Synopsis
PET/MR benefits neurological clinical care and research by providing spatially and temporally matched anatomic MR imaging, advanced MR physiologic imaging, and metabolic PET imaging. MR imaging sequences and PET tracers can be modified to target physiology specific to a neurological disease process, with applications in neuro-oncology, epilepsy, dementia, cerebrovascular disease, and psychiatric and neurological research. Simultaneous PET/MR provides efficient acquisition of multiple temporally matched datasets, and opportunities for motion correction and improved anatomic assignment of PET data. Current challenges include optimizing MR based attenuation correction and necessity for dual expertise in PET and MR.
Keywords: PET/MR, Neuro-oncology, Epilepsy, MR perfusion-weighted imaging, MR diffusion-weighted imaging, Dementia
Introduction
PET and MRI are both established imaging modalities for the study of disorders of the central nervous system, with applications in neuro-oncology, epilepsy, dementia syndromes, cerebrovascular disease, neurological disease, and psychiatric disorders. The two modalities offer complimentary information, with conventional MRI providing structural imaging of the brain with high spatial resolution and high tissue contrast and PET providing physiologic information about brain metabolism. In the last two decades, advanced MR imaging techniques, including perfusion-weighted imaging (PWI), functional magnetic resonance imaging (fMRI), magnetic resonance spectroscopy (MRS), and diffusion-weighted imaging (DWI), and several PET imaging agents targeting numerous metabolic pathways in the brain have expanded the tools available for studying the nervous system in the normal state and in various diseased states. Fusion of MRI and PET images obtained at different time points is commonly performed for clinical and research purposes, but the possibility that the physiologic processes studied by PET and by advanced MR techniques may differ slightly during the two different imaging sessions limits the ability to cross-validate emerging imaging techniques and may leave open questions about how the findings on the two imaging modalities relate to each other as there may be changes in the disease between imaging sessions. Brain imaging was one of the first applications of simultaneous PET/MR and the development of commercial simultaneous PET/MR units allows for true multiparametric analysis of the brain using the two modalities.1 This review will focus on applications suited to hybrid PET/MR scanners that acquire PET and MR imaging simultaneously.
PET/MR Imaging Protocols
Simultaneous PET/MR of the central nervous system is most beneficial when conventional MR imaging sequences are optimized for depicting the relevant structural anatomy, advanced MR imaging sequences are utilized to study the relevant physiological state of the brain, and the PET radiotracer and image acquisition are chosen to best depict the metabolic state of the brain. MR imaging protocols targeted to specific clinical or research questions, such as oncology, epilepsy, and dementia imaging, may be easily replicated on the simultaneous PET/MR unit allowing for straightforward comparison to MR examinations performed without simultaneous PET. Similarly, PET imaging protocols are easily replicated with results of PET brain imaging acquired on PET/MR units with MR attenuation correction performing similarly to PET brain imaging acquired with conventional CT attenuation correction.2 Initial studies showed good performance of hybrid PET/MR compared to PET/CT.2–5
Most commonly, PET/MR brain imaging will be performed alone without additional body imaging, although PET/MR may be used to study the remainder of the body, particularly for the workup of metastatic disease to the brain from a source outside the CNS.5–8 The minimum MR sequences necessary include the MR attenuation correction sequences, which depend on the MR attenuation correction technique used by the manufacturer and clinical or research site, and basic anatomic brain imaging sequences as dictated by the local site (Box 1). Commercial software for viewing simultaneous PET/MR imaging studies can fuse the PET images with any acquired conventional MR imaging sequence, including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), DWI, susceptibility-weighted imaging (SWI), and contrast-enhanced T1WI. At our institution, we optimize imaging for epilepsy by acquiring high spatial resolution imaging of the brain utilizing T1WI, fluid-attenuated inversion recovery (FLAIR), and T2WI sequences in the coronal plane, for tumors by acquiring high spatial resolution post-contrast T1WI and FLAIR sequences, and for dementia by acquiring high spatial resolution magnetization prepared rapid acquisition gradient echo (MPRAGE) sequences for quantitative volumetric analysis and PWI for assessment of cerebrovascular disease. Advanced imaging sequences including PWI, MRS, DTI, and fMRI may be obtained as well with results comparable to images acquired on a dedicated MRI.
Box 1. PET/MR Brain Imaging Protocols.
MR Acquisition
Dixon sequence for PET attenuation correction (modify if using an alternative method of attenuation correction)
Conventional brain MRI sequences
For tumor evaluation consider including PWI, MRS, fMRI, DTI
For epilepsy evaluation consider including coronal plane T1WI, T2WI, FLAIR
For dementia evaluation consider including MPRAGE for quantitative volumetric analysis and PWI
PET Acquisition
PET tracer may be injected either prior to scan or during scan depending on the indication
Simultaneously acquire PET data during MR acquisition
Tumor evaluation: 18F-FDG, amino acid analogs, 18F-FLT, 18F-MISO
Epilepsy evaluation: 18F-FDG
Dementia evaluation: 18F-FDG, amyloid-specific tracers, tau-specific tracers
Numerous PET imaging agents targeted to specific disease processes may be used with PET/MR. 2-[fluorine-18]fluoro-2-deoxy-D-glucose (18F-FDG) is the most well-known PET radiotracer and the most commonly used Food and Drug Administration (FDA)-approved radiotracer for clinical tumor imaging, epilepsy imaging, and dementia imaging. It is widely available in areas of the world that routinely perform clinical PET. There are currently three FDA-approved 18F -labeled amyloid-β PET imaging agents including 18F -florbetaben, 18F -florbetapir, and 18F -flutemetamol. Multiple other radiotracers, including those targeting tau protein in Alzheimer disease research and amino acid metabolites in brain tumor research, are not currently FDA approved. Limited access to cyclotron facilities capable of producing many of these PET tracers limits their use to research facilities.
The portion of the PET/MR exam specific to PET imaging includes the injection and uptake period for the PET tracer, acquisition of the MR images required for PET attenuation correction, and collection of PET data. Brain-only PET imaging may allow for the injection and uptake phase of the PET exam to occur while the targeted MR imaging is being acquired, thus increasing efficiency of the exam acquisition for both the patient and the PET/MR facility. Some PET studies benefit from the collection of dynamic PET data or PET images at more than one time point after injection which is easily coordinated within the sequence of MR image acquisition since PET data can be collected simultaneously. Long PET acquisition times are also possible, thus increasing total collected counts and signal to noise ratio.
PET/MR Benefits and Challenges
PET/MR offers several advantages over separately acquired conventional PET/CT and MRI (Box 2). The practical advantage of simultaneously acquiring PET and MRI during a single imaging session eliminates possible delays in scheduling two separate exams; potentially improves patient satisfaction as the patient only needs to travel to the imaging facility and take time away from other daily activities for a single exam rather than two exams; and patients requiring sedation for PET and MRI only need to undergo sedation a single time for a shorter total time length. A PET/MRI only utilizes ionizing radiation for the radiotracer used for the PET imaging, saving the added radiation necessary for attenuation correction and anatomic correlation utilized in the CT portion of a PET/CT which is beneficial in young patients, pregnant patients, and patients with a potentially long lifespan who may need multiple PET imaging studies during their remaining lifetime. PET/MR may add value by promoting multidisciplinary interpretations with PET and neuroimaging experts.6 While commercially available fusion software does a good job of fusing separately acquired PET/CT with MRI, only simultaneous PET/MR can provide temporally matched information for disease states that may change between the time it takes to acquire two separate imaging studies. Patient head motion during PET acquisition may interfere with appropriate attenuation correction or lead to misregistration of the PET data to the MR images. MR-based motion tracking may be used to realign the PET data.9,10 The PET spatial resolution obtained from the Siemens mMR Biograph simultaneous PET/MR (Siemens, Erlangen, Germany) has been measured at 4.3mm, which is greater than typical human cortical thicknesses.11 Activity in the thin cortical layer may be falsely localized to adjacent areas. The MR component of the scan may be used to create an anatomical mask for gray matter and white matter to improve assignment of PET activity to tissue on the MRI.10 Quantitative brain PET relies on an input function obtained from arterial sampling to estimate delivery of the tracer to the binding site. PET/MR provides a means for noninvasively measuring the arterial input function from the internal carotid artery, validated against conventional arterial blood sampling and 15O-H2O measurement of cerebral blood flow.12
Box 2. PET/MR Benefits and Challenges.
Benefits
Improved efficiency by acquiring PET and MR datasets during a single examination
MR-based attenuation correction reduces total radiation dose
Temporally and spatially matched anatomic MRI, PWI, DWI, and MRS data with PET metabolic data
Simultaneous acquisition reduces error in combined interpretation of MR physiologic and PET metabolic data which may fluctuate across time as disease states and therapies change
Opportunity for combined interpretations from PET and MR experts that may provide added benefit over separately interpreted PET and MR studies
Potential for MR-based motion correction for PET
Potential for MR-based gray-white matter mask to improve localization of PET activity
Noninvasive MR-based tools for measuring arterial input function for quantitative brain PET
Challenges
MR attenuation correction techniques require further validation
Availability of PET/MR scanners for clinical use remains limited to large medical centers
Two sets of technologists and physicians with expertise in PET and MR, or a single technologist and physician with dual training in PET and MR, are required to acquire and interpret PET/MR studies
PET/MR poses challenges for the PET and MR imaging communities (Box 2). MR-based attenuation correction strategies for the brain, discussed in detail elsewhere, are an ongoing area of research. The most common strategy used for pure MR-based attenuation correction in clinical scanners uses the Dixon MR technique creating an attenuation correction map consisting of tissue, air, fat, and water. This technique does not account for attenuation due to bone which is relevant to brain imaging since the brain is surrounded by the skull. Studies are ongoing evaluating the impact of this attenuation correction technique on brain imaging as well as developing alternative methods.13,14 Hitz and colleagues reported that the measured uptake of 18F-FDG in the brains of dementia patients was lower on a PET/MR scanner compared to a PET/CT scanner. This was true for both attenuation corrected and non-attenuation corrected data. A new cohort of normal control patients imaged with PET/MR may be necessary for future clinical trials which may require quantitative PET.15 There are few physicians with dual expertise in both modalities. The American College of Radiology and the Society of Nuclear Medicine and Molecular Imaging recommend that the MR component and PET component should be interpreted by physicians that meet qualifications standards in the two modalities.16 PET/MR is ideally interpreted as a single study utilizing both PET and MR information to arrive at a final interpretation. At some institutions, this may require joint interpretation by PET and MR experts. Cross-modality training should also be considered in residency and fellowship programs as well as professional societies offering continuing medical education. Similarly, the skill set and knowledge base required for meeting the technical and safety standards for acquiring PET and MRI differ, necessitating the crosstraining of PET and MRI technologists in the complimentary modality in order to safely acquire a PET/MR. The Society of Nuclear Medicine and Molecular Imaging Technologist Section and the Section for Magnetic Resonance Technologists recommend that technologists involved in acquiring PET/MR meet qualification standards for MR and PET individually which may either require MR and PET technologists, or a single dual trained technologist with advanced training in the field secondary to their area of expertise.17
Applications
Oncology
Contrast-enhanced MRI is the first line imaging study for diagnosis, presurgical planning, and treatment monitoring of both primary brain tumors and metastatic brain tumors. Advanced MR imaging techniques including perfusion-weighted imaging (PWI), diffusion-weighted imaging (DWI), MR spectroscopy (MRS), diffusion tractography (DTI), and functional MR imaging (fMRI) provide physiologic information about the tumor and adjacent brain which complements conventional MRI in both clinical and research settings.
PET utilizes radiotracers targeted to different aspects of tumor metabolism: glucose metabolism (18F-FDG), amino acid transport and protein synthesis (11C-MET, 18F -FET, 18F -DOPA), proliferation rate and DNA synthesis (18F -FLT), oxygen metabolism and hypoxia (18F -MISO). Although not practical for clinical use due to short half-life, 15O-H2O is used to study tumor perfusion.18 While PET does not have a primary clinical role in oncologic imaging of the brain, 18F-FDG PET of the brain is used clinically most commonly to differentiate radiation necrosis from recurrent tumor.19,20 The radiotracers targeting other metabolites are promising because of improved signal to background ratio and specificity for tumor compared to 18F-FDG. PET has been used in glioma research utilizing multiple tracers beyond 18F-FDG targeting different aspects of tumor metabolism to study tumor grading, tumor extension, treatment planning, treatment follow up, and prognosis, as well as to validate physiologic information gained from advanced MR imaging. The combination of MR-based anatomic and physiologic imaging with temporally and spatially matched PET-based molecular imaging allows us to answer questions pertaining to prognosis, plan therapeutic approaches, and assess response to treatment with greater confidence than is possible with either modality alone (Box 3).21
Box 3. Oncology Applications.
Simultaneous PET/MR provides benefit by ensuring that MR physiologic data and PET metabolic data reflect the same biological state, eliminating the chance that change in therapy between separate imaging sessions may cause discordant results
Glioma Grading
Contrast enhancement, elevated rCBV, lactate peak on MRS, and low ADC suggest HGG
18F-FDG, 11C-MET, 18F-DOPA, and 18F-MISO can differentiate HGG from LGG
Tumor Extension
PWI, DTI/DWI, MRS, and amino acid analog PET tracers aid in determining nonenhancing tumor margins
Treatment Follow Up
MRS, PWI, DWI, 18F-FDG PET, and amino acid PET aid in differentiating radiation necrosis and pseudoprogression from recurrent high grade glioma
Metastatic Disease
Conventional contrast-enhanced MRI is primarily used for detection of intracranial metastatic disease and follow up after treatment
Whole-body 18F-FDG PET/MR including dedicated contrast-enhanced brain imaging may be equivalent to PET/CT and brain MRI for staging metastatic lung cancer
Tumor Grading and Differential Diagnosis
The differential diagnosis of intracranial masses and initial estimation of tumor grade begins with conventional MRI. Contrast-enhancement due to breakdown of the blood brain barrier is the imaging marker used in MRI to differentiate high grade gliomas (HGG) from low grade gliomas (LGG), and to detect transformation of a LGG to a HGG. Histopathologic evaluation of brain tumors remains the gold standard for assessing tumor grade since as many as a third of HGG may not enhance.22 Additionally, stereotactic biopsy may undersample a tumor, leading to an inappropriately lower histologic grade.23 PWI, DWI, and MRS provide additional physiologic information and PET provides additional metabolic information to delineate regions of the tumor of highest grade for biopsy planning and to detect transformation of a LGG to a HGG with greater confidence.
PWI interrogates the vascularity of tumor and the surrounding tissue. Within the astrocytoma line of tumors, higher rCBV corresponded to higher tumor grade. rCBV aids in differentiating neoplastic from non-neoplastic lesions, provides an estimate of tumor grade, and may be used to direct biopsy towards the portion of the tumor with the potential highest grade.24–26 Caution is necessary in utilizing results as there is variability in techniques for processing and interpreting MR perfusion which influence results, and the major techniques do not yield the same values when applied to the same tumor.27,28 The apparent diffusion coefficient (ADC) value obtained from DWI corresponds to high cellularity seen at pathology in HGG.29 DWI can predict glioma grade, with higher grade tumors having lower ADC values.30,31 An altered metabolite spectrum with 1H-MRS suggests tumor grade. 1H-MRS demonstrates increased choline due to increased cellular membrane synthesis and reduced N-acetylaspartate due to neuronal loss in the setting of HGG. Elevated lactate suggests tumor hypoxia and lipid indicates necrosis as is seen in HGG histopathology.32 MR spectroscopy is technically challenging to acquire in clinical practice with overlap seen between tumors and tumor mimickers.
High grade tumors have a high rate of glucose metabolism and increased 18F-FDG uptake. 18F-FDG uptake correlates with high glioma grade and reduced survival.33 New elevated 18F-FDG uptake within a LGG signals conversion to HGG.33,34 Inherent high 18F-FDG background activity in the brain results in poor delineation of some primary brain tumors and small metastases compared to MRI, preventing routine use of 18F-FDG in brain tumor imaging.8,35,36 Coregistration of 18F-FDG PET with MRI is critical as 18F-FDG uptake of tissue must be correctly assigned to white matter, gray matter, or abnormal tissue for proper interpretation.37 Alternative PET tracers, including 18F-DOPA, 18F –MISO and 11C-MET, have been studied as well for predicting tumor grade and time to progression but are not in routine clinical use.38–41
Tumor Extension and Treatment Planning
MRI defines the margins of enhancing and nonenhancing primary and metastatic tumor for surgical resection, but there is overlap in the appearance of nonenhancing glioma margins and vasogenic edema on T2WI.22 Advanced MRI sequences, including diffusion tensor imaging, MRS, and PWI, and PET imaging with amino acid analogs aid in differentiating tumor margin from edema. PWI, particularly rCBV, and fractional anisotropy derived from diffusion tensor imaging differ between nonenhancing tumor and peritumoral edema.26,30 Amino acid analogs are well suited to this problem because they have high uptake in tumor and low uptake in normal brain which improves tumor-tissue contrast compared to 18F-FDG.42 11C -Methionine and 18F-FET PET have been shown to differentiate tumor margin from edema, with 18F -FET PET shown to be superior to 18F-FDG PET in determining extent of tumor.43–45 18F -DOPA also shows promise in ability to delineate tumor margins for surgical resection and radiotherapy planning (Fig. 1). Multiparametric maps including MRI and PET sequences may lead to maps of the brain delineating on a voxel by voxel basis the expected grade of tumor and delineate the full extent of tumor. 46 Simultaneous PET/MR efficiently combines structural MR imaging with advanced MR data and metabolic PET data for complete pretreatment evaluation of the tumor.3,6,38,47
Fig. 1.






18F-DOPA PET/MR obtained for defining extent of nonenhancing WHO grade III oligodendroglioma. Axial post-contrast T1WI (A) and FLAIR (B) shows nonenhancing tumor in the right frontal lobe. 18F-DOPA PET unfused (C) and fused to FLAIR (D) shows uptake in a portion of the abnormal tissue with a region of interest demarcating greatest 18F-DOPA update and suspected nonenhancing high grade tumor margin on FLAIR (B), unfused PET (B), and fused PET (C) images. The 18F-DOPA activity corresponds well to increased rCBV (E) and reduced ADC (F) which are additional MR markers of high grade tumor.
fMRI and tractography play a role in presurgical planning prior to biopsy or resection of gliomas and metastases by delineating eloquent cortex and the associated fiber tracts. Incorporating fMRI and DTI into the presurgical workup facilitates maximal resection with reduced risk of loss of neurologic function as a result surgery.48,49 Neuschmelting et al. recently reported using PET/MR including 18F -FET PET, contrast-enhanced MRI, tractography, and navigated transcranial magnetic stimulation to map the relationships between enhancing tumor, metabolically apparent tumor, and eloquent cortex and tracts for surgical planning.50
Treatment Follow Up
Contrast-enhanced MRI is the key imaging study for following response to therapy, recurrence, and development of treatment-related changes. Conventional MRI is fraught with problems in the post-treatment brain because of difficulties in assessing nonenhancing tumor, following response to anti-angiogenic therapy, differentiating pseudoprogression from true early progression, and differentiating recurrent tumor from delayed radiation necrosis.51–54 Although structural detail is superior with MRI, overlap in the appearance of treatment-related changes and recurrent high grade glioma or metastases supports a role for PET imaging. Simultaneous PET/MR is especially useful as it is a common conundrum in clinical practice that results from MR differ from the results of 18F-FDG PET. Differences may be due to changes in therapy initiated between the scans or reflective of complex tumor biology. Simultaneous acquisition of MR physiologic imaging and PET metabolic imaging provides more confidence that all imaging data reflects the same state.
rCBV has been shown to aid in differentiating delayed radiation necrosis from recurrent high grade tumor.55 Fink et al. showed that multivoxel MRS, specifically Choline/Creatine and Choline/N-acetylaspartate ratios, and rCBV ratios had equivalent performance in differentiating radiation necrosis from recurrent high grade glioma.56 Multiparametric maps incorporating ADC, rCBV have been shown to correlate with response to treatment.57,58 Prat et al. showed that MRS and PWI reached 100% positive and negative predictive values for detecting the presence of high grade glioma in treated patients, compared to a positive predictive value of 75% and negative predictive value of 61.1% for 18F-FDG PET.59
One of the earliest applications of 18F-FDG PET in the brain was differentiating radiation necrosis from recurrent tumor (Fig. 2).20 18F -FLT, 18F -DOPA, 18F -FLT, and 11C-MET with their more favorable tumor-to-background activity characteristics, may perform better than 18FFDG. 19,60–63 In a meta-analysis published in 2013, Nihashi et al. reported that 11C-MET had a summary sensitivity of 0.70 (95% confidence interval, 0.50–0.84) and specificity of 0.93 (95% confidence interval, 0.44–1.0) across 7 studies for detecting recurrence of HGG after treatment compared to a summary sensitivity of 0.77 (95% confidence interval, 0.66–0.85) and specificity of 0.78 (95% confidence interval, 0.54–0.91) across 16 studies for 18F-FDG PET.19 The mean lesion-to-normal tissue ratio of 11C-MET uptake was a good tool for differentiating tumor recurrence from radiation necrosis for both gliomas and metastatic disease.64 Two studies found that 18F -DOPA was more accurate than rCBV in detecting recurrent brain metastases from radiation necrosis.65,66 While the body of evidence comparing PET to other imaging modalities is growing, the body of knowledge is still small.
Fig. 2.

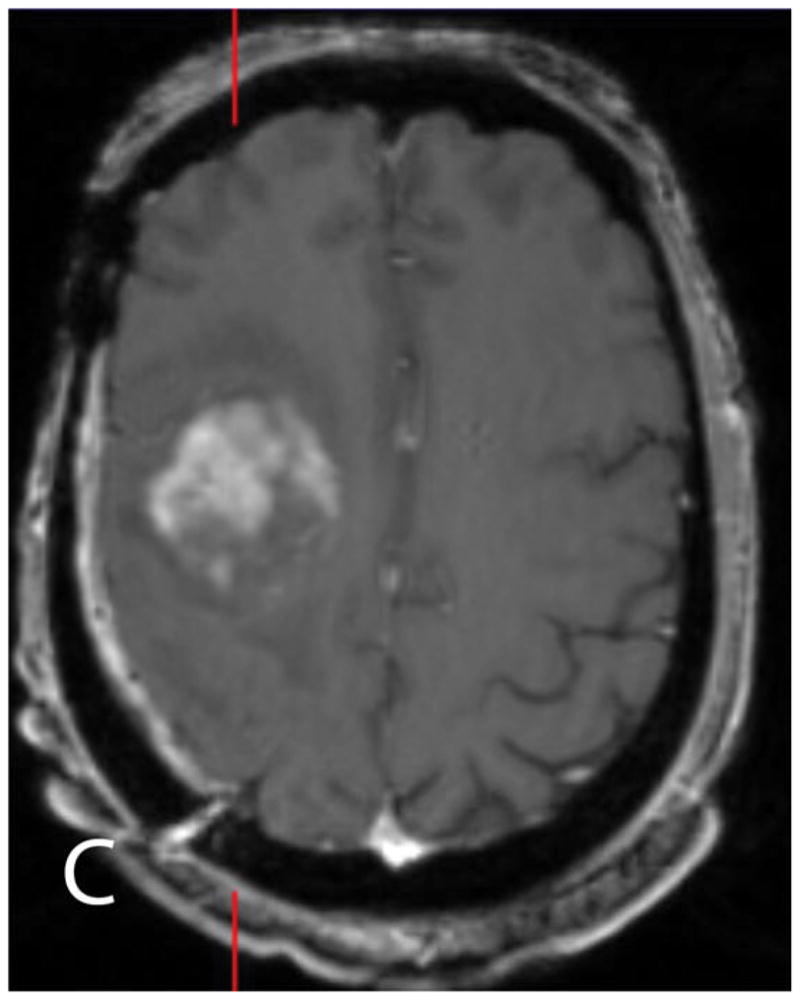
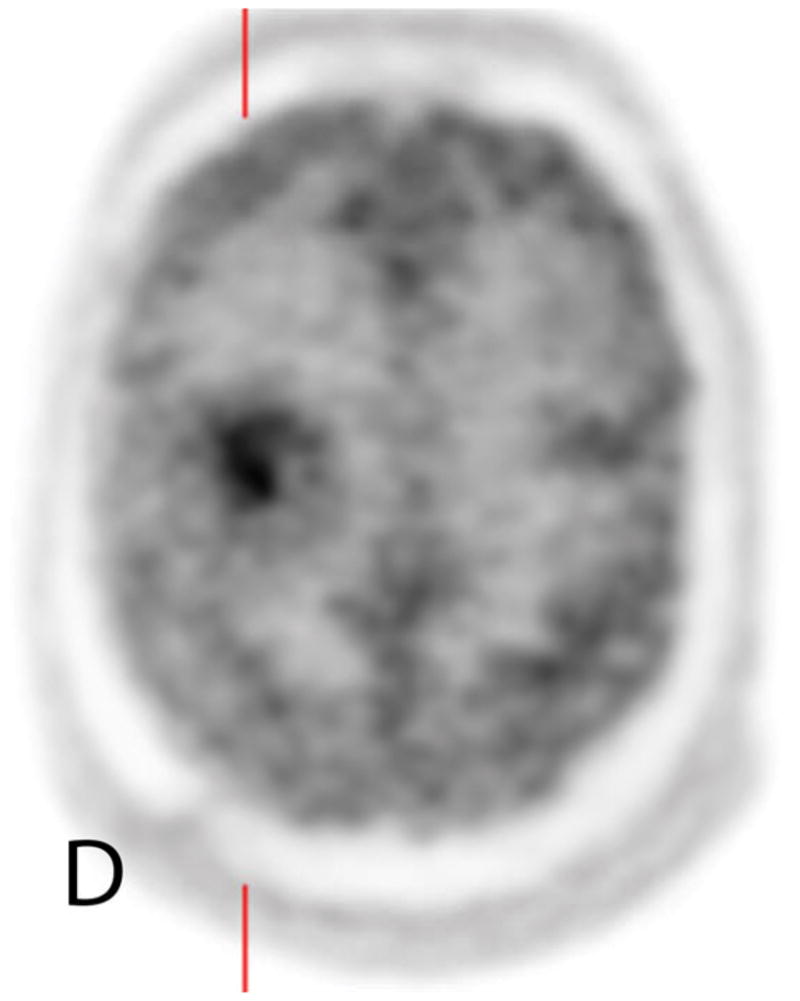

45 year old man with a history of a resected WHO grade IV GBM treated with chemotherapy and radiotherapy presents with worsening FLAIR (A) signal changes and new enhancement on contrast-enhanced T1WI (B, C) adjacent to the treatment site. Increased 18F-FDG uptake on unfused PET (D) and fused PET/MR (E), and elevated rCBV (F) support recurrent GBM.
Evaluation of Metastatic Disease
Brain metastases are best detected with contrast-enhanced MRI. Many brain metastases may be small and therefor masked by normal cortical 18F-FDG uptake. Whole body PET/MR has been proposed as a one-stop shop approach to screening for metastatic disease in newly diagnosed lung cancer, evaluating the body with 18F-FDG PET with MR for attenuation correction and anatomic correlation and the brain with dedicated contrast-enhanced brain MRI sequences.6–8 Other cancers requiring initial staging with brain MRI and whole-body PET/CT may be candidates for whole-body PET/MR as well.
Role of PET/MR in Neuro-Oncology
Multimodality imaging of brain tumors with MRI and PET at initial diagnosis and in follow up imaging adds information that may benefit treatment planning and decision making.21,67 PET/MR has emerged as an important tool in neuro-oncologic research providing spatially and temporally matched molecular, physiologic, and anatomic data for the study of tumor biology and therapies as well as a means for cross-validating advanced MR techniques with PET-derived data. Studies are emerging showing that multiparametric tumor maps incorporating PWI, DWI, and MRS with molecular PET data predict tumor grade, define tumor extent, and correlate with response to therapy.46,57,58 The combined data obtained from PET/MR may provide a better estimate of tumor extent, higher accuracy in tumor grading, more prognostic information, optimization of surgical, chemotherapeutic, and radiation-based therapy, and better delineation of tumor recurrence from radiation necrosis than either modality alone.
Challenges still remain for PET/MR in the field of neuro-oncology. Filss at al. found that areas of high grade tumor predicted by elevated rCBV differed from those predicted by 18F -FET uptake.68 Sacconi et al. studied accuracy of 18F-FDG and PWI for differentiating HGG from LGG and tumor recurrence from treatment-related changes. They found differing spatial distribution between regions of elevated rCBV and increased 18F-FDG uptake.69 Future challenges for the PET/MR community include defining the optimal set of MR imaging parameters and standardizing their acquisition and processing for reproducibility, identifying the best PET radiotracer for each clinical question, and justifying the clinical utility of multimodal imaging.
Epilepsy
Diagnostic imaging plays a crucial role in the evaluation of patients with medically refractory epilepsy, including patients with temporal lobe epilepsy (TLE), focal cortical dysplasia (FCD), and tuberous sclerosis complex (TSC). Both structural imaging with MRI and metabolic imaging with interictal 18F-FDG PET are utilized to confirm the location of epileptogenic cortical lesions suspected by clinical observation, EEG, and intracranial monitoring in preparation for surgical resection (Box 4). PET may provide additional diagnostic support for a suspected epileptogenic focus identified on MRI, or it may provide independent information regarding a potential epileptogenic focus in the setting of either a normal MRI or an MRI with multiple structural abnormalities. In addition to surgical planning, the combination of structural and PET imaging informs outcomes, alters treatment plans, and increases confidence in surgical therapy.
Box 4. Epilepsy Applications.
Structural imaging with MRI can identify lesions responsible for medically-refractory epilepsy
Combined MRI and 18F-FDG improves detection of focal cortical dysplasia over either modality alone
Unilateral reduced 18F-FDG uptake in temporal lobe epilepsy predicts a better outcome following surgery
Simultaneous PET/MR may show lesions not detected by PET/CT and MR evaluation alone
18F-FDG is the dominant PET radiotracer used in clinical practice, with epileptogenic foci demonstrating hypometabolism (Fig. 3).70,71 Fusion of separately acquired MRI with 18F-FDG PET improves detection of FCD over either modality alone and supports planning for surgical resection.72 Fusion of separately acquired high spatial resolution MRI to 18F-FDG allows limited cortical resection of metabolically abnormal foci.73 In a study of TLE patients, subjects with a positive 18F-FDG PET and a normal MRI did as well after temporal lobe resection, suggesting that a PET scan lateralizing clinically suspected TLE may obviate the need for intracranial monitoring when MRI is normal.74 Gok et al. demonstrated that TLE patients with unilateral reduced temporal lobe 18F-FDG uptake had excellent postsurgical outcomes, with 96% Engel class I and II.75
Fig. 3.

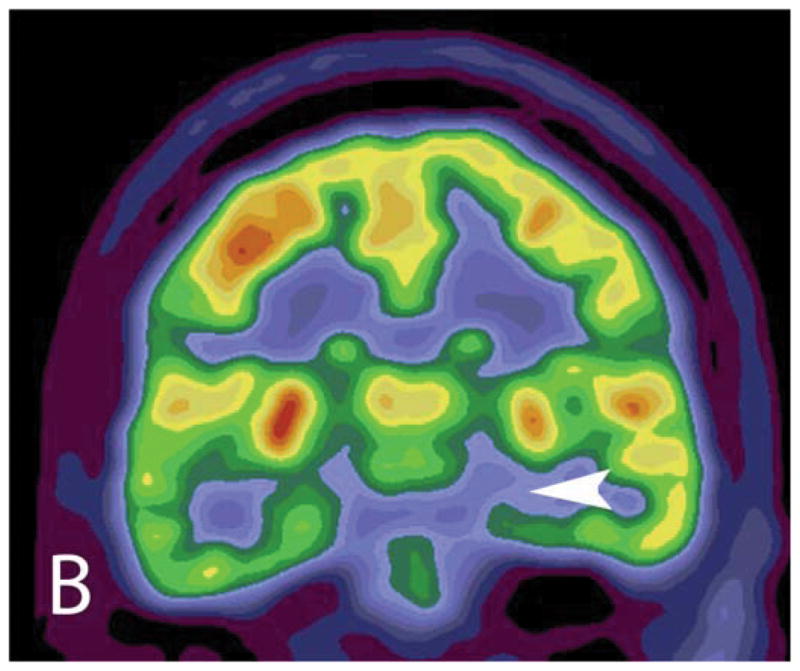

PET/MR for presurgical workup of a 47 year old woman with medically refractory temporal lobe epilepsy shows classic signs of hippocampal sclerosis on the coronal FLAIR images (A) including increased signal intensity and reduced size of the hippocampus (arrow) with reduced 18F-FDG uptake on the unfused PET (B) and fused PET/MR (C) (arrowhead).
While the vast majority of research has been performed with separately acquired PET and MRI, recently simultaneous PET/MR has been reported to perform well in detecting clinically confirmed epileptogenic foci.76 Simultaneous PET/MR has also been reported to show new lesions that were not detected on PET/CT and MR evaluation alone.77
Dementia
Dementia is a major cause of disability in the elderly. Alzheimer’s disease (AD) is present either alone or coexisting with another type of dementia in approximately 60–80% of patients with dementia, with cerebrovascular dementia (CVD), frontotemporal lobar dementia (FTLD), and dementia with Lewy bodies (DLB) accounting for the majority of the remaining causes of dementia.78 Mild cognitive impairment (MCI) is a state in which there is impairment in memory in combination with cognitive impairment, but it does not interfere with the patient’s ability to function in usual activities.79 MCI often precedes AD, but not all patients with MCI will progress to AD. Pathologic accumulation of amyloid-β plaques within the brain initiates a cascade that results in tau protein accumulation in the form of neurofibrillary tangles and eventual neuronal loss.80–83 The diagnosis of AD and MCI relies primarily on meeting core clinical criteria for AD without meeting core criteria for other disorders such as CVD, FTLD, or DLB. The diagnosis of AD may be supported by imaging and cerebrospinal fluid biomarkers.79 There are currently no therapies that can halt or reverse AD, but cholinesterase inhibitors and memantine are approved by the Food and Drug Administration to temporarily improve the cognitive symptoms of AD.
The evaluation of dementia begins with structural imaging to exclude causes other than AD. Advanced MR techniques, including volumetric MRI, susceptibility-weighted imaging (SWI), fMRI, and PWI, and PET molecular imaging with 18F-FDG, amyloid-specific radiotracers, and tau-specific radiotracers serve as biomarkers of the pathogenesis of AD (Box 5)79,84–97.
Box 5. Dementia Applications.
Quantitative volumetric MRI can provide patterns of atrophy that suggest AD, FTLD, or normal pressure hydrocephalus
PET provides imaging biomarkers of AD physiology within the brain
A negative amyloid-PET scan suggests that AD is unlikely to be the cause of dementia
Tau-PET tracer accumulation displays the spatial distribution of tau protein deposition in AD and correlates with clinical severity of dementia and degree of neurodegeneration seen at autopsy
18F-FDG PET reflects neuronal injury in AD, helps differentiate AD from other causes of dementia, and predicts decline from MCI to AD with good sensitivity and specificity
Simultaneous PET/MR provides both the convenience of a single imaging session as well as confidence that all imaging biomarker data reflects the same biological state of the brain
Structural MRI
Structural imaging first excludes imaging patterns that support other explanations for dementia including multiple infarcts in the cortex and deep gray nuclei (CVD), frontotemporal-predominant atrophy (FTLD), ventriculomegaly disproportionate to cerebral atrophy (normal pressure hydrocephalus), and marked intracranial mass effect (large intracranial tumors or subdural hygromas). The pattern of cortical volume loss reflects the pathophysiology of AD, with volume loss more prominent in the temporal and parietal lobes, with the entorhinal cortex and hippocampus most severely affected.88 Cortical atrophy is a normal part of aging, but the rate of cortical atrophy is accelerated in AD. Hippocampal atrophy is reported in 70–95% of patients with AD and has a sensitivity of 85% and specificity of 88% for distinguishing patients with AD from healthy control patients.88,93
Perfusion Weighted Imaging
There is a growing recognition that vascular disease influences the cognitive impairment of AD.98 PWI using dynamic-contrast enhanced technique (DCE) displays regional alterations in cerebral hemodynamics and blood-brain barrier integrity.99 As research into the role of cerebral hemodynamics in AD continues, assessment of cerebrovascular disease may be viewed alongside the metabolic imaging markers of AD pathophysiology (amyloid-PET, tau-PET, FDG-PET) and structural MR imaging data in determining progression of MCI to AD or guiding future therapy for AD.99
Amyloid-PET
Amyloid-β in the brain can be imaged by radiotracers that specifically bind amyloid-β, including 11C-labeled Pittsburgh compound B (PiB, GE Healthcare) and 18F-labeled florbetapir (Avid Radiopharmaceuticals), flutametamol (GE Healthcare), and florbetaben (Piramal Imaging).89 Amyloid-β accumulation demonstrated by a positive amyloid-PET study is not unique to AD and can be seen in cognitively normal adults, patients with dual causes for dementia, and patients with other amyloid-related disease such as DLB. A positive amyloid-PET study may not indicate the presence of AD or may not indicate that AD is the sole cause of cognitive impairment. A negative amyloid-PET scan does indicate that AD is unlikely to be the cause of the dementia disorder.86,87 In patients with MCI, a positive amyloid-PET study is associated with progression to dementia.90 Amyloid-PET is most commonly used in drug treatment trials and other research settings since it is not currently reimbursed by Medicare and Medicaid.
Tau-PET
Several PET radiotracers that bind tau with high affinity are available including 11C -PBB3, 18F -T807(AV-1451), 18F -1808, 18F -THK-5105, and 18F -THK-5117.85 Tau-PET displays the spatial distribution of tau protein deposition over time, which correlates with the neurodegeneration of AD. Tau accumulation begins in the entorhinal cortex and hippocampus, and then it spreads to the temporal and parietal cortices.80,91 Amyloid-PET may be positive before tau-PET since amyloid-β accumulation precedes tau-mediated damage to the brain.91,92 Tau-PET is closely associated with clinical severity of dementia and has a high association with neurodegeneration seen at autopsy.84,100
18F-FDG PET
18F-FDG preceded amyloid- and tau-specific PET imaging agents. It serves as a biomarker for neuronal injury in AD. A pattern of hypometabolism in the parietotemporal associate cortices, posterior cingulate, and precuneus differentiates AD from the healthy elderly brain and from FTLD, DLB, and CVD based on differing distribution of hypometabolism in the brain.96 As more and more complex patients are presenting for dementia workup, patients with dual diagnoses of CVD and AD may complicate interpretation of 18F-FDG PET as blood flow influences 18F-FDG PET imaging. Inclusion of PWI with simultaneous 18F-FDG PET/MR can help differentiate between altered cerebral blood flow impacting the distribution of 18F-FDG and it can assess for dual physiologic process of AD and CVD. 18F-FDG PET predicts a decline from MCI to AD with sensitivity and specificity ranging from 75% to 100% and predicts early AD with high sensitivity (approximately 90%).96 18F-FDG PET distinguishes AD from FTLD with 93–98% specificity.94 As the dementia disorders have differing clinical manifestations, fMRI has shown that AD, FTLD, semantic dementia, and progressive nonfluent dementia show disruption of differing brain networks. 18F-FDG PET, structural MRI, and fMRI acquired with PET/MR have shown that patterns of regional hypometabolism, network disruption, and cortical atrophy perform well in differentiating these neurodegenerative syndromes.97 Currently, 18F-FDG PET is reimbursed by the Centers for Medicare and Medicaid for differentiating AD from FTLD, and for patients participating in approved clinical trials utilizing 18F-FDG PET for the diagnosis and treatment of AD.89
PET/MR in Dementia Research
The key hallmarks of Alzheimer’s pathology are all captured by neuroimaging which can be used to classify patients as positive or negative for amyloid, tauopathy, and neurodegeneration (Fig. 4).101 PET/MR provides a convenient tool for acquiring high resolution structural imaging with concurrent data for detecting neurodegeneration with the molecular imaging for detecting amyloid and tau proteins in AD clinical trials, prediction of progression of MCI to AD, and differentiation of AD from other clinical dementia syndromes.
Fig 4.





Axial 18F-Florbetapir amyloid PET (A), axial 18F-Flortaucipir tau PET (B), and axial and coronal T1WI (C, D) of a cognitively normal 78-year old male. The amyloid PET scan is negative for amyloid pathology with visually greater uptake of tracer in white matter than in gray matter. The tau PET scan is normal with no specific uptake in the cortex to suggest tauopathy. The brain MRI is normal, without temporal lobe atrophy to suggest neurodegeneration. Axial 18F-Florbetapir amyloid PET (E), axial 18F-Flortaucipir tau PET (F), and axial and coronal T1WI (G, H) of a 76-year old female with AD. The amyloid PET scan is positive for amyloid pathology, with visually equal uptake of tracer in white matter and in gray matter. The tau PET scan is positive, with left parieto-occipital uptake (arrowhead) supporting an AD pattern of tauopathy. The brain MRI demonstrates hippocampal atrophy, with concominant enlargement of the adjacent temporal horn of the lateral ventricle (arrow), a pattern of neurodegeneration supportive of AD.
Cerebrovascular Disease
Cerebral Perfusion
In acute ischemic stroke, MRI utilizing semi-quantitative PWI and DWI are able to evaluate infarct volume, potentially reversible tissue ischemia, and collateral circulation. These MRI parameters have been used in clinical trials to select patients for intravenous medical therapy and endovascular mechanical thrombectomy for revascularization of acute infarct.102–105 15O-H2O PET, and subsequently PWI, have been used to study cerebral blood flow (CBF) in reversible cerebral ischemia.106,107 Validation of PWI techniques, including DSC and ASL, remain necessary as the lack of standardization and variability in values produced by the techniques contributes to the lack of clinical consensus in adopting them for routine use. PET/MRI is the ideal imaging tool for cerebrovascular perfusion research as it allows temporally matched correlation of DWI with quantitative 15O-H2O PET CBF, and evaluation of CBF with MRI and with the gold standard 15O-H2O PET.108 The practical application of 15O-H2O PET to stroke research is challenging, however, because of difficulties in supplying a radiotracer with a short half-life within the appropriate timeframe to study cerebral hemodynamics during an acute stroke.
Carotid Plaque Imaging
PET/MR has been used for vessel wall imaging in the setting ischemic stroke of cryptogenic origin with complicated MR features of a carotid plaque combined with evidence of vessel wall inflammation on 18F-FDG PET suggesting a causal role for these plaques in the stroke.109 Conventional MR coregistered to 18F-FDG PET/CT has been used to correct partial volume error in vessel walls, suggesting that PET/MR may add benefit to vessel wall imaging in the evaluation of plaques at risk for rupture.110
Neurological and Psychiatric Disorders
Advances in the understanding of psychiatric disorders, including autism, depression, and schizophrenia, brought about by fMRI and DTI, and the potential of novel radiotracers in PET to study neurotransmitters in the brain make PET/MR an attractive modality for the future study of mental disorders.111
Traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) are areas ripe for study with PET/MR. 18F-FDG PET has been used to study TBI in human subjects and animal models, correlating alterations in patterns of 18F-FDG uptake in the brain with cognitive and behavioral abnormalities.112 The effects of TBI on the functional connectivity and white matter tracts of the brain are well studied by fMRI and DTI.113
Conclusion
Prior to the introduction of hybrid PET/MR technology, the role of PET and MRI in disease processes in the brain were studied independently. Post hoc fusion of the two modalities provided good spatial registration, but the possibility of the physiological state of the disease process changing between MR and PET imaging sessions limited interpretation of some datasets. Hybrid PET/MR allows temporal and spatial matching of datasets displaying different physiological and metabolic information about a disease process. As the body of research incorporating the information derived from PET and from MR grows and the utility of multimodal diagnostic evaluation in neuro-oncology, neurodegenerative disease, epilepsy, cerebrovascular disease, psychiatry, and neurology is evaluated, PET/MR shows promise in providing an efficient means for acquiring these studies and may show added benefit beyond separate acquisition and interpretation of PET and MRI alone.
Key Points.
Simultaneous PET/MR acquires spatially and temporally matched anatomic MR imaging, MR-based physiological imaging, and PET metabolic imaging of the brain for the purposes of clinical care and research.
The combined data obtained from PET/MR may provide a better estimate of tumor extent, higher accuracy in tumor grading, tools for optimization of therapy, and better differentiation of tumor recurrence from radiation necrosis than either modality alone.
While MRI is the first line imaging modality for evaluating potential surgical therapy for medically-refractory epilepsy, PET may provide additional diagnostic support for a suspected epileptogenic focus identified on MRI, or it may provide independent information regarding a potential epileptogenic focus in the setting of either a normal MRI or an MRI with multiple structural abnormalities.
18F-FDG PET, amyloid-PET, tau-PET, and volumetric MRI analysis provide imaging biomarkers of the pathological processes involved in Alzheimer’s disease, aiding in the prediction of progression to Alzheimer’s disease and confirmation of Alzheimer’s disease for the purpose of clinical trials.
Acknowledgments
Funding statements:
Work published in this article was supported NIH grants P50 AG05681, P01 AG003991, P30NS048056, P01AG026276, R01AG043434 and UL1TR000448, through the generous support of the Hope Center for Neurological Disorders and the Barnes-Jewish Hospital Foundation, and through by grants from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly), including provision of the 18F-Florbetapir and of the technology for synthesis and precursor for 18F-AV1451.
Footnotes
Disclosure statements:
Michelle M. Miller-Thomas, MD has no commercial or financial conflicts of interest. Tammie L.S. Benzinger, MD PhD receives grant support from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly), participates in clinical trials for Avid Radiopharmaceuticals, Eli Lilly, and Roche, prepares continuing medical education materials for Medscape and Quintiles, and receives travel support from the National Multiple Sclerosis Society.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle M. Miller-Thomas, Mallinckrodt Institute of Radiology, Washington University School of Medicine, 510 S. Kingshighway Blvd. Campus Box 8131, St. Louis, Missouri 63110.
Tammie L.S. Benzinger, Mallinckrodt Institute of Radiology, Washington University School of Medicine, 510 S. Kingshighway Blvd. Campus Box 8131, St. Louis, Missouri 63110.
References
- 1.Werner P, Barthel H, Drzezga A, Sabri O. Current status and future role of brain PET/MRI in clinical and research settings. European journal of nuclear medicine and molecular imaging. 2015 Mar;42(3):512–526. doi: 10.1007/s00259-014-2970-9. [DOI] [PubMed] [Google Scholar]
- 2.Boss A, Bisdas S, Kolb A, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010 Aug;51(8):1198–1205. doi: 10.2967/jnumed.110.074773. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DL, Barthel H, Beuthin-Baumann B, et al. Combined PET/MR: Where are we now? Summary report of the second international workshop on PET/MR imaging April 8–12, 2013, Tubingen, Germany. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2014 Jun;16(3):295–310. doi: 10.1007/s11307-014-0725-4. [DOI] [PubMed] [Google Scholar]
- 4.Schlemmer HP, Pichler BJ, Schmand M, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008 Sep;248(3):1028–1035. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- 5.Schwenzer NF, Stegger L, Bisdas S, et al. Simultaneous PET/MR imaging in a human brain PET/MR system in 50 patients--current state of image quality. European journal of radiology. 2012 Nov;81(11):3472–3478. doi: 10.1016/j.ejrad.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Garibotto V, Heinzer S, Vulliemoz S, et al. Clinical applications of hybrid PET/MRI in neuroimaging. Clinical nuclear medicine. 2013 Jan;38(1):e13–18. doi: 10.1097/RLU.0b013e3182638ea6. [DOI] [PubMed] [Google Scholar]
- 7.Drzezga A, Souvatzoglou M, Eiber M, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2012 Jun;53(6):845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 8.Lee SM, Goo JM, Park CM, et al. Preoperative staging of non-small cell lung cancer: prospective comparison of PET/MR and PET/CT. European radiology. doi: 10.1007/s00330-016-4255-0. ePub Feb 16 2016. [DOI] [PubMed] [Google Scholar]
- 9.Catana C, Benner T, van der Kouwe A, et al. MRI-assisted PET motion correction for neurologic studies in an integrated MR-PET scanner. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011 Jan;52(1):154–161. doi: 10.2967/jnumed.110.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catana C, Drzezga A, Heiss WD, Rosen BR. PET/MRI for neurologic applications. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2012 Dec;53(12):1916–1925. doi: 10.2967/jnumed.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delso G, Furst S, Jakoby B, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011 Dec;52(12):1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 12.Su Y, Arbelaez AM, Benzinger TL, et al. Noninvasive estimation of the arterial input function in positron emission tomography imaging of cerebral blood flow. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013 Jan;33(1):115–121. doi: 10.1038/jcbfm.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malone IB, Ansorge RE, Williams GB, Nestor PJ, Carpenter TA, Fryer TD. Attenuation correction methods suitable for brain imaging with a PET/MRI scanner: a comparison of tissue atlas and template attenuation map approaches. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011 Jul;52(7):1142–1149. doi: 10.2967/jnumed.110.085076. [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Rubin B, McConathy J, et al. Impact of MR based attenuation correction on neurological PET studies. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016 Jun;57(6):913–917. doi: 10.2967/jnumed.115.164822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitz S, Habekost C, Furst S, et al. Systematic Comparison of the Performance of Integrated Whole-Body PET/MR Imaging to Conventional PET/CT for (1)(8)F-FDG Brain Imaging in Patients Examined for Suspected Dementia. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2014 Jun;55(6):923–931. doi: 10.2967/jnumed.113.126813. [DOI] [PubMed] [Google Scholar]
- 16.Jadvar H, Subramaniam RM, Berman CG, et al. American College of Radiology and Society of Nuclear Medicine and Molecular Imaging Joint Credentialing Statement for PET/MR Imaging: Brain. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2015 Apr;56(4):642–645. doi: 10.2967/jnumed.115.155218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore CD, Comeau CR, Alessi AM, et al. PET/MR imaging consensus paper: a joint paper by the Society of Nuclear Medicine and Molecular Imaging Technologist Section and the Section for Magnetic Resonance Technologists. Journal of nuclear medicine technology. 2013 Jun;41(2):108–113. doi: 10.2967/jnmt.113.123869. [DOI] [PubMed] [Google Scholar]
- 18.la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-oncology. 2011 Aug;13(8):806–819. doi: 10.1093/neuonc/nor054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR. American journal of neuroradiology. 2013 May;34(5):944–950. S941–911. doi: 10.3174/ajnr.A3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patronas NJ, Di Chiro G, Brooks RA, et al. Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology. 1982 Sep;144(4):885–889. doi: 10.1148/radiology.144.4.6981123. [DOI] [PubMed] [Google Scholar]
- 21.Fink JR, Muzi M, Peck M, Krohn KA. Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2015 Oct;56(10):1554–1561. doi: 10.2967/jnumed.113.131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology. 2002 Sep 24;59(6):947–949. doi: 10.1212/wnl.59.6.947. [DOI] [PubMed] [Google Scholar]
- 23.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-oncology. 2001 Jul;3(3):193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999 Jun;211(3):791–798. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 25.Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR. American journal of neuroradiology. 2004 May;25(5):746–755. [PMC free article] [PubMed] [Google Scholar]
- 26.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR. American journal of neuroradiology. 2003 Nov-Dec;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 27.Ludemann L, Warmuth C, Plotkin M, et al. Brain tumor perfusion: comparison of dynamic contrast enhanced magnetic resonance imaging using T1, T2, and T2* contrast, pulsed arterial spin labeling, and H2(15)O positron emission tomography. European journal of radiology. 2009 Jun;70(3):465–474. doi: 10.1016/j.ejrad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology. 2008 Nov;249(2):601–613. doi: 10.1148/radiol.2492071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR. American journal of neuroradiology. 2001 Jun-Jul;22(6):1081–1088. [PMC free article] [PubMed] [Google Scholar]
- 30.Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR. American journal of neuroradiology. 2006 Oct;27(9):1969–1974. [PMC free article] [PubMed] [Google Scholar]
- 31.White ML, Zhang Y, Yu F, Jaffar Kazmi SA. Diffusion tensor MR imaging of cerebral gliomas: evaluating fractional anisotropy characteristics. AJNR. American journal of neuroradiology. 2011 Feb;32(2):374–381. doi: 10.3174/ajnr.A2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwock L, Smith JK, Castillo M, et al. Clinical applications of proton MR spectroscopy in oncology. Technology in cancer research & treatment. 2002 Feb;1(1):17–28. doi: 10.1177/153303460200100103. [DOI] [PubMed] [Google Scholar]
- 33.Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. Journal of neuro-oncology. 2003 Sep;64(3):227–237. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 34.De Witte O, Levivier M, Violon P, et al. Prognostic value positron emission tomography with [18F]fluoro-2-deoxy-D-glucose in the low-grade glioma. Neurosurgery. 1996 Sep;39(3):470–476. doi: 10.1097/00006123-199609000-00007. discussion 476–477. [DOI] [PubMed] [Google Scholar]
- 35.Olivero WC, Dulebohn SC, Lister JR. The use of PET in evaluating patients with primary brain tumours: is it useful? Journal of neurology, neurosurgery, and psychiatry. 1995 Feb;58(2):250–252. doi: 10.1136/jnnp.58.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR. American journal of neuroradiology. 1998 Mar;19(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- 37.Wong TZ, Turkington TG, Hawk TC, Coleman RE. PET and brain tumor image fusion. Cancer J. 2004 Jul-Aug;10(4):234–242. doi: 10.1097/00130404-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Bisdas S, Ritz R, Bender B, et al. Metabolic mapping of gliomas using hybrid MR-PET imaging: feasibility of the method and spatial distribution of metabolic changes. Invest Radiol. 2013 May;48(5):295–301. doi: 10.1097/RLI.0b013e31827188d6. [DOI] [PubMed] [Google Scholar]
- 39.Hirata K, Terasaka S, Shiga T, et al. (1)(8)F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. European journal of nuclear medicine and molecular imaging. 2012 May;39(5):760–770. doi: 10.1007/s00259-011-2037-0. [DOI] [PubMed] [Google Scholar]
- 40.Pafundi DH, Laack NN, Youland RS, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro-oncology. 2013 Aug;15(8):1058–1067. doi: 10.1093/neuonc/not002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spence AM, Muzi M, Swanson KR, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008 May 1;14(9):2623–2630. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2001 Mar;42(3):432–445. [PubMed] [Google Scholar]
- 43.Pauleit D, Stoffels G, Bachofner A, et al. Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nuclear medicine and biology. 2009 Oct;36(7):779–787. doi: 10.1016/j.nucmedbio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Galldiks N, Kracht LW, Dunkl V, et al. Imaging of non- or very subtle contrast-enhancing malignant gliomas with [(1)(1)C]-methionine positron emission tomography. Molecular imaging. 2011 Dec;10(6):453–459. [PubMed] [Google Scholar]
- 45.Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. European journal of nuclear medicine and molecular imaging. 2010 Jan;37(1):84–92. doi: 10.1007/s00259-009-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prior FW, Fouke SJ, Benzinger T, et al. Predicting a multi-parametric probability map of active tumor extent using random forests. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2013. pp. 6478–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preuss M, Werner P, Barthel H, et al. Integrated PET/MRI for planning navigated biopsies in pediatric brain tumors. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2014 Aug;30(8):1399–1403. doi: 10.1007/s00381-014-2412-9. [DOI] [PubMed] [Google Scholar]
- 48.Petrella JR, Shah LM, Harris KM, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006 Sep;240(3):793–802. doi: 10.1148/radiol.2403051153. [DOI] [PubMed] [Google Scholar]
- 49.Ulmer JL, Salvan CV, Mueller WM, et al. The role of diffusion tensor imaging in establishing the proximity of tumor borders to functional brain systems: implications for preoperative risk assessments and postoperative outcomes. Technology in cancer research & treatment. 2004 Dec;3(6):567–576. doi: 10.1177/153303460400300606. [DOI] [PubMed] [Google Scholar]
- 50.Neuschmelting V, Weiss Lucas C, Stoffels G, et al. Multimodal Imaging in Malignant Brain Tumors: Enhancing the Preoperative Risk Evaluation for Motor Deficits with a Combined Hybrid MRI-PET and Navigated Transcranial Magnetic Stimulation Approach. AJNR. American journal of neuroradiology. 2016 Feb;37(2):266–273. doi: 10.3174/ajnr.A4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. The Lancet. Oncology. 2008 May;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 52.Young RJ, Gupta A, Shah AD, et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011 May 31;76(22):1918–1924. doi: 10.1212/WNL.0b013e31821d74e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Apr 10;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 54.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000 Nov;217(2):377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 55.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR. American journal of neuroradiology. 2009 Mar;30(3):552–558. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fink JR, Carr RB, Matsusue E, et al. Comparison of 3 Tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. Journal of magnetic resonance imaging: JMRI. 2012 Jan;35(1):56–63. doi: 10.1002/jmri.22801. [DOI] [PubMed] [Google Scholar]
- 57.Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nature medicine. 2009 May;15(5):572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamstra DA, Galban CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008 Jul 10;26(20):3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prat R, Galeano I, Lucas A, et al. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2010 Jan;17(1):50–53. doi: 10.1016/j.jocn.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 60.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2005 Jun;46(6):945–952. [PubMed] [Google Scholar]
- 61.Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2006 Jun;47(6):904–911. [PubMed] [Google Scholar]
- 62.Lee IH, Piert M, Gomez-Hassan D, et al. Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. International journal of radiation oncology, biology, physics. 2009 Feb 1;73(2):479–485. doi: 10.1016/j.ijrobp.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. European journal of nuclear medicine and molecular imaging. 2004 Nov;31(11):1464–1470. doi: 10.1007/s00259-004-1590-1. [DOI] [PubMed] [Google Scholar]
- 64.Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008 May;49(5):694–699. doi: 10.2967/jnumed.107.048082. [DOI] [PubMed] [Google Scholar]
- 65.Cicone F, Minniti G, Romano A, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. European journal of nuclear medicine and molecular imaging. 2015 Jan;42(1):103–111. doi: 10.1007/s00259-014-2886-4. [DOI] [PubMed] [Google Scholar]
- 66.Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2014 Jan;55(1):30–36. doi: 10.2967/jnumed.113.121418. [DOI] [PubMed] [Google Scholar]
- 67.Walter F, Cloughesy T, Walter MA, et al. Impact of 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine PET/CT on managing patients with brain tumors: the referring physician’s perspective. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2012 Mar;53(3):393–398. doi: 10.2967/jnumed.111.095711. [DOI] [PubMed] [Google Scholar]
- 68.Filss CP, Galldiks N, Stoffels G, et al. Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2014 Apr;55(4):540–545. doi: 10.2967/jnumed.113.129007. [DOI] [PubMed] [Google Scholar]
- 69.Sacconi B, Raad RA, Lee J, et al. Concurrent functional and metabolic assessment of brain tumors using hybrid PET/MR imaging. Journal of neuro-oncology. 2016 Apr;127(2):287–293. doi: 10.1007/s11060-015-2032-6. [DOI] [PubMed] [Google Scholar]
- 70.Lee KK, Salamon N. [18F] fluorodeoxyglucose-positron-emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. AJNR. American journal of neuroradiology. 2009 Nov;30(10):1811–1816. doi: 10.3174/ajnr.A1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Brien TJ, Hicks RJ, Ware R, Binns DS, Murphy M, Cook MJ. The utility of a 3-dimensional, large-field-of-view, sodium iodide crystal--based PET scanner in the presurgical evaluation of partial epilepsy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2001 Aug;42(8):1158–1165. [PubMed] [Google Scholar]
- 72.Salamon N, Kung J, Shaw SJ, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008 Nov 11;71(20):1594–1601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chassoux F, Rodrigo S, Semah F, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010 Dec 14;75(24):2168–2175. doi: 10.1212/WNL.0b013e31820203a9. [DOI] [PubMed] [Google Scholar]
- 74.LoPinto-Khoury C, Sperling MR, Skidmore C, et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012 Feb;53(2):342–348. doi: 10.1111/j.1528-1167.2011.03359.x. [DOI] [PubMed] [Google Scholar]
- 75.Gok B, Jallo G, Hayeri R, Wahl R, Aygun N. The evaluation of FDG-PET imaging for epileptogenic focus localization in patients with MRI positive and MRI negative temporal lobe epilepsy. Neuroradiology. 2013 May;55(5):541–550. doi: 10.1007/s00234-012-1121-x. [DOI] [PubMed] [Google Scholar]
- 76.Ding YS, Chen BB, Glielmi C, Friedman K, Devinsky O. A pilot study in epilepsy patients using simultaneous PET/MR. American journal of nuclear medicine and molecular imaging. 2014;4(5):459–470. [PMC free article] [PubMed] [Google Scholar]
- 77.Shin HW, Jewells V, Sheikh A, et al. Initial experience in hybrid PET-MRI for evaluation of refractory focal onset epilepsy. Seizure. 2015 Sep;31:1–4. doi: 10.1016/j.seizure.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Alzheimer’s Disease Facts and Figures. 2014. [DOI] [PubMed] [Google Scholar]
- 79.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011 May;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braak H, Braak E. Staging of Alzheimer-related cortical destruction. International psychogeriatrics/IPA. 1997;9( Suppl 1):257–261. discussion 269–272. [PubMed] [Google Scholar]
- 81.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of neuropathology and experimental neurology. 2011 Nov;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 82.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2012 Jul;2(7):a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santa-Maria I, Varghese M, Ksiezak-Reding H, Dzhun A, Wang J, Pasinetti GM. Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes. The Journal of biological chemistry. 2012 Jun 8;287(24):20522–20533. doi: 10.1074/jbc.M111.323279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. Journal of Alzheimer’s disease: JAD. 2013;34(2):457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 85.James OG, Doraiswamy PM, Borges-Neto S. PET Imaging of Tau Pathology in Alzheimer’s Disease and Tauopathies. Frontiers in neurology. 2015;6:38. doi: 10.3389/fneur.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013 Jan;9(1):e-1–16. doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2013 Mar;54(3):476–490. doi: 10.2967/jnumed.113.120618. [DOI] [PubMed] [Google Scholar]
- 88.Laakso MP, Soininen H, Partanen K, et al. MRI of the hippocampus in Alzheimer’s disease: sensitivity, specificity, and analysis of the incorrectly classified subjects. Neurobiology of aging. 1998 Jan-Feb;19(1):23–31. doi: 10.1016/s0197-4580(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 89.McConathy J, Sheline YI. Imaging biomarkers associated with cognitive decline: a review. Biological psychiatry. 2015 Apr 15;77(8):685–692. doi: 10.1016/j.biopsych.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Archives of neurology. 2009 Dec;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okamura N, Harada R, Furumoto S, Arai H, Yanai K, Kudo Y. Tau PET imaging in Alzheimer’s disease. Current neurology and neuroscience reports. 2014 Nov;14(11):500. doi: 10.1007/s11910-014-0500-6. [DOI] [PubMed] [Google Scholar]
- 92.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009 Oct 15;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus. 2009 Nov;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 94.Ishii K. PET approaches for diagnosis of dementia. AJNR. American journal of neuroradiology. 2014 Nov-Dec;35(11):2030–2038. doi: 10.3174/ajnr.A3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jack CR., Jr Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012 May;263(2):344–361. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. European journal of nuclear medicine and molecular imaging. 2005 Apr;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 97.Tahmasian M, Shao J, Meng C, et al. Based on the Network Degeneration Hypothesis: Separating Individual Patients with Different Neurodegenerative Syndromes in a Preliminary Hybrid PET/MR Study. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016 Mar;57(3):410–415. doi: 10.2967/jnumed.115.165464. [DOI] [PubMed] [Google Scholar]
- 98.Gordon BA, Najmi S, Hsu P, Roe CM, Morris JC, Benzinger TL. The effects of white matter hyperintensities and amyloid deposition on Alzheimer dementia. NeuroImage. Clinical. 2015;8:246–252. doi: 10.1016/j.nicl.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta neuropathologica. 2016 May;131(5):687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okamura N, Furumoto S, Fodero-Tavoletti MT, et al. Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain: a journal of neurology. 2014 Jun;137(Pt 6):1762–1771. doi: 10.1093/brain/awu064. [DOI] [PubMed] [Google Scholar]
- 101.Jack CR, Jr, Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016 Aug;87(5):539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008 Sep 25;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 103.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Annals of neurology. 2006 Nov;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 104.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. The New England journal of medicine. 2013 Mar 7;368(10):914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine. 2015 Mar 12;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 106.Heiss WD, Grond M, Thiel A, et al. Tissue at risk of infarction rescued by early reperfusion: a positron emission tomography study in systemic recombinant tissue plasminogen activator thrombolysis of acute stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998 Dec;18(12):1298–1307. doi: 10.1097/00004647-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 107.Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Marks MP, Albers GW. Relationship between severity of MR perfusion deficit and DWI lesion evolution. Neurology. 2001 Oct 9;57(7):1205–1211. doi: 10.1212/wnl.57.7.1205. [DOI] [PubMed] [Google Scholar]
- 108.Werner P, Saur D, Zeisig V, et al. Simultaneous PET/MRI in stroke: a case series. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 Sep;35(9):1421–1425. doi: 10.1038/jcbfm.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hyafil F, Schindler A, Sepp D, et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. European journal of nuclear medicine and molecular imaging. 2016 Feb;43(2):270–279. doi: 10.1007/s00259-015-3201-8. [DOI] [PubMed] [Google Scholar]
- 110.Izquierdo-Garcia D, Davies JR, Graves MJ, et al. Comparison of methods for magnetic resonance-guided [18-F]fluorodeoxyglucose positron emission tomography in human carotid arteries: reproducibility, partial volume correction, and correlation between methods. Stroke; a journal of cerebral circulation. 2009 Jan;40(1):86–93. doi: 10.1161/STROKEAHA.108.521393. [DOI] [PubMed] [Google Scholar]
- 111.Catana C, Guimaraes AR, Rosen BR. PET and MR imaging: the odd couple or a match made in heaven? Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2013 May;54(5):815–824. doi: 10.2967/jnumed.112.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Byrnes KR, Wilson CM, Brabazon F, et al. FDG-PET imaging in mild traumatic brain injury: a critical review. Frontiers in neuroenergetics. 2014;5:13. doi: 10.3389/fnene.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain imaging and behavior. 2012 Jun;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]







