Abstract
CD151, one of the tetraspanins, forms a stable complex with integrin α3β1, the major laminin receptor on the cell surface. We found that 8C3, an anti-CD151 mAb obtained by screening for reactivity with integrin α3β1–CD151 complexes, was capable of dissociating CD151 from integrin α3β1, thereby allowing us to deplete CD151 from purified integrin α3β1–CD151 complexes. The CD151-free integrin α3β1 thus obtained showed a significant reduction in its ability to bind to laminin-10/11, a high-affinity ligand for integrin α3β1, with a concomitant reduction in its reactivity with mAb AG89, which recognizes activated β1-containing integrins. These results raised the possibility that the association of integrin α3β1 with CD151 potentiates the ligand-binding activity of the integrin through sustaining its activated conformation. In support of this possibility, the ligand-binding activity was restored when CD151-free integrin α3β1 was reassociated with purified CD151. 8C3-induced dissociation of CD151 from integrin α3β1 was also demonstrated on the surface of living cells by fluorescent resonance energy transfer imaging, accompanied by a concomitant reduction in the cell adhesion to laminin-10/11-coated substrates. CD151 knock-down by RNA interference also resulted in a reduction in the adhesive activity of the cells. Taken together, these results indicate that CD151 association modulates the ligand-binding activity of integrin α3β1 through stabilizing its activated conformation not only with purified proteins but also in a physiological context.
Keywords: cell adhesion, laminin
Integrins are heterodimeric adhesion receptors for extracellular matrix proteins, consisting of an α-subunit and a β-subunit. In mammals, 18 α-subunits and 8 β-subunits have been identified, combinations of which yield >20 different integrins with distinct ligand-binding specificities (1). Among these, integrins α3β1, α6β1, α7β1, and α6β4 specifically bind to laminins, the major cell-adhesive proteins in basement membranes. Unlike other integrins, the laminin-binding integrins form complexes with tetraspanins (2). Tetraspanins are widely expressed membrane proteins characterized by four transmembrane segments and well conserved cysteine residues in a large extracellular loop (2). More than 30 tetraspanin proteins have been identified in mammals. Among these tetraspanins, CD151 forms a stable complex with integrin α3β1, the major laminin-binding integrin ubiquitously expressed in many cell types (3–5). Tetraspanins also bind to each other, yielding distinct membrane microdomains, referred to as the “tetraspanin web,” in which integrins and other membrane proteins such as Ig superfamily proteins and growth factor receptors are incorporated (6).
The association of CD151 with integrin α3β1 occurs early in their biosynthesis and has been implicated in sorting of integrin α3β1 to the cell surface (2). CD151 also interacts with phosphatidylinositol 4-kinase and protein kinase C, thereby linking integrin α3β1 to these signaling molecules (3, 7). Several lines of evidence have indicated that CD151 is involved in integrin α3β1-mediated adhesive events, including cell spreading, migration and cellular cable formation (8, 9), and anti-CD151 antibodies have been shown to inhibit the α3β1-dependent motility of neutrophils and endothelial cells (3, 10) as well as neurite outgrowth (11). Furthermore, a CD151 mutant defective in association with integrins, due to substitution of the Gln-Arg-Asp sequence in the large extracellular loop with Ile-Asn-Phe, blocks cell spreading on Matrigel-coated substrata and cellular cable formation on thick Matrigel layers (8), although it has minimal effects on integrin-mediated cell adhesion per se (2, 8). However, it remains to be defined whether the association of CD151 with laminin-binding integrins is involved in modulation of their ligand-binding activities or the conformations relevant to their activities, because the mutant CD151 did not prevent the association of endogenous CD151 with integrin α3β1.
In the present study, we obtained an anti-human CD151 mAb that was capable of dissociating CD151 from integrin α3β1, and succeeded in isolating CD151-free integrin α3β1 after incubation of purified integrin α3β1–CD151 complexes with this mAb immobilized on agarose beads. The binding activity of CD151-free integrin α3β1 to laminin-10/11, the highest affinity ligand for integrin α3β1 (12), was significantly reduced, and could be restored to the level of integrin α3β1–CD151 complexes upon reassociation with purified CD151. We also provide evidence that association with CD151 modulates the conformation of integrin α3β1 in an activation-dependent manner.
Materials and Methods
Cell Lines, Reagents, and Antibodies. A549 human lung adenocarcinoma cells, U251 human glioma cells, and Cos7 monkey kidney cells were cultured in DMEM containing 10% FBS. Laminin-10/11 was purified from conditioned medium of A549 cells (13). Human fibronectin was purchased from Sigma–Aldrich. mAbs against human integrin α3 (3G8) and CD151 (8C3) were produced as described (14). Other antibodies used were as follows: anti-human integrin α3 (P1B5) from Chemicon; anti-human CD151 (11G5) from Serotec; control mouse IgG and goat anti-human integrin α3 (I-19) from Santa Cruz Biotechnology; an activating anti-human integrin β1 mAb (TS2/16) from American Type Culture Collection (ATCC); anti-human CD151 (SFA1.2B4), anti-human integrin β1 (AG89) recognizing activated β1 integrins, FITC-conjugated AG89, and FITC-conjugated TS2/16 were produced as described (15, 16).
Purification of Integrin α3β1–CD151 Complexes and CD151. Integrin α3β1–CD151 complexes were purified from human placenta (12). Integrin-free CD151 was purified from a detergent extract of human placenta by immunoaffinity chromatography by using the anti-CD151 mAb 8C3. The column was washed with TBS (+) (20 mM Tris·HCl, pH 7.5/0.13 M NaCl/1 mM CaCl2/1 mM MgCl2) containing 1 mM PMSF and 50 mM n-octyl-β-d-glucoside, and the bound CD151 was eluted with 0.1 M triethylamine containing 50 mM n-octyl-β-d-glucoside and neutralized with 2 M NaHPO4.
Immunoprecipitation. Surface biotinylation of U251 cells and subsequent immunoprecipitation were performed as described (17).
Depletion of CD151 from Integrin α3β1–CD151 Complexes. The integrin α3β1–CD151 complexes purified from placenta were incubated with 8C3-conjugated agarose beads overnight at 4°C on a rotary shaker. The slurry was poured into an empty column, and CD151-free integrin α3β1 was collected in the flow-through fractions. The bound proteins were eluted with 0.1 M triethylamine containing 50 mM n-octyl-β-d-glucoside and neutralized with 2 M NaHPO4. The CD151-free integrin α3β1 and purified CD151 were stored at 4°C.
Integrin Binding Assays. Solid-phase binding assays using purified integrins (10–20 μg/ml) reconstituted into 3H-labeled liposomes were performed as described (12), except that the assays were performed in the presence of 1 mM MgCl2 and 1 mM CaCl2.
Fluorescent Resonance Energy Transfer (FRET) Imaging. Expression vectors for cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) were constructed as described (18). The CFP and YFP expression vectors were cleaved by NheI/KpnI and HindIII/KpnI, respectively, to insert the coding sequences for human integrin α3 chain and CD151, yielding the expression vectors for CFP-tagged integrin α3 and YFP-tagged CD151. A 6-aa spacer (Gly-Gly-Thr-Val-Pro-Arg) was introduced before the fluorescent protein tags. Cos7 cells were cotransfected with these expression vectors by using Lipofectamine 2000 (Invitrogen) and replated after 48 h on glass-bottom dishes coated with 50 μg/ml fibronectin in phenol red-free DMEM containing 5% FBS. Twelve hours after replating, the cells were imaged at 1-min intervals by using an Olympus IX71 microscope equipped with a cooled charge-coupled device camera (CoolSNAP HQ, Roper Scientific, Trenton, NJ) controlled by metamorph software (Universal Imaging, West Chester, PA) as described (18). The medium was replaced with medium containing control IgG (20 μg/ml) and then with medium containing one of the anti-CD151 mAbs (20 μg/ml each) at 5 and 15 min after the onset of recording, respectively. After background subtraction, a YFP/CFP ratio image was produced by metamorph software and used to represent the FRET efficiency.
Cell Adhesion Assays. Cell adhesion assays were performed as described (14). A549 cells were preincubated with the indicated mAbs (20 μg/ml) for 20 min at room temperature and plated on 24-well plates coated with 1 nM laminin-10/11. The cells were incubated for 20 min at 37°C, washed, fixed with 3.7% formaldehyde, and stained with 0.5% toluidine blue. The attached cells were counted in three independent fields per well.
CD151 Knock-Down by RNA Interference (RNAi). Double-stranded small interfering RNAs (siRNAs) were purchased from Dharmacon (Lafayette, CO), and used to knock-down CD151 expression. The target sequence of the most effective siRNA was 5′-ccagacagcugcugcaag-3′. Nonspecific Control Duplex VII RNA (Dharmacon) was used as a control. The siRNAs were transfected into A549 cells by using Lipofectamine 2000, and the cells were used for cell adhesion assays at 96 h after transfection. RNAi-mediated knock-down of the CD151 expression was verified by flow cytometry by using mAb 8C3.
Results
Production of an Anti-CD151 mAb That Dissociates CD151 from Integrin α3β1. Previously, we produced a panel of mAbs against integrin α3β1 based on their reactivity to integrin α3β1 purified from human placenta (14). One of these mAbs, named 8C3, immunoprecipitated a 30-kDa protein from surface-labeled U251 human glioma cells, instead of the 150-kDa/125-kDa integrin α3β1 (Fig. 1A). Because integrin α3β1 has been shown to associate with tetraspanin family proteins (3, 4), we also performed immunoprecipitation with a panel of anti-tetraspanin mAbs. Among these mAbs, anti-CD151 mAbs SFA1.2B4 and 11G5 precipitated a 30-kDa protein migrating to the same position as the protein precipitated with 8C3 (Fig. 1 A), indicating that the 30-kDa protein recognized by 8C3 is CD151. The identity of the 30-kDa protein as CD151 was verified by determination of the N-terminal amino acid sequence of the protein.
Fig. 1.
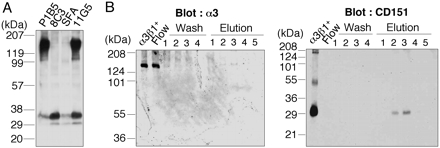
Dissociation of CD151 from integrin α3β1 by mAb 8C3. (A) U251 cells surface-labeled by biotinylation were lysed and immunoprecipitated with mAbs 8C3, SFA1.2B4, 11G5 (anti-CD151), or P1B5 (anti-α3). Precipitates were resolved by 4–20% SDS/PAGE under nonreducing conditions, followed by detection of the labeled proteins with streptavidin-horseradish peroxidase. (B) Integrin α3β1–CD151 complexes purified from placenta (α3β1+) were incubated with mAb 8C3-conjugated agarose beads overnight, followed by chromatographic separation of the unbound and bound proteins. The flow-through (Flow), wash (Wash), and 0.1 M triethylamine-eluted (Elution) fractions were collected separately and analyzed by immunoblotting with anti-α3 (I-19; Left) and anti-CD151 (8C3; Right) antibodies. The minor band in α3β1+ migrating at ≈55 kDa was identified as the CD151 dimer by N-terminal amino acid sequencing.
The tetraspanin CD151 has been shown to form a stable complex with integrin α3β1 (3, 4). Indeed, the purified integrin α3β1 used in our screening for anti-integrin α3β1 mAbs contained a significant amount of CD151 (data not shown), thus resulting in the isolation of mAb 8C3 recognizing CD151. Interestingly, many anti-CD151 mAbs have been shown to coimmunoprecipitate integrin α3β1 and CD151 as a complex, as was the case with mAb 11G5 (Fig. 1 A). Given the stability of integrin α3β1–CD151 complexes under stringent detergent conditions (i.e., 1% Triton X-100), the sole precipitation of CD151 with mAb 8C3 raised the possibility that this mAb was capable of dissociating CD151 from integrin α3β1. To explore this possibility, we incubated integrin α3β1–CD151 complexes purified from placenta with 8C3-conjugated agarose beads, followed by chromatographic separation into unbound and bound proteins (Fig. 1B). Western blot analyses showed that the flow-through fractions contained only integrin α3β1, whereas the bound fractions contained only CD151, supporting the possibility that 8C3 is capable of dissociating CD151 from integrin α3β1. Although the precise mechanism by which 8C3 dissociates CD151 from integrin α3β1 remains to be defined, 8C3 can be used to deplete CD151 from integrin α3β1–CD151 complexes, thereby allowing us to examine the role of CD151 association in integrin α3β1 functions.
CD151 Potentiates the Ligand-Binding Activity of Integrin α3β1. The ligand-binding activity of CD151-free integrin α3β1 (designated α3β1–) was compared with that of CD151-bound integrin α3β1 (designated α3β1+) by integrin-liposome binding assays by using laminin-10/11 as an adhesive ligand. We reconstituted α3β1+ and α3β1– in 3H-labeled phosphatidylcholine liposomes, and examined their binding to surface-adsorbed laminin-10/11 (Fig. 2A). Binding of integrin liposomes to a surface-adsorbed anti-α3 mAb (P1B5) was used as a reference to normalize the amount of bound integrin liposomes. The validity of P1B5 as a reference was confirmed by comparable binding of the integrin liposomes to surface-adsorbed mAb TS2/16, an anti-integrin β1 mAb. Notably, significantly less α3β1– bound to laminin-10/11 than α3β1+, indicating that depletion of CD151 decreases the ligand-binding activity of integrin α3β1. The ligand-binding activity of integrins has been shown to be dependent on their conformation (19, 20). To probe the activation-dependent conformations of α3β1+ and α3β1–, we examined their reactivity toward mAb AG89, an mAb that selectively recognizes activated β1-containing integrins (16, 21). Consistent with the reduced laminin-10/11 binding, α3β1– showed significantly reduced binding to mAb AG89 compared with α3β1+ (Fig. 2B). These results indicate that CD151 stabilizes the activated conformation of integrin α3β1, thereby potentiating its binding to laminin-10/11.
Fig. 2.
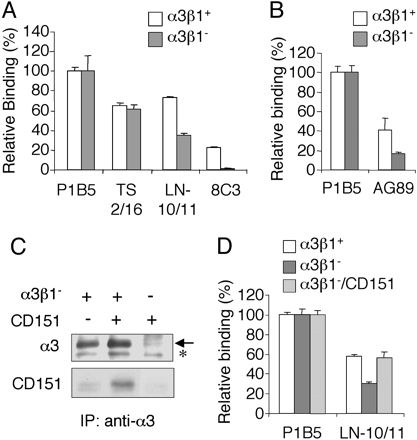
Modulation of the ligand-binding activity of integrin α3β1 by CD151 association. (A) Integrin α3β1–CD151 complexes (α3β1+; open bars) and CD151-free integrin α3β1(α3β1–; filled bars) were reconstituted in 3H-labeled liposomes and incubated with laminin-10/11 (LN-10/11) or mAbs against integrin α3 (P1B5), β1 (TS2/16), or CD151 (8C3), each coated on 96-well plates at 5 nM, for 6 h at room temperature. The amounts of bound liposomes were quantified by using a liquid scintillation counter and are expressed as relative values to the amount bound to P1B5. Each bar represents the mean of triplicate assays ± SD. (B) 3H-labeled liposomes containing α3β1+ and α3β1– were incubated with P1B5 (5 nM) or mAb AG89 recognizing activated β1 integrins (100 nM) coated on 96-well plates, followed by quantification of the bound liposomes. (C) Reassociation of purified CD151 with α3β1–. α3β1– was incubated with purified CD151 at an equimolar ratio overnight at 4°C, and then subjected to precipitation with 3G8-conjugated agarose beads. Precipitates were immunoblotted with anti-α3 (I-19) or anti-CD151 (8C3) antibodies under nonreducing conditions. The arrow indicates the α3 band. The minor band below the α3 band (asterisk) was derived from the 3G8-conjugated beads, and is considered to be nonspecific. (D) α3β1+ (open bars), α3β1– (filled bars), and α3β1– reassociated with purified CD151 at an equimolar ratio (α3β1–/CD151; shaded bars) were reconstituted in liposomes and incubated with laminin-10/11 (LN-10/11) or P1B5 coated on 96-well plates at 5 nM, followed by quantification of the bound liposomes as described above. The amounts of bound liposomes are expressed as relative values to the amount bound to P1B5. Each bar represents the mean of triplicate assays ± SD.
To confirm that CD151 association potentiates the ligand-binding activity of integrin α3β1, we purified CD151 from human placenta by 8C3-immunoaffinity chromatography, and examined whether reassociation with CD151 could restore CD151-free α3β1 to the full ligand-binding activity. Incubation of α3β1– with the purified CD151, followed by immunoprecipitation with an anti-integrin α3 mAb, demonstrated that α3β1– was capable of reassociating with purified CD151 (Fig. 2C). No CD151 was precipitated by the anti-α3 mAb in the absence of α3β1–, confirming the specificity of the immunoprecipitation assays. When reconstituted in 3H-labeled liposomes and examined for its ligand-binding activity, α3β1– reassociated with CD151 was significantly more potent than α3β1– in binding to laminin-10/11, and exhibited comparable activity to that of α3β1+ (Fig. 2D). Liposomes reconstituted with the purified CD151 alone did not show any significant binding to laminin-10/11 (Fig. 6, which is published as supporting information on the PNAS web site), excluding the possibility that CD151 per se could bind to laminin-10/11 and thereby exert an additive effect on the ligand-binding activity of α3β1– upon reassociation with CD151.
MAb 8C3-Induced Dissociation of CD151 from Integrin α3β1 on the Cell Surface. To examine whether mAb 8C3-induced dissociation of CD151 from integrin α3β1 could occur on the cell surface, we used FRET imaging assays using Cos7 cells cotransfected with CFP-tagged integrin α3 chain and YFP-tagged CD151. The FRET efficiency was monitored by the YFP/CFP emission ratio. Photobleaching of YFP resulted in an increase in CFP emission, demonstrating the presence of basal FRET in living cells (data not shown). Although the YFP/CFP emission ratio, indicative of FRET, remained unaffected when the cells were exposed to medium containing control IgG, a significant decrease in the ratio was observed within 1 min upon exposure to medium containing mAb 8C3 (Fig. 3A; see also Fig. 7, which is published as supporting information on the PNAS web site). Another mAb, 11G5, which is incapable of dissociating CD151 from integrin α3β1, did not induce any significant decrease in the ratio. These results, reproduced in three separate experiments, indicated that 8C3, but not 11G5, was capable of dissociating CD151 from integrin α3β1 on the surface of living cells. In support of this possibility, the amount of CD151 coprecipitated with integrin α3β1 from cell lysates was significantly reduced after pretreatment of the cells with 8C3 (data not shown).
Fig. 3.
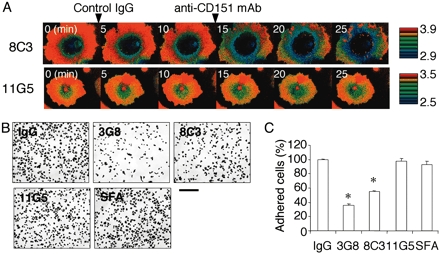
mAb 8C3 dissociates CD151 from integrin α3β1 on the cell surface. (A) Cos7 cells coexpressing CFP-tagged integrin α3 chain and YFP-tagged CD151 were serially treated with control IgG and an anti-CD151 mAb (8C3 or 11G5) at the indicated time points, and fluorescence images for CFP and YFP were recorded at 1-min intervals by time-lapse microscopy. YFP/CFP ratio images were used to represent the FRET efficiency and are expressed in eight colors in the intensity-modulated display mode (red for highest and blue for lowest). YFP/CFP ratio images at 5-min intervals are shown. Complete sets of images are included in Fig. 7. (B) A549 cells were preincubated with the indicated mAbs for 20 min at room temperature, and plated on 24-well plates coated with laminin-10/11. After incubation for 20 min at 37°C, the attached cells were fixed and stained. (Scale bar, 200 μm.) SFA, anti-CD151 mAb SFA1.2B4. (C) The numbers of attached cells were counted in three separate fields and expressed as percentages of the number after preincubation with mouse control IgG. Each bar represents the mean ± SD. Statistical analyses were performed by Student's t test. *, P < 0.001 versus IgG.
Because dissociation of CD151 from integrin α3β1 was expected to destabilize the active conformation of the integrin, we examined the reactivity of mAb AG89 to A549 cells pretreated with 8C3. Flow cytometric analyses showed that 8C3 pretreatment decreased the reactivity of cells toward AG89, whereas pretreatment with 11G5 did not (Fig. 8, which is published as supporting information on the PNAS web site). The decrease in AG89 reactivity was not due to a decrease in the surface expression of β1-containing integrins, because the reactivity toward TS2/16, another anti-integrin β1 mAb, remained unaffected after 8C3 pretreatment (Fig. 8).
We also examined the effect of 8C3 on cell adhesion to substrates coated with laminin-10/11. A549 cells have been shown to adhere to laminin-10/11 in an integrin α3β1-dependent manner (13, 14). Cells pretreated with 8C3 were significantly less active in adhering to laminin-10/11 than untreated cells, as was the case for cells pretreated with a function-blocking mAb against the integrin α3 chain (Fig. 3 B and C). In contrast, pretreatments with 11G5 or SFA1.2B4 did not exert any significant effects on the cell adhesion to laminin-coated substrates. These results provide further support for the 8C3-mediated dissociation of CD151 from integrin α3β1 and resulting destabilization of the activated conformation of the integrin.
Effect of CD151 Knock-Down on Cell Adhesion to Laminin-10/11. Finally, we examined the role of CD151 in modulating integrin α3β1 activity by RNA interference (RNAi)-mediated knock-down of CD151. Flow cytometric analyses showed that the expression level of CD151 on A549 cells was reduced by ≈90% upon introduction of siRNA targeted against CD151, whereas that of integrin α3β1 remained unaffected (Fig. 4A; also Fig. 9, which is published as supporting information on the PNAS web site). CD151 knock-down by RNAi resulted in ≈20% reduction in the number of cells adhering to laminin-10/11, whereas cells treated with control siRNA did not exhibit any significant decrease. Although the effect of CD151 knock-down on cell adhesion was only partial, it was reproducible and statistically significant, supporting our conclusion that CD151 stabilizes the activated conformation of integrin α3β1 in a physiological context.
Fig. 4.
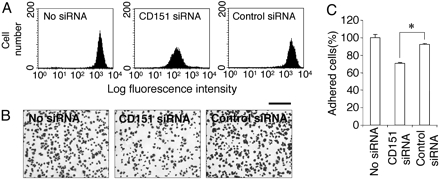
Effect of CD151 knock-down on cell adhesion to laminin-10/11. (A) The CD151 expression levels in A549 cells transfected with siRNA against CD151 (CD151 siRNA) or control siRNA were examined by flow cytometry by using mAb 8C3. The expression of CD151 is knocked down by ≈90% by CD151 siRNA. (B) A549 cells transfected with control or CD151 siRNA were plated on 24-well plates coated with laminin-10/11. After a 20-min incubation at 37°C, the attached cells were fixed and stained. (Scale bar, 200 μm.) (C) The numbers of attached cells were counted in three separate fields and expressed as percentages of the number without siRNA transfection (No siRNA). Each bar represents the mean ± SD. *, P < 0.001.
Discussion
Despite the stable and stoichiometric association between CD151 and integrin α3β1, the significance of their complex formation has been poorly understood at the molecular level. This result is largely due to the absence of biochemical analyses that directly compare the activities of the integrin with and without CD151 association. Due to the stability of integrin α3β1–CD151 complexes, it is difficult to dissociate CD151 from the integrin under physiological conditions. Hence, treatment with antibodies against CD151 as well as transfection with CD151 mutants that are unable to associate with integrins have been used to address the role of CD151 association in integrin-dependent cellular functions (8–10, 22). Several lines of evidence have indicated that CD151 is involved in integrin α3β1/α6β1-dependent adhesive events, including migration, spreading, and mechanical force generation, but not in static cell adhesion (8, 22), although it has not been proven whether these agents can dissociate endogenous CD151 from α3β1 and other laminin-binding integrins. For example, although the QRD mutant of CD151 lacks the ability to associate with integrins (8), there is no reason to assume that exogenous expression of this mutant can prevent the association of endogenous CD151 with α3β1 and other integrins. Furthermore, no anti-CD151 antibodies have previously been demonstrated to dissociate CD151 from integrin α3β1 on the cell surface. In the present investigation, we demonstrated that mAb 8C3 actively dissociates CD151 from integrin α3β1 not only in purified CD151-integrin α3β1 complexes but also on the surface of living cells, thereby allowing us to compare the ligand-binding activities as well as conformational states of integrin α3β1 with and without CD151 association. Our results clearly showed that the ligand-binding activity of integrin α3β1 was potentiated by association with CD151 through stabilization of the active conformation of the integrin. To the best of our knowledge, we present a previously uncharacterized demonstration of a regulatory role for CD151 in the adhesive interactions of integrin α3β1 with its physiological ligands.
There is accumulating evidence that the ligand-binding activity of integrins is intimately linked with their conformational state (1). The crystal structure of the extracellular domain of integrin αvβ3 revealed a compact conformation with a severe bend in the middle of the receptor (23). Subsequent electron-microscopic as well as biochemical studies showed that the compact conformation represents the low-affinity default state, whereas activation and ligand binding result in global unbending of the receptor, leading to an extended conformation (19). mAb AG89 binds to the midregion of the β1 chain, which is partially buried in the bent conformation, and thereby preferentially recognizes activated β1-containing integrins (16, 21). Our results show that the reactivity of integrin α3β1 toward AG89 was significantly reduced after depletion of CD151, with a concomitant reduction in the ligand-binding activity. This observation strongly suggests that integrin α3β1 assumes a more extended conformation when it is associated with CD151. It is important to note that both the AG89 reactivity and the laminin-binding activity of CD151-free integrin α3β1 were reduced, but not abolished. Integrins on cells can exist in multiple conformational states, and there seems to be a rather fast equilibrium among them (24). Therefore, CD151 may modulate the activity of integrin α3β1 by shifting the position of the conformational equilibrium toward the more extended form, rather than strictly controlling the ligand-binding affinity (Fig. 5).
Fig. 5.
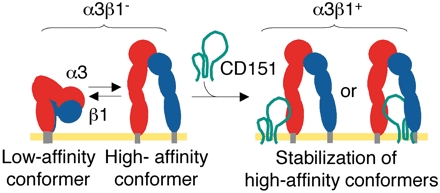
Schematic model for conformational modulation of integrin α3β1by CD151 association. The extracellular domains of the α3-(red) and β1-(blue) subunits are schematically depicted as connected ovals, with membrane-anchoring regions at their ends (gray rods). Integrin α3β1 depleted of CD151 (α3β1–) assumes both low-affinity (bent) and high-affinity (extended) conformers, which are in fast equilibrium. Lateral association of CD151 (represented by a green line) with α3β1– stabilizes the high-affinity conformers, thereby potentiating the ligand-binding activity of integrin α3β1.
The association of integrin α3β1 with CD151 is mediated by interaction of the α3 chain with the large extracellular loop of CD151 (5). The region of the α3 chain involved in the association with CD151 has been mapped to amino acid residues 570–705 (5), which correspond to the “thigh” and “calf-1” domains of the αv chain (23). This portion corresponds to the region where the integrin is most severely bent in its putative low-affinity conformation, and is thus likely to be the fulcrum point during the “switchblade-opening.” Therefore, it is tempting to speculate that the laterally associated CD151 acts as a “prop” to stabilize the extended high-affinity conformation of α3β1 and render the bending unfavorable (Fig. 5).
The observation that mAb 8C3 can dissociate CD151 from integrin α3β1 was the strategic basis of our investigation into the biological significance of the stable association of CD151 with integrin α3β1. Although the precise mechanism by which 8C3 dissociates CD151 from integrin α3β1 remains to be defined, two possible scenarios can be postulated. One is the “wedge” model, in which 8C3 binds at or near the integrin-binding site on CD151, and sterically prevents CD151 from associating with integrin α3β1. The other is the “allosteric” model, in which binding of 8C3 induces a conformational change in CD151, converting it to a low-affinity conformation for the integrin. According the wedge model, 8C3 would deplete CD151 from integrin α3β1–CD151 complexes by capturing only the small fraction of CD151 that spontaneously dissociates from the integrin, and therefore would not deplete CD151 to completion in a short period. In contrast, the allosteric model predicts rapid dissociation of the integrin α3β1–CD151 complexes, because the rate-limiting step would be binding of 8C3 to the complexes. Given that 8C3 was capable of dissociating CD151 from integrin α3β1 on the surface of living cells within 1 min, the allosteric model seems more likely, although detailed kinetic analyses as well as epitope mapping of 8C3 will be needed to consolidate this model.
One approach for addressing the in vivo functions of CD151 is to produce animals deficient in CD151 expression and analyze their phenotypes. Wright et al. (25) successfully produced CD151-null mice and demonstrated that these mice were apparently healthy and fertile except for some minor abnormalities, including impaired outside-in signaling in platelets and reduced keratinocyte migration in skin explant cultures (25, 26). Despite the role of CD151 in the adhesive interactions of integrin α3β1/α6β1 integrins with laminins (8, 22), no apparent abnormalities in organogenesis of the skin and kidneys, in which cell-basement membrane interactions play critical roles, were observed in the CD151-null mice (25). This finding is in striking contrast to mice deficient in integrin α3 and/or α6 chains, which exhibit severe phenotypes including malformation of the kidneys and lungs and skin blistering (27, 28). The absence of such severe phenotypes in CD151-null mice could result from functional compensation by other tetraspanins that are also capable of associating with integrin α3β1 and other laminin-binding integrins. Recently, CD151 was found to be identical to the MER2 blood group antigen (29). MER2-negative individuals exhibited hereditary nephritis and pretibial epidermolysis bullosa, both of which were associated with thickening and splitting of the basement membranes (29). These abnormalities partially resemble the phenotypes of integrin α3- or α6-deficient mice (27, 28), consistent with a regulatory role for CD151 in the interactions of integrins α3β1/α6β1 with laminins, which in turn regulate the basement membrane assembly of laminins.
Tetraspanins have been considered to be molecular facilitators that organize integrins and other proteins into a network of membrane microdomains, called the “tetraspanin web,” with which some signaling molecules, such as protein kinase C, are also associated (2, 30). Besides this organizational role, CD151 and possibly other tetraspanins play a regulatory role in the ligand binding of integrin α3β1 (and possibly other laminin-binding integrins) through stabilizing its activated conformation. Although the structural basis of the CD151-dependent modulation of the ligand-binding activity of laminin-binding integrins remains to be elucidated, CD151 can be regarded as a “conformational facilitator” of integrin α3β1 and other laminin-binding integrins. Further studies on the mechanism by which CD151 modulates the ligand-binding of integrin α3β1 should provide clues for better understanding of the conformational regulation of laminin-binding integrin functions as well as the integrin-mediated outside-in signaling.
Supplementary Material
Acknowledgments
We thank Yoshiko Yagi and Yumi Yoshimura for expert assistance in the amino acid sequencing and Dr. Masashi Yamada for invaluable suggestions regarding the manuscript.
Author contributions: R.N. and K.S. designed research; R.N., S.N., and Y.S. performed research; R.N., S.N., J.T., and K.S. analyzed data; R.N. and K.S. wrote the paper; and N.S., S.N., Y.S., Y.W., M.O., J.T., H.H., and K.S. contributed new reagents/analytic tools.
Abbreviations: α3β1+, integrin α3β1 complexed with CD151; α3β1–, CD151-free integrinα3β1; FRET, fluorescent resonance energy transfer; siRNA, short interference RNA; CFP, cyan fluorescent protein; YFP, yellow fluorescent protein.
References
- 1.Hynes, R. O. (2002) Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- 2.Stipp, C. S., Kolesnikova, T. V. & Hemler, M. E. (2003) Trends Biochem. Sci. 28, 106–112. [DOI] [PubMed] [Google Scholar]
- 3.Yauch, R. L., Berditchevski, F., Harler, M. B., Reichner, J. & Hemler, M. E. (1998) Mol. Biol. Cell 9, 2751–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serru, V., Le Naour, F., Billard, M., Azorsa, D. O., Lanza, F., Boucheix, C. & Rubinstein, E. (1999) Biochem. J. 340, 103–111. [PMC free article] [PubMed] [Google Scholar]
- 5.Yauch, R. L., Kazarov, A. R., Desai, B., Lee, R. T. & Hemler, M. E. (2000) J. Biol. Chem. 275, 9230–9238. [DOI] [PubMed] [Google Scholar]
- 6.Hemler, M. E. (2001) J. Cell Biol. 155, 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, X. A., Bontrager, A. L. & Hemler, M. E. (2001) J. Biol. Chem. 276, 25005–25013. [DOI] [PubMed] [Google Scholar]
- 8.Kazarov, A. R., Yang, X., Stipp, C. S., Sehgal, B. & Hemler, M. E. (2002) J. Cell Biol. 158, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohno, M., Hasegawa, H., Miyake, M., Yamamoto, T. & Fujita, S. (2002) Int. J. Cancer 97, 336–343. [DOI] [PubMed] [Google Scholar]
- 10.Yanez-Mo, M., Alfranca, A., Cabanas, C., Marazuela, M., Tejedor, R., Ursa, M. A., Ashman, L. K., de Landazuri, M. O. & Sanchez-Madrid, F. (1998) J. Cell Biol. 141, 791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stipp, C. S. & Hemler, M. E. (2000) J. Cell Sci. 113, 1871–1882. [DOI] [PubMed] [Google Scholar]
- 12.Nishiuchi, R., Murayama, O., Fujiwara, H., Gu, J., Kawakami, T., Aimoto, S., Wada, Y. & Sekiguchi, K. (2003) J. Biochem. (Tokyo) 134, 497–504. [DOI] [PubMed] [Google Scholar]
- 13.Kikkawa, Y., Sanzen, N. & Sekiguchi, K. (1998) J. Biol. Chem. 273, 15854–15859. [DOI] [PubMed] [Google Scholar]
- 14.Kikkawa, Y., Sanzen, N., Fujiwara, H., Sonnenberg, A. & Sekiguchi, K. (2000) J. Cell Sci. 113, 869–876. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa, H., Nomura, T., Kishimoto, K., Yanagisawa, K. & Fujita, S. (1998) J. Immunol. 161, 3087–3095. [PubMed] [Google Scholar]
- 16.Takagi, J., Isobe, T., Takada, Y. & Saito, Y. (1997) J. Biochem. (Tokyo) 121, 914–921. [DOI] [PubMed] [Google Scholar]
- 17.Shigeta, M., Sanzen, N., Ozawa, M., Gu, J., Hasegawa, H. & Sekiguchi, K. (2003) J. Cell Biol. 163, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuoka, H., Nada, S. & Okada, M. (2004) J. Biol. Chem. 279, 5975–5983. [DOI] [PubMed] [Google Scholar]
- 19.Takagi, J., Petre, B. M., Walz, T. & Springer, T. A. (2002) Cell 110, 599–611. [DOI] [PubMed] [Google Scholar]
- 20.Takagi, J., Strokovich, K., Springer, T. A. & Walz, T. (2003) EMBO J. 22, 4607–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi, J., Erickson, H. P. & Springer, T. A. (2001) Nat. Struct. Biol. 8, 412–416. [DOI] [PubMed] [Google Scholar]
- 22.Lammerding, J., Kazarov, A. R., Huang, H., Lee, R. T. & Hemler, M. E. (2003) Proc. Natl. Acad. Sci. USA 100, 7616–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong, J. P., Stehle, T., Diefenbach, B., Zhang, R., Dunker, R., Scott, D. L., Joachimiak, A., Goodman, S. L. & Arnaout, M. A. (2001) Science 294, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carman, C. V. & Springer, T. A. (2003) Curr. Opin. Cell Biol. 15, 547–556. [DOI] [PubMed] [Google Scholar]
- 25.Wright, M. D., Geary, S. M., Fitter, S., Moseley, G. W., Lau, L. M., Sheng, K. C., Apostolopoulos, V., Stanley, E. G., Jackson, D. E. & Ashman, L. K. (2004) Mol. Cell. Biol. 24, 5978–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau, L. M., Wee, J. L., Wright, M. D., Moseley, G. W., Hogarth, P. M., Ashman, L. K. & Jackson, D. E. (2004) Blood 104, 2368–2375. [DOI] [PubMed] [Google Scholar]
- 27.Kreidberg, J. A., Donovan, M. J., Goldstein, S. L., Rennke, H., Shepherd, K., Jones, R. C. & Jaenisch, R. (1996) Development (Cambridge, U.K.) 122, 3537–3547. [DOI] [PubMed] [Google Scholar]
- 28.Georges-Labouesse, E., Messaddeq, N., Yehia, G., Cadalbert, L., Dierich, A. & Le Meur, M. (1996) Nat. Genet. 13, 370–373. [DOI] [PubMed] [Google Scholar]
- 29.Karamatic Crew, V., Burton, N., Kagan, A., Green, C. A., Levene, C., Flinter, F., Brady, L. R., Daniels, G. & Anstee, D. J. (2004) Blood 104, 2217–2223. [DOI] [PubMed] [Google Scholar]
- 30.Maecker, H. T., Todd, S. C. & Levy, S. (1997) FASEB J. 11, 428–442. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


