Abstract
Previous work demonstrated that efficient RNA Polymerase sigma S-subunit (RpoS) translation requires the N6-isopentenyladenosine i6A37 transfer RNA (tRNA) modification for UUX-Leu decoding. Here we investigate the effect of two additional tRNA modification systems on RpoS translation; the analysis was also extended to another High UUX-leucine codon (HULC) protein, Host Factor for phage Qβ (Hfq). One tRNA modification, the addition of the 2’-O-methylcytidine/uridine 34 (C/U34m) tRNA modification by tRNA (cytidine/uridine-2’O)-ribose methyltransferase L (TrmL), requires the presence of the N6-isopentenyladenosine 37 (i6A37) and therefore it seemed possible that the defect in RpoS translation in the absence of i6A37 prenyl transferase (MiaA) was in fact due to the inability to add the C/U34m modification to UUX-Leu tRNAs. The second modification, addition of 2-thiouridine (s2U), part of (mnm5s2U34), is dependent on tRNA 2-thiouridine synthesizing protein A (TusA), previously shown to affect RpoS levels. We compared expression of PBAD-rpoS990-lacZ translational fusions carrying wild-type UUX leucine codons with derivatives in which UUX codons were changed to CUX codons, in the presence and absence of TrmL or TusA. The absence of these proteins, and therefore presumably the modifications they catalyze, both abolished PBAD-rpoS990-lacZ translation activity. UUX-Leu to CUX-Leu codon mutations in rpoS suppressed the trmL requirement for PBAD-rpoS990-lacZ expression. Thus, it is likely that the C/U34m and s2U34 tRNA modifications are necessary for full rpoS translation. We also measured PBAD-hfq306-lacZ translational fusion activity in the absence of C/U34m (trmL) or i6A37 (miaA). The absence of i6A37 resulted in decreased PBAD-hfq306-lacZ expression, consistent with a role for i6A37 tRNA modification for hfq translation.
Keywords: s2U34 tRNA modification, C/U34m tRNA modification, i6A37 tRNA modification, RpoS, Hfq, MiaA, TrmL, TusA, leucine, codon bias
1. Introduction
Escherichia coli RpoS (σS) is an alternative sigma factor that facilitates physiological adaptation to general starvation and stationary phase growth [1,2]. RpoS levels are tightly regulated, particularly at the post-transcriptional level. Several small regulatory RNAs modulate translational initiation in response to various environmental conditions [3,4,5,6,7,8]. In addition, RpoS stability is decreased by the activity of the ATP-dependent Protease ClpXP with the assistance of the Regulator of sigma S B (RssB) adaptor protein [9]. Conversely, RpoS is stabilized by three anti-adaptor proteins: Inhibitor of RssB activity in response to phosphate starvation (IraP), Inhibitor of RssB activity in response to magnesium starvation (IraM), and Inhibitor of RssB activity in response to DNA damage (IraD) [10,11,12,13].
RpoS expression is also regulated at the level of translation within the open reading frame (ORF). SsrA (tmRNA) is a small RNA that enters the A-site of stalled ribosomes and is translated into a C-terminal tag that targets truncated proteins for degradation by several cellular proteases [14]. SsrA is necessary for full RpoS translation [14]. Translation of RpoS is significantly affected by at least one tRNA modification, N6-isopentyl adenosine (i6A) [15]. tRNA dimethylallyltransferase (MiaA) catalyzes the prenylation (i6) of adenine 37 (A37) to tRNAs that read UXX codons [16,17,18]. The mechanism of action of the i6A37 tRNA modification on RpoS expression is, at least in part, promotion of efficient UUX-leucine decoding (Figure 1) [19]. Here, we examined whether two other tRNA modification systems played roles in RpoS translation.
Figure 1.
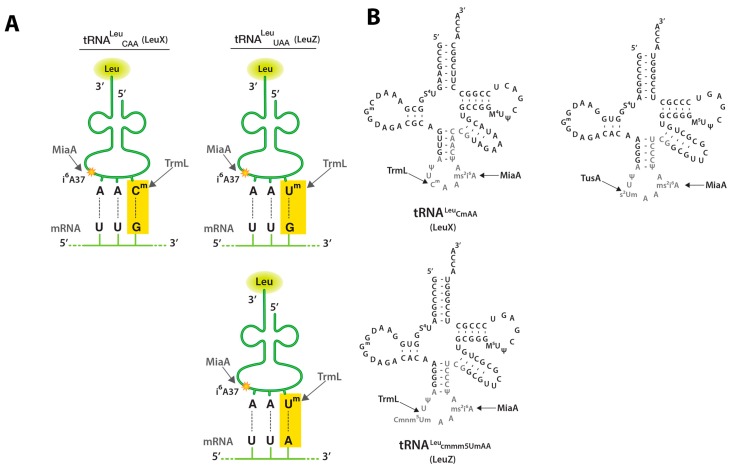
tRNA (cytidine/uridine-2’O)-ribose methyltransferase L (TrmL), tRNA dimethylallyltransferase (MiaA), and tRNA 2-thiouridine synthesizing protein A (TusA) tRNA modifications and UUX-Leu codon recognition; (A) Schematic depicting tRNALeuCAA (LeuX) and tRNALeuUAA (LeuZ) anticodons and their cognate mRNA codons (UUG or UUA). This schematic is modified from a leucine tRNA schematic obtained from the following source [20]; (B) tRNALeuCAA (LeuX) and tRNALeuUAA (LeuZ) secondary structure secondary structure. Nucleotides subject to modification are shown in grey and the sites of the MiaA, TrmL, and TusA modifications studied here are shown. This schematic is modified from Figure 3C of [21].
A major role for tRNA modifications is the promotion of translational fidelity, through suppression of frameshifting and ribosome stalling [22,23,24]. Modifications also improve reading frame maintenance in response to rare or underutilized codons [25,26]. In this work, we investigated the role of several tRNA modifications on rpoS or hfq expression: 2′-O-methylation of Cytidine or Uridine, at the wobble position (C/Um), 2-thiouridine at the wobble position (s2U), as well as isopentyl adenosine 37 (i6A) (Figure 1).
C/U34m is catalyzed by the tRNA methyl transferase enzyme TrmL enzyme in E. coli [21,27,28]. The C/U34m modification occurs in leucine tRNACmAA and tRNAcmnm5UmAA (Figure 1) [21]. Both of these leucine tRNA isoacceptors recognize UUA-Leu and UUG-Leu codons, and also carry the i6A37 tRNA modification that assists in the optimal decoding of the Leu codons during rpoS translation (Figure 1) [19]. The TrmL-catalyzed C/U34m modification of these leucine tRNAs isoacceptors requires i6A37 modification [21]. Taken together, this suggests that miaA and trmL may work together to affect rpoS expression and we investigated the role the trmL in rpoS translation in this work.
The 2-thiolation (s2) tRNA modification occurs in the anticodon stem loop (ASL) on uridines at the wobble position 34 (Figure 1) [29]. The 2-thiouridine (s2U34) wobble tRNA modification, like other tRNA modifications within the ASL, improves translational fidelity by suppressing translational frameshifting, promoting tight codon-anticodon interactions for tRNAGlnUUG with CAA and CAG glutamine codons [30]. tRNA 2-thiouridine synthesizing protein A (TusA) is necessary for the synthesis of the s2U34 modification [31]. TusA point mutations were identified in a genetic screen for mutations affecting rpoS expression, decreasing σS levels at the level of stability [32]. In addition, the s2U34 modification is also a component of a more complex modification present on C/U34m modified leucine tRNA isoacceptors, one of which is implicated in rpoS translation [19].
Host Factor for phage Qβ (Hfq) is a co-factor necessary for the vast majority of trans-acting small regulatory RNAs in E. coli and other bacteria, and has many pleotropic roles in the cell [33,34]. It was first discovered as a host factor for Bacteriophage Qβ replication [35]. The hfq gene is in a complex operon with multiple promoters and is immediately downstream of the i6A37 prenyl transferase miaA [36]. There is an elevated UUX-Leu to CUX-Leu ratio in the hfq open reading frame, characterizing it as a HULC protein that may, like RpoS, be sensitive to the i6A37 tRNA modification [19]. There is little known about the translational regulation of the hfq ORF, in particular the contribution of tRNA modifications.
Here we used a genetic approach to test the role of the TrmL-catalyzed and TusA-catalyzed tRNA modifications during rpoS expression. We also further tested our previous predictions on the role of the i6A37 modification on expression of proteins with High UUX leucine Codon (HULC) content, using hfq as a model gene. Hfq was an attractive candidate, due to its elevated UUX-Leu codon usage ratio (Table S7, [19]) and its phylogenetically conserved cotranscription with miaA. Here we demonstrate that both TrmL and TusA are necessary for full RpoS translation and MiaA-catalyzed-i6A37 is necessary for hfq expression.
2. Results
2.1. Wobble Base tRNA Modifications Enzymes TrmL and TusA Are Necessary for RpoS Expression
In order to determine if mnm5s2U34 and C/U34m tRNA modifications may play a role in facilitating proper rpoS translation, we measured the effect of mutations in the enzymes necessary for these modifications on rpoS expression, using two different rpoS-lacZ translational fusion strains. Here, we will assume that phenotypes associated with absence of the enzyme are due to lack of the modification, although it is possible these enzymes have other roles in the cell.
The first translational fusion strain contains the rpoS promoter, the 5′ untranslated region, and 750 nucleotides of the open reading frame (rpoS750-lacZ) fused, in frame, to the ninth codon of the lacZ ORF. The second translational fusion strain has the arabinose inducible PBAD promoter in place of the rpoS promoter and 5′ untranslated region (5’ UTR), as well as the complete open reading frame, except the termination codon (PBAD-rpoS990-lacZ) fused, in frame, to the ninth codon of the lacZ ORF. Both of these fusions were previously used to characterize the contribution of the MiaA-catalyzed i6A37 tRNA modification in rpoS translation [15,19]. We transduced zeomycin-linked null mutations in trmL (ΔtrmL::zeo) and tusA (ΔtusA::zeo) into both the rpoS750-lacZ and PBAD-rpoS990-lacZ translational fusion strains and measured β-galactosidase expression compared to the wild type control strain (Figure 1B and Figure 2A). For the rpoS750-lacZ translational fusion—at Optical Density 600nm (OD600) 0.5, 1.0, 1.5, and 2.0—the activity of the rpoS-lacZ fusion was virtually undetectable in the absence of trmL or tusA at <1.0 machine units (Figure 2A). Arabinose led to significant induction of the wild type PBAD-rpoS990-lacZ, as expected and previously observed (Figure 2B). However, in the absence of trmL or tusA this fusion also had virtually undetectable activity, with specific activities <1.0 machine units throughout the 30 min following induction (Figure 2B). Taken together, the decreased activity of the rpoS fusions in the absence of tusA and trmL suggest that the presence of the s2U34 and C/U34m tRNA modifications are necessary for rpoS expression. Any effects seen with both fusions cannot be attributed to the native promoter or the 5′ UTR (and thus sRNA regulation of RpoS), since neither the native promoter nor the 5′ UTR are present in the PBAD fusion. Effects on RpoS-LacZ levels could reflect differences in translation, in mRNA stability, or in protein degradation; these possibilities are explored in the discussion.
Figure 2.
The effect of s2U34 and C/U34m tRNA modifications on rpoS-lacZ expression; (A) Wild type (EM1050), trmL− (KMT767), and tusA− (KMT766) rpoS750-lacZ translational fusion strains were grown in Luria Bertani (LB) Lennox media at 37 °C and 200 rpm. Aliquots were taken at Optical Density 600 nm (OD600) of 0.5, 1.0, 1.5, and 2.0 for β-galactosidase assay; (B) Wild type (KMT30003), trmL− (KMT30003), and tusA− (KMT30003) PBAD-rpoS990-lacZ translational fusion strains (in rssB− backgrounds) were grown in LB media, supplemented with glucose to a final concentration of 0.2%, at 37 °C to an OD600 of 0.5. Cells were harvested by centrifugation and resuspended in LB media, supplemented with arabinose to a final concentration of 0.2%, and further incubated at 37 °C. Aliquots were taken at 5 min intervals for 30 min for β-galactosidase assay.
2.2. TrmL tRNA Modification is Necessary for Decoding of UUX-Leucine Decoding in RpoS
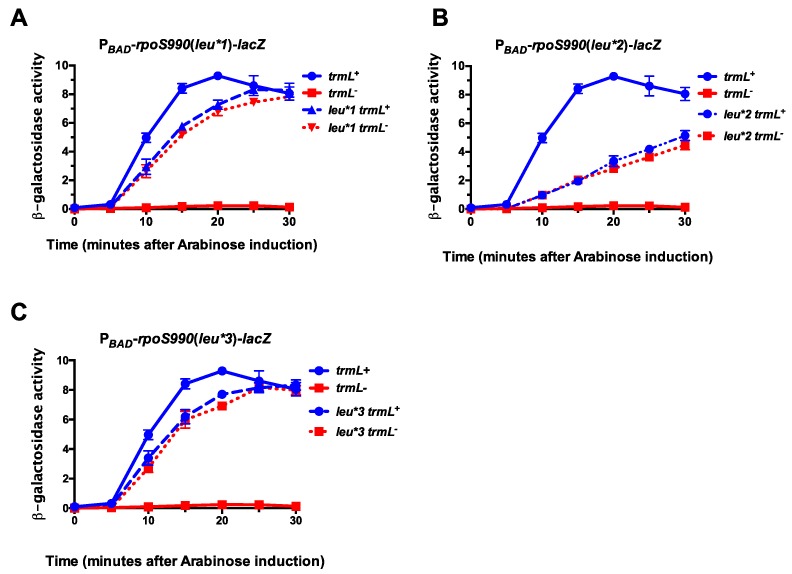
We previously demonstrated that the i6A37 tRNA modification was required for UUX-leu decoding, with silent UUX-Leu to CUX-Leu codon mutations in the rpoS reading frame partially suppressing the i6A37 requirement for expression [19]. In addition, since the C/U34m occurs in certain leucine tRNAs, requires the MiaA-catalyzed i6A37 tRNA modification, and may be necessary for rpoS translation (Figure 1), we asked whether the trmL requirement for RpoS expression may be related to UUX-leucine decoding, using our previously described UUX to CUX mutant derivatives of the PBAD-rpoS990-lacZ fusions [19]. We measured the activity of PBAD-rpoS990-lacZ translational fusions in which UUA-Leu codons with the rpoS portion of the fusion ORF have been changed to CUX-Leu (leu*1), UUG-Leu to CUX-Leu (leu*2), or UUA-Leu and UUG-Leu to CUX-Leu (leu*3) mutations, in the presence and absence of trmL (Figure 3). Either the UUA-Leu to CUX-Leu codon construct (leu*1) or the UUG-Leu to CUX-Leu construct (leu*2) in the rpoS ORF completely suppressed the trmL requirement for rpoS expression (Figure 2B and Figure 3A). Finally, combined UUA-Leu to CUX-Leu and UUG-Leu to CUX-Leu codon mutations within the rpoS ORF also completely suppressed the trmL requirement (Figure 3C). Taken together, this strongly supports the idea that the TrmL-catalyzed C/U34m tRNA modification is necessary for rpoS translation, dependent upon the presence of UUX-Leu codons.
Figure 3.
trmL is necessary for leucine decoding during RpoS expression. trmL+ and trmL− PBAD-rpoS990-lacZ translational fusions (in rssB− backgrounds) were grown in LB, supplemented with glucose to a final concentration of 0.2%, at 37 °C to an OD600 of 0.5. Cells were harvested by centrifugation and res-suspended in LB supplemented with arabinose to a final concentration of 0.2%. Finally, 100 mL aliquots of culture were isolated at 5-min intervals for 30 min. This process was executed for PBAD-rpoS990-lacZ translational fusions with rpoS UUA-Leu CUX-Leu mutations (leu*1)–strains KMT36002 and KMT36010 (A); rpoS UUG-Leu to CUX-leu mutation (leu*2)–strains KMT37002 and KMT37010 (B); rpoS with both UUA-Leu to CUX-Leu and UUG-Leu to CUX-Leu mutations (leu*3)–strains KMT33001 and KMT33013 (C). All time points represent the average of three independent experiments (biological replicates) and the error bars represent the standard error of the mean.
2.3. MiaA Is Necessary, While TrmL Is Dispensable, for Hfq Expression
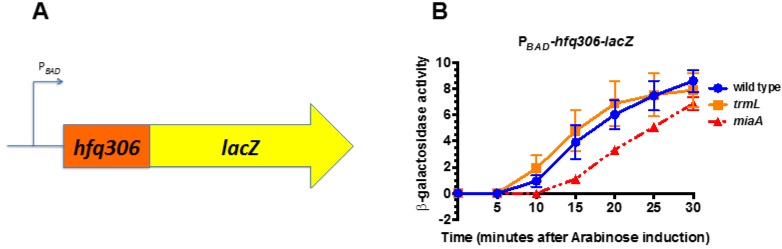
We previously identified the UUX-Leu ratios of all open reading frames within the E. coli genome and proposed using this ratio as a predictor of i6A37 sensitivity during translation of the open reading frame [19]. We measured the effect of mutations in miaA on expression of another gene known to be involved in the RpoS regulatory circuitry, that also has a UUX-Leu ratio suggestive of i6A37 sensitivity, the RNA-chaperone Hfq. We hypothesized that additional HULC proteins may be sensitive to the presence of the C/U34m modification, since the expression of at least two predicted HULC were defective in the miaA mutant and the C/U34m modification requires the MiaA-catalyzed i6A37 modification. An arabinose inducible hfq fusion containing the hfq open reading frame, except the termination codon, was fused in-frame, with the ninth codon of the lacZ ORF (PBAD-hfq306-lacZ—Figure 4A). Next, we transduced in zeomycin-linked null mutations in trmL (ΔtrmL::zeo) and miaA (ΔmiaA::zeo), and measured PBAD-hfq306-lacZ activity in the wild-type, trmL, and miaA mutants following arabinose induction. The activity of the wild-type fusion increased in a time-dependent manner following arabinose induction (Figure 4). The trmL mutation had no difference in the activity of the fusion following induction. However, there were some differences in the activity of the hfq fusion in the absence of the absence of the MiaA-catalyzed i6A37 tRNA modification. Overall, expression of the fusion was slower to increase in the miaA mutant mutant, with significantly lower levels most obvious at 10 and 15′ (Figure 4 and Table 1).
Figure 4.
miaA, not trmL, is necessary for full hfq expression. (A) Schematic depicting the PBAD-hfq306-lacZ translational fusion used for this experiment. (B) miaA+ trmL+ (KMT38000), miaA+ trmL-(KMT38004), and miaA− trmL+ (KMT38002) PBAD-hfq306-lacZ translational fusion strains were grown in LB media, supplemented with glucose to a final concentration of 0.2% at 37 °C to an OD600 of 0.5. Cells were harvested by centrifugation and resuspended in LB media, supplemented with arabinose to a final concentration of 0.2%, and further incubated at 37 °C. Aliquots were taken at 5 min intervals for 30 min for β-galactosidase assay. All time points represent the average of three independent experiments (biological replicates) and the error bars represent the standard error of the mean.
Table 1.
PBAD-hfq306-lacZ translational fusion miaA+/miaA− activity ratio.
| Time (Min after Ara Induction) | Strain (Mean β-Gal Activity) | ||
|---|---|---|---|
| hfq+ | hfq- | hfq+/hfq- Fold Change | |
| 10 | 0.94 | 0.00 | ∞ |
| 15 | 3.91 | 1.11 | 3.52 |
| 20 | 6.04 | 3.35 | 1.80 |
| 25 | 7.50 | 5.10 | 1.47 |
| 30 | 8.62 | 6.89 | 1.25 |
Taken together, this suggests that while the TrmL-catalyzed C/U34m tRNA modification is dispensable for hfq translation, the MiaA catalyzed i6A37 is necessary for efficient hfq translation. This also provides experimental evidence that a 3rd HULC protein, in addition to rpoS and iraP, requires the i6A37 tRNA modification, although further confirmation of a direct effect of the miaA mutant on hfq translation will require testing a version of the hfq fusion in which the UUX codons have been changed to CUX codons.
3. Discussion
3.1. Expanded Network of tRNA Modifications Affecting rpoS Expression and Physiological Implications
Prior to this work, there was only one report of a tRNA modification, i6A37, directly influencing rpoS translation [15,19]. Since there are multiple post-transcriptional regulators of RpoS, we hypothesized that additional tRNA modifications may be necessary for efficient translation of RpoS. Here we examined two additional tRNA modifications as possible regulators of rpoS expression, TusA-catalyzed s2U and TrmL-catalyzed C/Um.
Both of these tRNA modifications occur at the wobble position and are likely to influence rpoS expression through improving proper codon-anticodon interactions at the wobble position where non-canonical RNA–RNA interactions can occur. The C/Um modification occurs on leucine tRNA isoacceptor tRNALeucmnm5s2AA, which also contains the mnm5s2U34 tRNA modification and requires the ms2i6A37 tRNA modification [21]. The TusA catalyzed s2U34 modification is also a precursor for the 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U34) tRNA hypermodification or the 5-methylaminomethyl-2-thiouridine (mnm5s2U34) tRNA modification via the Methylaminomethyl modification G/E (MnmG/E) pathway [37,38].
Our previous experiments suggested that the requirement for MiaA (assumed in this discussion to reflect a requirement for the i6A37 modification) was due to direct effects on decoding of rpoS. That evidence started from the observation that rpoS, unlike rpoD, was enriched for UUX leucine codons (termed here HULC for High UUX-leucine codon) [15,19]. The tRNA, tRNALeuCAA (encoded by leuX) that is the target for these modifications, acts as a multi-copy suppressor of the i6A37 requirement for optimal rpoS expression [19], consistent with UUX leucine codons limiting translation. Finally, rpoS codon swapping experiments, specifically changing UUX-Leu to CUX-Leu, demonstrated partial suppression of the MiaA requirement during rpoS expression [19], ruling out more indirect effects on translation. The presence of the TrmL-catalyzed C/Um modification on the tRNALeuCAA isoacceptor and the necessity of both TrmL and MiaA for complete rpoS translation suggest that TrmL and MiaA-catalyzed tRNA modifications could work together to optimize rpoS translation. This was tested here; the trmL requirement for rpoS translation in our experimental model were much more dramatic than the miaA requirement for rpoS expression for reasons that are not yet clear. However, because the trmL defect was fully suppressed when UUX codons in rpoS were changed to CUX codons (Figure 2), indirect effects of the trmL mutant on RpoS expression can be ruled out.
The precise physiological implications of the TrmL and MiaA effects on rpoS translation are still under investigation. However, previous reports offer some clues as to how these modifications have a more global impact on the cell. In long-term survival experiments, trmL mutants were less competitive than wild type cells, suggesting a role for trmL in stationary phase recovery [21]. That report is consistent with an important role for trmL in rpoS expression, since rpoS is necessary for stationary phase stress responses. In E. coli, the miaAP3(HS) transcript is elevated under extreme heat shock, 50 °C [36]. In Salmonella typhimurium, miaA mutants lack the ability to survive at 42 °C and are sensitive to oxidative stress [39]. These reports both suggest that MiaA levels are important during heat shock. Leucine supplementation or suppression of the leu operon were able to suppress the sensitivity of miaA mutants to heat shock and oxidative stress [39]. Therefore, heat shock and/or leucine starvation may be critical conditions under which the i6A37, s2U34, and C/Um are necessary for rpoS translation. Heat shock could increase the translation requirement for leucine amino acids due to global protein denaturation. Under these limiting leucine conditions, proper incorporation of leucine tRNAs into the less commonly utilized UUX-Leu codons would be critical.
3.2. TusA Catalyzed s2U34 and rpoS Translation
The s2U requirement for rpoS expression, at the level of translation, adds to the current knowledge of the role that TusA plays on rpoS expression. Mutations in tusA were previously shown to decrease rpoS expression at the level of protein stability [32]. In this study, our rpoS translational fusions were in cells containing a deletion of the adaptor protein, RssB, which targets RpoS for degradation by the ClpXP protease; RpoS is stable in this strain background. Therefore, our observations suggest that tusA is necessary for optimal translation as well. Taken together, it would appear that TusA decreases RpoS expression at the level of stability and translation. It is also possible that defective rpoS translation in the absence of trmL leads to increased RpoS degradation. Similar to the trmL effect on the rpoS fusion, the tusA effect on the rpoS fusion was much more dramatic than the miaA effect for reasons that are not clear.
3.3. The i6A37 Requirement for Hfq and Implications for HULC Protein Predictive Model and Small RNA Biology
Hfq’s critical role in the action of bacterial small regulatory RNAs makes it a pleotropic effector of cellular physiology [34,40]. In this work, we examine the role of tRNA modifications in hfq translation at the level of the reading frame. We observed a two-fold decrease in Hfq levels in the absence of miaA (Figure 4), suggesting a role for i6A37 tRNA modification during hfq translation. Since Hfq levels are affected by the presence of i6A37, and sRNA steady state levels are affected by Hfq levels, we hypothesize that the presence of the i6A37 tRNA modification may be indirectly implicated in sRNA steady state levels.
Transcriptional regulation of hfq has been more extensively characterized than post-transcriptional regulation of hfq [41]. There are several hfq promoters within the miaA gene, each with different activity levels; these include promoters for vegetative growth and heat shock [36,41]. The transcripts from this superoperon appear to undergo post-transcriptional regulation in an RNaseE dependent manner [41]. Based on these transcriptional mapping studies, hfq should contain more than one 5′ untranslated region [36,41]. Proteins or small regulatory RNAs could target these 5′ UTRs for post-transcriptional regulation. The further examination of the hfq reading frame translation will also contribute additional understanding of hfq regulation and small RNA action. This is the first report on the role of tRNA modifications in Hfq expression.
While the identification of hfq as a HULC protein suggested that it may be sensitive to the presence of the MiaA, this has not previously been tested [19]. In addition, there have only been a few predicted HULC proteins tested for MiaA sensitivity [19]. Demonstrating the i6A37 sensitivity for an additional HULC protein, hfq, strengthens the predictive power of this model [19]. Interestingly, unlike rpoS, trmL mutants had no effect on hfq translation, suggesting that not all miaA effects are due to inability to add the TrmL-dependent modification (Figure 1 and Figure 3). Based on our results (Figure 4), small regulatory RNA levels and activity may be influenced by i6A37 tRNA modification levels. Hfq is limiting under some conditions [42]. Therefore, decreased levels of Hfq under conditions that may decrease the i6A37 tRNA modification, may limit small regulatory RNA levels and activity.
3.4. Implications for the Prokaryotic and Eukaryotic Organisms
In eukaryotic cells, tRNA modifications have been shown to play a regulatory role in cellular physiology and stress responses [25,26,43,44,45,46,47]. This includes cell cycle progression and metabolic deficiencies [25,47]. The stress responses that require tRNA modifications include global translational stress, oxidative stress, and DNA damage [25,43,44]. Since RpoS is considered a general stress adaptor for E. coli and other bacteria, the role of tRNA modifications in modulating its expression draws some parallels. Mycobacterial survival during hypoxia was recently shown to require tRNA modifications [48]. This suggests a broad role for tRNA modifications in allowing cells to respond to oxidative stress across the biological domains. Mitochondrial tRNA modifications are critical for the prevention of the rare genetic and neurodegenerative disorder Mitochondrial, Encephalopathy, Lactic acidosis, and Stroke (MELAS) syndrome [49]. Those with this disease lack the 5-taurinomethyl-2-thiouridine (τm5s2) on the wobble position of tRNALeuUUR [49]. Furthermore, miRNA-9/9* expression results in the post-transcriptional repression of several tRNA modification enzymes [49]. As a parallel, our observations with Hfq suggest bacterial small RNA involvement in tRNA modification biology. Interestingly, the tRNA modification in MELAS syndrome is associated with leucine decoding.
4. Materials and Methods
4.1. Strains and Oligonucleotide Primers
All strains were derivatives of Escherichia coli K12 MG1655. All strains used in this study are listed in Table S1. All oligonucleotide primers are used in this study are listed in Table S2.
4.2. Growth Conditions and Media
All bacteria were grown in Luria Bertani (LB) Media unless otherwise stated. In experiments requiring rapid induction of arabinose inducible fusions, LB was supplemented with 0.2% Glucose (w/v) to repress the fusion, or 0.2% Arabinose (w/v) to induce it. LB agar plates supplemented with zeomycin to a final concentration of 25 μg/mL were used to select for ΔtusA::zeo and ΔtrmL::zeo recombinants or transductants. M63 minimal media supplemented with glycerol, 5% sucrose, and 80 μg/mL of Xgal (M63-Gly-Suc-XG) agar plates were used for the positive selection of PBAD-hfq306-lacZ.
4.3. Genetic Constructions
The ΔtusA::zeo and ΔtrmL::zeo mutations were created in DJ480 mini-λ::tet via recombineering [50] and transduced into wild type or leu* versions of rpoS750-lacZ, PBAD-rpoS990-lacZ, PBAD-hfq306-lacZ translational fusions using Bacteriophage P1. Briefly, allelic exchange substrates for ΔtusA::zeo and ΔtrmL::zeo mutations were created by PCR amplification of zeomycin resistance cassette using oligonucleotide primers KT1188 and KT1189 (Table S2) as well as KT1192 and KT1193 (Table S2), containing 40 nucleotides of flanking homology to tusA and trmL, respectively. The PCR products were analyzed by agarose gel electrophoresis and purified. An overnight culture of DJ480 mini-λ::tet was resuspended in fresh LB and grown at 30 °C to an OD600 of 0.5. The culture was then shifted to 43.5 °C for 15 min. Finally, these induced cells were washed with ice-cold H2O and resuspended in ice-cold 10% glycerol. Approximately 100 ng of purified PCR products, corresponding to ΔtusA::zeo and ΔtrmL::zeo allelic exchange substrates, were electroporated into induced DJ480 mini-λ::tet cells, as well as a no DNA control, using 0.1 mm cuvettes and an electroporator at setting Ec1 (Biorad). Cells were recovered in 1 mL of LB for 30–60 min and a 100 μL aliquot of cells were spread on LB agar plates supplemented with zeomycin to a final concentration of 25 μg/mL (LB-zeo). Zeomycin resistant recombinants or transductants were purified once on LB-zeo plates and twice on LB plates.
PBAD-hfq306-lacZ translational fusions were constructed using PM1800 as previously described [19]. Briefly, allelic exchange substrates corresponding to PBAD-hfq306-lacZ were PCR amplified using synthetic gBlock DNA (IDT DNA) as a template and specific oligonucleotide primers (KT1160 and KT1161). The PCR products were analyzed by agarose gel electrophoresis and purified. An overnight culture of PM1800 was resuspended in fresh LB and grown at 30 °C to an OD600 of 0.5. The culture was then shifted to 43.5 °C for 15 min. Finally, these induced cells were washed with ice-cold H2O and resuspended in ice-cold 10% glycerol. Approximately 100 ng of purified PCR products, corresponding to PBAD-hfq306-lacZ allelic exchange substrates were electroporated into induced PM1800 cells, as well as a no DNA control, using 0.1 mm cuvettes and an electroporator at setting Ec1 (Biorad). Cells were recovered in 10 mL of LB for 18 h. Recovered cultures were serially diluted down to 10−6 and 100 μL aliquot of serial dilutions were spread on M63-Gly-Suc-XG. Colonies that were blue on XG were purified once on M63-Gly-Suc-XG plates once and twice on LB. Then, they were screened for chloramphenicol sensitivity and confirmed by PCR.
4.4. β-Galactosidase Assays
High-throughput kinetic β-galactosidase assays were carried out in 96-well plates as previously described [19]. The Filtermax F5 (Molecular Devices, Sunnyvale, CA 94089 USA) multimode microplate reader was used to read microtiter plates. β-galactosidase specific activity units are defined as the slope of OD420 reading divided by OD600 and are approximately 25-fold lower than Miller Units.
Experimental design for assays executed following arabinose induction. Briefly, samples to be assayed were grown in 5 mL of LB-Glu overnight at 37 °C in a roller drum. Overnight cultures were diluted 1:1000 in 30 mL of fresh LB-Glu in a 125-mL Erlenmeyer flask and grown at 37 °C in a shaking water bath at 200 rpm. When cultures reached an OD600 of 1.0, cells were harvested by centrifugation, and resuspended in 30 mL of fresh LB supplemented 0.2% arabinose and 100 μL aliquots of each culture were taken every 5 min for β-galactosidase assays. Samples were collected in triplicate for each individual experiment and averages were taken as a representative sample for each experiment. The data presented represent the mean and standard error of the mean of at least three independent replicates.
5. Conclusions
TrmL catalyzed tRNA methylation C/U34m and TusA catalyzed tRNA thiouridinylation are critical for rpoS expression in E. coli. Removal of UUX-codons from the rpoS open reading frame suppresses the rpoS requirement for trmL during translation. The MiaA catalyzed i6A37 tRNA modification is required for full hfq translation in E. coli.
Acknowledgments
We would like to thank Susan Gottesman for her comments on this manuscript. We would like to thank Bev Victor/Africa on Paper for assisting with the creation of the tRNA schematics in Figure 1. This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number SC2 GM105419 (K.M.T.). This research was also supported by the National Science Foundation under the LSAMP program (HRD-1000286—supporting M.O.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Institute of Health or the National Science Foundation.
Supplementary Materials
The following are available online at http://www.mdpi.com/2218-273X/7/2/39/s1, Table S1: Strain list, Table S2: Oligonucleotide list.
Author Contributions
K.M.T. conceived and designed the experiments; J.I.A. and M.O. performed the experiments; K.M.T. analyzed the data; K.M.T. wrote the paper.
Conflicts of Interest
The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Tanaka K., Takayanagi Y., Fujita N., Ishihama A., Takahashi H. Heterogeneity of the principal σ factor in Escherichia coli: The rpoS gene product, σ38, is a second principal σ factor of RNA polymerase in stationary-phase Escherichia coli. Proc. Natl. Acad. Sci. USA. 1993;90:8303. doi: 10.1073/pnas.90.17.8303a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hengge-Aronis R. Survival of hunger and stress: The role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-A. [DOI] [PubMed] [Google Scholar]
- 3.Sledjeski D.D., Gupta A., Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of rpoS during exponential growth in Escherichia coli. EMBO J. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang A., Altuvia S., Tiwari A., Argaman L., Hengge-Aronis R., Storz G. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 1998;17:6061–6068. doi: 10.1093/emboj/17.20.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majdalani N., Cunning C., Sledjeski D.D., Elliott T., Gottesman S. Dsra RNA regulates translation of rpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majdalani N., Hernandez D., Gottesman S. Regulation and mode of action of the second small RNA activator of rpoS translation, RprA. Mol. Microbiol. 2002;46:813–826. doi: 10.1046/j.1365-2958.2002.03203.x. [DOI] [PubMed] [Google Scholar]
- 7.Majdalani N., Chen S., Murrow J., St. John K., Gottesman S. Regulation of rpoS by a novel small RNA: The characterization of RprA. Mol. Microbiol. 2001;39:1382–1394. doi: 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- 8.Mandin P., Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29:3094–3107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Gottesman S. Regulation of proteolysis of the stationary-phase σ factor rpoS. J. Bacteriol. 1998;180:1154–1158. doi: 10.1128/jb.180.5.1154-1158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bougdour A., Wickner S., Gottesman S. Modulating RssB activity: IraP, a novel regulator of σs stability in Escherichia coli. Genes Dev. 2006;20:884–897. doi: 10.1101/gad.1400306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battesti A., Tsegaye Y.M., Packer D.G., Majdalani N., Gottesman S. H-NS regulation of IraD and IraM antiadaptors for control of rpos degradation. J. Bacteriol. 2012;194:2470–2478. doi: 10.1128/JB.00132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bougdour A., Cunning C., Baptiste P.J., Elliott T., Gottesman S. Multiple pathways for regulation of sigmas (rpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 2008;68:298–313. doi: 10.1111/j.1365-2958.2008.06146.x. [DOI] [PubMed] [Google Scholar]
- 13.Battesti A., Majdalani N., Gottesman S. Stress σ factor rposS degradation and translation are sensitive to the state of central metabolism. Proc. Natl. Acad. Sci. USA. 2015;112:5159–5164. doi: 10.1073/pnas.1504639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman S., Roche E., Zhou Y., Sauer R.T. The ClpXP and ClpaAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson K.M., Gottesman S. The MiaA tRNA modification enzyme is necessary for robust rpoS expression in Escherichia coli. J. Bacteriol. 2014;196:754–761. doi: 10.1128/JB.01013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caillet J., Droogmans L. Molecular cloning of the Escherichia coli miaA gene involved in the formation of delta 2-isopentyl adenosine in tRNA. J. Bacteriol. 1988;170:4147–4152. doi: 10.1128/jb.170.9.4147-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly D.M., Winkler M.E. Structure of Escherichia coli K-12 miaa and characterization of the mutator phenotype caused by miaa insertion mutations. J. Bacteriol. 1991;173:1711–1721. doi: 10.1128/jb.173.5.1711-1721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly D.M., Winkler M.E. Genetic and physiological relationships among the miaA gene, 2-methylthio-N6-(delta 2-isopentenyl)-adenosine tRNA modification, and spontaneous mutagenesis in Escherichia coli K-12. J. Bacteriol. 1989;171:3233–3246. doi: 10.1128/jb.171.6.3233-3246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubee J.I., Olu M., Thompson K.M. The i6a37 tRNA modification is essential for proper decoding of UUX-Leucine codons during rpoS and iraP translation. RNA. 2016;22:729–742. doi: 10.1261/rna.053165.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wobble. [(accessed on 24 April 2017)]; Available online: http://www.mun.ca/biology/scarr/iGen3_06-08.html.
- 21.Benitez-Paez A., Villarroya M., Douthwaite S., Gabaldon T., Armengod M.E. Yibk is the 2-o-methyltransferase trml that modifies the wobble nucleotide in Escherichia coli tRNA(leu) isoacceptors. RNA. 2010;16:2131–2143. doi: 10.1261/rna.2245910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klassen R., Bruch A., Schaffrath R. Independent suppression of ribosomal +1 frameshifts by different tRNA anticodon loop modifications. RNA Biol. 2016:1–8. doi: 10.1080/15476286.2016.1267098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tukenmez H., Xu H., Esberg A., Bystrom A.S. The role of wobble uridine modifications in +1 translational frameshifting in eukaryotes. Nucleic Acids Res. 2015;43:9489–9499. doi: 10.1093/nar/gkv832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbonavicius J., Qian Q., Durand J.M., Hagervall T.G., Bjork G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20:4863–4873. doi: 10.1093/emboj/20.17.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil A., Dyavaiah M., Joseph F., Rooney J.P., Chan C.T., Dedon P.C., Begley T.J. Increased tRNA modification and gene-specific codon usage regulate cell cycle progression during the DNA damage response. Cell Cycle. 2012;1:3656–3665. doi: 10.4161/cc.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endres L., Dedon P.C., Begley T.J. Codon-biased translation can be regulated by wobble-base tRNA modification systems during cellular stress responses. RNA Biol. 2015;12:603–614. doi: 10.1080/15476286.2015.1031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R.J., Zhou M., Fang Z.P., Wang M., Zhou X.L., Wang E.D. The tRNA recognition mechanism of the minimalist SPOUT methyltransferase, TrmL. Nucleic Acids Res. 2013;41:7828–7842. doi: 10.1093/nar/gkt568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M., Long T., Fang Z.P., Zhou X.L., Liu R.J., Wang E.D. Identification of determinants for tRNA substrate recognition by Escherichia coli C/U34 2′-O-methyltransferase. RNA Biol. 2015;12:900–911. doi: 10.1080/15476286.2015.1050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers K.C., Crescenzo A.T., Soll D. Aminoacylation of transfer RNAs with 2-thiouridine derivatives in the wobble position of the anticodon. Biochimie. 1995;77:66–74. doi: 10.1016/0300-9084(96)88106-5. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Hernandez A., Spears J.L., Gaston K.W., Limbach P.A., Gamper H., Hou Y., Kaiser R., Agris P.F., Perona J.J. Structural and mechanistic basis for enhanced translational efficiency by 2-thiouridine at the tRNA anticodon wobble position. J. Mol. Biol. 2013;425:3888–3906. doi: 10.1016/j.jmb.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Yamashino T., Isomura M., Ueguchi C., Mizuno T. The yhhp gene encoding a small ubiquitous protein is fundamental for normal cell growth of Escherichia coli. J. Bacteriol. 1998;180:2257–2261. doi: 10.1128/jb.180.8.2257-2261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsui H.C., Leung H.C., Winkler M.E. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol. Microbiol. 1994;13:35–49. doi: 10.1111/j.1365-2958.1994.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 34.De Lay N., Schu D.J., Gottesman S. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J. Biol. Chem. 2013;288:7996–8003. doi: 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajitani M., Ishihama A. Identification and sequence determination of the host factor gene for bacteriophage Q beta. Nucleic Acids Res. 1991;19:1063–1066. doi: 10.1093/nar/19.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsui H.C., Feng G., Winkler M.E. Transcription of the mutL repair, miaA tRNA modification, hfq pleiotropic regulator, and hflA region protease genes of Escherichia coli K-12 from clustered Eσ32-specific promoters during heat shock. J. Bacteriol. 1996;178:5719–5731. doi: 10.1128/jb.178.19.5719-5731.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armengod M.E., Meseguer S., Villarroya M., Prado S., Moukadiri I., Ruiz-Partida R., Garzon M.J., Navarro-Gonzalez C., Martinez-Zamora A. Modification of the wobble uridine in bacterial and mitochondrial tRNAs reading NNA/NNG triplets of 2-codon boxes. RNA Biol. 2014;11:1495–1507. doi: 10.4161/15476286.2014.992269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armengod M.E., Moukadiri I., Prado S., Ruiz-Partida R., Benitez-Paez A., Villarroya M., Lomas R., Garzon M.J., Martinez-Zamora A., Meseguer S., et al. Enzymology of tRNA modification in the bacterial mnmeg pathway. Biochimie. 2012;94:1510–1520. doi: 10.1016/j.biochi.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Blum P.H. Reduced leu operon expression in a miaA mutant of Salmonella typhimurium. J. Bacteriol. 1988;170:5125–5133. doi: 10.1128/jb.170.11.5125-5133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papenfort K., Vogel J. Multiple target regulation by small noncoding RNAs rewires gene expression at the post-transcriptional level. Res Microbiol. 2009;160:278–287. doi: 10.1016/j.resmic.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Tsui H.C., Winkler M.E. Transcriptional patterns of the mutl-miaA superoperon of Escherichia coli K-12 suggest a model for posttranscriptional regulation. Biochimie. 1994;76:1168–1177. doi: 10.1016/0300-9084(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 42.Moon K., Gottesman S. Competition among hfq-binding small RNAs in Escherichia coli. Mol. Microbiol. 2011;82:1545–1562. doi: 10.1111/j.1365-2958.2011.07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patil A., Chan C.T., Dyavaiah M., Rooney J.P., Dedon P.C., Begley T.J. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biol. 2012;9:990–1001. doi: 10.4161/rna.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan C.T., Dyavaiah M., DeMott M.S., Taghizadeh K., Dedon P.C., Begley T.J. A quantitative system approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010;6:e1001247. doi: 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Begley T.J., Dyavaiah M., Patil A., Rooney J.P., Direnzo D., Young C.M., Conklin D.S., Zitomer R.S., Begley T.J. Trm-9 catalized tRNA modifications link translation to the DNA damage response. Mol. Cell. 2007;28:860–870. doi: 10.1016/j.molcel.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamichhane T.N., Blewett N.H., Crawford A.K., Cherkasova V.A., Iben J.R., Begley T.J., Farabaugh P.J., Maraia R.J. Lack of tRNA modification isopentenyl-a37 alters mRNA decoding and causes metabolic deficiences in fission yeast. Mol. Cell. Biol. 2013;33:2918–2929. doi: 10.1128/MCB.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chionh Y.H., McBee M., Babu I.R., Hia F., Lin W., Zhao W., Cao J., Dziergowska A., Malkiewicz A., Begley T.J., et al. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat. Commun. 2016;11:13302. doi: 10.1038/ncomms13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meseguer S., Martinez-Zamora A., Garcia-Arumi E., Andreu A.L., Armengod M.E. The ROS-sensitive microRNA-9/9* controls the expression of mitochondrial tRNA modifyng enzymes and is involved in the molecular mechanism of melas syndrome. Hum. Mol. Genet. 2015;24:167–184. doi: 10.1093/hmg/ddu427. [DOI] [PubMed] [Google Scholar]
- 50.Court D.L., Swaminathan S., Yu D., Wilson H., Baker T., Bubunenko M., Sawitzke J., Sharan S.K. Mini-λ: A tractable system for chromosome and bac engineering. Gene. 2003;315:63–69. doi: 10.1016/S0378-1119(03)00728-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.