Abstract
There is increasing evidence for intercellular trafficking of macromolecules through plasmodesmata (PD) during plant development. Here we study the ability of PD to traffic proteins during embryogenesis and early seedling development in Arabidopsis. Transgenic lines that induce GFP expression only in meristems, MSG (meristem-specific GFP), were used to monitor GFP movement. Cell-to-cell movement of different-sized GFP reporters reveals that embryos and young seedlings traffic proteins at least 54 kDa in size. Although 27-kDa soluble GFP (1×sGFP) freely moves between cells throughout the entire embryo during all stages analyzed, 2×sGFP movement becomes more restricted as development proceeds. After germination, cells near the apical meristem in seedlings show a higher size exclusion limit (SEL), whereas the SEL becomes more restricted as surrounding tissues develop identities. Although 1×sGFP moves throughout leaf primordia, as the leaf develops only the basal part of leaf petioles, main vascular tissues, and leaf veins (not blades) allow 1×sGFP movement. Although previous studies showed that embryos allow movement of small symplastic tracers (0.5 kDa), the present data demonstrate that the embryo constitutes a single symplast that allows transport of macromolecules as well. Even 2×sGFP moves from its site of expression at the apical meristem in embryos and seedlings, yet the extent of movement is more limited than 1×sGFP. Thus, PD have distinct SELs in different subregions of the embryo and seedling. These studies support the general concept that PD in younger tissues are more dilated and less restrictive than PD in older (nonvascular) tissues.
Keywords: plasmodesmata, symplast
Evidence for cell-to-cell movement of proteins and RNAs during plant development has increased dramatically during the last few years. Plasmodesmata (PD) are dynamic channels that interconnect the cytoplasm between adjacent plant cells and provide a route not only for nutrients and small molecules but also for macromolecular trafficking (1–4). PD are bounded by the plasma membrane and have a core of modified endoplasmic reticulum (ER) in their axial centers. Transport through PD is thought to occur primarily through the cytoplasmic space between the plasma membrane and modified ER. An important measure of PD function is their size exclusion limit (SEL), the upper limit in the size of macromolecules that can freely diffuse from cell to cell. The aperture of PD is regulated temporally, spatially, and physiologically throughout the development of a plant, although SEL may be fixed in certain cell types or at specific stages of development.
Historically, PD have been studied at the ultrastructural level (5), as a function of plant physiology (6), and during plant viral spread (7). However, the presence of PD structure per se does not ensure their functionality, because PD aperture fluctuates from closed to open to dilated during different stages of postgermination development (8–13). Furthermore, PD have roles during complex morphogenesis, selectively allowing movement of some but not all proteins including transcription factors critical in cell-fate determination (14–21). In addition, plant RNAs are trafficked through PD (22–24). Thus, PD in different tissues may be regulated differently, possibly by the involvement of developmentally regulated factors.
Embryogenesis is a critical developmental stage, because it directs the basic blueprint for the adult plant body (25). To date, few studies have assessed PD function during Arabidopsis embryogenesis (26, 27), mainly because of the technical difficulty of introducing tracers into embryonic tissues. Embryos are enclosed in maternal tissues and thus not easily available for standard methods such as microprojectile bombardment (9) and microinjection (28). In addition, embryos become symplastically isolated from the mother plant as they develop (26, 29), thus phloem loading (8), another well used technique to introduce tracers into plant cells, also is not applicable to study PD function during the course of embryogenesis.
Here we construct GFP-transgenic lines, called meristem-specific GFP (MSG), in which the expression of different-sized soluble GFP (sGFP) tracers is induced only in a subset of cells at the shoot and root apical meristems (SAMs and RAMs, respectively). These lines were used to monitor the extent of GFP movement in embryos and early seedlings during their development. In embryos, 1×sGFP (27 kDa) freely moves throughout the whole embryo, from the early heart to midtorpedo stages, confirming that the embryo is a single symplast (27). The 2×sGFP (54 kDa) also moves during these stages but less extensively. The movement pattern of 1×sGFP and 2×sGFP also was monitored in the shoot apex during early seedling development. Generally, the data reveal that 1×sGFP moved more extensively than 2×sGFP, and cells with less-determined fate or with specific vascular fate show higher SELs, allowing 2×sGFP movement.
Materials and Methods
Construction of Plasmids and Transgenic Arabidopsis. Single sGFP (1×sGFP) and double sGFP (2×sGFP) sequences were excised from pRTL2 (10) after EcoRI digestion and introduced into the EcoRI site on pAS61 just downstream of 12 copies of the GAL4 upstream activation sequence (UAS) and a minimal promoter (www.plantsci.cam.ac.uk/Haseloff/Home.html). pAS61 carrying 1×sGFP or 2×sGFP was then cut with XhoI and XbaI for insertion into the Agrobacterium binary vector pMON200 (30). Agrobacterium carrying these constructs were used to transform Arabidopsis thaliana J2341 and wild-type C24 by the floral dipping method (31).
Microscopy. Seedlings and embryos were grown under greenhouse conditions and observed by epifluorescence and confocal laser scanning microscopy as described (27).
Tissue Preparation and in Situ Hybridization. Siliques from 10-week-old Arabidopsis were used to obtain midtorpedo embryos. Apical and basal ends of siliques were cut off to improve the fixative infiltration. Excised siliques were fixed in 3.7% formaldehyde/5% acetic acid/50% ethanol. After 20 min of vacuum treatment (20–25 Hg) at room temperature, siliques then were incubated for 2 h at room temperature without vacuum. Serial dehydration, paraffin embedding, and in situ hybridization were performed as described (http://carnegiedpb.stanford.edu/research/barton/in_situ_protocol.html). A 723-bp fragment (SpeI–NotI) of the GFP-coding region (32) was used as a probe, and hybridization was performed for 40 h at 55°C.
Results and Discussion
MSG-Expressing Transgenic Lines. J2341 is a transgenic Arabidopsis line reported to induce ER-tethered GFP expression at SAMs (Fig. 1C) and RAMs (www.plantsci.cam.ac.uk/Haseloff/Home.html) in early seedlings. J2341 carries a cell-type-specific enhancer driving expression of the yeast transcriptional activator GAL4 in meristems. The presence of GAL4 then leads to expression of a linked ER-GFP insert controlled by a tandem array of five copies of GAL4-specific UASs (Fig. 1 A). In line J2341, GFP expression also is localized to the SAM in embryos (Fig. 2). ER-GFP and GAL4 proteins behave cell-autonomously (10, 33, 34) so that green fluorescence marks the cells in which GFP is synthesized.
Fig. 1.
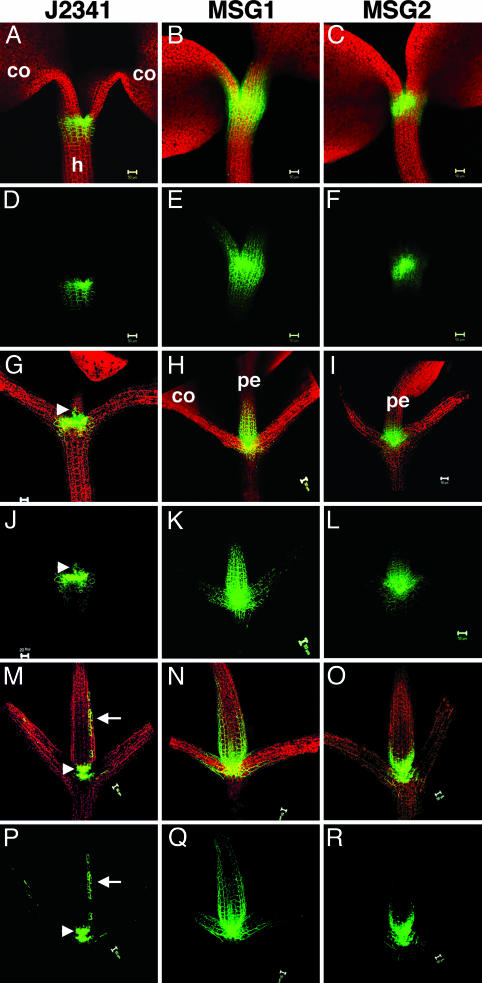
Construction of MSG lines. (A) Schematic representation of enhancer-trap line J2341 that drives GAL4 expression and expression of a linked ER-GFP by means of GAL4 UASs. (B) J2341 was transformed with a vector carrying UAS::1×sGFP or UAS::2×sGFP to create MSG1 and MSG2. (C–F) Expression of ER-GFP and sGFP movement in the shoot apex of 4-dpg seedlings in J2341 (C), control apices from plants containing UAS::sGFP only (D), MSG1 (E), and MSG2 (F) lines. co, cotyledon; h, hypocotyl. Arrows indicate dome-shaped emerging leaf primordia. Red indicates chlorophyll autofluorescence, and green indicates GFP signal captured by epifluorescence microscopy.
Fig. 2.
sGFP movement in embryos. The expression pattern of ER-GFP and the extent of sGFP movement are monitored during embryogenesis in early heart (A–C), late heart (D–F), and midtorpedo (G–I) embryos in J2341(A, D, and G), MSG1(B, E, and H), and MSG2 (C, F, and I) lines. Apex-specific localization of sGFP mRNA in midtorpedo embryos in MSG1(K) and MSG2 (L). (J) Green circles represent the sites of GFP synthesis (both ER and soluble), and green arrows indicate the movement of soluble proteins. Arrows in D, F, G, and I indicate comparable regions of protodermal cells. Arrowheads in G and I indicate expression in the RAM. (I) * and + indicate the gradient of sGFP movement in the hypocotyl (h) and cotyledons (co), respectively. (Scale bars, 50 μm.)
To study the movement of nontethered sGFP after meristem-specific transcription, we transformed the J2341 line with constructs to express single- or double-sized sGFP (1×sGFP or 2×sGFP). These sGFP constructs contain a tandem array of 12 optimized GAL4-specific UASs upstream of their coding sequences and lack GAL4-coding sequences (Fig. 1B). The meristem-specific enhancer driving GAL4 expression activates expression of both ER-GFP as well as unlinked sGFP in the same cells; however, only sGFP is available for intercellular movement through PD (Figs. 1, 2, 3, 4, 5). Heterozygous initially transformed lines were allowed to self-pollinate, and homozygous lines carrying 1× or 2×sGFP that did not segregate the sGFP inserts were selected for additional analyses and designated MSG1 (single sGFP) and MSG2 (double sGFP), respectively.
Fig. 3.
sGFP movement in the shoot apex of seedlings. ER-GFP expression and sGFP movement in seedlings 3 dpg (A–F), 8 dpg (G–L), and 14 dpg (M–R) in J2341 (A, D, G, J, M, and P), MSG1 (B, E, H, K, N, and Q), and MSG2 (C, F, I, L, O, and R) lines are shown. For each time frame the three upper panels show overlapping red autofluorescence and green GFP fluorescence, whereas the lower panels show GFP fluorescence alone. co, cotyledon; pe, petiole; h, hypocotyl. Arrowheads in G, J, M, and P indicate the slight expression of ER-GFP at the base of the petioles of J2341. Arrows in M and P indicate expression of ER-GFP along the length of the petioles. (Scale bars, 50 μm.)
Fig. 4.
sGFP movement in developing leaves. Different-stage leaves from MSG1 at 14 dpg were monitored for sGFP movement. A and B compare GFP signals in the overlapping red autofluorescent and green fluorescent images versus bright field. sGFP movement in the basal petiole region (C) or blade region (D) of a mature leaf is shown. +, leaf primordia; *, vascular tissues in leaf petioles; v, leaf veins. (Scale bars, 50 μm.)
Fig. 5.
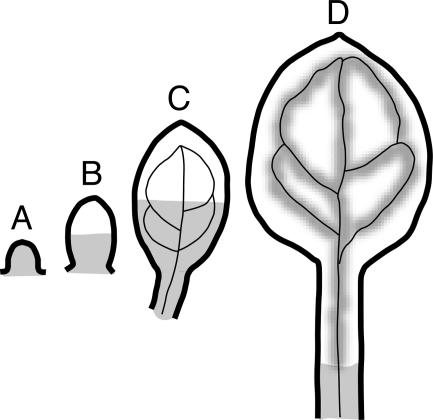
Diagram of sGFP movement pattern during leaf development. (A) Leaf primordia allow the movement of 1×sGFP from the SAM. Younger leaves without (B) and with (C) visible vein structures allow 1×sGFP movement throughout the basal half of leaf blades. (D) Older leaves with elongated petioles allow the sGFP (1× and 2×) movement through vascular tissues and leaf veins and the basal part of petioles closer to the SAM.
The GFP expression/movement pattern in seedlings 4 days postgermination (dpg) exemplifies the distinct differences in the patterns of movement between MSG1 and MSG2 lines. In MSG1, 1×sGFP moves from the SAM to surrounding tissues, including the petioles of the cotyledons, the hypocotyls, and emerging leaf primordia (Fig. 1E). In contrast, in MSG2 there was only limited movement of 2×sGFP to cells immediately surrounding the SAM, and leaf primordia were devoid of GFP (Fig. 1F, arrow). Control wild-type plants transformed with the same constructs to express sGFP never show GFP expression because of lack of GAL4 expression (Fig. 1D).
GFP Movement During Embryo Development. Early heart embryos of J2341 show ER-GFP in the central regions in a band-like pattern including and surrounding the SAM (Fig. 2 A). 1×sGFP moves throughout the whole embryo (Fig. 2B). 2×sGFP also moves quite extensively outward from the SAM through ground tissues to the cells in protodermal layers as well (Fig. 2C).
Late heart embryos of J2341 show clear SAM-specific expression of ER-GFP (Fig. 2D). The ER-GFP signal is most intense in the center of the apex and forms a gradient in intensity in cells toward the periphery of the SAM. Because ER-GFP behaves cell-autonomously, this gradient cannot reflect nontargeted movement (11). Instead, the gradient of ER-GFP expression reflects the developmental control of the cell-type-specific enhancer in line J2341, revealing that enhancer activity is strongest in undifferentiated meristematic cells and weaker in more differentiated cells on the flanks of the meristem (see also Fig. 2G). In MSG1, 1×sGFP was detected in all cells (Fig. 2E). The bright-green dots in each cell represent nuclear localization of 1×sGFP. In MSG2, 2×sGFP movement was limited but clearly detected; 2×sGFP was detected in protodermal cells in MSG2 (Fig. 2F, arrow), whereas ER-GFP was absent in J2341 (Fig. 2D, arrow).
In midtorpedo embryos, ER-GFP is expressed in the SAM in a “dumbbell shape,” which trails off into a gradient in signal intensity (Fig. 2G, asterisk). Very weak expression also was detected in the RAM (Fig. 2G, arrowhead); the signal was too weak for photographic documentation but was visible by direct observation under the microscope. In MSG1 midtorpedo embryos, 1×sGFP moves from cell to cell throughout the entire embryo (Fig. 2H). In MSG2, 2×sGFP still exhibited limited movement, predominantly downward to the hypocotyl but also upward to the cotyledons (Fig. 2I). Like the late heart stage, 2×sGFP also moved into protodermal layers (Fig. 2I, arrow). 2×sGFP expression in the RAM is detected more easily in MSG2 than in J2341 (Fig. 2I, arrowhead). This increased GFP intensity likely is caused by the higher number of UASs used in MSG lines; J2341 contains 5 UAS repeats, whereas MSG1 and MSG2 each contain 12 UAS repeats for sGFP.
To ensure that we detected sGFP protein movement versus sGFP expression after mRNA movement, we performed in situ hybridization to localize sGFP mRNA in MSG1 and MSG2 midtorpedo embryos. In the SAM, we either detected small foci of expression (Fig. 2K, arrow) or U-shaped expression (Fig. 2L, arrow) depending on the longitudinal section of the embryo under examination. sGFP mRNA appeared as foci in a few cells in the RAM as well (Fig. 2 K and L, arrowheads). Thus, the sGFP detected in midtorpedo embryos of MSG1 and MSG2 represents bona fide movement of protein that originates in the SAM or RAM and subsequently moves outward (Fig. 2 J).
The data with MSG1 indicate that the embryo constitutes a single symplast with all cells interconnected through open PD channels. Previously we showed that the small symplastic tracer, 8-hydroxpyrene-1,3,6-trisulfonic acid (524 Da), traffics throughout the embryo (27). The present data extend these studies to reveal that proteins of at least 27 kDa also move throughout the embryonic symplast.
The data with MSG2 indicate that the SEL of different regions of the embryo changes throughout embryogenesis and support our previous finding that there is a down-regulation of PD aperture by the midtorpedo stage (27). In early stages such as early heart, 2×sGFP moves quite extensively so that the SEL of the early embryo is at least 54 kDa. By the late heart stage, 2×sGFP moves less and is restricted to the region around the SAM. Early movement of 2×sGFP supports the general principle that intercellular movement through PD is more extensive in young versus older tissues (9, 11, 35).
In previous studies, 10-kDa FITC-conjugated dextrans (F-dextrans) did not move cell to cell in midtorpedo embryos (27), yet here 27-kDa sGFP moved freely in midtorpedo embryos. There are several explanations for these different results. First, tracers were introduced into embryo cells differently in the two studies. Dextrans were added to the external medium for uptake and then assayed for subsequent movement, whereas sGFP was expressed endogenously. Dextrans are taken up by protodermal cells and then move inward. sGFP is expressed in a cup-shaped pattern that includes meristematic cells from the protodermal L1 layer to the underlying L2 and L3 layers (see in situ mRNA patterns in Fig. 2 K and L), and then it moves inward and outward. Thus, the nature of the cells initially containing/expressing tracer may affect tracer movement.
Second, these different data may reflect dissimilarities in the effective hydrodynamic size between the “expandable coil-like” polysaccharide 10-kDa F-dextran (Sigma FD-10S) and the cylinder-shaped GFP (36). It may be misleading to consider that GFP has a larger predicted Stokes radius (2.8 nm) than 10-kDa dextran (2.3 nm) (reviewed in ref. 4). Because 10-kDa F-dextran has no precise secondary or tertiary structure, the predicted “diameter” is only a statistical average of unbranched random coils, many of which might have larger diameters. In contrast, the structures of proteins are precise. It is very likely that the 3 × 4-nm barrel-shaped GFP (36) migrates through PD end to end versus sideways; as such, GFP may have an effective narrower dimension than 10-kDa F-dextran. Thus, size and molecular mass alone do not predict PD SELs accurately. Other factors such as hydrophobicity and molecular charge of cargo macromolecules (4) likely play significant roles in their transportability through PD. Nevertheless, data with dextrans and 2×sGFP confirm that there is a down-regulation of PD aperture during Arabidopsis embryogenesis at the late heart stage for 2×sGFP and at the midtorpedo stage for 10-kDa F-dextran.
The data showing that PD are innately dilated during embryogenesis have important practical implications for the use of GFP as a reporter in embryos. Tissue-specific localization of GFP translational fusions to proteins of interest should be interpreted with caution, because fusion proteins of at least 54 kDa (similar in size to 2×sGFP) can move from their initial site of transcription in early embryos. Thus, fusion to an ER-GFP should be used to monitor specific promoter activity and the original site of gene expression. Furthermore, nuclear localization of sGFP fusion proteins also should be interpreted with caution, because 54-kDa 2×sGFP without a nuclear localization signal moves into nuclei (see Fig. 2).
GFP Movement in Seedling Shoots. Shoot apices of seedlings were observed at 3, 8, and 14 dpg. The overall sGFP expression level in MSG lines is stronger than ER-GFP in J2341, as discussed above for embryos.
Seedlings of J2341 and MSG lines 3 dpg typically have one pair of expanded cotyledons under the growth conditions used (Fig. 3 A–F). ER-GFP in J2341 shows a gradient in expression at the shoot apex, from stronger in the center to less in cells near the hypocotyl (Fig. 3 A and D), similar to the pattern observed in midtorpedo embryos (Fig. 2G). In MSG1, 1×sGFP moves equally as a gradient up toward the cotyledons and down to the hypocotyl (Fig. 3 B and E). In MSG2, 2×sGFP moves only a few cell layers (Fig. 3 C and F).
Eight-dpg seedlings have one pair of expanded cotyledons and one pair of small rosette leaves (Fig. 3 G–L). ER-GFP expression remains tight at the shoot apex, with a slight gradient in expression pattern. In addition, a small group of cells in the petioles of rosette leaves show ER-GFP expression (Fig. 3 G and J, arrowheads). In MSG1, the movement pattern of 1×sGFP has changed; upward movement toward the petioles of rosette leaves predominates (Fig. 3 H and K), whereas downward movement to hypocotyls and upward movement to cotyledons are limited. In MSG2, 2×sGFP shows the same pattern of movement as 1×sGFP, albeit to a lesser extent, with predominate upward movement toward to the petioles of young rosette leaves (Fig. 3 I and L).
Fourteen-dpg seedlings have one pair of expanded cotyledons and two to four pairs of leaves (Fig. 3 M–R). Interestingly, ER-GFP expression now occurs not only in the SAM and basal petioles (Fig. 3 M and P, arrowheads) but expands to the upper part of leaf petioles (Fig. 3 M and P, arrows). The upward movement pattern of 1×sGFP is sustained (Fig. 3 N and Q) as well as that of 2×sGFP to a lesser extent (Fig. 3 O and R). In both MSG lines, movement toward rosettes leaves (younger tissues) prevails over movement toward cotyledons (older tissues).
That 1×sGFP moved throughout leaf primordia (Fig. 1E, arrow) prompted us to monitor its movement pattern during different stages of leaf development, exemplified by 14-dpg seedlings of MSG1. As in earlier stages, sGFP traffics throughout primordia (Fig. 4 A and B, +). However, in very young leaves (arrowheads) as well as slightly older leaves (arrows), sGFP moves only into approximately half of the leaf blade. Furthermore, whereas small younger leaves with visible vein structure allow 1×sGFP movement through the leaf blades (Fig. 4 A and B, arrows), as petioles elongate and leaves get bigger, 1×sGFP moves only through the central vascular tissues of petioles (Fig. 4C) and leaf veins (Fig. 4D). Although 2×sGFP does not move in primordia or young leaves (Fig. 1F), it does move into the vascular tissues of petioles and the veins of mature leaves, revealing a pattern identical to that shown for 1×sGFP in Fig. 4 C and D (data not shown). This latter result reflects that the SEL of vascular conduits is much larger than in nonvascular tissues. Fig. 5 diagrams the patterns of 1×sGFP movement in leaf primordia and developing leaves.
In summary, seedlings allow significant movement of 1×sGFP from the SAM into leaf primordia, petioles, and hypocotyls. These movement patterns reiterate that PD are more dilated and less restricted in younger, less differentiated tissues than in more mature tissues. The data also show definite differences in polarity of movement within the leaf; as primordia develop, PD in the distal region of the developing leaf blade close down, whereas PD proximal to the petiole (close to the SAM) retain the ability to traffic GFP. Even 2×sGFP exhibits moderate movement from the SAM. Finally, cells with specific fate to be conduits, i.e., vascular tissues and leaf veins, sustain/acquire a large SEL, allowing extensive movement of 1×sGFP and 2×sGFP, whereas nonvascular cells down-regulate their SEL as they mature.
Conclusions
MSG lines provide useful tools for assessing sGFP movement, and thereby PD SEL, from the SAM during different stages of development in Arabidopsis from embryogenesis to postgermination. The data presented here reinforce that intercellular macromolecular transport through PD occurs to the most significant extent in undifferentiated tissues and provide insight into the patterns of such movement as tissues mature. The data further provide evidence for directionality of symplastic movement in specific regions of developing leaves.
Acknowledgments
We thank Jim Haseloff (University of Cambridge, Cambridge, U.K.) for J2341 seeds and Steve Ruzin and Denise Schichnes (Biological Imaging Facility, University of California, Berkeley) for technical support with microscopy. This work was supported by National Institutes of Health Grant GM45244.
Author contributions: I.K., E.C., K.C., and F.D.H. performed research; and I.K. and P.C.Z. wrote the paper.
Abbreviations: PD, plasmodesmata; ER, endoplasmic reticulum; SEL, size exclusion limit; MSG, meristem-specific GFP; sGFP, soluble GFP; SAM, shoot apical meristem; RAM, root apical meristem; UAS, upstream activation sequence; dpg, days postgermination; F-dextran, FITC-conjugated dextran.
See Commentary on page 1817.
References
- 1.Zambryski, P. (2004) J. Cell Biol. 164, 165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oparka, K. J. (2004) Trends Plant Sci. 9, 33-41. [DOI] [PubMed] [Google Scholar]
- 3.Lucas, W. J. & Lee, J. Y. (2004) Nat. Rev. Mol. Cell. Biol. 5, 712-726. [DOI] [PubMed] [Google Scholar]
- 4.Heinlein, M. & Epel, B. L. (2004) Int. Rev. Cytol. 235, 93-164. [DOI] [PubMed] [Google Scholar]
- 5.Ding, B., Turgeon, R. & Parthasarathy, M. V. (1992) Protoplasma 169, 28-41. [Google Scholar]
- 6.Schultz, A. (1999) in Plasmodesmata: Structure, Function, Role in Cell Communication, eds. Van Bel, A. & Van Kesteren, W. (Springer, Berlin), pp. 173-204.
- 7.Carrington, J. C., Kasschau, K. D., Mahajan, S. K. & Schaad, M. C. (1996) Plant Cell 8, 1669-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duckett, C. M., Oparka, K. J., Prior, D. A. M., Dolan, L. & Roberts, K. (1994) Development (Cambridge, U.K.) 120, 3247-3255. [Google Scholar]
- 9.Oparka, K. J., Roberts, A. G., Boevink, P., Santa Cruz, S., Roberts, I., Pradel, K. S., Imlau, A., Kotlizky, G., Sauer, N. & Epel, B. (1999) Cell 97, 743-754. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, K. M. & Zambryski, P. C. (2000) Curr. Biol. 10, 1032-1040. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, K. M. & Zambryski, P. C. (2001) Plant Physiol. 125, 1802-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts, I. M., Boevink, P., Roberts, A. G., Sauer, N., Reichel, C. & Oparka, K. J. (2001) Protoplasma 218, 31-44. [DOI] [PubMed] [Google Scholar]
- 13.Gisel, A., Hempel, F. D., Barella, S. & Zambryski, P. (2002) Proc. Natl. Acad. Sci. USA 99, 1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas, W. J., Bouche-Pillon, S., Jackson, D. P., Nguyen, L., Baker, L., Ding, B. & Hake, S. (1995) Science 270, 1980-1983. [DOI] [PubMed] [Google Scholar]
- 15.Hantke, S. S., Carpenter, R. & Coen, E. S. (1995) Development (Cambridge, U.K.) 121, 27-35. [DOI] [PubMed] [Google Scholar]
- 16.Sessions, A., Yanofsky, M. F. & Weigel, D. (2000) Science 289, 779-782. [DOI] [PubMed] [Google Scholar]
- 17.Wu, X., Dinnemy, J. R., Crawford, K. M., Rhee, Y., Citovsky, V., Zambryski, P.C. & Weigel, D. (2003) Development (Cambridge, U.K.) 130, 3735-3745. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, K., Sena, G., Nawy, T. & Benfey, P. N. (2001) Nature 413, 307-311. [DOI] [PubMed] [Google Scholar]
- 19.Schellmann, S., Schnittger, A., Kirik, V., Wada, T., Okada, K., Beermann, A., Thumfahrt, J., Jurgens, G. & Hulskamp, M. (2002) EMBO J. 21, 5036-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada, T., Kurata, T., Tominaga, R., Koshino-Kimura, Y., Tachibana, T., Goto, K., Marks, M. D., Shimura, Y. & Okada, K. (2002) Development (Cambridge, U.K.) 129, 5409-5419. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J.-Y., Yuan, Z. & Jackson, D. (2003) Development (Cambridge, U.K.) 130, 4351-4362. [DOI] [PubMed] [Google Scholar]
- 22.Kim, M., Canio, W., Kessler, S. & Sinha, N. (2001) Science 293, 287-289. [DOI] [PubMed] [Google Scholar]
- 23.Xoconostle-Cazares, B., Xiang, Y., Ruiz-Medrano, R., Wang, H. L., Monzer, J., Yoo, B. C., McFarland, K. C., Franceschi, V. R. & Lucas, W. J. (1999) Science 283, 94-98. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Medrano, R., Xoconostle-Cazares, B. & Lucas, W. J. (1999) Development (Cambridge, U.K.) 126, 4405-4419. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg, R. B., De Paiva, G. & Yadegari, R. (1994) Science 266, 605-614. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield, S. G. & Briarty, L. G. (1991) Can. J. Bot. 69, 461-476. [Google Scholar]
- 27.Kim, I., Hempel, F. D., Sha, K., Pfluger, J. & Zambryski, P. C. (2002) Development (Cambridge, U.K.) 129, 1261-1272. [DOI] [PubMed] [Google Scholar]
- 28.Palevitz, B. A. & Hepler, P. K. (1985) Planta 164, 473-479. [DOI] [PubMed] [Google Scholar]
- 29.Jurgens, G. (1994) Pattern Formation in the Embryo (Cold Spring Harbor Lab. Press, Woodbury, NY).
- 30.Fraley, R. T., Rogers, S. G., Horsch, R. B., Eichholtz, D. A., Flick, J. S., Fink, C. L., Hoffmann, N. L. & Sanders, P. R. (1985) Bio/Technology 3, 629-635. [Google Scholar]
- 31.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 32.Delagrave, S., Hawtin, R. E., Silva, C. M., Yang, M. M. & Youvan, D. C. (1995) Bio/Technology 13, 151-154. [DOI] [PubMed] [Google Scholar]
- 33.Kim, J. Y., Yuan, Z., Cilia, M., Khalfan-Jagani, Z. & Jackson, D. (2002) Proc. Natl. Acad. Sci. USA 99, 4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oparka, K. J. & Turgeon, R. (1999) Plant Cell 11, 739-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi, K., Kim, I., Cho, E. & Zambryski, P. (2005) in Plasmodesmata, ed. Oparka, K. (Blackwell, Oxford), in press.
- 36.Ormo, M., Cubitt, A. B., Kallio, K., Gross, L. A., Tsien, R. Y. & Remington, S. J. (1996) Science 273, 1392-1395. [DOI] [PubMed] [Google Scholar]