immunized with the specific recombinant human cytokine. Negative controls were carried out by adding the detection antibody to a non-incubated plate. To quantify unspecific bindings each serum sample was incubated concurrently in a coated as well as a non-coated well.
STAT3 phosphorylation assay
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by using density gradient centrifugation and then washed in PBS (Sigma, US). 105 PBMCs were re-suspended in RPMI 1640 (Sigma-Aldrich).
Prior to adding 50 ng/ml Il-21, patients’ sera were added to the cell culture at a 1:200 dilution and incubated for 30 min. After another 20 min the cells were fixed with 200 μl Cytofix Buffer (BD Bioscience). Once the cells were centrifuged, the supernatant was discarded and 500 μl Permbuffer III (BD Bioscience) added to permeabilize the cells. After thorough washing, the cells were stained with 5 μl of a FITC p-STAT3 antibody and incubated protected from light for 60 min. Lastly the cells were centrifuged, the supernatant discarded and the pellet resuspended in 500 μl Stain-Buffer (BD Pharmingen™) for FACS analysis.
Survival assay
Using a 24-well plate (Thermo Fisher Scientific) 105 PBMCs were cultivated in 1 ml cell medium, consisting of RPMI 1640 supplemented with 10% heat-inactivated FBS and Streptavidin. Cells were then stimulated with BAFF at a concentration of 0.2 μg/ml. Selected patient sera were then added at a dilution of 1:200.
An incubation-period of 9 days was found to provide the best ratio of dead/alive cells. Cells transferred into FACS-Tubes, washed using 3 ml of FACS-Buffer and then centrifuged for 8 min at 1200 rpm at 4 °C. After discarding the supernatant, the step was repeated. Binding buffer was added and the cells stained with 5 μl DAPI und 5 μl APC anti-CD19. After a 15 min incubation period away from light, the cells were analyzed by FACS (LSR II flow cytometer). The data was evaluated using FACSDiva Software (BD Biosciences).
Statistical analysis
In line with literature, we assumed a normal distribution of immunoglobulins [49], allowing us to execute a parametric statistical analysis using a t-test. Each PAD-group was compared to the healthy donor data set. Two-tailed p-values < 0 · 01 were considered statistically significant.
Acknowledgements
We would like to thank the following laboratory members for their important contributions: A. Sassi, Karin R. Engelhardt, G. Lopez Herrera.
Funding
This work has been supported by the EC Marie-Curie Grant MEXT-CT-2006-042316, as well as by funds from the EU 7th framework programme (FP7 2007–2013 No. 223293 and FP7 2007–2013 EURO-PADnet HEALTH-F2-2008-201549) and by the German Federal Ministry of Education and Research (BMBF 01 EO 0803). The authors are responsible for the contents of this publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MCP carried out the ELISA, Survival Assay and STAT3 phosphorylation assays. EOG, CG, NF, JB and AS participated in the design of the study and helped to draft and revise the manuscript. LH contributed all sIgAD samples. BG conceived of the study, and participated in its design and coordination and helped to write the manuscript. All authors read and approved the final manuscript.
Competing interests
The author(s) declare that they have no competing interests.
Ethics approval and consent to participate
All patients gave their informed consent; approval was granted by the local ethics committee.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- APRIL
A proliferation inducing ligand
- APS-I
Autoimmune polyendocrinopathy I
- BAFF
B cell activating factor
- CMC
Chronic mucocutaneous candidiasis
- CVID
Common variable immunodeficiency
- IFN-gamma
Interferon gamma
- IL-21
Interleukin 21
- sIgAD
selective IgA deficiency
References
- 1.Tanner JE, Diaz-Mitoma F, Rooney CM, Alfieri C. Anti-interleukin-10 antibodies in patients with chronic active Epstein-Barr virus infection. J Infect Dis. 1997;176:1454–1461. doi: 10.1086/514141. [DOI] [PubMed] [Google Scholar]
- 2.Bendtzen K, Svenson M, Jonsson V, Hippe E. Autoantibodies to cytokines--friends or foes? Immunol Today. 1990;11:167–169. doi: 10.1016/0167-5699(90)90068-K. [DOI] [PubMed] [Google Scholar]
- 3.Sauerborn M, Schellekens H. B-1 cells and naturally occurring antibodies: influencing the immunogenicity of recombinant human therapeutic proteins? Curr Opin Biotechnol. 2009;20:715–721. doi: 10.1016/j.copbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian J, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, Varet B, Mayeux P. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 6.Macdougall IC, Rossert J, Casadevall N, Stead RB, Duliege A, Froissart M, Eckardt K. A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia. N Engl J Med. 2009;361:1848–1855. doi: 10.1056/NEJMoa074037. [DOI] [PubMed] [Google Scholar]
- 7.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres P, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova J. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisand K, Bøe W, Anette S, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn, Kai JE, Pura M, Schalke B, Ströbel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meager A, Visvalingam K, Peterson P, Möll K, Murumägi A, Krohn K, Eskelin P, Perheentupa J, Husebye E, Kadota Y, Willcox N. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puel A, Picard C, Lorrot M, Pons C, Chrabieh M, Lorenzo L, Mamani-Matsuda M, Jouanguy E, Gendrel D, Casanova J. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180:647–654. doi: 10.4049/jimmunol.180.1.647. [DOI] [PubMed] [Google Scholar]
- 11.Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, Nicol M, Schölvinck E, Relman D, Waddell S, Langford P, Sheehan B, Semple L, Wilkinson KA, Wilkinson RJ, Ress S, Hibberd M, Levin M. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115:2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puel A, Casanova J. Autoantibodies against cytokines: back to human genetics. Blood. 2013;121:1246–1247. doi: 10.1182/blood-2013-01-474213. [DOI] [PubMed] [Google Scholar]
- 13.Wood PM. Primary antibody deficiency syndromes. Curr Opin Hematol. 2010;17:356–361. doi: 10.1097/MOH.0b013e328338f69e. [DOI] [PubMed] [Google Scholar]
- 14.Liadaki K, Sun J, Hammarström L, Pan-Hammarström Q. New facets of antibody deficiencies. Curr Opin Immunol. 2013;25:629–638. doi: 10.1016/j.coi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Losi CG, Salzer U, Gatta R, Lougaris V, Cattaneo G, Meini A, Soresina A, Grimbacher B, Plebani A. Mutational analysis of human BLyS in patients with common variable immunodeficiency. J Clin Immunol. 2006;26:396–399. doi: 10.1007/s10875-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 16.Losi CG, Silini A, Fiorini C, Soresina A, Meini A, Ferrari S, Notarangelo LD, Lougaris V, Plebani A. Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-R) in patients with common variable immunodeficiency. J Clin Immunol. 2005;25:496–502. doi: 10.1007/s10875-005-5637-2. [DOI] [PubMed] [Google Scholar]
- 17.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Dräger R, Eibel H, Fischer B, Schäffer AA, Mages HW, Kroczek RA, Peter HH. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Herrera G, Tampella G, Pan-Hammarström Q, Herholz P, Trujillo-Vargas CM, Phadwal K, Simon AK, Moutschen M, Etzioni A, Mory A, Srugo I, Melamed D, Hultenby K, Liu C, Baronio M, Vitali M, Philippet P, Dideberg V, Aghamohammadi A, Rezaei N, Enright V, Du L, Salzer U, Eibel H, Pfeifer D, Veelken H, Stauss H, Lougaris V, Plebani A, Gertz EM, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90:986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Zelm Menno C, Reisli I, van der Burg M, van Noesel CD, van Tol CJM, Maarten JD, Woellner C, Grimbacher B, Patiño PJ, van Dongen Jacques JM, Franco JL. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol (Orlando, Fla) 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 21.Wang N, Hammarström L. IgA deficiency: what is new? Curr Opin Allergy Clin Immunol. 2012;12:602–608. doi: 10.1097/ACI.0b013e3283594219. [DOI] [PubMed] [Google Scholar]
- 22.Kralovicova J, Hammarström L, Plebani A, Webster A, David B, Vorechovsky I. Fine-scale mapping at IGAD1 and genome-wide genetic linkage analysis implicate HLA-DQ/DR as a major susceptibility locus in selective IgA deficiency and common variable immunodeficiency. J Immunol (Baltimore, Md: 1950) 2003;170:2765–2775. doi: 10.4049/jimmunol.170.5.2765. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira RC, Pan-Hammarström Q, Graham RR, Gateva V, Fontán G, Lee AT, Ortmann W, Urcelay E, Fernández-Arquero M, Núñez C, Jorgensen G, Ludviksson BR, Koskinen S, Haimila K, Clark HF, Klareskog L, Gregersen PK, Behrens TW, Hammarström L. Association of IFIH1 and other autoimmunity risk alleles with selective IgA deficiency. Nat Genet. 2010;42:777–780. doi: 10.1038/ng.644. [DOI] [PubMed] [Google Scholar]
- 24.Brandt D, Gershwin ME. Common variable immune deficiency and autoimmunity. Autoimmun Rev. 2006;5:465–470. doi: 10.1016/j.autrev.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Gathmann B, Mahlaoui N, Gérard L, Oksenhendler E, Warnatz K, Schulze I, Kindle G, Kuijpers TW, van Beem Rachel T, Guzman D, Workman S, Soler-Palacín P, de Gracia J, Witte T, Schmidt RE, Litzman J, Hlavackova E, Thon V, Borte M, Borte S, Kumararatne D, Feighery C, Longhurst H, Helbert M, Szaflarska A, Sediva A, Belohradsky BH, Jones A, Baumann U, Meyts I, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 26.Cancro MP. Living in context with the survival factor BAFF. Immunity. 2008;28:300–301. doi: 10.1016/j.immuni.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Mackay F, Silveira P, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009;158:155–163. doi: 10.1111/j.1365-2249.2009.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, Plebani A, Lougaris V, Quinti I, Thon V, Litzman J, Schlesier M, Warnatz K, Thiel J, Rolink AG, Eibel H. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol (Baltimore, Md: 1950) 2012;188:497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 30.Knight AK, Radigan L, Marron T, Langs A, Zhang L, Cunningham-Rundles C. High serum levels of BAFF, APRIL, and TACI in common variable immunodeficiency. Clin Immunol. 2007;124:182–189. doi: 10.1016/j.clim.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 32.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 33.Borte S, Pan-Hammarström Q, Liu C, Sack U, Borte M, Wagner U, Graf D, Hammarström L. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood. 2009;114:4089–4098. doi: 10.1182/blood-2009-02-207423. [DOI] [PubMed] [Google Scholar]
- 34.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki K. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 36.Bendtzen K, Hansen MB, Ross C, Poulsen LK, Svenson M. Cytokines and autoantibodies to cytokines. Stem Cells. 1995;13:206–222. doi: 10.1002/stem.5530130303. [DOI] [PubMed] [Google Scholar]
- 37.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 38.Fawzy SM, Gheita TA, El-Nabarawy E, El-Demellawy HH, Shaker OG. Serum BAFF level and its correlations with various disease parameters in patients with systemic sclerosis and systemic lupus erythematosus. Egypt Rheumatologist. 2011;33:45–51. doi: 10.1016/j.ejr.2010.12.001. [DOI] [Google Scholar]
- 39.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, Vlkova M, Hernandez M, Detkova D, Bos PR, Poerksen G, von Bernuth H, Baumann U, Goldacker S, Gutenberger S, Schlesier M, der Cruyssen Florence B-v, Le Garff M, Debré P, Jacobs R, Jones J, Bateman E, Litzman J, van Hagen PM, Plebani A, Schmidt RE, Thon V, Quinti I, Espanol T, Webster AD, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, MACKAY F, Bixler SA, Zafari M, Liu ZY, Woodcock SA, Qian F, Batten M, Madry C, Richard Y, Benjamin CD, Browning JL, Tsapis A, Tschopp J, Ambrose C. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/S1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 42.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 43.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 44.Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry. 2008;74B:261–271. doi: 10.1002/cyto.b.20432. [DOI] [PubMed] [Google Scholar]
- 45.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, MACKAY F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol. 2011;73:1–7. doi: 10.1111/j.1365-3083.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- 47.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24:203–215. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ESID - European Society for Immunodeficiencies: Diagnostic criteria for PID//Clinical//ESID - European Society for Immunodeficiencies. https://esid.org/Working-Parties/Registry/Diagnosis-criteria. Accessed 20 Jan 2017.
- 49.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


