Abstract
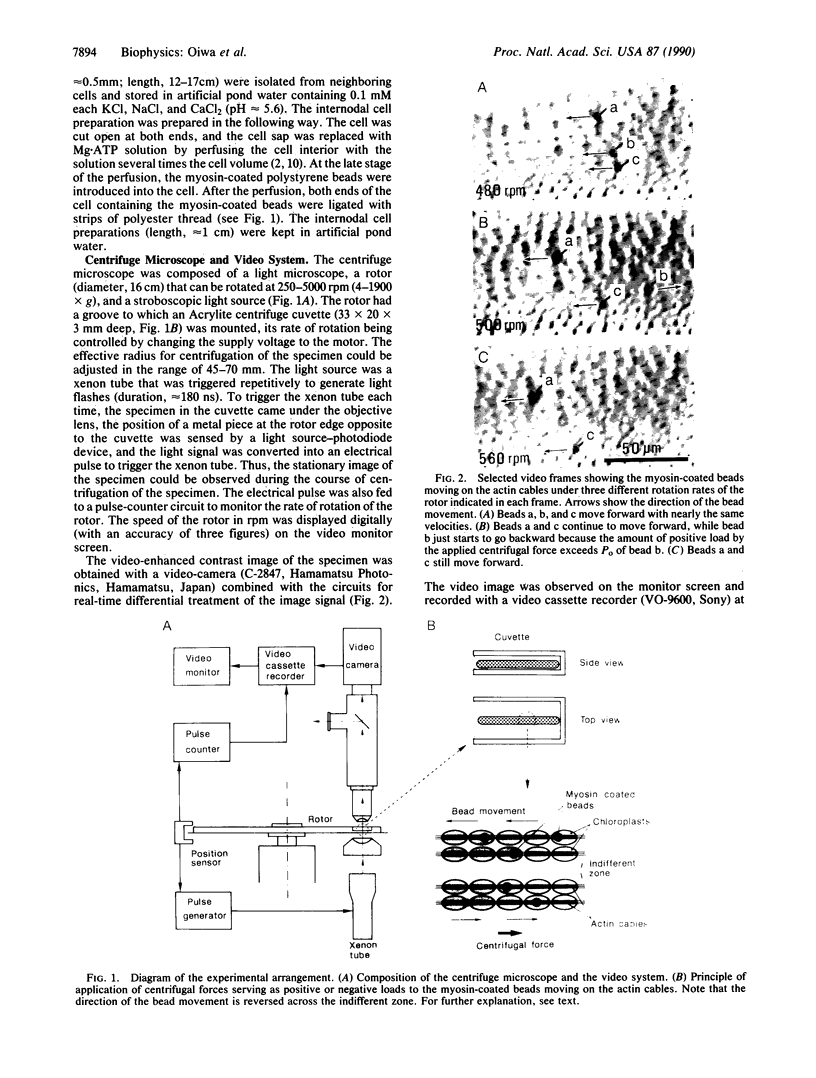
To eliminate the gap between the biochemistry of actomyosin in solution and the physiology of contracting muscle, we developed an in vitro force-movement assay system in which the steady-state force-velocity relation in the actin-myosin interaction can be studied. The assay system consists of the internodal cells of an alga, Nitellopsis obtusa, containing well-organized actin filament arrays (actin cables); tosyl-activated polystyrene beads (diameter, 2.8 microns; specific gravity, 1.3) coated with skeletal muscle myosin; and a centrifuge microscope equipped with a stroboscopic light source and a video system. The internodal cell preparation was mounted on the rotor of the centrifuge microscope, so that centrifugal forces were applied to the myosin-coated beads moving along the actin cables in the presence of ATP. Under constant centrifugal forces directed opposite to the bead movement ("positive" loads), the beads continued to move with constant velocities, which decreased with increasing centrifugal forces. The steady-state force-velocity curve thus obtained was analogous to the double-hyperbolic force-velocity curve of single muscle fibers. The unloaded velocity of bead movement was 1.6-3.6 microns/s (20-23 degrees C), while the maximum "isometric" force generated by the myosin molecules on the bead was 1.9-39 pN. If, on the other hand, the beads were subjected to constant centrifugal forces in the direction of bead movement ("negative" loads), the bead also moved with constant velocities. Unexpectedly, the velocity of bead movement did not increase with increasing negative loads but first decreased by 20-60% and then increased towards the initial unloaded velocity until the beads were eventually detached from the actin cables.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaen S., Oiwa K., Shimmen T., Iwamoto H., Sugi H. Simultaneous recordings of force and sliding movement between a myosin-coated glass microneedle and actin cables in vitro. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1510–1514. doi: 10.1073/pnas.86.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988 Oct;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Harada Y., Noguchi A., Kishino A., Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987 Apr 23;326(6115):805–808. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- Iwamoto H., Sugaya R., Sugi H. Force-velocity relation of frog skeletal muscle fibres shortening under continuously changing load. J Physiol. 1990 Mar;422:185–202. doi: 10.1113/jphysiol.1990.sp017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T., Ohshima H. A self-induced translation model of myosin head motion in contracting muscle. I. Force-velocity relation and energy liberation. J Muscle Res Cell Motil. 1988 Jun;9(3):248–260. doi: 10.1007/BF01773895. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Spudich J. A. Movement of myosin-coated fluorescent beads on actin cables in vitro. Nature. 1983 May 5;303(5912):31–35. doi: 10.1038/303031a0. [DOI] [PubMed] [Google Scholar]







